Abstract
BACKGROUND:
Guidelines regarding the use of infliximab in Crohn’s disease were previously published by the Canadian Association of Gastroenterology in 2004. However, recent clinical findings and drug developments warrant a review and update of these guidelines.
OBJECTIVE:
To review and update Canadian guidelines regarding the use of tumour necrosis factor-alpha antibody therapy in both luminal and fistulizing Crohn’s disease.
METHODS:
A consensus group of 25 voting participants developed a series of recommendation statements that addressed pertinent clinical questions and gaps in existing knowledge. An iterative voting and feedback process was used in advance of the consensus meeting in conjunction with a systematic literature review to refine the voting statements. These statements were brought to a formal consensus meeting held in Montreal, Quebec (March 2008), wherein each statement underwent discussion, reformulation, voting and subsequent revision until group consensus was obtained (at least 80% agreement).
OUTCOME:
The 47 voting statements addressed three themes: induction therapy, maintenance therapy and safety issues. As a result of the iterative process, 23 statements achieved consensus and were submitted for publication.
CONCLUSION:
In the past five years, tumour necrosis factor-alpha antagonist therapy has become a cornerstone in the management of moderate-to-severe Crohn’s disease refractory to conventional treatment algorithms. The evidentiary base supporting the use of these drugs in Crohn’s disease is substantial and strengthened by results from long-term clinical and molecular studies. However, significant gaps in knowledge exist, particularly with regard to treatment failure. Confidence in the safety of these drugs is increasing, provided that therapy is administered in a clinical setting in which potential complications can be readily recognized and treated.
Keywords: Adalimumab, Antibodies, Certolizumab, Crohn’s disease, Infliximab, Monoclonal, Practice guideline
Abstract
HISTORIQUE :
En 2004, l’Association canadienne de gastroentérologie a publié des lignes directrices au sujet de l’utilisation de l’infliximab pour le traitement de la maladie de Crohn. Cependant, de récentes observations cliniques et le développement des médicaments en justifient la révision et la mise à jour.
OBJECTIF :
Réviser et mettre à jour les lignes directrices canadiennes au sujet de l’utilisation du traitement par l’antagoniste du facteur de nécrose tumorale alpha en cas de maladie de Crohn luminale ou fistulisante.
MÉTHODOLOGIE :
Un groupe consensuel de 25 participants ayant droit de vote a élaboré une série de recommandations portant sur des questions et des lacunes cliniques pertinentes à l’égard des connaissances à jour. On a fait appel à un processus itératif de votes et de commentaires avant la réunion consensuelle, conjointement avec une analyse bibliographique systématique pour préciser les déclarations retenues par vote. Ces affirmations ont été présentées à une réunion consensuelle officielle tenue à Montréal, au Québec, en mars 2008, où chaque affirmation a fait l’objet de discussions, d’une reformulation, d’un vote et d’une révision subséquente jusqu’à l’obtention du consensus du groupe (entente d’au moins 80 %).
RÉSULTATS :
Les 47 affirmations pour lesquelles le groupe avait voté portaient sur trois thèmes : la thérapie par induction, la thérapie d’entretien et les questions d’innocuité. Par suite du processus itératif, 23 ont obtenu un consensus et ont été soumises à la publication.
CONCLUSION :
Depuis cinq ans, le traitement par l’antagoniste du facteur de nécrose tumorale alpha est une pierre angulaire de la prise en charge de la maladie de Crohn modérée à grave réfractaire à des algorithmes de traitement classiques. Les données probantes étayant l’utilisation de ces médicaments contre la maladie de Crohn sont imposantes et renforcées par les résultats d’études cliniques et moléculaires à long terme. Cependant, il existe d’importantes lacunes à l’égard des connaissances, notamment pour ce qui est de l’échec du traitement. La confiance augmente quant à l’innocuité de ces médicaments, pourvu que le traitement soit administré en milieu clinique où il est possible de dépister et de traiter immédiatement les complications potentielles.
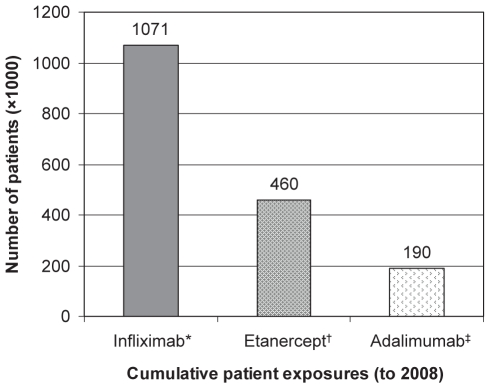
Approximately 81,000 Canadians currently suffer from Crohn’s disease. While not usually lethal, the debilitating symptoms associated with this disease frequently cause patients to experience a severely compromised quality of life (1–4). Treatment for severe Crohn’s disease has traditionally involved the use of corticosteroids and immunosuppressive drugs such as azathioprine and methotrexate. More recently, monoclonal antibody drugs directed at tumour necrosis factor (TNF)-alpha have been demonstrated to be effective for achieving and maintaining remission in Crohn’s disease. Increasing confidence in the effectiveness and safety of this drug class has resulted in their regular use in other conditions such as rheumatoid arthritis and ulcerative colitis. By 2009, more than 1.5 million patients worldwide will have been exposed to an anti-TNF agent (Figure 1).
Figure 1).
Worldwide patient exposures to tumour necrosis factor antagonists (to 2008). * Data from reference 165; †Data from reference 166; ‡Data from reference 167
Guidelines on the use of infliximab for Crohn’s disease were published by the Canadian Association of Gastroenterology (CAG) in 2004 (5,6). Since that time, a number of advances have occurred including the development of newer TNF antagonist drugs (eg, adalimumab and certolizumab pegol), as well as an increased clinical understanding of the role and safety of these products in the management of Crohn’s disease. These advances merit an evidence-based, comprehensive revision of the guidelines to more effectively inform Canadian adult and pediatric gastroenterologists, their patients, health care payers and regulatory authorities. The current guidelines address relevant clinical questions regarding the appropriate use of TNF antagonist therapies for achieving and maintaining remission of Crohn’s disease. As well, significant gaps in knowledge are identified and addressed via the discussion and consensus of expert opinion.
METHODS
During the planning and implementation of the present initiative, the organizers endeavoured to be compliant with the CAG policies surrounding the development of clinical practice guidelines and consensus statements (7).
Determination of the need for a guideline update
The need for an updated clinical guideline for the management of inflammatory bowel disease was determined by a needs assessment forum conducted annually by the CAG. This process is part of the requirements for the CAG to be an accredited educational provider on behalf of the Royal College of Physicians and Surgeons of Canada. A review of the most recent CAG guidelines (5), in juxtaposition with the results of a literature review, was performed for the years 2004 through 2007. As a result of these investigations, it was determined that the most practicable option was to update the 2004 guidelines regarding TNF antagonist therapy for the treatment of Crohn’s disease in both adult and pediatric patients.
Membership of the Consensus Group
The co-chairs of the Consensus Conference (DS and RF) selected a steering committee (AB, CB, KC and AG) in consultation with the Executive Committee of the CAG. In turn, the steering committee invited voting members who were experts in either adult or pediatric inflammatory bowel disease, as well as representatives from family medicine and nursing (Appendix). Two international members also served as expert resources (CE and ST). Nonparticipatory observers included interested physicians and representatives from the pharmaceutical industry (n=28). Provincial drug formulary representatives were also invited to attend.
Development of voting statements
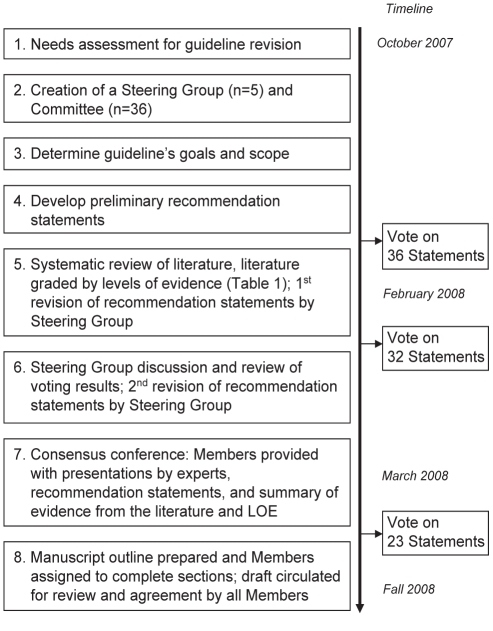
The consensus process was modelled on previous CAG initiatives in which relevant clinical questions and gaps in knowledge were identified in an a priori fashion (Figure 2) (8,9). The steering committee identified a series of clinically relevant issues derived from the needs assessment. From this, 47 initial voting statements were formulated. Iterative changes to each statement were made based on feedback from voting members on two separate occasions and the ongoing systematic literature review.
Figure 2).
Guideline development process. LOE Levels of evidence
The iterative voting process
At four months before and again at one month before the consensus meeting, members voted on each statement via electronic mail (Table 1) and were encouraged to comment on wording and validity. Members were also given access to the systematic literature review pertinent to each statement and the resulting Grading of Recommendations, Assessment, Development and Evaluation level of evidence (see below). Results were compiled by the CAG to preserve voter anonymity. Following each round of voting, the steering committee revised each statement to best reflect the input received from the voters.
TABLE 1.
Schemata for voting and grading the evidence in the literature
|
Voting options for the Consensus Committee | |
| A | Agree strongly |
| B | Agree with minor reservation |
| C | Agree with major reservation |
| D | Disagree with minor reservation |
| E | Disagree with major reservation |
| F | Disagree strongly |
|
Levels of evidence using the GRADE approach | |
| High | Additional research is unlikely to change the Committee’s confidence in the estimate of the effect |
| Moderate | Additional research is likely to add important information thereby impacting the Committee’s confidence in the estimate of the effect. In turn, this may lead to a change in the estimate of the effect |
| Low | Additional research is likely to impact both the Committee’s confidence in the estimate of the effect and change their estimate of the effect |
| Very Low | Any estimate of the effect is uncertain |
GRADE Grading of Recommendations, Assessment, Development and Evaluation
Systematic literature review
Identification of the relevant literature was performed systematically using the following search terms to retrieve all papers regarding Crohn’s disease: “Crohn’s disease”, “ulcerative colitis”, “intestinal inflammation” and “colitis”. Results were then searched for the terms “adalimumab”, “certolizumab”, “infliximab”, “etanercept” or “visilizumab” to retrieve the subset of papers concerning TNF-antagonist drugs. Where possible, the database’s controlled vocabulary system was used for each search term. Searches were conducted in EMBASE, MEDLINE, CINAHL and PubMed. The limiting parameters were the time of print or electronic publication (January 1, 2004 to May 1, 2008) and that the articles were written in English. Retrieved articles were subsequently manually selected to identify original, well conducted, peer-reviewed research trials or meta-analyses that focused specifically on Crohn’s disease and either infliximab, adalimumab or certolizumab. Approximately 480 papers were selected and then categorized according to their level of evidence using the the GRADE approach (Table 1) (10).
Organization of the Conference
A two-day Consensus Conference was held in March 2008 under the auspices of the CAG. Each of the three conference sessions, Induction Therapy, Maintenance Therapy and Safety Issues began with a summary of the literature and important issues presented by an invited expert. Subsequently, each statement and the level of evidence of the supporting literature were discussed by the committee under the direction of the moderator; delegates were asked to vote for each statement via anonymous electronic keypads according to the following possible choices:
A = Agree strongly
B = Agree with minor reservation
C = Agree with major reservation
D = Disagree with minor reservation
E = Disagree with major reservation
F = Disagree strongly
Consensus was deemed to have been achieved when at least 80% of the delegates voted either ‘agree strongly’ or ‘agree with minor reservation’ to a particular statement. Several rounds of voting with subsequent statement modifications were often required before consensus could be achieved.
Financial support and disclosures
The entire consensus process was administrated by the CAG. External funding was secured after approaching a large number of potential sponsors from the Canadian Institutes of Health Research Institute of Nutrition, Metabolism and Diabetes, and from multiple industry sponsors. The funds were administered through an unrestricted educational grant. Conflict of interest statements were obtained from all voting members and the moderator before the meeting (see Disclosure of Potential Conflict of Interest below). Honoraria for participation were not provided.
Future directions
Revision of the present guideline will be conducted as new information becomes available through clinical trials, and as Canadian clinical experience with these drugs increases. The Board of the CAG had already determined that a Consensus Conference on the clinical management of ulcerative colitis will be conducted in 2010.
Preparation of the manuscript
A draft manuscript was prepared according to the Appraisal of Guidelines Research and Evaluation (AGREE) principles (11) and circulated to members for review and completion. The final draft was circulated for approval by all voting participants and the nonvoting chair. Before submission for peer review and publication, the manuscript was posted on a members-only section of the CAG Web site (www.cag-acg.org) for review and comments by all members of the CAG.
STATEMENTS
Each statement is reflective of the consensus developed during the conference. The statement numbers represent the numbering scheme devised after the consensus meeting. Some of the terminology used in the statements is explained below for clarification:
‘Clinical response’ is commonly defined in clinical trials as a reduction of 70 or more points in the Crohn’s Disease Activity Index (CDAI).
‘Clinical remission’ is commonly defined as a CDAI of less than 150 points.
○ For pediatrics, this is defined as a PCDAI of less than or equal to 10 points.
‘Disease relapse’ or ‘loss of response’ is usually defined as an increase in the CDAI of at least 70 points.
○ For pediatrics, ‘disease relapse’ is defined as an increase of 15 or more points in the PCDAI.
‘Moderate-to-severe’ disease is usually defined by CDAI scores of between 220 and 400 points. For pediatrics, this is defined as a PCDAI score greater than 30 points.
○ ‘Conventional therapies’ commonly refers to immunosuppressives, such as purine antimetabolites (eg, 6-mercaptopurine or azathioprine) or methotrexate and/or corticosteroids.
A ‘corticosteroid-dependent’ patient is defined as one who will experience relapse or flare if their steroid dose is tapered.
Caveat: Before commencing any TNF antagonist therapy, the clinician is responsible for ensuring that the necessary clinical and laboratory resources (eg, for tuberculosis screening) are available to effectively manage any possible serious adverse event associated with the use of these drugs.
At the time of publication, certolizumab has not received regulatory approval in Canada. In April 2008, it was approved for therapeutic use in Crohn’s disease, in the United States.
SECTION 1: INDUCTION THERAPY
STATEMENT 1
Biologic therapy with infliximab, adalimumab or certolizumab is clinically effective for the induction of remission in patients who demonstrate continuing Crohn’s disease symptoms despite conventional therapy (immunosuppressives [purine antimetabolites/methotrexate] and/or corticosteroids). GRADE: High; Vote: A 96%, B 4%.
Discussion of statement 1
In 1997, a multicentre, double-blind (DB) randomized controlled trial (RCT) compared the efficacy of a single infusion of infliximab with placebo in 108 adult Crohn’s disease patients with moderate-to-severe disease activity (12). Four weeks after the infusion, clinical response was observed in 81% of those given 5 mg/kg of infliximab (22 of 27 patients), 50% of those given 10 mg/kg (14 of 28 patients) and 64% of those given 20 mg/kg (18 of 28 patients), compared with 17% of those given placebo (four of 24 patients). Similar results were later obtained in the open-label induction portion of the A Crohn’s Disease Clinical Trial Evaluating Infliximab in a New Long-Term Treatment Regimen (ACCENT I) trial (13) in which 335 of 573 patients (58%) achieved a clinical response two weeks after receiving a single infliximab infusion. In a European trial (14), significant healing of endoscopic lesions was observed in patients on infliximab while there was no change reported for those in the placebo group (n=8).
The Clinical Assessment of Adalimumab Safety and Efficacy Studies as Induction Therapy in Crohn’s Disease (CLASSIC-I) DB RCT (15) investigated the efficacy of adalimumab subcutaneous injections in 299 patients with moderate-to-severe Crohn’s disease. After two injections at weeks 0 and 2, clinical remission rates at week 4 were significantly higher in two adalimumab treatment arms (80 mg/40 mg and 160 mg/80 mg) compared with placebo (n=74) (24% and 36%, respectively versus 12%; P=0.004). The open-label induction portion of the Candesartan in Heart Failure – Assessment of Mortality and Morbidity (CHARM) trial (16) resulted in 499 of 854 patients (58%) experiencing a clinical response at week 4.
Certolizumab was also shown to improve clinical response rates in patients with moderate-to-severe Crohn’s disease (17). The Pegylated Antibody Fragment Evaluation in Crohn’s Disease: Safety and Efficacy (PRECISE 1) trial was a DB RCT in which patients received three subcutaneous injections of certolizumab (400 mg) or placebo at weeks 0, 2 and 4. Patients in the active treatment arm had a response rate of 35% (115 of 327) compared with 27% (87 of 325) in the placebo group at week 6 (P=0.02). Additional supporting evidence for certolizumab comes from the open-label induction portion of PRECISE 2 in which 428 of 668 patients (64%) experienced a clinical response at week 6 (18).
For patients failing corticosteroid treatment, an open-label trial (19) examined the efficacy of infliximab induction therapy. Twelve weeks after initiating infliximab induction therapy, 322 of 382 patients (84%) experienced a clinical response and 228 (60%) also achieved clinical remission (12).
Commentary on statement 1
To date, Crohn’s disease induction trials have characteristically enrolled patients with moderate-to-severe disease, most of whom have already failed treatment with aminosalicylates, corticosteroids and immunosuppressive drugs. This step-wise progression through conventional therapies before administering anti-TNF drugs, is also a commonly practiced clinical strategy. However, conference delegates stated that a lengthy trial-and-error treatment algorithm was inappropriate, particularly in situations in which rapid onset of remission was required, such as in a severe acute flare (see Statement 4).
Despite the absence of rigorous study, both the 2004 CAG (5) and the Dutch (20) guidelines stated that infliximab could be used when immunosuppressors were contraindicated. As evidence, a limited case series indicated that infliximab induction and maintenance therapy was effective in excess of two years for patients who were intolerant to immunosuppressive therapy (21). The conference delegates reconfirmed their support for this treatment as an alternate approach.
To date, there have been few prospective RCTs conducted in children. However, infliximab induction data are available from the recently completed A Randomized, Multicenter, Open-Label Study to Evaluate the Safety and Efficacy of Anti-TNF alpha Chimeric Monoclonal Antibody in Pediatric Subjects with Moderate to Severe Crohn’s Disease (REACH) multicentre pediatric clinical trial. As reported by Hyams et al (22), 112 children and adolescents six to 17 years of age with moderately-to-severely active Crohn’s disease were treated with a standard three-dose (5 mg/kg) infliximab regimen. To be eligible for inclusion in the trial, all patients were required to have active disease, despite immunomodulatory therapy. When assessed at week 10, 59% of patients (95% CI 50% to 68%) had achieved clinical remission (a score of 10 or less as defined by the PCDAI). In 88% of patients (95% CI 82% to 94%), the PCDAI had declined by 15 points or more to a value of 30 or less, the a priori definition of clinical response. These data confirm the efficacy of infliximab for inducing clinical remission in pediatric Crohn’s disease. Multicentre clinical trials using adalimumab and certolizumab for the treatment of active Crohn’s disease in children have not yet been performed.
STATEMENT 2: DOSING REGIMENS (BOX 1)
Box 1: Induction Regimens.
For luminal Crohn’s disease:
Infliximab (5 mg/kg, intravenously)
At weeks 0, 2 and 6
Adalimumab (subcutaneously)
At week 0 (160 mg)
At week 2 (80 mg)
Certolizumab* (400 mg, subcutaneously)
At weeks 0, 2 and 4
*From reference 165
For luminal Crohn’s disease, the dosing regimen for induction therapy with infliximab is 5 mg/kg (intravenously) at weeks 0, 2 and 6. Single-dose induction therapy is not recommended. GRADE: High; Vote: A 84%, B 12%, C 4%.
For luminal Crohn’s disease, the dosing regimen for induction therapy with adalimumab is 160 mg (subcutaneously) at week 0 and 80 mg (subcutaneously) at week 2. GRADE Moderate; Vote: A 84%, B 16%.
For luminal Crohn’s disease, the dosing regimen for induction therapy with certolizumab is 400 mg (subcutaneously) at weeks 0, 2 and 4. GRADE: High; Vote: A 84%, B 16%.
Discussion of statement 2a
Infliximab:
For infliximab, a dose-response relationship was not found between infliximab induction doses (5 mg/kg, 10 mg/kg or 20 mg/kg) and clinical response (12). An earlier open-label study (12,23) reported transient induction results with 1 mg/kg compared with those achieved with 5 mg/kg, 10 mg/kg or 20 mg/kg, thereby indicating that a 5 mg/kg dose of infliximab is adequate.
Since the publication of the 2004 CAG guidelines (5), the optimum number of induction doses of infliximab (5 mg/kg) have increased from two to three for immunogenicity reasons (24). A subgroup analysis (24) of patients in the ACCENT I trial compared the efficacy of a three-dose induction regimen of infliximab at weeks 0, 2 and 6 versus a single-dose induction regimen at week 0. By week 10, the three-dose induction regimen resulted in 69% of the patients (266 of 385) having a clinical response, of which 42% also achieved remission. Conversely, of the 188 patients receiving a single induction dose, only 59% had a response and 32% attained remission (P=0.014 and P=0.019, respectively). Pearce and Lawrence (25) provided supporting evidence in their observational study. At eight weeks, the three-dose induction regimen resulted in 93% of patients (13 of 14) achieving remission versus 75% (24 of 32) in those who received a single dose of infliximab (5 mg/kg).
Commentary on statement 2a
Conference delegates noted that optimal intervals for administration of the three induction doses have not been investigated. Additional studies are required to assess the efficacy of the commonly used zero-, two-, and six-week dosing intervals. The delegates also identified that it is currently unknown if patients who do not respond to the first two induction doses of infliximab should receive a third dose because the likelihood of response is probably less than 3%.
Discussion of statement 2b
Adalimumab:
For adalimumab, the dose-finding CLASSIC I trial (15) found that the clinical remission rate at week 4 for the adalimumab 160 mg/80 mg cohort (n=74) was significantly higher than the placebo cohort (36% versus 12%, respectively; P=0.001). No significant differences were found between the lower-dose adalimumab cohorts (40 mg/20 mg [n=74]; 80 mg/40 mg [n=75]) and the placebo cohort (n=74).
Commentary on statement 2b
Subsequent trials, such as CHARM (16), have not investigated the efficacy of higher doses of adalimumab for inducing remission. The delegates also drew attention to the subgroup of ‘induction-delayed’ responders in the CLASSIC II trial (26). As part of the open-label portion of the trial, patients who did not have a response at weeks 0 and 4 continued either their weekly or every-other-week injections of adalimumab (40 mg); 42% and 49%, respectively, went on to successfully achieve remission by week 56. This study highlights the variability in response time to adalimumab induction therapy. For adalimumab, pediatric dosing is not well established. An ongoing multicentre pediatric trial uses a loading dose of 160 mg/80 mg for patients weighing 40 kg or more, and 80 mg/40 mg for those weighing less than 40 kg.
Discussion of statement 2c
Certolizumab:
In a dose-finding, phase II study, three treatment groups received subcutaneous certolizumab injections of 100 mg, 200 mg or 400 mg at weeks 0, 4 and 8, and were compared with a placebo control group (27). The clinical response rates were highest for certolizumab 400 mg, particularly at week 10 (certolizumab 400 mg 52.8%; placebo 30.1%: P=0.006). Subsequently, in the PRECISE 1 (17) study, the certolizumab (400 mg) induction treatment arm had significantly superior response rates compared with the placebo (35% and 27%, repectively; P=0.02).
Commentary on statement 2c
To the present time, the experience of conference delegates with certolizumab has been limited mainly to clinical trials. Pediatric dosing with this drug is not well established.
STATEMENT 3
For patients who do not respond to one of the suggested multiple-dose induction regimens for luminal Crohn’s disease, additional doses of the same agent are not recommended. Switching to another TNF antagonist may be considered on a case-by-case basis. GRADE: Low; Vote: A 60%, B 36%, C 4%.
For patients who have a partial response to one of the suggested multiple-dose induction regimens for luminal Crohn’s disease, alternative strategies (which may include dose escalation or switching to another TNF antagonist) may be considered on a case-by-case basis. GRADE: Low; Vote: A 64%, B 32%, C 4%.
Discussion of statement 3a
In a recent study (28), 25 patients with fistulizing Crohn’s disease and primary nonresponse to the three-dose induction regimen for infliximab, were given a second round of induction therapy with the same drug. After repeating the induction, 28% achieved a complete response. In a second, smaller case series (29), benefit was seen in three of six patients who were switched from infliximab to adalimumab because of primary nonresponse to infliximab. Although the patient numbers are low, there is evidence that in instances of initial primary non-response, it may be beneficial to initiate the use of an alternative TNF antagonist.
Discussion of statement 3b
In the setting of primary nonresponse to infliximab, dose escalation to 10 mg/kg has not been studied in clinical trials. To date, studies have been restricted to either primary or partial responders to infliximab induction, and who subsequently lost the response during maintenance therapy or involved patients who became intolerant to infliximab due to acute or delayed reactions.
Commentary on statements 3a and 3b
Conference delegates believed that the lack of response to standard induction therapy should initiate the consideration of other potential conditions that could mimic acute Crohn’s exacerbation (eg, small bowel obstruction due to fibrotic strictures and intra-abdominal abscess). In the clinical experience of the conference attendees, further therapy with the same agent in the setting of primary nonresponse is associated with a low probability of success. The ideal time to initiate a switch to a different TNF antagonist is unclear. To date, no studies have investigated switching from infliximab or adalimumab to certolizumab or vice versa. Similarly, the benefits of switching from adalimumab to infliximab are also unknown. The delegates further noted that the efficacy of dose escalation for both adalimumab and certolizumab is unknown.
STATEMENT 4
A TNF antagonist may be used in hospitalized patients with luminal or fistulizing Crohn’s disease for situations in which a rapid onset of action is desired. GRADE: Moderate; Vote: A 68%, B 28%, C 4%.
Discussion of statement 4
For patients with severe Crohn’s symptoms requiring hospitalization, intravenous steroids are commonly prescribed, and a therapeutic effect is expected within 10 days (30). In an open-label study (31), infliximab use was associated with a reduction in mean hospital stay by three days compared with patients treated with intravenous hydrocortisone. The use of TNF antagonist drugs in hospitalized patients should only be considered after sepsis has been excluded. A retrospective study (32) of 226 consecutive patients identified that preoperative infliximab therapy did not affect overall postsurgical healing rates for perianal fistulas.
STATEMENT 5
Infliximab and adalimumab are effective for patients with active fistulizing Crohn’s disease. GRADE: High; Vote: A 56%, B 32%, C 12%.
Discussion of statement 5
Infliximab is the only agent to date that has been demonstrated to completely close Crohn’s disease fistulas in a dedicated RCT (33). In the CHARM trial (16), a subgroup of 70 patients with Crohn’s disease fistulas had a significantly higher healing rate in the adalimumab treatment arms versus placebo at weeks 26 and 56 (P=0.043 and P=0.016, respectively). Fistula closure with a TNF antagonist appears to be most effective for perianal fistulas. Abdominal, rectovaginal and enterovesical fisutulas are associated with lower rates of closure (16,34).
Commentary on statement 5
The majority of the consensus members believed that fistula closure was an effect that could most likely be observed with all drugs in the TNF antagonist class, although there are no studies of fistula closure with certolizumab to date. Management of all Crohn’s fistulas should be multimodal – with judicious use of antibiotics, surgical consultation and drainage of abscesses. Importantly, rapid closure of fistulas with infliximab was not associated with an increase in abscess development (35).
STATEMENT 6 (BOX 2)
Box 2: Induction Regimens.
For fistulizing Crohn’s disease:
Infliximab (5 mg/kg, intravenously)
Weeks 0, 2 and 6
Adalimumab (subcutaneously)
Week 0 (160 mg)
Week 2 (80 mg)
For fistulizing Crohn’s disease, the dosing regimen for induction therapy with infliximab is 5 mg/kg intravenously at weeks 0, 2 and 6. Single-dose induction therapy is not recommended.
For fistulizing Crohn’s disease, the dosing regimen for induction therapy with adalimumab is 160 mg at week 0 (subcutaneously) and 80 mg at week 2. GRADE: High; Vote: A 68%, B 32%.
Discussion of statement 6
An early dose-finding study (33) found no dose-response association with the therapeutic benefit of either 5 mg/kg or 10 mg/kg infliximab in the three-dose induction regimen for patients with fistulizing Crohn’s disease. However, a significantly greater number of patients treated with infliximab experienced a 50% or more reduction in the number of draining fistulas that persisted for at least four weeks. The median length of time to response was two weeks.
In the ACCENT II trial, 71.1% of the perianal fistulas responded to infliximab induction therapy at week 14, during which time the fistulas ceased draining. Also, 56.4% and 64.0% of the abdominal and rectovaginal fistulas, respectively, responded to infliximab induction therapy at week 14 (36). A substudy (34) of ACCENT II examined the long-term effect of infliximab therapy in rectovaginal fistulas. Interestingly, the response was not durable, evidenced by the 15.9% decrease in the number of closed, nondraining fistulas between weeks 10 and 14.
In the CLASSIC I trial (15), 32 patients had either draining enterocutaneous or perianal fistulas. Because these individuals were not evenly distributed across the treatment groups, the only conclusion to be drawn was that the rates of fistula improvement and remission were not significantly different between patients receiving adalimumab and those receiving placebo. The appropriate dosing and interval of adalimumab for fistulas was not identified.
Commentary on statement 6
In the setting of Crohn’s disease with fistula, conference delegates believed that induction therapy with a TNF antagonist must be followed by appropriate maintenance therapy. The delegates acknowledged that a patient with fistulizing Crohn’s disease represents a complex treatment scenario, and it is recommended that treatment algorithms be developed alongside multidisciplinary consultations that should include a surgeon.
STATEMENT 7
Patients who do not respond to one of the suggested multiple-dose induction regimens for fistulizing Crohn’s disease present a special problem and must be managed on a case-by-case basis. GRADE: Low; Vote: A 36%, B 52%, C 12%.
Discussion of statement 7
Crohn’s disease patients who have fistulas refractory to initial induction therapy with a TNF antagonist represent a special management problem. Treatment options with TNF-antagonist therapy include repetition of the induction cycle and dose escalation, specifically with infliximab (28,36). No clinical trials have investigated the therapeutic benefit of switching to a second TNF antagonist in the setting of fistulas. TNF antagonist therapy in combination with surgical intervention is safe and does not increase the risk of infection (37). Surgical intervention, such as seton drainage or fistulotomy, is often necessary.
SECTION 2: MAINTENANCE THERAPY
STATEMENT 8 (BOX 3)
Box 3: Maintenance Regimens.
For patients who responded to induction regimens:
Infliximab (5 mg/kg, intravenously)
Every 8 weeks
Adalimumab (40 mg, subcutaneously)
Every 2 weeks
Certolizumab* (400 mg, subcutaneously)
Every 4 weeks
*From reference 165
In patients who have responded to an induction regimen, maintenance therapy with infliximab (5 mg/kg every eight weeks), adalimumab (40 mg subcutaneously every two weeks) or certolizumab (400 mg subcutaneously every four weeks) has been shown to maintain remission (Box 3). GRADE: High; Vote: A 72%, B 28%.
Selected patients can be successfully maintained with an immunosuppressive drug alone following induction therapy with a TNF-antagonist. GRADE: Medium; Vote: A 40%, B 44%, C 12%, D 4%.
Discussion of statement 8a
Infliximab:
In the ACCENT I trial (13), all patients received an induction dose of 5 mg/kg of infliximab at week 0. Patients were randomly assigned at week 2 (based on their initial response) to one of three groups that received subsequent induction doses at weeks 2 and 6, and every eight weeks thereafter with either placebo, 5 mg/kg infliximab or 5 mg/kg infliximab for induction, followed by 10 mg/kg maintenance doses. At week 30, patients in the infliximab treatment arms had a higher rate of sustaining remission (41.8%) than those in the placebo control group (21%). Long-term analyses showed that the median time to loss of response to the single induction dose in the placebo group was 19 weeks. Conversely, patients receiving 5 mg/kg or 10 mg/kg infliximab maintenance therapy maintained their response for a median time of 38 weeks (P=0.002) or in excess of 54 weeks (P=0.0002), respectively. In the pediatric REACH (38) study, all responders (n=104) to the open three-dose induction regimen were randomly assigned to receive infliximab maintenance therapy (5 mg/kg) at either eight- or 12-week intervals. At week 54, 33 of 52 (63.5%) and 29 of 52 (55.8%) patients receiving infliximab every eight weeks had a clinical response and were in clinical remission, respectively, compared with 17 of 51 (33.3%) and 12 of 51 (23.5%) patients receiving treatment every 12 weeks (P=0.002 and P<0.001, respectively).
Adalimumab:
In the CHARM study (16), 778 patients were randomly assigned at week 4 based on their response to an open-label, two-injection induction regimen with adalimumab (80 mg/40 mg). At week 26, there were significantly more patients in the two adalimumab treatment arms, 40 mg every week or every other week, in remission (40% and 47%, respectively) than those in the placebo group (17%; P<0.001). By week 56, 36% and 41% of the every week and every other week treatment arms, repectively, were still in remission compared with 12% receiving placebo (P<0.001). No significant differences in clinical remission rates were identified between the two treatment arms.
The smaller CLASSIC II trial (n=299) investigated CLASSIC I patients who were in remission at weeks 4 and 6 following adalimumab induction therapy, plus one injection of 40 mg adalimumab maintenance therapy. These patients were then randomly assigned to receive 40 mg adalimumab every week or every other week, or placebo. Of the 55 randomly assigned patients, significantly more individuals in the adalimumab groups (79% and 83%, repectively) were in remission by week 56 than those in the placebo group (44%; P<0.05).
Certolizumab:
The efficacy of certolizumab maintenance therapy was investigated in patients who received three induction doses at weeks 0, 2 and 4, after which they were randomly assigned according to their baseline C-reactive protein level at week 6 (18). Patients receiving injections of 400 mg of certolizumab pegol every four weeks had a remission rate of 48% at week 26 compared with 29% in the placebo group (P<0.001).
Discussion of statement 8b
Both azathioprine and 6-mercaptopurine have been shown to induce remission and to act as steroid-sparing agents in Crohn’s disease (39,40). However, the slow onset of action of these drugs limits their effectiveness for patients with acute symptoms in whom a rapid therapeutic response is required. A common clinical strategy is to use corticosteroids as a bridge to maintenance with an immunosuppressive. For patients intolerant or resistant to corticosteroids, induction with a TNF antagonist can also function as a therapeutic bridge in patients who are immunosuppressant naive (41). Small open-label trials (42,43) have also demonstrated that infliximab induction therapy can be used as a bridge to maintenance therapy with methotrexate for both luminal and fistulizing disease.
Commentary on statement 8b
Delegates who disagreed with voting statement 8b expressed concern that this strategy would result in intermittent exposure to anti-TNF drugs thereby stimulating antibody formation and sensitizing the patient to future use of TNF antagonist agents.
STATEMENT 9
During maintenance therapy with infliximab, a diminished or suboptimal response can be managed by: I. shortening the interval between infliximab dosing; or II. increasing the dose to 10 mg/kg. GRADE: Moderate; Vote: A 80%, B 20%.
During maintenance therapy with adalimumab, a diminished or suboptimal response can be managed by weekly dosing. GRADE: High; Vote: A 64%, B 36%.
During maintenance therapy with certolizumab, a diminished or suboptimal response can be managed by a supplemental dose. GRADE: Very low; Vote: A 16%, B 60%, C 24%.
Discussion of statement 9a
The clinical efficacy of infliximab in both rheumatoid arthritis and Crohn’s disease has been associated with trough concentrations above 1 μg/mL. Crohn’s disease and rheumatoid arthritis patients who require dose escalations appear to clear infliximab more rapidly than other patients (44,45). Regular maintenance infusions with infliximab appear to maintain therapeutic blood levels resulting in improved clinical outcomes including mucosal healing and fewer hospitalizations compared with episodic ‘on-demand’ therapy (46). In a single-centre retrospective review (47), 64% of patients undergoing maintenance infliximab therapy required dose intensification, either by a dose increase or an interval decrease. Of those patients, 76% were able to regain response and remain on infliximab maintenance. A small observational study (48) also suggested that infliximab dose escalation to 10 mg/kg resulted in an improvement in Crohn’s disease activity as assessed by the Harvey-Bradshaw index.
Discussion of statement 9b
Although the CLASSIC II and CHARM trials (16,26) found that adalimumab maintenance dosing every other week was effective therapy compared with placebo, both had treatment arms that received adalimumab (40 mg) every week. Although no significant difference in remission rates between these two treatment arms were identified, the weekly injections were well-tolerated and may be a treatment option for patients who are failing the every other week maintenance regimen. Two studies indicated that the successful dose escalation from every other week to every week occurs in approximately one-half of the patients who responded to open-label induction therapy but then lose response to every other week dosing regimen (26,49).
Discussion of statement 9c
In the PRECISE trial (50), patients who experienced loss of response to maintenance therapy underwent re-induction with a single supplemental dose of certolizumab (400 mg). This resulted in 44% of patients achieving remission.
Commentary on statement 9
Up to 30% of primary responders to TNF antagonist therapy lose response over the following six to 12 months (13,16,36).
Possible causes of secondary failure include a change in the natural history of disease, antibody formation and loss of response to the TNF antagonist mechanism of action. The clinician should also be alert to noninflammatory causes of refractory symptoms such as the presence of fibrostenotic disease, small bowel bacterial overgrowth, intra-abdominal abscess, intestinal infections (particularly those due to Clostridium difficile) and irritable bowel syndrome.
Delegates expressed concern that there is currently no clinical trial evidence examining dose intensification with adalimumab for patients losing response to once every other week maintenance dosing. Clinical experience among the delegates with supplemental dosing of certolizumab was limited.
STATEMENT 10
For Crohn’s disease patients with a diminished or suboptimal response or for those who are intolerant to a particular TNF antagonist, maintenance therapy may be continued by switching to a different TNF antagonist. GRADE: Low; Vote: A 28%, B 64%, C 4%, D 4%.
Discussion of statement 10
Previous exposure to a TNF antagonist agent is associated with a reduced response to a new TNF antagonist drug compared with those who are TNF antagonist naive (16). However, in a group of patients specifically selected for loss of response to infliximab maintenance therapy, switching to adalimuab resulted in a 14% increase in the remission rate at week 4 compared with placebo (21% versus 7%; P<0.001) (51). This trend was also observed in two earlier small pilot studies (52,53) as well as an open-label trial (54).
To date, no study has directly compared the strategy of dose escalation with a within-class change of drug. A cost analysis comparing infliximab dose escalation with switching to adalimumab suggested that dose escalation will yield more quality-adjusted life-years compared with switching to adalimumab; however, the cost was considerable (55).
Commentary on statement 10
The consensus panel believed that while there were no RCTs to address this issue, the evidence from the rheumatological literature suggested that resumption of clinical efficacy after switching to a new agent is an effect inherent to the TNF antagonist drug class (56–58). Delegates disagreeing with the statement believed that the clinical effect associated with within-class switching was modest and not demonstrably better than dose escalation. In switching to a different anti-TNF agent, it is important to be cognizant of each drug’s unique safety and efficacy profile (59).
STATEMENT 11
Sensitization to TNF antagonists can occur and is characterized by antidrug antibody formation, hypersensitivity reactions and/or a loss of clinical response. The incidence of these events may be reduced by:
regular maintenance therapy;
the use of concomitant immunosuppressive therapy with azathioprine, 6-mercaptopurine or methotrexate; or
in the case of infliximab, pretreatment with corticosteroids. GRADE: High; Vote: A 52%, B 44%, C 4%.
Discussion of statement 11
All biologic agents are foreign proteins and have the potential to induce an immunogenic response. While more data exist for infliximab, antibody formation is likely a class effect common to all TNF antagonist drugs (60). Patients who develop antibodies to biologic agents theoretically have a higher likelihood of infusion reactions, shorter duration of response to infusions and the potential for eventual loss of response to that agent (61,62).
Regular maintenance infusions of infliximab instead of episodic (ie, ‘on-demand’) infusions have been rigorously demonstrated to reduce the formation of antibodies to it (13,24). Also, pretreatment with intravenous hydrocortisone has been shown to lower antibody levels compared with placebo (63). In patients receiving intermittent infliximab therapy, concomitant therapy with either azathioprine or methotrexate was associated with reduced antibody formation, higher serum infliximab levels and fewer infusion reactions (44,64). While antibody formation can be inhibited with the use of azathioprine or methotrexate, there is no evidence that this strategy will either maintain or improve the effectiveness of the TNF antagonist during regularly scheduled, maintenance dosing (see Statement 12). A recent two-year trial in stable patients receiving scheduled maintenance infliximab demonstrated that continuation of immunosuppressive therapy beyond six months offered no clear benefit over scheduled infliximab monotherapy. However, infliximab monotherapy was associated with lower median infliximab trough levels and increased C-reactive protein levels (65).
Adalimumab should theoretically exhibit less immunogenicity than infliximab because of its ‘humanized’ structure. However, antibodies to adalimumab have been documented in Crohn’s disease and are associated with decreased clinical response (26,52,66). Antibody formation was also observed with certolizumab during both induction (9%) and maintenance therapy (8%) (17,18).
Commentary on statement 11
Conference delegates expressed concern that some patients undergoing scheduled maintenance therapy with infliximab experienced interruption in their treatments because of insurance coverage issues (eg, when changing insurance providers or due to policies of some third-party payers that do not fund full maintenance therapy).
Corticosteroid pretreatment can be accomplished with prednisone (50 mg orally 24 h before infusion), or either hydro-cortisone (100 mg intravenously) or methylprednisone (20 mg to 40 mg intravenously) given 20 min before infusion (67).
STATEMENT 12
Patients with Crohn’s disease who are receiving regular maintenance TNF antagonist therapy may derive clinical benefit with the use of concomitant immunosuppressive therapy (eg, azathioprine, 6-mercaptopurine or methotrexate). The magnitude of this benefit is unknown at the present time and must be balanced against the risk of additional immunosuppression. GRADE: Low; Vote: A 72%, B 28%.
Discussion of statement 12
Gastroenterologists have broad clinical experience with the use of immunosuppressant drugs (azathioprine, methotrexate, 6-mercaptopurine) because they are effective steroid-sparing agents and can maintain remission in a subset of patients with Crohn’s disease (68). As a result, a common clinical strategy is to combine an immunosuppressive agent and an anti-TNF drug for Crohn’s disease maintenance therapy. However, mounting evidence suggests that combining immunosuppressants and biologics for maintenance carries added risk and may not be necessary, provided that regular anti-TNF maintenance dosing is performed (69). Along with an increased risk of infection, cases of rare hepatosplenic T cell lymphoma have been reported in young males treated with azathioprine or 6-mercaptopurine and infliximab (see Statement 18) (70). An observational study (71) concluded that immunomodulators did not have an effect on response or duration of response to regular infusion of infliximab in either luminal or fistulizing Crohn’s disease (n=200). A recent study (72) in patients with stable Crohn’s disease in remission, found that there was no clear benefit of immunosuppressives over regular maintenance infliximab up to two years. For situations in which episodic or ‘on-demand’ infliximab therapy is provided, the concomitant use of immunosuppressants have reduced antibody formation to infliximab and improved its pharmacokinetics (64). With adalimumab, response rates did not differ significantly between patients receiving or not receiving concomitant immunosuppressants (26).
Commentary on statement 12
Conference delegates believed that the potential and as yet unproven benefits of concomitant immunosuppressive drugs and anti-TNF drugs must be balanced against the potential risks of infection and malignancy. No study has specifically examined the use of concurrent immunosuppressive therapy with anti-TNF drugs in patients who were already refractory to the same immunosuppressant drug.
At the time of consensus, the results of two potentially pivotal clinical trials (The Community Intervention Trial for Smoking Cessation [COMMIT] and The Study of Biologic and Immunomodulator Naive Patients in Crohn’s Disease [SONIC]) were unavailable (73,74). Because both studies are particularly relevant to the present consensus statement, they are discussed here at the request of the Steering Committee. The COMMIT clinical trial investigated the effectiveness of infliximab with or without concomitant immunosuppressant therapy (methotrexate, 25 mg/week) in patients with active disease in spite of prednisone treatment for six weeks. The outcome of this 50-week DB RCT found no difference in efficacy or tolerance of infliximab with or without methotrexate. In the SONIC trial, 508 Crohn’s disease patients naive to both immunomodulators and biologic therapy were treated with azathioprine alone, azathioprine in combination with infliximab, or infliximab alone. At week 26, steroid-free remission was achieved by 31% of the azathioprine cohort. Conversely, the infliximab and azathioprine and infliximab cohorts achieved 57% and 44% remission, respectively (P<0.001); there was no significant difference among these cohorts regarding mucosal healing at 26 weeks. Week 52 data are still pending. This study provides evidence that infliximab monotherapy is a reasonable option for treatment of active Crohn’s disease in patients who have not received a previous course of azathioprine.
STATEMENT 13
For the patient who has responded favourably to 52 weeks of TNF antagonist maintenance therapy, the benefits of continuing therapy appear to outweigh the risks of discontinuation. GRADE: Low; Vote: A 44%, B 52%, C 4%.
Discussion of statement 13
There are no studies comparing the efficacy of more than one year of TNF antagonist therapy with placebo. In the rheumatological literature, TNF antagonist drugs appear to have clinical effect for as long as they are given (75).
Commentary on statement 13
Despite the lack of evidence, the Consensus Group maintained that for patients with Crohn’s disease severe enough to require therapy, discontinuation of the TNF antagonist agent after one year was associated with a significant risk of relapse. Also, discontinuation of treatment could potentially lead to sensitization should retreatment be required in the future.
SECTION 3: SAFETY ISSUES
While serious events associated with TNF-antagonist therapy are rare, patients should be properly informed of the potential risks of infection, malignancy, autoimmunity and demyelinating neurological disorders. Given the relatively recent development of these drugs, the long-term risks associated with their use are currently unknown. Before initiation of treatment, physicians should diligently investigate risk factors predictive of adverse events. In particular, screening for tuberculosis should be performed before initiation of therapy. Prescribing physicians should practice in an environment where appropriate laboratory, diagnostic imaging and consultation resources are available to safely manage this complex group of patients.
STATEMENT 14
TNF antagonist therapy is usually contraindicated in the following situations:
Patients with a clinically significant bacterial infection; and
Patients with moderate to severe (New York heart Association class III or IV) congestive heart failure. GRADE: High; Vote: A 92%, B 8%.
Discussion of statement 14a
Clinically significant bacterial infections include pneumonia, urinary tract infections and cellulitis, which require the use of antibiotic therapy and/or hospitalization (71,72). TNF has a central role in host defense mechanisms to bacterial and opportunistic infections. In a meta-analysis (76,77) of nine randomized, placebo-controlled trials (n=5000), TNF antagonists (adalimumab and infliximab) used for rheumatoid arthritis demonstrated a pooled OR of 2.0 (95% CI 1.3 to 3.1) for serious infection. However, in a large, observational registry of Crohn’s disease, The Crohn’s Therapy, Resource, Evaluation and Assessment Tool (TREAT), infliximab was not an independent risk factor for serious infection. In this registry, independent risk factors for infection included prednisone use, narcotic analgesic use and moderate-to-severe disease activity (78). The most common infections included respiratory tract (ie, sinusitis, pharyngitis and bronchitis) and urinary tract infections as well as cellulitis, abscess and skin ulceration (79). Opportunistic infections (eg, herpetic, pneumocystis and histoplasmosis) have been reported both with infliximab and adalimumab, although the incidence rates are extremely low (80–89). In a case-control analysis (90), inflammatory bowel disease patients treated with infliximab had an OR of 4.4 (95% CI 1.2 to 17.1) for developing an opportunistic infection; concomitant immunosuppression therapy dramatically increased the OR to 14.5 (95% CI 4.9 to 43).
Discussion of statement 14b
The association between infliximab use and worsening of congestive heart failure was first reported in a randomized, placebo-controlled trial (85) assessing infliximab therapy in 150 patients with New York Heart Association class III or IV congestive heart failure (left ventricular ejection fraction of 35% or less). High doses of infliximab (10 mg/kg) were associated with an increase in all-cause mortality and congestive heart failure hospitalizations (hazard ratio 2.84; 95% CI 1.01 to 7.97; nominal P=0.043). Congestive heart failure has been reported in a retrospective study (91) of United States health care claims data of 4018 patients with either rheumatoid arthritis or Crohn’s disease. Overall, 0.2% presumed cases of heart failure were identified following treatment with infliximab, which was not statistically significant compared with unexposed individuals. New-onset heart failure in 10 relatively young individuals (younger than 50 years of age) has been associated with TNF antagonist therapy (92).
STATEMENT 15
Patients who have experienced a severe hypersensitivity reaction to a TNF antagonist should not be retreated with the same agent. GRADE: High; Vote: A 84%, B 16%.
Discussion of statement 15
Acute infusion reactions to infliximab occur in up to 5% of patients, with 1% of the reactions being severe (93). Subcutaneously administered adalimumab (40 mg every other week or 40 mg every week) and certolizumab can be associated with mild-to-moderate injection site reactions in 3% to 6% of patients (eg, irritation, erythema) (16,17).
Acute infusion reactions occur within 24 h after administration of the drug (usually between 10 min and 4 h). Most symptoms are mild and include pain or itching at the infusion or injection site, rash, urticaria and fever. Severe infusion reactions producing hypotension, chest pain, dyspnea, laryngospasm and angioedema are rare acute infusion reactions that may occur after a dose of infliximab. Milder symptoms associated with infliximab can be abated by temporarily halting infusion and readministering the drug at a lower rate along with acetaminophen and diphenhydramine. The benefit of pretreatment with acetaminophen, diphenhydramine and/or steroids to prevent infusion reactions remains to be determined. However, there are limited data suggesting that premedication in individuals who have had infusion reactions may reduce subsequent reactions (94). Concomitant immunomodulator therapy may reduce the risk of infusion reactions, particularly if infliximab is used episodically (95).
Delayed infusion reactions with infliximab occur from 24 h to 14 days after administration and include symptoms of arthralgia, fever, malaise, urticaria, myalgia, angioedema, lymphadenopathy and itching. Life-threatening serum sickness reactions have been reported (13,80,96–99). In the event of a severe hypersensitivity reaction, switching to a different TNF antagonist agent (adalimumab or certolizumab) may prevent recurrence but should be approached with caution (100).
Commentary on statement 15
In this setting, some conference delegates would advocate a desensitization regimen (101).
STATEMENT 16
In patients with pre-existing demyelinating disorders, the risks and benefits of TNF antagonist therapy should be carefully considered in consultation with a neurologist. GRADE: High; Vote: A 76%, B 24%.
Discussion of statement 16
A rare complication of TNF antagonist agents is new onset or the exacerbation of central nervous system demyelinating disorders including multiple sclerosis, optic neuritis and transverse myelitis (102–106). The product monographs for both infliximab and adalimumab reflect this clinical association (107,108). However, demyelinating diseases have also been demonstrated to be more common in patients with inflammatory bowel disease independent of TNF antagonist drug use (109). For infliximab, one patient of 879 patients in the ACCENT I and II trials (18,45) developed multiple sclerosis attributed to the therapeutic drug. With certolizumab, two patients in the same clinical trial experienced substantially decreased visual acuity that was attributed to the trial drug (27). In both of the PRECISE 1 and 2 trials, there were no adverse events of this nature with certolizumab (17,18).
Commentary on statement 16
Some delegates have used TNF antagonist therapy in patients with remote stable or mild multiple sclerosis without worsening neurological symptoms. TNF antagonist treatment of patients with pre-existing demyelinating disease should only be considered in collaboration with a neurologist.
STATEMENT 17
Live attenuated vaccine is contraindicated in patients during treatment with TNF antagonists. GRADE: Moderate; Vote: A 81%, B 19%.
Discussion of statement 17
Although no data on the effect of live vaccines on patients receiving concurrent TNF antagonist therapy are available, these vaccines should not be administered to such patients. Immunosuppressants can increase the risk of disseminated infection from the vaccine virus or bacteria.
Commentary on statement 17
Commonly administered live vaccines include mumps, measles, rubella, yellow fever, Bacille Calmette-Guerin, oral polio and varicella.
STATEMENT 18
Any patient being considered for TNF antagonist therapy with a current or past malignancy, including lymphoma, should be managed in consultation with an oncologist. GRADE: Low; Vote: A 73%, B 27%.
Discussion of statement 18
In 2006, a meta-analysis (77) of nine RCTs reported a threefold increased risk (OR 3.3; 95% CI 1.2 to 9.1) of malignancies observed in patients with rheumatoid arthritis receiving either infliximab or adalimumab therapy. A United States observational national registry study (110) in rheumatoid arthritis reported an increased risk of dermatological malignancies (melanoma and nonmelanoma) but not other malignancies with the use of anti-TNF agents. A meta-analysis (111) of placebo-controlled trials (21 trials in the safety analysis) of TNF antagonist agents for luminal and fistulizing Crohn’s disease reported no increase in malignancy compared with placebo. The TREAT registry, which followed patients with Crohn’s disease, showed no increased risk of malignancy, including lymphoma, in patients (over a period of 15,000 patient-years) receiving infliximab (112).
More specifically, initial concerns were raised about the increased incidence of lymphoma with the use of anti-TNF agents. Confounding factors such as type and severity of chronic disease, as well as concomitant immunsuppressives, make it difficult to quantify this risk (113). A national registry study (114) in rheumatoid arthritis reported no increased risk of lymphoma with anti-TNF use. The TREAT registry (3272 infliximabexposed versus 2001 unexposed patients) reported no difference in the incidence of lymphoma in infliximab-treated patients (0.06 per 100 patient-years) compared with non-infliximab-treated patients (0.05 per 100 patient-years; RR 1.3; 95% CI 0.4 to 5.0) (112). The incidence rate ranges from 0.2% to 1.4% (80,81).
Hepatosplenic T cell lymphoma (HSTCL), a rare and aggressive form of non-Hodgkin’s lymphoma, has been reported in inflammatory bowel disease patients receiving concomitant thiopurines and infliximab. As of September 2008, HSTCL has been reported mostly in young adult males (12 to 58 years of age) with Crohn’s disease (13 cases) or ulcerative colitis (three cases). Two of the above cases had also been exposed to adalimumab. An additional case of HSTCL with adalimumab in a rheumatoid arthritis patient (61 years of age) has been reported (115–117).
There are few data regarding anti-TNF treatment in patients with past or concurrent malignancy. One trial (118) observed no cancer progression during therapy with infliximab in a group of individuals with advanced disease. Nonetheless, use of TNF antagonist drugs in the setting of previous or treated malignancy should not be attempted, apart from a multidisciplinary approach in consultation with an expert oncological team.
STATEMENT 19
Caution should be exercised before administering TNF antagonist therapy in the following situations:
in patients with a suspected abscess (perianal or intra-abdominal); or
in patients with a suspected intestinal obstruction. GRADE: Low; Vote: A 72%, B 16%, C 12%.
Discussion of statement 19
A perianal or intra-abdominal abscess will not respond to TNF antagonist therapy alone, and could potentially lead to sepsis in this setting. Suspected abscesses should be investigated and drained when appropriate, and treated in combination with antibiotic therapy. Small intra-abdominal abscesses that are not amenable to drainage may respond to antibiotic therapy and do not necessarily preclude the subsequent use of a TNF antagonist agent.
There has been some concern about potentially precipitating obstructive symptoms due to stricturing Crohn’s disease following anti-TNF therapy (119,120). An analysis of the TREAT registry and ACCENT 1 trial data found no such association with infliximab; however, the authors reported an association of strictures or bowel obstruction with disease severity, disease duration, ileal location and new corticosteroid use by multivariate analysis (121). Fibrostenotic small bowel disease resulting in subacute bowel obstruction will not respond to TNF antagonist therapy and should be treated surgically. However, clinical uncertainty as to the degree of inflammatory component in stricturing disease often exists and TNF antagonist therapy may be attempted provided that close clinical monitoring is performed (122). In several case reports (123–125), systemic infliximab therapy successfully resolved stricturing. In one report (126), systemic treatment failed but the stricture responded well to local injections of infliximab via the sclerotherapy technique. There is limited experience with this technique in Canada. TNF antagonist therapy appears to neither promote a profibrotic transcriptional response or affect existing fibrosis (127,128).
STATEMENT 20
TNF antagonist therapy should be administered with caution to patients who have a history of recurrent bacterial or viral infections. GRADE: High; Vote: A 60%, B 32%, C 8%.
Discussion of statement 20
Patients with Crohn’s disease may have a higher background risk for acquiring tuberculosis infection, possibly due to the use of immunosuppressive medications (129,130). Additional risk factors include the use of steroids and immunosuppressive drugs. Before starting TNF antagonist therapy, an appropriate clinical history of risk factors for tuberculosis exposure needs to be completed in addition to the tuberculin skin test (TST) and a chest x-ray. The interferon-gamma release assay (eg, QuantiFERON, Cellestis, USA) is being used in some centres as a screening test for tuberculosis and is more specific for latent tuberculosis. Individuals with evidence of latent infection should be evaluated by an infectious disease consultant for appropriate medical therapy. It should be noted that individuals with a negative TST and chest x-ray have still developed tuberculosis after TNF antagonist therapy (131,132). In a patient with a normal chest x-ray and positive TST, antituberculosis prophylaxis should be commenced before initiating the TNF antagonist therapy (133).
Commentary on statement 20
Conference delegates noted that patients with recurrent urinary tract infections, skin infections, human papilloma virus or herpes simplex virus (HSV 1 or 2) should receive the appropriate antibiotic or antiviral prophylaxis.
STATEMENT 21
TNF antagonist therapy should be administered with caution to patients after consultation with the appropriate specialist in the following instances:
HIV infection;
hepatitis B and C; and
organtransplant recipients on multiple immunosuppressives.
GRADE: Low; Vote: A 80%, B 16%, C 4%.
Discussion of statement 21
HIV infection is not an absolute contraindication to the use of TNF antagonist therapy. While extreme caution is required, several case reports have demonstrated the successful use of infliximab for induction and maintenance therapy of Crohn’s disease in patients with controlled HIV. In one case report (134), a Crohn’s disease-HIV patient underwent induction and maintenance therapy with infliximab for a minimum of two months. In a second case report (135), the patient developed an allergic reaction to infliximab maintenance therapy and treatment was stopped after only two months. There is no published information regarding adalimumab or certolizumab in this setting.
Life-threatening flares of hepatitis B resulting in fulminant hepatic failure and death have been reported with TNF antagonist therapy, particularly when immunosuppression is withdrawn. These situations have occurred in patients who were previously asymptomatic hepatitis B virus carriers (136,137). Serological testing for the hepatitis B virus should be conducted before initiation of TNF antagonist therapy in patients with risk factors for the infection (138). Prophylactic therapy with lamivudine may prevent hepatitis B progression (139,140). Conference delegates noted that consultation with a hepatologist should be considered in the setting of hepatitis B infection.
To date, TNF antagonist therapy has not been associated with increased hepatitis C viremia. An ongoing Phase IIIB randomized, open-label trial (141) is investigating the effect of infliximab induction on the efficacy of pegylated-interferon/ribavirin in patients with genotype 1 hepatitis C virus infection. However, the long-term effects of TNF-antagonist therapy in the setting of hepatitis C virus infection are unknown.
The use of infliximab in organ transplant recipients on multiple immunosuppressants should be approached with caution due to the increased risk of opportunistic infections. The effect of TNF antagonist therapy on graft function remains to be determined. There is some evidence that TNF antagonist therapy may help prevent ischemia/reperfusion rejection (126). TNF antagonists have been used successfully to treat and prevent acute rejection in organ transplant recipients and to treat acute graft versus host disease (142,143). There are reports of patients developing inflammatory bowel disease following organ transplantation who were successfully treated with TNF inhibitors (144,145). As such, a TNF antagonist may assume a dual role in organ transplant recipients. However, in the absence of long-term studies conducted with a large cohort, the safety and efficacy of TNF antagonists in this clinical setting are largely unknown.
STATEMENT 22
TNF antagonist therapy should be administered with caution to patients who are planning pregnancy, are pregnant or lactating. GRADE: Low; Vote: A 80%, B 20%.
Discussion of statement 22
To date, there are limited data reporting pregnancy outcomes in TNF antagonist-exposed patients. Most available information pertains to infliximab exposure. The rates of spontaneous abortions and neonatal complications do not appear to differ in infliximab-treated Crohn’s disease patients compared with non-infliximab-treated patients or the general population (146–148). Fewer data are available regarding adalimumab and pregnancy but the trend is similar to that reported for infliximab (149). No data on certolizumab are available, but there is no reason to believe that this agent will affect pregnancy outcomes differently from the other TNF antagonists. Larger registries (that are ongoing) are necessary to better assess and quantify the risk of TNF antagonists during pregnancy.
For new mothers, a single case report (150) found no evidence of infliximab in breast milk. The mother continued infliximab maintenance therapy (10 mg/kg) throughout her pregnancy, with the last infusion completed two weeks before delivery. The breastfed infant’s high serum level of infliximab was attributed to placental transfer. The levels slowly declined during a period of six months, indicating that the half-life of infliximab was prolonged, similar to other maternally acquired antibodies. No information has been reported for adalimumab or certolizumab in these circumstances.
Commentary on statement 22
In all patients, the risk of TNF antagonist therapy in pregnancy needs to be balanced against control of the underlying Crohn’s disease. TNF antagonists (infliximab, adalimumab and certolizumab) are categorized in pregnancy as class B drugs by the United States Food and Drug Administration (no documented fetal toxicity). Discussion of the risks and benefits of TNF antagonist therapy with patients of child-bearing age should be conducted before conception if they are planning or considering pregnancy. If maintenance therapy with a TNF antagonist drug is planned during pregnancy, the timing of the last dose before delivery should be based on the half-life of the drug because potential for placental transfer of the drug is increased during the third trimester and at the time of delivery. For example, the last dose of infliximab before the delivery should be given at week 32 of pregnancy.
STATEMENT 23
TNF antagonist therapy may be beneficial in certain inflammatory conditions associated with Crohn’s disease (Table 2). GRADE: Moderate; Vote: A 68%, B 28%, C 4%.
TABLE 2.
Studies in which tumour necrosis factor (TNF) antagonists (infliximab) were used to treat patients with Crohn’s disease (CD) who were experiencing debilitating extraintestinal manifestations associated with CD in spite of conventional therapy (2002 to 2008)
| Extraintestinal manifestation | Reference, year | Trial design | Outcomes |
|---|---|---|---|
| Ankylosing spondylitis | Herfarth et al (167), 2002 | Open-label | At week 12, 35 of 69 patients (61%) experienced significant improvement while 41% had complete resolution of arthritis |
| Kaufman et al (168), 2005 | Case series (n=11) | Overall improvement experienced by seven patients; two partial responders and two nonresponders | |
| Rednic et al (169), 2006 | Case report | Overall improvement | |
| Peripheral arthritis | Generini et al (170), 2004 | Controlled trial comparing outcomes in active CD (n=16) versus inactive CD (n=8) | In both cohorts, therapy was associated with significant improvement compared with baseline scores (P<0.01) |
| Pyoderma gangrenosum | Batres et al (171), 2002 | Case report (pediatric; peristomal variant) | Complete healing |
| Brooklyn et al (172), 2006 | Randomized, double-blind, placebo-controlled trial (n=30) | By week 2, 46% of the patients in the infliximab group experienced improvement compared with 6% in the placebo group (P=0.025). There was no difference in response between patients with or without inflammatory bowel disease | |
| Ferkolj et al (173), 2006 | Case report | Complete healing after five weeks | |
| Hewitt et al(174), 2007 | Case report | Complete healing | |
| Grange et al (175), 2002 | Case report | Complete healing by week 11 | |
| Kaufman et al (168), 2005 | Case series (n=4) | Improvement in all patients after a single infusion; complete healing of ulcers in three patients after repeated infusions | |
| Kugasthasan et al (176), 2003 | Case report (pediatric) | Complete healing with no recurrence after one year | |
| Ljung et al (177), 2002 | Case series (n=8) | Complete healing in three patients; partial healing in three; temporary improvement observed in two. | |
| Mimouni et al (178), 2003 | Case series (n=3) (peristomal variant) | Complete healing in two patients; one had a partial response | |
| Regueiro et al (179), 2003 | Retrospective, multicentre study (n=13) | Complete healing in three patients; 10 responded to induction and continued with maintenance infusions; 100% discontinued corticosteroid treatment | |
| Sapienza et al (180), 2004 | Case series (n=4) | Complete healing at week 4 | |
| Triantafillidis et al (181), 2002 | Case report | Improvement of skin lesions at week 2 with complete healing thereafter; no recurrence | |
| Zaccagna et al (182), 2003 | Case report | Complete healing | |
| Uveitis | Fries et al (183), 2002 | Case report | Complete resolution after first infusion |
Discussion of statement 23
For a list of the most common extraintestinal manifestations associated with Crohn’s disease that have been treated with TNF-antagonist therapy, refer to Table 2. During a 24-week RCT (151), infliximab was well tolerated and effective for the treatment of ankylosing spondylitis. In pediatric patients, the cause of growth failure is considered to be multifactorial due to malnutrition, side effects of drugs and inflammation-associated anorexia (152). An early study (153) found that successful infliximab induction and maintenance therapy resulted in significant height and weight increases within six months. Infliximab therapy has been associated with improvement in both height velocity and height centile increases, but only if the patient is treated before or at the cusp of puberty (154).
Corticosteroid treatment was believed to have been a major contributor to bone mineral loss in patients with Crohn’s disease; however, recent studies (155–159) suggest that another significant contributor is inflammatory cytokine-induced bone resorption. Both infliximab and adalimumab therapies have been demonstrated to provide steroid-sparing effects and reduce inflammation, and have been associated with the reduction of osteoporosis-related issues (13,16,160–163).
CONCLUSIONS
Infliximab, adalimumab and certolizumab collectively represent a significant advance in the treatment of moderate-to-severe Crohn’s disease. Canadian gastroenterologists have developed a large body of clinical experience with these drugs. As such, TNF antagonists have been accepted into the mainstream treatment armamentarium for a specifically identified subgroup of patients. In spite of recent advances, important clinical questions remain and further research is required in areas such as immunogenicity, predictors of response, long-term outcomes and the use of these drugs in early disease. Future guideline development will undoubtedly be required to integrate new knowledge as it is integrated into clinical practice.
Acknowledgments
The conference delegates thank Louise Hope and Paul Sinclair from the CAG office for their superior organization of this meeting. The authors thank Kathleen Ismond for her expertise in conducting the literature review and for her editorial assistance.
APPENDIX:
LIST OF ATTENDEES: Co-chairs: Richard N Fedorak (RF), Daniel Sadowski (DS). Steering Committee: Charles N Bernstein (CB), Alain Bitton (AB), Ken Croitoru (KC), and Anne-Marie Griffiths (AG). Non-voting moderator: Richard Hunt (RH). Voting participants: Adult Gastroenterology: Guy Aumais (GA), Naoki Chiba (NC), Robert Enns (RE), Brian Feagan (BF), E. Jan Irvine (EI), John Marshall (JM), Remo Panaccione (RP), Pierre Paré (PP), Sunil Patel (SP), Craig Render (CR), and Hillary Steinhart (HS) Pediatric Gastroenterology: Decker Butzner (DB), Hien Huynh (HH), Kevin Jacobson (KJ), and Ernest Seidman (ES). International Experts: Simon Travis (ST) (England) and Charles Elson (CE) (USA). Nursing: Patricia Rawsthorne (PR). Family Medicine: John Stewart (JS). Nonvoting observers: Paul Sinclair (CAG), Dr Michael Beyak, Dr Pushpa Sathya, Dr Eric Benchimol, Dr Sylvaine Forget, Dr Terry Ponich, Dr Cynthia Seow, Dr Hoda El Aggan (Faculty of Medicine, Egypt), Dr Kevin Glasgow (CCFC), Dr George Tolomiczenko (CCFC), Mr Paul Belanger (CIHR), Mr Nick Makris (Abbott Laboratories Ltd), Mr Kevin McHugh (Abbott Laboratories Ltd), Ms Josee Bernier (Abbott Laboratories Ltd), Ms Adle Georgi (Abbott Laboratories Ltd), Mr Bruce McTavish (Axcan Pharma Inc), Dr JoEllen Schweinle (Axcan Pharma Inc), Ms Caroline Gagnon, (Axcan Pharma Inc), Ms Erica Leung (Procter & Gamble), Ms Edith Garon (Schering), Mr Brent Pullen (Schering). Mr Dory Solomon (Shire), Dr Adel Gehshan (UCB Pharmaceuticals Inc), Mr Rob Hamilton (UCB Phamaceuticals Inc).
Footnotes
DISCLOSURE OF POTENTIAL CONFLICT OF INTEREST: Please note: Unless listed below, faculty disclosure information was not provided. GA, DB, SP, JS and PR do not have any industry or government relationships to report. Advisory Board: Abbott Laboratories Ltd (DS, CB, AB, KC, RE, RF, KJ, JM, RP, PP, HS, ST), AstraZeneca (RE, BF, JM, PP), Axcan Pharma Inc (CB, AB, RF, JM, HS), Celgene (BF), Celltech (BF, RF), Elan/Biogen (BF, RF, RP), Ferring Pharamceuticals (RP), Given (ES), Janssen-Ortho Inc (JM), Mead Johnson (KJ), Nestlé (ES), Novartis (PP), Procter & Gamble (AB, RF, JM, RP, PP, HS), Prometheus (ES), Protein Design Labs (BF), Schering (AB, RE, BF, RF, JM, RP, CR, ES, DS, HS, ST), Shire (AB, CB, NC, KC, RF, JM, RP, PP, HS), Solvay (JM), Synta (BF), UCB Canada (CB, AB, KC, RF, JM, RP, PP, ES, HS, ST) and VSL3 (RF). Consultation fees: Abbott Laboratories Ltd (BF, RF, PP, ES), Allergan Inc (RH), AstraZeneca (BF, RH, RP), Axcan Pharma Inc (RF, RH), Berlex (BF, RF), Bristol-Myers Squibb (CB, BF, RF, RP), Canadian Agency for Drugs and Technology in Health (JM), Celgene (BF), Celltech (BF), Centocor (BF, RF, AG, RP), Cerimon (BF), Combinatrox (BF), Elan/Biogen (BF, RP), Ferring Pharmaceuticals Inc (RF, RP), GeneLogic (BF), Genetech (BF), GlaxoSmithKline (RP), ISIS (BF), Janssesn-Ortho (BF), Merck & Co (RH), Millenium (BF), Napo (BF), Negma (RH), Novartis (RF, RH, PP), Nycomed (RF, RH), Ontario Ministry of Health and Long-term Care (JM), Osiris (BF), Otsuka (RF), Pfizer (RH), Procter & Gamble (BF, RF, AG, RP), Protein Design Labs (BF, RF), Santarus (BF, RH), Schering (CE, BF, RF, AG, RP), Serono (BF), Shire (RE, RP), Synta (BF, RF), Teva (BF), Tioga (BF), UCB Pharmaceuticals Inc (RF, AG, RP), VSL3 (RF). Educational grants: Abbott Laboratories Ltd (EI), Axcan Pharma Inc (CR, RP), Ferring Pharmaceuticals (RP), Janssen-Ortho Inc (RP) and Schering (RP, CR). Research grants/Clinical trial funding: Abbott Laboratories Ltd (AB, CE, BF, RF, AG, JM, RP, PP, HS, ST), Allergan Inc (RH), AstraZeneca (RF, RH, PP), Axcan Pharma Inc (RF, RH), Berlex (BF, RF, JM), Boehringer-Ingelheim (BF), Bristol-Myers Squibb (CE, BF, RF, JM, RP, HS, ST), Centocor (BF, RF, HH, RP, PP, HS), Chemocentryx (HS), Dynogen (JM), Elan/Biogen (BF, RF, JM, RP), Ferring Pharmaceuticals (RF), Given (ES), Millenium (BF, RF, RP), Napo (BF), Negma (RH), Nestlé (ES), Novartis (BF, RF, JM, PP), Nycomed (RF), Ocera (JM), Otsuka (BF, RF, HS), PDL (JM), Procter & Gamble (BF, RF, RP, PP, HS), Prometheus (ES), Protein Design Labs (BF, RF), Schering (AB, BF, RF, AG, RP, PP, HS, ST), Solvay (JM), Synta (BF, RF), TAP Pharmaceuticals (RH), Tillots (BF, RF), UCB Pharmaceuticals Inc (CE, BF, RF, HS), VSL3 (RF). Speaker’s bureau: Abbott Laboratories Ltd (AB, RE, NC, RF, JM, RP, PP, HS, ST), AstraZeneca (RE, BF, RH, RP, PP), Axcan (RF, JM, RP, HS), Centocor (RF, RP), Elan (RP), Ferring Pharmaceuticals (RF), Given (ES), Janssen-Ortho Inc (RP), Merck & Co (RH), Nestlé (ES), Novartis (RH, PP), Nycomed (RE, RF, RP), Procter & Gamble (AB, NC, RF, JM, RP, PP, HS), Prometheus (RP, ES), Santarus (RH), Schering (AB, RE, RF, AG, JM, RP, CR, HS, ES, ST), Shire (RF, RP), Solvay (JM), TAP Pharmaceuticals (RH), UCB Pharmaceuticals Inc (RF, ST), VSL3 (RF). Teaching: Abbott Laboratories Ltd (AB). Significant shareholdings: None reported. Other: Strategic Consultants International (RH). Grant support: This consensus conference was endorsed and organized by the CAG, and was held in Montreal, Quebec, on March 3 to 4, 2008. Consensus members did not receive compensation from the CAG or industry (eg, honoraria or travel expenses) for their participation.
REFERENCES
- 1.Statistics Canada Table 102-0531 – Deaths, by cause, Chapter XI: Diseases of the digestive system (K00 to K93), age group and sex, Canada, annual number, 2000–2004. <http://cansim2.statcan.ca/cgi-win/cnsmcgi.exe?Lang=E&CANSIMFile=CII\CII_1_E.htm&RootDir=CII/> (Version current at May 5, 2008).
- 2.Cohen RD. The quality of life in patients with Crohn’s disease. Aliment Pharmacol Ther. 2002;16:1603–9. doi: 10.1046/j.1365-2036.2002.01323.x. [DOI] [PubMed] [Google Scholar]
- 3.Bernklev T, Jahnsen J, Schulz T, et al. Course of disease, drug treatment and health-related quality of life in patients with inflammatory bowel disease 5 years after initial diagnosis. Eur J Gastroenterol Hepatol. 2005;17:1037–45. doi: 10.1097/00042737-200510000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein CN, Wajda A, Svenson LW, et al. The epidemiology of inflammatory bowel disease in Canada: A population-based study. Am J Gastroenterol. 2006;101:1559–68. doi: 10.1111/j.1572-0241.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- 5.Panaccione R, Fedorak RN, Aumais G, et al. Canadian Association of Gastroenterology clinical practice guidelines: The use of infliximab in Crohn’s disease. Can J Gastroenterol. 2004;18:503–8. doi: 10.1155/2004/670161. [DOI] [PubMed] [Google Scholar]
- 6.Panaccione R, for the Canadian Consensus Group on the use of Infliximab in Crohn’s Disease Infliximab for the treatment of Crohn’s disease: Review and indications for clinical use in Canada. Can J Gastroenterol. 2001;15:371–5. doi: 10.1155/2001/490921. [DOI] [PubMed] [Google Scholar]
- 7.Canadian Association of Gastroenterology. Policy on the application for, and implementation of, clinical practice guidelines. <http://www.cag-acg.org/uploads/cpg%20guidelines%20v17june2008rev.pdf> (Version current at September 22, 2008). [DOI] [PMC free article] [PubMed]
- 8.Armstrong D, Marshall JK, Chiba N, et al. Canadian Consensus Conference on the management of gastroesophageal reflux disease in adults – update 2004. Can J Gastroenterol. 2005;19:15–35. doi: 10.1155/2005/836030. [DOI] [PubMed] [Google Scholar]
- 9.Hunt R, Fallone C, Veldhuyzan van Zanten S, et al. Canadian Helicobacter Study Group Consensus Conference: Update on the management of Helicobacter pylori – an evidence-based evaluation of six topics relevant to clinical outcomes in patients evaluated for H pylori infection. Can J Gastroenterol. 2004;18:547–54. doi: 10.1155/2004/326767. [DOI] [PubMed] [Google Scholar]
- 10.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490–4. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Targan SR, Hanauer SB, Van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029–35. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 13.Hanauer SB, Feagan B, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: The ACCENT I randomised trial. Lancet. 2002;359:1541–9. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 14.D’Haens G, van Deventer S, Van Hogezand RA, et al. Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn’s disease: A European multicenter trial. Gastroenterology. 1999;116:1029–34. doi: 10.1016/s0016-5085(99)70005-3. [DOI] [PubMed] [Google Scholar]
- 15.Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–33. doi: 10.1053/j.gastro.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 16.Colombel J, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: The CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 17.Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357:228–38. doi: 10.1056/NEJMoa067594. [DOI] [PubMed] [Google Scholar]
- 18.Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357:239–50. doi: 10.1056/NEJMoa062897. [DOI] [PubMed] [Google Scholar]
- 19.Orlando A, Colombo E, Kohn A, et al. Infliximab in the treatment of steroid resistant/dependant and fistulating Crohn’s disease: An analysis of predictors of response in a Italian multicentric open study in 573 patients. Eur Rev Med Pharmacol Sci. 2004;8:230. [Google Scholar]
- 20.Hommes DW, Oldenburg B, van Bodegraven AA, et al. Guidelines for treatment with infliximab for Crohn’s disease. Neth J Med. 2006;64:219–29. [PubMed] [Google Scholar]
- 21.Kao KT, McClune AC, Conteas CN. Long-term remission of Crohn’s disease using infliximab without human anti-chimeric antibody (HACA) in absence of anti-metabolites: A series of three case reports. Pract Gastroenterol. 2006;30:106–11. [Google Scholar]
- 22.Hyams J, Crandall W, Kugathasan S, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology. 2007;132:863–73. doi: 10.1053/j.gastro.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 23.McCabe RP, Woody J, van Deventer S, et al. A multicenter trial of cA2 anti-TNF chimeric monoclonal antibody in patients with active Crohn’s disease Gastroenterology 1996110A962(Abst). [Google Scholar]
- 24.Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology. 2004;126:402–13. doi: 10.1053/j.gastro.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Pearce CB, Lawrance IC. Careful patient selection may improve response rates to infliximab in inflammatory bowel disease. J Gastroenterol Hepatol. 2007;22:1671–7. doi: 10.1111/j.1440-1746.2006.04739.x. [DOI] [PubMed] [Google Scholar]
- 26.Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: Results of the CLASSIC II trial. Gut. 2007;56:1232–9. doi: 10.1136/gut.2006.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schreiber S, Rutgeerts P, Fedorak RN, et al. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn’s disease. Gastroenterology. 2005;129:807–18. doi: 10.1053/j.gastro.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigo L, Perez-Pariente JM, Fuentes D, et al. Retreatment and maintenance therapy with infliximab in fistulizing Crohn’s disease. Rev Esp Enfermedades Digest. 2004;96:548–58. doi: 10.4321/s1130-01082004000800004. [DOI] [PubMed] [Google Scholar]
- 29.Ho GT, Smith L, Aitken S, et al. The use of adalimumab in the management of refractory Crohn’s disease. Aliment Pharmacol Ther. 2008;27:308–15. doi: 10.1111/j.1365-2036.2007.03583.x. [DOI] [PubMed] [Google Scholar]
- 30.Chun A, Chadi RM, Korelitz BI, et al. Intravenous corticotrophin vs. hydrocortisone in the treatment of hospitalized patients with Crohn’s disease: A randomized double-blind study and follow-up. Inflamm Bowel Dis. 1998;4:177–81. doi: 10.1097/00054725-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Bhatia JK, Korelitz BI, Panagopoulos G, et al. A prospective open-label trial of Remicade in patients with severe exacerbation of Crohn’s disease requiring hospitalization: A comparison with outcomes previously observed in patients receiving intravenous hydrocortisone. J Clin Gastroenterol. 2007;41:677–81. doi: 10.1097/MCG.0b013e31802c2a23. [DOI] [PubMed] [Google Scholar]
- 32.Gaertner WB, Decanini A, Mellgren A, et al. Does infliximab infusion impact results of operative treatment for Crohn’s perianal fistulas? Dis Colon Rectum. 2007;50:1754–60. doi: 10.1007/s10350-007-9077-3. [DOI] [PubMed] [Google Scholar]
- 33.Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398–405. doi: 10.1056/NEJM199905063401804. [DOI] [PubMed] [Google Scholar]
- 34.Sands BE, Blank MA, Patel K, Van Deventer SJ. Long-term treatment of rectovaginal fistulas in Crohn’s disease: Response to infliximab in the ACCENT II study. Clin Gastroenterol Hepatol. 2004;2:912–20. doi: 10.1016/s1542-3565(04)00414-8. [DOI] [PubMed] [Google Scholar]
- 35.Sands BE, Blank MA, Diamond RH, Barrett JP, Van Deventer SJ. Maintenance infliximab does not result in increased abscess development in fistulizing Crohn’s disease: Results from the ACCENT II study. Aliment Pharmacol Ther. 2006;23:1127–36. doi: 10.1111/j.1365-2036.2006.02878.x. [DOI] [PubMed] [Google Scholar]
- 36.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–85. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 37.Gaertner WB, Decanini A, Mellgren A, et al. Does infliximab infusion impact results of operative treatment for Crohn’s perianal fistulas? Dis Colon Rectum. 2007;50:1754–60. doi: 10.1007/s10350-007-9077-3. [DOI] [PubMed] [Google Scholar]
- 38.Hyams JS. Infliximab treatment of Crohn’s disease in children: It’s time to not go retro(spective) Am J Gastroenterol. 2003;98:5–6. doi: 10.1111/j.1572-0241.2003.07170.x. [DOI] [PubMed] [Google Scholar]
- 39.Sandborn W, Sutherland L, Pearson D, May G, Modigliani R, Prantera C. Azathioprine or 6-mercaptopurine for inducing remission of Crohn’s disease. Cochrane Database Syst Rev. 2000:CD000545. doi: 10.1002/14651858.CD000545. [DOI] [PubMed] [Google Scholar]
- 40.Markowitz J, Grancher K, Kohn N, Lesser M, Daum F. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn’s disease. Gastroenterology. 2000;119:895–902. doi: 10.1053/gast.2000.18144. [DOI] [PubMed] [Google Scholar]
- 41.D’Haens G, Baert F, Van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: An open randomised trial. Lancet. 2008;371:660–7. doi: 10.1016/S0140-6736(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 42.Schroder O, Blumenstein I, Schulte-Bockholt A, Stein J. Combining infliximab and methotrexate in fistulizing Crohn’s disease resistant or intolerant to azathioprine. Aliment Pharmacol Ther. 2004;19:295–301. doi: 10.1111/j.1365-2036.2004.01850.x. [DOI] [PubMed] [Google Scholar]
- 43.Schroder O, Blumenstein I, Stein J. Combining infliximab with methotrexate for the induction and maintenance of remission in refractory Crohn’s disease: A controlled pilot study. Eur J Gastroenterol Hepatol. 2006;18:11–6. doi: 10.1097/00042737-200601000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Klotz U, Teml A, Schwab M. Clinical pharmacokinetics and use of infliximab. Clin Pharmacokinet. 2007;46:645–60. doi: 10.2165/00003088-200746080-00002. [DOI] [PubMed] [Google Scholar]
- 45.Mori S. A relationship between pharmacokinetics (PK) and the efficacy of infliximab for patients with rheumatoid arthritis: Characterization of infliximab-resistant cases and PK-based modified therapy. Mod Rheumatol. 2007;17:83–91. doi: 10.1007/s10165-006-0544-9. [DOI] [PubMed] [Google Scholar]
- 46.Rutgeerts P, Diamond RH, Bala M, et al. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn’s disease. Gastrointest Endosc. 2006;63:433–42. doi: 10.1016/j.gie.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 47.Regueiro M, Siemanowski B, Kip KE, Plevy S. Infliximab dose intensification in Crohn’s disease. Inflamm Bowel Dis. 2007;13:1093–9. doi: 10.1002/ibd.20177. [DOI] [PubMed] [Google Scholar]
- 48.Menachem Y, Avidan B, Lavy A, et al. Increasing the infliximab dose is beneficial in Crohn’s disease patients who responded to a lower dose and relapsed. Digestion. 2005;72:124–8. doi: 10.1159/000088367. [DOI] [PubMed] [Google Scholar]
- 49.Ho GT, Smith L, Aitken S, et al. The use of adalimumab in the management of refractory Crohn’s disease. Aliment Pharmacol Ther. 2008;27:308–15. doi: 10.1111/j.1365-2036.2007.03583.x. [DOI] [PubMed] [Google Scholar]
- 50.Schreiber S, Panes J, Mason D, Lichtenstein GR, Sandborn WJ. Efficacy and Tolerability of Certolizumab Pegol Are Sustained Over 18 Months: Data from Precise 2 and Its Extension Studies (Precise 3 and 4) Gastroenterology. 2008;134:A-490. (Abst T1133) [Google Scholar]
- 51.Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: A randomized trial. Ann Intern Med. 2007;146:829–38. doi: 10.7326/0003-4819-146-12-200706190-00159. [DOI] [PubMed] [Google Scholar]
- 52.Papadakis KA, Shaye OA, Vasiliauskas EA, et al. Safety and efficacy of adalimumab (D2E7) in Crohn’s disease patients with an attenuated response to infliximab. Am J Gastroenterol. 2005;100:75–9. doi: 10.1111/j.1572-0241.2005.40647.x. [DOI] [PubMed] [Google Scholar]
- 53.Sandborn WJ, Hanauer S, Loftus EV, Jr, et al. An open-label study of the human anti-TNF monoclonal antibody adalimumab in subjects with prior loss of response or intolerance to infliximab for Crohn’s disease. Am J Gastroenterol. 2004;99:1984–9. doi: 10.1111/j.1572-0241.2004.40462.x. [DOI] [PubMed] [Google Scholar]
- 54.Hinojosa J, Gomollon F, Garcia S, et al. Efficacy and safety of short-term adalimumab treatment in patients with active Crohn’s disease who lost response or showed intolerance to infliximab: A prospective, open-label, multicentre trial. Aliment Pharmacol Ther. 2007;25:409–18. doi: 10.1111/j.1365-2036.2006.03232.x. [DOI] [PubMed] [Google Scholar]
- 55.Kaplan GG, Hur C, Korzenik J, Sands BE. Infliximab dose escalation versus initiation of adalimumab for loss of response in Crohn’s disease: A cost-effectiveness analysis. Aliment Pharmacol Ther. 2007;26:1509–20. doi: 10.1111/j.1365-2036.2007.03548.x. [DOI] [PubMed] [Google Scholar]
- 56.Nikas SN, Drosos AA. Onercept. Serono Curr Opin Invest Drugs. 2003;4:1369–76. [PubMed] [Google Scholar]
- 57.van Vollenhoven R, Harju A, Brannemark S, Klareskog L. Treatment with infliximab (Remicade) when etanercept (Enbrel) has failed or vice versa: Data from the STURE registry showing that switching tumour necrosis factor alpha blockers can make sense. Ann Rheum Dis. 2003;62:1195–8. doi: 10.1136/ard.2003.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wick JY, Zanni GR. Rheumatoid arthritis – Crohn’s disease connection. Consult Pharm. 2005;20:110–22. doi: 10.4140/tcp.n.2005.110. [DOI] [PubMed] [Google Scholar]
- 59.Gottlieb AB. Tumor necrosis factor blockade: Mechanism of action. J Invest Dermatol Sym Proc. 2007;12:1–4. doi: 10.1038/sj.jidsymp.5650029. [DOI] [PubMed] [Google Scholar]
- 60.Benucci M, Saviola G, Baiardi P, Cammelli E, Manfredi M. Anti-nucleosome antibodies as prediction factor of development of autoantibodies during therapy with three different TNF-alpha blocking agents in rheumatoid arthritis. Clin Rheumatol. 2008;27:91–5. doi: 10.1007/s10067-007-0728-5. [DOI] [PubMed] [Google Scholar]
- 61.Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348:601–8. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 62.Hanauer SB, Wagner CL, Bala M, et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn’s disease. Clin Gastroenterol Hepatol. 2004;2:542–53. doi: 10.1016/s1542-3565(04)00238-1. [DOI] [PubMed] [Google Scholar]
- 63.Farrell RJ, Alsahli M, Jeen Y-T, Falchuk KR, Peppercorn MA, Michetti P. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn’s disease: A randomized controlled trial. Gastroenterology. 2003;124:917–24. doi: 10.1053/gast.2003.50145. [DOI] [PubMed] [Google Scholar]
- 64.Vermeire S, Noman M, Van Assche G, Baert F, D’Haens G, Rutgeerts P. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut. 2007;56:1226–31. doi: 10.1136/gut.2006.099978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Assche G, Magdelaine-Beuzelin C, D’Haens G, et al. Withdrawal of immunosuppression in Crohn’s disease treated with scheduled infliximab maintenance: A randomized trial. Gastroenterology. 2008;134:1861–8. doi: 10.1053/j.gastro.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 66.West RL, Zelinkova Z, Wolbink GJ, Kuipers EJ, Stokkers PC, Van Der Woude CJ. Immunogenicity negatively influences the outcome of adalimumab treatment in Crohn’s disease. Aliment Pharmacol Ther. 2008;28:1122–6. doi: 10.1111/j.1365-2036.2008.03828.x. [DOI] [PubMed] [Google Scholar]
- 67.Cheifetz A, Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab: A large center experience. Am J Gastroenterol. 2003;98:1315–24. doi: 10.1111/j.1572-0241.2003.07457.x. [DOI] [PubMed] [Google Scholar]
- 68.Lichtenstein GR, Abreu MT, Cohen R, Tremaine W. American gastroenterological association institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:940–87. doi: 10.1053/j.gastro.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 69.Hanauer SB. Risks and benefits of combining immunosuppressives and biological agents in inflammatory bowel disease: Is the synergy worth the risk? Gut. 2007;56:1181–3. doi: 10.1136/gut.2006.115980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mackey AC, Green L, Liang L-C, Dinndorf P, Avigan M. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:265–7. doi: 10.1097/MPG.0b013e31802f6424. [DOI] [PubMed] [Google Scholar]
- 71.Fefferman DS, Lodhavia PJ, Alsahli M, et al. Smoking and immunomodulators do not influence the response or duration of response to infliximab in Chrohn’s disease. Inflamm Bowel Dis. 2004;10:346–51. doi: 10.1097/00054725-200407000-00004. [DOI] [PubMed] [Google Scholar]
- 72.Van Assche G, Magdelaine-Beuzelin C, D’Haens G, et al. Withdrawal of immunosuppression in Crohn’s disease treated with scheduled infliximab maintenance: A randomized trial. Gastroenterology. 2008;134:1861–8. doi: 10.1053/j.gastro.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 73.Feagan BG, McDonald JW, Panaccione R, et al. A randomized trial of methotrexate in combination with infliximab for the treatment of Crohn’s disease (Late breaking abstract) Gastroenterology. 2008;135:294–5. [Google Scholar]
- 74.Sandborn W, Rutgeerts P, Reinisch W, et al. SONIC: A randomized, double blind, controlled trial comparing infliximab and infliximab and azathioprine to azathioprine in patients with Crohn’s disease naive to immunomodulators and biologic therapy. (Late-breaking abstract, 29) Am J Gastroenterol. 2008;103:1117. [Google Scholar]
- 75.Ducoulombier V, Solau E, Coquerelle P, et al. Long-term results of infliximab therapy in rheumatoid arthritis: Experience acquired by the North-Pas-de-Calais hospital network. Joint Bone Spine. 2007;74:56–9. doi: 10.1016/j.jbspin.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 76.Listing J, Strangfeld A, Kary S, et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum. 2005;52:3403–12. doi: 10.1002/art.21386. [DOI] [PubMed] [Google Scholar]
- 77.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: Systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–85. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 78.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4:621–30. doi: 10.1016/j.cgh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 79.Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4:621–30. doi: 10.1016/j.cgh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 80.Colombel J-F, Loftus EV, Jr, Tremaine WJ, et al. The safety profile of infliximab in patients with Crohn’s disease: The Mayo Clinic experience in 500 patients. Gastroenterology. 2004;126:19–31. doi: 10.1053/j.gastro.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 81.Ljung T, Karlen P, Schmidt D, et al. Infliximab in inflammatory bowel disease: Clinical outcome in a population based cohort from Stockholm County. Gut. 2004;53:849–53. doi: 10.1136/gut.2003.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tai TL, O’Rourke KP, McWeeney M, Burke CM, Sheehan K, Barry M. Pneumocystis carinii pneumonia following a second infusion of infliximab. Rheumatology (Oxford) 2002;41:951–2. doi: 10.1093/rheumatology/41.8.951. [DOI] [PubMed] [Google Scholar]
- 83.Nakelchik M, Mangino JE. Reactivation of histoplasmosis after treatment with infliximab. Am J Med. 2002;112:78. doi: 10.1016/s0002-9343(01)00945-7. [DOI] [PubMed] [Google Scholar]
- 84.Kamath BM, Mamula P, Baldassano RN, Markowitz JE. Listeria meningitis after treatment with infliximab. J Pediatr Gastroenterol Nutr. 2002;34:410–2. doi: 10.1097/00005176-200204000-00018. [DOI] [PubMed] [Google Scholar]
- 85.Morelli J, Wilson FA. Does administration of infliximab increase susceptibility to listeriosis? Am J Gastroenterol. 2000;95:841–2. doi: 10.1111/j.1572-0241.2000.01872.x. [DOI] [PubMed] [Google Scholar]
- 86.Gluck T, Linde HJ, Scholmerich J, Muller-Ladner U, Fiehn C, Bohland P. Anti-tumor necrosis factor therapy and Listeria monocytogenes infection: Report of two cases. Arthritis Rheum. 2002;46:2255–7. doi: 10.1002/art.10374. [DOI] [PubMed] [Google Scholar]
- 87.Warris A, Bjorneklett A, Gaustad P. Invasive pulmonary aspergillosis associated with infliximab therapy. N Engl J Med. 2001;344:1099–100. [PubMed] [Google Scholar]
- 88.Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunothrapy with tumour necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum. 2002;46:2565–70. doi: 10.1002/art.10583. [DOI] [PubMed] [Google Scholar]
- 89.McNamara DA, Brophy S, Hyland JMP. Perianal Crohn’s disease and infliximab therapy. Surgeon. 2005;2:258–63. doi: 10.1016/s1479-666x(04)80094-5. [DOI] [PubMed] [Google Scholar]
- 90.Toruner M, Loftus EV, Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–36. doi: 10.1053/j.gastro.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 91.Curtis JR, Kramer JM, Martin C, et al. Heart failure among younger rheumatoid arthritis and Crohn’s patients exposed to TNF-{alpha} antagonists. Rheumatology (Oxford) 2007;46:1688–93. doi: 10.1093/rheumatology/kem212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kwon HJ, Cote TR, Cuffe MS, Kramer JM, Braun MM. Case reports of heart failure after therapy with a tumor necrosis factor antagonist. Ann Intern Med. 2003;138:807–81148. doi: 10.7326/0003-4819-138-10-200305200-00008. [DOI] [PubMed] [Google Scholar]
- 93.Cheifetz A, Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab: A large center experience. Am J Gastroenterol. 2003;98:1315–24. doi: 10.1111/j.1572-0241.2003.07457.x. [DOI] [PubMed] [Google Scholar]
- 94.Jacobstein DA, Markowitz JE, Kirschner BS, et al. Premedication and infusion reactions with infliximab: Results from a pediatric inflammatory bowel disease consortium. Inflamm Bowel Dis. 2005;11:442–6. doi: 10.1097/01.mib.0000158166.88238.ea. [DOI] [PubMed] [Google Scholar]
- 95.Moss AC, Fernandez-Becker N, Jo KK, Cury D, Cheifetz AS. The impact of infliximab infusion reactions on long-term outcomes in patients with Crohn’s disease. Aliment Pharmacol Ther. 2008;28:221–7. doi: 10.1111/j.1365-2036.2008.03734.x. [DOI] [PubMed] [Google Scholar]
- 96.Miheller P, Muzes G, Lakatos G, Mihaly E, Tulassay Z. Repeated infliximab therapy after serum sickness-like reaction in Crohn’s disease. J Emerg Med. 2007;32:209–10. doi: 10.1016/j.jemermed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 97.Centocor, Inc . Package Insert. Malvern: Centocor, Inc; 2002. Remicade (infliximab) for IV injection. [Google Scholar]
- 98.Seiderer J, Goke B, Ochsenkuhn T. Safety aspects of infliximab in inflammatory bowel disease patients. A retrospective cohort study in 100 patients of a German University Hospital. Digestion. 2004;70:3–9. doi: 10.1159/000080075. [DOI] [PubMed] [Google Scholar]
- 99.Gamarra RM, McGraw SD, Drelichman VS, Maas LC. Serum sickness-like reactions in patients receiving intravenous infliximab. J Emerg Med. 2006;30:41–4. doi: 10.1016/j.jemermed.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 100.Rutgeerts P, Van Assche G, Vermeire S. Review article: Infliximab therapy for inflammatory bowel disease – Seven years on. Aliment Pharmacol Ther. 2006;23:451–63. doi: 10.1111/j.1365-2036.2006.02786.x. [DOI] [PubMed] [Google Scholar]
- 101.Duburque C, Lelong J, Iacob R, et al. Successful induction of tolerance to infliximab in patients with Crohn’s disease and prior severe infusion reactions. Aliment Pharmacol Ther. 2006;24:851–8. doi: 10.1111/j.1365-2036.2006.03026.x. [DOI] [PubMed] [Google Scholar]
- 102.Freeman HJ, Flak B. Demyelination-like syndrome in Crohn’s disease after infliximab therapy. Can J Gastroenterol. 2005;19:313–6. doi: 10.1155/2005/358658. [DOI] [PubMed] [Google Scholar]
- 103.Thomas CW, Jr, Weinshenker BG, Sandborn WJ. Demyelination during anti-tumor necrosis factor alpha therapy with infliximab for Crohn’s disease. Inflamm Bowel Dis. 2004;10:28–31. doi: 10.1097/00054725-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 104.Vadikolias K, Kouklakis G, Heliopoulos I, et al. Acute paraplegia after the initiation of anti-tumour necrosis factor-alpha therapy for Crohn’s disease. Eur J Gastroenterol Hepatol. 2007;19:159–62. doi: 10.1097/01.meg.0000250589.45984.b4. [DOI] [PubMed] [Google Scholar]
- 105.Chung JH, Van Stavern GP, Frohman LP, Turbin RE. Adalimumab-associated optic neuritis. J Neurol Sci. 2006;244:133–6. doi: 10.1016/j.jns.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 106.Shin IJ, Baer AN, Kwon HJ, Papadopoulos EJ, Siegel JN. Guillain-Barre and Miller Fisher syndromes occurring with tumor necrosis factor alpha antagonist therapy. Arthritis Rheum. 2006;54:1429–34. doi: 10.1002/art.21814. [DOI] [PubMed] [Google Scholar]
- 107.Centocor Inc Product Monograph Remicade November22007. 2007Malvern: Centocor Inc [Google Scholar]
- 108.Abbott Laboratories Limited . Product Monograph. Humira. St-Laurent: Abbott Laboratories Limited; Jan 23, 2008. [Google Scholar]
- 109.Gupta G, Gelfand JM, Lewis JD. Increased risk for demyelinating diseases in patients with inflammatory bowel disease. Gastroenterology. 2005;129:1117–20. doi: 10.1053/j.gastro.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 110.Wolfe F, Michaud K. Biologic treatment of rheumatoid arthritis and the risk of malignancy: Analyses from a large US observational study. Arthritis Rheum. 2007;56:2886–95. doi: 10.1002/art.22864. [DOI] [PubMed] [Google Scholar]
- 111.Peyrin-Biroulet L, Deltenre P, de Suray N, Branche J, Sandborn WJ, Colombel JF. Efficacy and safety of tumor necrosis factor antagonists in Crohn’s disease: Meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol. 2008;6:644–53. doi: 10.1016/j.cgh.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 112.Lichtenstein GR, Cohen RD, Feagan BG, et al. Safety of infliximab and other Crohn’s disease therapies – Treat™ registry data with nearly 15,000 patient-years of follow-up. Gastroenterology. 2006;130:A-71. [Google Scholar]
- 113.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: A population-based study. Cancer. 2001;91:854–62. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 114.Wolfe F, Michaud K. The effect of methotrexate and anti-tumor necrosis factor therapy on the risk of lymphoma in rheumatoid arthritis in 19,562 patients during 89,710 person-years of observation. Arthritis Rheum. 2007;56:1433–9. doi: 10.1002/art.22579. [DOI] [PubMed] [Google Scholar]
- 115.Shale M, Kanfer E, Panaccione R, Ghosh S. Hepatosplenic T-Cell lymphoma in inflammatory bowel disease. Gut. 2008;57:1639–41. doi: 10.1136/gut.2008.163279. [DOI] [PubMed] [Google Scholar]
- 116.Rosh JR, Gross T, Mamula P, Griffiths A, Hyams J. Hepatosplenic T-cell lymphoma in adolescents and young adults with Crohn’s disease: A cautionary tale? Inflamm Bowel Dis. 2007;13:1024–30. doi: 10.1002/ibd.20169. [DOI] [PubMed] [Google Scholar]
- 117.Mackey AC, Green L, Liang LC, Dinndorf P, Avigan M. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:265–7. doi: 10.1097/MPG.0b013e31802f6424. [DOI] [PubMed] [Google Scholar]
- 118.Brown ER, Charles KA, Hoare SA, et al. A clinical study assessing the tolerability and biological effects of infliximab, a TNF-alpha inhibitor, in patients with advanced cancer. Ann Oncol. 2008;19:1340–6. doi: 10.1093/annonc/mdn054. [DOI] [PubMed] [Google Scholar]
- 119.Vasilopoulos S, Kugathasan S, Saeian K, et al. Intestinal strictures complicating initially successful infliximab treatment for luminal Crohn’s disease Am J Gastroenterol 2000952503(Abst). [Google Scholar]
- 120.Toy LS, Scherl EJ, Kornbluth A, et al. Complete bowel obstruction following initial response to infliximab therapy for Crohn’s disease: A series of a newly described complication. Gastroenterology. 2000;118:A2974. [Google Scholar]
- 121.Lichtenstein GR, Olson A, Travers S, et al. Factors associated with the development of intestinal strictures or obstructions in patients with Crohn’s disease. Am J Gastroenterol. 2006;101:1030–8. doi: 10.1111/j.1572-0241.2006.00463.x. [DOI] [PubMed] [Google Scholar]
- 122.Louis E, Boverie J, Dewit O, Baert F, De VM, D’Haens G. Treatment of small bowel subocclusive Crohn’s disease with infliximab: An open pilot study. Acta Gastroenterol Belg. 2007;70:15–9. [PubMed] [Google Scholar]
- 123.Burke JP, Ferrante M, Dejaegher K, et al. Transcriptomic analysis of intestinal fibrosis-associated gene expression in response to medical therapy in Crohn’s disease. Inflamm Bowel Dis. 2008 doi: 10.1002/ibd.20482. [DOI] [PubMed] [Google Scholar]
- 124.Knapp AB, Mirsky FJ, Dillon EH, Korelitz BI. Successful infliximab therapy for a duodenal stricture caused by Crohn’s disease. Inflamm Bowel Dis. 2005;11:1123–5. doi: 10.1097/01.mib.0000191612.43584.94. [DOI] [PubMed] [Google Scholar]
- 125.Sorrentino D, Avellini C, Beltrami CA, Pasqual E, Zearo E. Selective effect of infliximab on the inflammatory component of a colonic stricture in Crohn’s disease. Int J Colorectal Dis. 2006;21:276–81. doi: 10.1007/s00384-005-0739-0. [DOI] [PubMed] [Google Scholar]
- 126.Swaminath A, Lichtiger S. Dilation of colonic strictures by intralesional injection of infliximab in patients with Crohn’s colitis. Inflamm Bowel Dis. 2008;14:213–6. doi: 10.1002/ibd.20318. [DOI] [PubMed] [Google Scholar]
- 127.Burke JP, Ferrante M, Dejaegher K, et al. Transcriptomic analysis of intestinal fibrosis-associated gene expression in response to medical therapy in Crohn’s disease. Inflamm Bowel Dis. 2008 doi: 10.1002/ibd.20482. [DOI] [PubMed] [Google Scholar]
- 128.Sorrentino D, Avellini C, Beltrami CA, Pasqual E, Zearo E. Selective effect of infliximab on the inflammatory component of a colonic stricture in Crohn’s disease. Int J Colorectal Dis. 2006;21:276–81. doi: 10.1007/s00384-005-0739-0. [DOI] [PubMed] [Google Scholar]
- 129.Aberra FN, Stettler N, Brensinger C, Lichtenstein GR, Lewis JD. Risk for active tuberculosis in inflammatory bowel disease patients. Clin Gastroenterol Hepatol. 2007;5:1070–5. doi: 10.1016/j.cgh.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 130.Gomez-Reino JJ, Carmona L, Angel DM. Risk of tuberculosis in patients treated with tumor necrosis factor antagonists due to incomplete prevention of reactivation of latent infection. Arthritis Rheum. 2007;57:756–61. doi: 10.1002/art.22768. [DOI] [PubMed] [Google Scholar]
- 131.Carmona L, Gomez-Reino JJ, Rodriguez-Valverde V, et al. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum. 2005;52:1766–72. doi: 10.1002/art.21043. [DOI] [PubMed] [Google Scholar]
- 132.Matulis G, Juni P, Villiger PM, Gadola SD. Detection of latent tuberculosis in immunosuppressed patients with autoimmune diseases: Performance of a Mycobacterium tuberculosis antigen-specific interferon gamma assay. Ann Rheum Dis. 2008;67:84–90. doi: 10.1136/ard.2007.070789. [DOI] [PubMed] [Google Scholar]
- 133.Centers for Disease Control and Prevention (CDC) Tuberculosis associated with blocking agents against tumor necrosis factor-alpha California, 2002–2003. Morb Mortal Wkly Rep. 2004;53:683–6. [PubMed] [Google Scholar]
- 134.Beltran B, Nos P, Bastida G, Iborra M, Hoyos M, Ponce J. Safe and effective application of anti-TNF-alpha in a patient infected with HIV and concomitant Crohn’s disease. Gut. 2006;55:1670–1. doi: 10.1136/gut.2006.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Filippi J, Roger P-M, Schneider SM, et al. Infliximab and human immunodeficiency virus infection: Viral load reduction and CD4+ T-cell loss related to apoptosis. Arch Intern Med. 2006;166:1783–4. doi: 10.1001/archinte.166.16.1783. [DOI] [PubMed] [Google Scholar]
- 136.Colbert C, Chavarria A, Berkelhammer C. Fulminant hepatic failure in chronic hepatitis B on withdrawal of corticosteroids, azathioprine and infliximab for Crohn’s disease. Inflamm Bowel Dis. 2007;13:1453–4. doi: 10.1002/ibd.20216. [DOI] [PubMed] [Google Scholar]
- 137.Michel M, Duvoux C, Hezode C, Cherqui D. Fulminant hepatitis after infliximab in a patient with hepatitis B virus treated for an adult onset Still’s disease. J Rheumatol. 2003;30:1624–5. [PubMed] [Google Scholar]
- 138.Carroll MB, Bond MI. Use of tumor necrosis factor-alpha inhibitors in patients with chronic hepatitis B infection. Semin Arth Rheum. 2008;38:208–17. doi: 10.1016/j.semarthrit.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 139.Nathan DM, Angus PW, Gibson PR. Hepatitis B and C virus infections and anti-tumor necrosis factor-alpha therapy: Guidelines for clinical approach. J Gastroenterol Hepatol. 2006;21:1366–71. doi: 10.1111/j.1440-1746.2006.04559.x. [DOI] [PubMed] [Google Scholar]
- 140.Sakellariou GT, Chatzigiannis I. Long-term anti-TNFalpha therapy for ankylosing spondylitis in two patients with chronic HBV infection. Clin Rheumatol. 2007;26:950–2. doi: 10.1007/s10067-006-0392-1. [DOI] [PubMed] [Google Scholar]
- 141.Schering Plough Effect of Infliximab on the Efficacy of Peg-Intron/Ribavirin in Patients With Hepatitis C (Study P04257AM1) < http://clinicaltrials.gov> (Version current at January 9, 2009)
- 142.Pascher A, Klupp J, Langrehr JM, Neuhaus P. Anti-TNF alpha therapy for acute rejection in intestinal transplantation. Transplant Proc. 2005;37:1635–6. doi: 10.1016/j.transproceed.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 143.Jacobsohn DA, Hallick J, Anders V, McMillan S, Morris L, Vogelsang GB. Infliximab for steroid-refractory acute GVHD: A case series. Am J Hematol. 2003;74:119–24. doi: 10.1002/ajh.10392. [DOI] [PubMed] [Google Scholar]
- 144.Lal S, Steinhart HA. Infliximab for ulcerative colitis following liver transplantation. Eur J Gastroenterol Hepatol. 2007;19:277–80. doi: 10.1097/MEG.0b013e3280116ccc. [DOI] [PubMed] [Google Scholar]
- 145.Tamaz R, Bitton A, Seidman EG, Deschenes M, Bouin M, Marleau D. New-onset IBD occurring after solid-organ transplantation. Gastroenterology. 2008;134:A-501. (Abstr T1177) [Google Scholar]
- 146.Hudson M, Flett G, Sinclair TS, Brunt PW, Templeton A, Mowat NA. Fertility and pregnancy in inflammatory bowel disease. Int J Gynaecol Obstet. 1997;58:229–37. doi: 10.1016/s0020-7292(97)00088-x. [DOI] [PubMed] [Google Scholar]
- 147.Ventura SJ, Mosher WD, Curtin SC, et al. Trends in pregnancies and pregnancy rates by outcome: Estimates for the United States, 1976–96. Vital Health Stat. 2000;21:1–47. [PubMed] [Google Scholar]
- 148.Katz JA, Antoni C, Keenan GF, Smith DE, Jacobs SJ, Lichtenstein GR. Outcome of pregnancy in women receiving infliximab for the treatment of Crohn’s disease and rheumatoid arthritis. Am J Gastroenterol. 2004;99:2385–92. doi: 10.1111/j.1572-0241.2004.30186.x. [DOI] [PubMed] [Google Scholar]
- 149.Chambers CD, Johnson DL, Jones KL. Pregnancy outcome in women exposed to adalimumab: The OTIS autoimmune diseases in pregnancy project. Arthritis Rheum. 2006;54:S251. (Abst) [Google Scholar]
- 150.Vasiliauskas EA, Church JA, Silverman N, Barry M, Targan SR, Dubinsky MC. Case report: Evidence for transplacental transfer of maternally administered infliximab to the newborn. Clin Gastroenterol Hepatol. 2006;4:1255–8. doi: 10.1016/j.cgh.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 151.van der Heijde D, Dijkmans B, Geusens P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: Results of a randomized, placebo-controlled trial (ASSERT) Arthritis Rheum. 2005;52:582–91. doi: 10.1002/art.20852. [DOI] [PubMed] [Google Scholar]
- 152.Faubion WA, Tung J. Growth failure in pediatric inflammatory bowel disease: Prevalence, risk factors, and treatment. Pract Gastroenterol. 2006;30:14–29. [Google Scholar]
- 153.Borrelli O, Bascietto C, Viola F, et al. Infliximab heals intestinal inflammatory lesions and restores growth in children with Crohn’s disease. Digest Liv Dis. 2004;36:342–7. doi: 10.1016/j.dld.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 154.Walters TD, Gilman AR, Griffiths AM. Linear growth improves during infliximab therapy in children with chronically active severe Crohn’s disease. Inflamm Bowel Dis. 2007;13:424–30. doi: 10.1002/ibd.20069. [DOI] [PubMed] [Google Scholar]
- 155.Bartram SA, Peaston RT, Rawlings DJ, Walshaw D, Francis RM, Thompson NP. Mutifactorial analysis of risk factors for reduced bone mineral density in patients with Crohn’s disease. World J Gastroenterol. 2006;12:5680–6. doi: 10.3748/wjg.v12.i35.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gilman J, Shanahan F, Cashman KD. Altered levels of biochemical indices of bone turnover and bone-related vitamins in patients with Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1007–16. doi: 10.1111/j.1365-2036.2006.02835.x. [DOI] [PubMed] [Google Scholar]
- 157.Sylvester FA, Wyzga N, Hyams JS, et al. Natural history of bone metabolism and bone mineral density in children with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:42–50. doi: 10.1002/ibd.20006. [DOI] [PubMed] [Google Scholar]
- 158.von Scheven E, Gordon CM, Wypij D, Wertz M, Gallagher KT, Bachrach L. Variable deficits of bone mineral despite chronic glucocorticoid therapy in pediatric patients with inflammatory diseases: A Glaser Pediatric Research Network study. J Pediatr Endocrinol Metab. 2006;19:821–30. doi: 10.1515/jpem.2006.19.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Paganelli M, Albanese C, Borrelli O, et al. Inflammation is the main determinant of low bone mineral density in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:416–23. doi: 10.1002/ibd.20039. [DOI] [PubMed] [Google Scholar]
- 160.Bartram SA, Peaston RT, Rawlings DJ, Walshaw D, Francis RM, Thompson NP. Mutifactorial analysis of risk factors for reduced bone mineral density in patients with Crohn’s disease. World J Gastroenterol. 2006;12:5680–6. doi: 10.3748/wjg.v12.i35.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gilman J, Shanahan F, Cashman KD. Altered levels of biochemical indices of bone turnover and bone-related vitamins in patients with Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1007–16. doi: 10.1111/j.1365-2036.2006.02835.x. [DOI] [PubMed] [Google Scholar]
- 162.Sylvester FA, Wyzga N, Hyams JS, et al. Natural history of bone metabolism and bone mineral density in children with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:42–50. doi: 10.1002/ibd.20006. [DOI] [PubMed] [Google Scholar]
- 163.Paganelli M, Albanese C, Borrelli O, et al. Inflammation is the main determinant of low bone mineral density in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:416–23. doi: 10.1002/ibd.20039. [DOI] [PubMed] [Google Scholar]
- 164.Centocor Inc . PSUR17 Data File. Malvern: Centocor; 2008. [Google Scholar]
- 165.Amgen and Wyeth Pharmaceuticals Enbrel - Etanercept. < http://www.enbrel.com/> (Version current at September 22 2008).
- 166.Abbott Laboratories Limited Humira - Adalimumab. < http://www.humirapro.com/> (Version current at September 22 2008).
- 167.Herfarth H, Obermeier F, Andus T, et al. Improvement of arthritis and arthralgia after treatment with infliximab (Remicade) in a German prospective, open-label, multicenter trial in refractory Crohn’s disease. Am J Gastroenterol. 2002;97:2688–90. doi: 10.1111/j.1572-0241.2002.06064.x. [DOI] [PubMed] [Google Scholar]
- 168.Kaufman I, Caspi D, Yeshurun D, Dotan I, Yaron M, Elkayam O. The effect of infliximab on extraintestinal manifestations of Crohn’s disease. Rheumatol Int. 2005;25:406–10. doi: 10.1007/s00296-004-0467-8. [DOI] [PubMed] [Google Scholar]
- 169.Rednic S, Marinescu C, Chira R, Rogojan L, Rednic N. Treatment with infliximab in a patient with ankylosing spondylitis and Crohn’s disease. J Gastrointestin Liver Dis. 2006;15:379–82. [PubMed] [Google Scholar]
- 170.Generini S, Giacomelli R, Fedi R, et al. Infliximab in spondyloarthropathy associated with Crohn’s disease: An open study on the efficacy of inducing and maintaining remission of musculoskeletal and gut manifestations. Ann Rheum Dis. 2004;63:1664–9. doi: 10.1136/ard.2003.012450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Batres LA, Mamula P, Baldassano RN. Resolution of severe peristomal pyoderma gangrenosum with infliximab in a child with Crohn’s disease. J Pediatr Gastroenterol Nutr. 2002;34:558–60. doi: 10.1097/00005176-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 172.Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: A randomised, double blind, placebo controlled trial. Gut. 2006;55:505–9. doi: 10.1136/gut.2005.074815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ferkolj I, Hocevar A, Golouh R, Voljc MD. Infliximab for treatment of resistant pyoderma gangrenosum associated with Crohn’s disease. Acta Dermatovenerol Alp Panonica Adriat. 2006;15:173–7. [PubMed] [Google Scholar]
- 174.Hewitt D, Tait C. Use of infliximab in pyoderma gangrenosum. Australas J Dermatol. 2007;48:95–8. doi: 10.1111/j.1440-0960.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- 175.Grange F, Djilali-Bouzina F, Weiss AM, Polette A, Guillaume JC. Corticosteroid-resistant pyoderma gangrenosum associated with Crohn’s disease: Rapid cure with infliximab. Dermatology. 2002;205:278–80. doi: 10.1159/000065860. [DOI] [PubMed] [Google Scholar]
- 176.Kugathasan S, Miranda A, Nocton J, Drolet BA, Raasch C, Binion DG. Dermatologic manifestations of Crohn disease in children: Response to infliximab. J Pediatr Gastroenterol Nutr. 2003;37:150–4. doi: 10.1097/00005176-200308000-00013. [DOI] [PubMed] [Google Scholar]
- 177.Ljung T, Staun M, Grove O, Fausa O, Vatn MH, Hellstrom PM. Pyoderma gangrenosum associated with Crohn disease: Effect of TNF-alpha blockade with infliximab. Scand J Gastroenterol. 2002;37:1108–10. doi: 10.1080/003655202320378338. [DOI] [PubMed] [Google Scholar]
- 178.Mimouni D, Anhalt GJ, Kouba DJ, Nousari HC. Infliximab for peristomal pyoderma gangrenosum. Br J Dermatol. 2003;148:813–6. doi: 10.1046/j.1365-2133.2003.05294.x. [DOI] [PubMed] [Google Scholar]
- 179.Regueiro M, Valentine J, Plevy S, Fleisher MR, Lichtenstein GR. Infliximab for treatment of pyoderma gangrenosum associated with inflammatory bowel disease. Am J Gastroenterol. 2003;98:1821–6. doi: 10.1111/j.1572-0241.2003.07581.x. [DOI] [PubMed] [Google Scholar]
- 180.Sapienza MS, Cohen S, Dimarino AJ. Treatment of pyoderma gangrenosum with infliximab in Crohn’s disease. Dig Dis Sci. 2004;49:1454–7. doi: 10.1023/b:ddas.0000042245.20042.4f. [DOI] [PubMed] [Google Scholar]
- 181.Triantafillidis JK, Cheracakis P, Sklavaina M, Apostolopoulou K. Favorable response to infliximab treatment in a patient with active Crohn disease and pyoderma gangrenosum. Scand J Gastroenterol. 2002;37:863–5. [PubMed] [Google Scholar]
- 182.Zaccagna A, Bertone A, Puiatti P, et al. Anti-tumor necrosis factor alpha monoclonal antibody (infliximab) for the treatment of pyoderma gangrenosum associated with Crohn’s disease. Eur J Dermatol. 2003;13:258–60. [PubMed] [Google Scholar]
- 183.Fries W, Giofre MR, Catanoso M, Lo GR. Treatment of acute uveitis associated with Crohn’s disease and sacroileitis with infliximab. Am J Gastroenterol. 2002;97:499–500. doi: 10.1111/j.1572-0241.2002.05514.x. [DOI] [PubMed] [Google Scholar]