Abstract
Synaptotagmins are a family of proteins that function in membrane fusion events, including synaptic vesicle exocytosis. Within this family, synaptotagmin IV (Syt IV) is unique in being a depolarization-induced immediate early gene (IEG). Experimental perturbation of Syt IV modulates neurotransmitter release in mice, flies, and PC12 cells, and modulates learning in mice. Despite these features, induction of Syt IV expression by a natural behavior has not been previously reported. We used the zebra finch, a songbird species, to investigate Syt IV because song is a naturally learned behavior whose neuro-anatomical basis is largely identified. We observed that, similar to rodents, Syt IV is inducible in songbirds. This induction was selective and depended on the nature of neuronal depolarization. Generalized seizures caused by the GABAA receptor antagonist, metrazole, induced the IEG, ZENK, in zebra finch brain. However, these same seizures failed to induce Syt IV in song control areas. In contrast, when nontreated birds sang, three song control areas showed striking Syt IV induction. Further, this induction appeared sensitive to the social context in which song was sung. Together, these data suggest that neural activity during singing can drive Syt IV expression within song circuitry whereas generalized seizure activity fails to do so even though song control areas are depolarized. Our findings indicate that, within this neural circuit for a procedurally learned sensorimotor behavior, Syt IV is selective and requires precisely patterned neural activity and/or neuro-modulation associated with singing.
Keywords: immediate early gene, motor-driven, social regulation, birdsong, vocal
INTRODUCTION
Immediate early genes (IEGs) offer a promising entry point to detect early changes in the molecular makeup of neural circuits that underlie learning. Unlike many IEG-encoded proteins that serve as transcription factors and thus can influence cellular events only through downstream genes, synaptotagmin IV (Syt IV) is a membrane trafficking-related protein and thus can directly alter the cell biology of neurons (reviewed in Sudhof, 2002). The synaptotagmin family of proteins is characterized by a transmembrane domain and two C2 calcium-binding domains. Synaptotagmin family members are thought to function in membrane fusion events. Accordingly, synaptotagmins I and II (Syt I and Syt II) participate in presynaptic calcium-mediated vesicle exocytosis. In contrast, the localization and function of Syt IV is less clear. In various studies, anti-Syt IV antibodies have localized the protein to presynaptic membranes, to postsynaptic membranes, to endosomal compartments, or to glia (Berton et al., 2000; Fukuda et al., 2001; Ibata et al., 2002; Zhang et al., 2004; Yoshihara et al., 2005).
Similarly, various functions have been proposed for Syt IV. Like all synaptotagmins, Syt IV contains the highly conserved C2A and C2B calcium-binding domains, but an amino acid substitution found in the C2A domain of Syt IV (position 244 in mammals and birds, and position 284 in fly) decreases Syt IV's affinity for calcium under some conditions (von Poser et al., 1997; Littleton et al., 1999; Dai et al., 2004). Unlike other synaptotagmins, Syt IV is an IEG; its mRNA is induced by seizure-producing agents in rodent hippocampus, and by forskolin treatment or potassium depolarization of PC12 pheochromocytoma cells exposed to a protein synthesis inhibitor (Vician et al., 1995). Together, these observations form the basis for the hypothesis that Syt IV is a short-term regulator of synaptic transmission. However, whether Syt IV antagonizes or mimics the function of Syt I as a presynaptic calcium sensor for neurotransmitter release remains to be resolved. The answer may depend on the system or organism studied and, in Syt IV transgenic studies, on the levels of transgene expression relative to levels of other synaptotagmins and of endogenous Syt IV (Ferguson et al., 1999; Littleton et al., 1999; Wang et al., 2001; Robinson et al., 2002; Dai et al., 2004; Machado et al., 2004; Yoshihara et al., 2005; Ting et al., 2006). Syt IV null mutant mice exhibit heightened forms of hippocampal short-term plasticity and are impaired in some forms of hippocampus-dependent memory including social transmission of food preference (Ferguson et al., 2000a, 2004). Despite these intriguing observations, behavioral induction of endogenous Syt IV expression has not been previously demonstrated in any species. Identification of a naturally occurring behavior that induces endogenous Syt IV to physiological levels and within a physiological context may thus provide an experimental system in which Syt IV function could be more reliably elucidated.
We used adult male zebra finches, which are oscine songbirds, to investigate Syt IV induction by neural activity associated with pharmacological versus behavioral events. These birds exhibit a well-characterized form of procedural, sensorimotor learning, namely learning to produce their courtship song, and they possess a sexually dimorphic neural circuit, known as the song circuit, which underlies this behavior (Fig. 1; Nottebohm and Arnold, 1976; Reiner et al., 2004). The song circuit consists of two interconnected pathways, a vocal motor pathway and an anterior forebrain pathway (AFP). The vocal motor pathway includes a cortical-like pallial nucleus known as HVC, which connects to another pallial region called the robust nucleus of the arcopallium (RA) (Nottebohm et al., 1982; Wild, 1993; Sturdy et al., 2003). The firing of neurons in these areas is directly premotor to song output (Yu and Margoliash, 1996; Hahnloser et al., 2002). The AFP forms a pallio-basal ganglia–thalamo-pallial loop with input from HVC and output to RA of the vocal motor pathway by way of specialized sub-regions (reviewed in Bottjer and Arnold, 1997; Farries, 2001; Jarvis, 2004). The AFP is necessary for song modification during development and for song maintenance in adulthood when neurons in this pathway still exhibit vigorous firing patterns during singing (Bottjer et al., 1984; Sohrabji et al., 1990; Scharff and Nottebohm, 1991; Hessler and Doupe, 1999a,b; Williams and Mehta, 1999; Brainard and Doupe, 2000; Kao et al., 2005; Olveczky et al., 2005).
Figure 1.
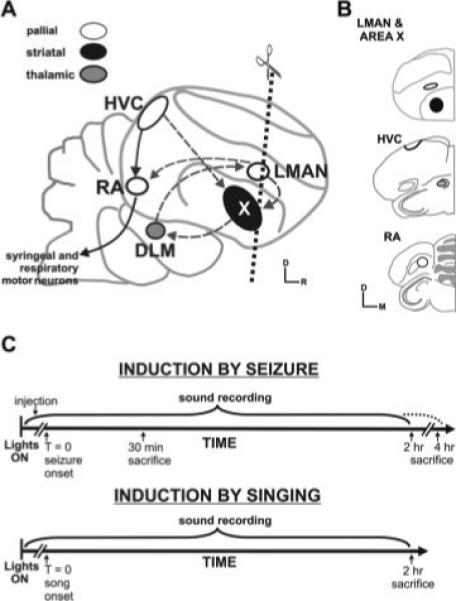
Avian song circuit and experimental set up. (A) Schematic sagittal diagram of the avian song circuit depicts two interconnected pathways. The vocal motor pathway (solid black arrows connecting white circles) controls song production and is composed of the cortical-like pallial nuclei HVC and RA and the brainstem motor neurons that innervate the vocal organ and respiratory muscles. The AFP (dashed arrows) allows song modification and is composed of a subset of HVC neurons, striatal Area X (black), thalamic DLM (gray), and pallial LMAN (white). Projections of LMAN neurons join the two pathways at RA, and these same neurons send axon collaterals back to Area X. Dotted line indicates the angle of coronal plane used to generate brain sections. (B) Drawings made from Nissl-stained material show representative hemicoronal sections used for gene expression analysis. Bold outlines indicate song nuclei that were the focus of study. D, dorsal; DLM, medial portion of the dorsolateral nucleus of the anterior thalamus; HVC, HVC (used as the proper name), LMAN, lateral magnocellular nucleus of the anterior nidopallium; M, medial; R, rostral; RA, robust nucleus of the arcopallium; St, striatum; X, song nucleus Area X within the medial striatum. (C) Experimental time-lines used to test for seizure-driven or singing-driven gene induction. T = 0 indicates the onset of either seizure or song in experimental groups. Birds were continuously monitored for singing activity. Birds were sacrificed at one of three time-points: 30 min, 2 or 4 h.
Zebra finch singing behavior has been classified into two broad categories (Zann, 1996): “directed” singing refers to when males sing towards a female conspecific during social interactions; “undirected” singing is when males sing alone or in the presence of, but not towards, conspecifics (Jarvis et al., 1998). These two socially distinct yet acoustically similar behaviors are accompanied by distinct brain activation patterns (Hessler and Doupe, 1999a), and expression of the IEG ZENK (acronym for zif-268, egr-1, NGFI-A, Krox-24) is robust within Area X of birds singing undirected songs compared with those singing directed songs (Jarvis et al., 1998).
We examined Syt IV induction in adult birds by pharmacological seizure, and by singing, including singing in different social contexts. We used the GABAA receptor antagonist, metrazole, to induce seizures and used resultant expression patterns of ZENK to confirm that depolarization occurred broadly throughout the telencephalon, as in Mello and Clayton (1995). As expected from rodent studies (Vician et al., 1995), we find that Syt IV gene expression is inducible in birds following seizures. Moderate Syt IV, but no Syt I, induction occurs in non-song control areas following seizures. Surprisingly, in the song circuit, we did not observe changes in Syt IV gene expression in response to metrazole-induced depolarization, even though these regions contain GABAA receptors (Schmidt and Perkel, 1998; Spiro et al., 1999; Rosen and Mooney, 2000). In contrast, when adult male zebra finches are allowed to sing, Syt IV expression is induced in three song circuit nuclei: the lateral magnocellular nucleus of the anterior nidopallium (LMAN), HVC, and RA. Further, we find that induction in LMAN depends upon the social context of singing. Our examination of Syt IV expression levels as a function of multiple forms of neural activation (seizure, directed singing, or undirected singing) reveals that Syt IV induction is selective and depends upon the nature of neuronal depolarization. The specific requirement for singing-related, but not seizure-related, neural activity for Syt IV induction within vocal control neurons demonstrates active regulation of Syt IV during production of learned vocalizations in adulthood.
METHODS
Figure 1 shows the experimental timeline used to investigate Syt IV induction by seizure or by singing as well as a schematic of the neuroanatomical areas relevant to these experiments.
Animals and Tissues
Adult (101−700 days of age, mean = 209 days) male zebra finches (Taeniopygia guttata) from our closed breeding colony were maintained on a 14:10 h light/dark photoperiod and fed ad libitum. Thirty two birds were analyzed. The University of California at Los Angeles Institutional Animal Care and Use Committee approved all animal use.
Metrazole Administration
Metrazole was administered following previously established protocols (Mello and Clayton, 1995). Briefly, in the morning after ‘lights on’, birds (12.8 ± 0.3 g) were injected intrapectorally either with saline or with metrazole (0.06 mg/g of bird weight; 0.02 mg/μL), a GABAA receptor antagonist and convulsant. Injection volumes ranged from 30 to 55 μL. Metrazole-injected birds exhibited convulsions characterized by loss of balance, rapid, uncoordinated movement and then stiffening, and unstructured (non-song) vocalizations. These seizures generally began 2 min after injection and lasted for a few minutes. On two occasions, seizure onset occurred 7−8 min after the injection and lasted for ∼10 min. Following recovery, most birds exhibited normal daily activity or long periods of quiescence. In some birds, occasional additional seizures occurred. This variable response to metrazole between individuals was not correlated with the amount of metrazole birds received. To restrict this portion of the study to the effects of pharmacological depolarization, birds that sang during or after seizure induction were subsequently excluded from the study.
Behavioral Monitoring
For investigating the effects of seizure activity, males were sacrificed at 30 min, 2 or 4 h after seizure onset (metrazole-injected birds) or saline injection (control birds). For the 30 min and 2 h time-points, males were kept in an undisturbed place and continuously monitored for general activity and for singing. For the 4-h time-point, males were individually placed in a sound attenuation chamber (Acoustic Systems, Austin, TX) where all sounds were recorded for detection of any singing.
For investigating the effects of singing-related activity, males were acclimated to the experimental environment for 1−2 days by individually housing them in cages placed inside a sound attenuation chamber. Introduction of a female zebra finch to the chamber the night prior to behavioral recording was used to encourage the male to sing the following morning. Initially, we used females simply for this purpose and were not concerned about whether males directed all of their songs to the females. However, later analysis (see below) revealed that, although housed with a female, males sang some undirected songs. We thus refer to this group of singing males as ‘mixed-singers’ to indicate that they sang both directed and undirected songs. On the morning of the experiment, sound recording began at ‘lights on’, as previously described (Livingston et al., 2000). To maximize likelihood of observing motor-driven gene expression (Jarvis and Nottebohm, 1997), only birds that sang a minimum of 70 song bouts were included in the comparison with non-singing birds. Non-singing birds were housed like the mixed-singers but without females and were inhibited from singing by the investigator sitting near the cage during behavioral monitoring. Only males that did not sing or sang two bouts maximum were included.
For investigation of whether the social context of singing affects Syt IV expression patterns, we tested two additional groups of males: those that sang 100% of their songs to females (‘directed-singers’) and those that sang alone (‘undirected-singers’). For the directed-singers, individual males were presented with a different female every 3−4 min for 2 h while their singing behavior, including body orientation and posture (Zann, 1996), was closely observed. This frequent replacement of females resulted in performance of 100% directed songs. For undirected singing, individually housed males were allowed to sing for 2 h.
All birds were sacrificed 2 h after the onset of their first song bout (singing) or ‘lights on’ (non-singing controls). A bout was defined as one or a series of motifs separated from other motifs by more than 1 s.
Tissue Preparation
Birds were sacrificed by decapitation and their brains were rapidly extracted, frozen on aluminum floats on liquid nitrogen, and stored at −80°C until use. Brains were sectioned at 20 μm coronally using a cryostat (Microm HM560, Micron, Germany). Sections were thaw mounted onto seven sets of microscope slides (Superfrost plus, Fisher, Pittsburgh, PA) in a manner that created essentially replicate sets, allowing for multiple histological comparisons within one bird. Slides were kept at −80°C until processing.
Isolation of Zebra Finch Syt I cDNAs
Poly(A+) RNA was isolated from the brains of ≥40-day-old zebra finches using Oligotex mRNA Maxi kit (QIAGEN, Valencia, CA) and reversed transcribed using the Marathon cDNA Amplification kit (Beckman Dickinson Biosciences, Palo Alto, CA). The entire coding region of Syt I was obtained through PCR with primers designed based on the chicken Syt I 5′-untranslated region (UTR) and 3′UTR sequences (sense 5′- CGGCAAGCTGACTGTTGTC-3′; antisense 5′-GGCTGGAAATGAAAGGACCTA-3′). PCR cycling conditions using BD Advantage™ 2 PCR kit (Beckman Dickinson Biosciences) were (i) 2 min at 94°C for 1 cycle; (ii) 15 s at 94°C, 30 s at 64°C, and 1 min at 72°C for 45 cycles; and (iii) 1 min at 72°C for 1 cycle.
Amplified cDNAs were subcloned into pCR TOPO-4 vector (Invitrogen Corp., Carlsbad, CA) and sequenced in sense and antisense directions. Three independent clones were sequenced and the consensus sequence was deposited in the GenBank (accession: DQ267216).
Isolation of Zebra Finch Syt IV cDNAs
We screened a cDNA library made from 1–day-old male zebra finch telencephalon. Approximately 5 × 105 pfu were plated on XL1 Blue MRF’ cells, lifted onto nitrocellulose membranes (Schleicher & Schuell BioScience Inc., Keene, New Hampshire) and screened with a randomly primed, α32P-dCTP-labeled, probe prepared from a full length rat Syt IV clone (Vician et al., 1995) (Pharmacia Kit for Random Prime Probe Generation, Amersham Pharmacia Biotech, Piscataway, NJ). Individual positive plaques were isolated after three rounds of screening and in vivo excision was performed to extract (−) p-Bluescript (pBSII-SK, Stratagene, La Jolla, CA) from the phage. Clones were bi-directionally sequenced using T7 and M13 reverse primers. The putative Syt IV clone was then sequenced with an internal primer (5′-CTGCCAGCAATGGATGA-3′) to reconfirm that the Asp → Ser substitution characteristic of orthologous Syt IV C2A domains was present. The clone was then sequenced in sense and antisense directions with six different overlapping primers to confirm that there were no mismatches (GenBank accession: DQ267217).
Zebra Finch Syt I and Syt IV Probe Synthesis
The full length zebra finch Syt I was digested with Spe I to produce the sense cDNA template, or with Not I to produce the antisense cDNA template through in vitro transcription. A Syt IV DNA template, containing full coding sequence plus 3′-untranslated regions, was digested with Not I to produce the sense cDNA template, or with Bgl II to produce the antisense cDNA template. A second Syt IV DNA template consisting of only 3′UTR was obtained through PCR with primers designed based on the zebra finch Syt IV 3′UTR sequence (sense 5′- CAGGTTAGAGAGGGGAT-3′; antisense 5′-CAAATATGAGTCACTAATAAGTTAC-3′). Two identical clones with opposite orientations were digested with Bgl II to produce both the sense and antisense cDNA template of 341 bp. The linearized templates were then labeled with [33P] uridine triphosphate (GE Health-care, Piscataway, NJ) using Riboprobe Combination System-T3/T7 (Promega Corp, Madison, WI). The riboprobes were purified using BD Chroma spin columns (Beckman Dickinson Biosciences).
ZENK Probe Synthesis
A 1887 bp fragment encoding the canary ZENK cDNA (Mello et al., 1992) was digested with EcoR1 and then subcloned into pGEM7 vectors (Promega Corp.) in opposite orientations. These two clones were used to generate the sense and the antisense probes as described above.
In situ Hybridization
To analyze Syt I, Syt IV, and ZENK expression, we performed in situ hybridizations essentially as described in Teramitsu et al. (2004). Briefly, one of seven sets of brain sections was hybridized with one of six [33P] UTP-labeled riboprobes: Syt I sense, Syt I antisense, Syt IV sense, Syt IV antisense, ZENK sense, or ZENK antisense. The seventh set was stained with thionin blue to reveal Nissl substance (Tolivia and Tolivia, 1985) for identification of neuroanatomical structures and to guide localization of the expression patterns for each gene with reference to a songbird brain atlas (Stokes et al., 1974). Neuroanatomical terms for brain regions used current avian nomenclature (Reiner et al., 2004; Jarvis et al., 2005). Each in situ hybridization experiment contained slides from the two groups of birds under comparison (i.e. either metrazole-injected and saline-injected, mixed singing and non-singing, or undirected singing and directed singing). Following hybridization, slides were exposed to autoradiographic film (BioMax MR film, Eastman Kodak, Rochester, NY) for 18−24 h for Syt I or 24−48 h for Syt IV and ZENK. Slides were then dipped in liquid emulsion (Kodak NTB-2) and exposed at 4°C in lightproof boxes for approximately 2−4 weeks, depending on the relative abundance of the gene and on probe radioactivity levels. Emulsion-coated slides were developed in Kodak D19 developer, fixed in Kodak fixer, and counter stained with thionin blue.
Several criteria were applied in order to assign the observed radioactive labeling to specific neuroanatomical regions. For each anatomical designation: (1) labeling patterns were reliably detected within each group of birds, (2) labeling was observed bilaterally in each area, (3) labeling was observed in consecutive sections, (4) labeling was detected with antisense, but not with sense, probes.
Quantification of Gene Expression and Statistical Analyses
A trained observer blind to the treatment groups performed the initial round of quantification of gene expression. To quantify gene expression intensity for each brain region sampled, photomicrographs of autoradiograms were captured with a MTI DC-330E camera (Dage-MTI, inc. Michigan City, IN) attached to a Leica MZ APO microscope (Leica, Heerbrugg, Switzerland) and converted into an 8-bit digital image, using Adobe Photoshop® 7.0. For each experimental comparison (metrazole to saline, mixed singing to non-singing, or undirected singing to directed singing) images were captured with identical microscope settings and illumination conditions. An image from an adjacent Nissl-stained section was overlaid on photomicrographs to indicate the targeted area for quantification. Once an area was targeted, the Nissl image was removed. Mean pixel density (optical density, OD), was used as a measurement of labeling intensity. To minimize variability between in situ hybridizations, the OD measurement of a song nucleus or other region of interest [e.g. striatum (St) outside of Area X; (Fig. 1)] was normalized by dividing it by the OD of an outlying brain area chosen for its stable gene expression across treatment groups (selected areas of nidopallium; see Supplementary Table). Normalizing a signal from a region of interest using a stable signal from the same brain section removes variability across experiments due to the length of autoradiogram exposures. The Supplementary Table indicates the regions of interest and the corresponding nidopallial areas selected for normalization as well as the statistical tests that indicate that gene activity did not change in the latter areas as a function of treatment. Normalized measurements from consecutive sections were then averaged per bird. Thus, each bird generated one value per brain region. These values were averaged among birds in the same treatment group. The final value represents averaged normalized OD per brain region. A ratio value of 1 indicates that the mRNA in the region of interest and the outlying areas used for normalization are expressed at similar levels.
Quantification of emulsion-coated slides yielded more variable but comparable results to those obtained from film autoradiograms (simple linear regression of data subset; n = 23, r = 0.826, p = 0.001). Emulsion-coated slides require longer processing times; for this reason optical density values from autoradiograms are reported throughout the study.
JMP™ statistical analysis software (SAS Institute Inc., Cary, NC) was used to generate an analysis of variance and compute linear regressions. The dependent variable was expressed as the average normalized OD for each song region. The independent variable was treatment type: saline versus metrazole, non-singing versus mixed singing, directed singing versus undirected singing. Nonparametric Wilcoxon, with treatment type as a grouping variable, was used to test whether treatment had an effect on gene expression. Non-parametric statistics were chosen because the data set did not conform to parametric assumptions. Simple linear regressions were used to test whether gene expression levels correlated with amount of singing. Statistical values are reported in the Results and Discussion sections as average normalized OD ± standard error of the mean (SEM) unless otherwise noted. In graphs, error bars show SEM, while triangles indicate average values obtained for each subject. All tests are 1-way based on published observations that depolarization should increase Syt IV mRNA (e.g. Vician et al., 1995; n.s., nonsignificant).
LALIGN was used to generate percent identity scores for phylogenetic comparison of synaptotagmin sequences (Huang and Miller, 1991).
RESULTS
Isolation and Identification of Zebra Finch Syt I and Syt IV
Full length zebra finch Syt I and Syt IV cDNAs were isolated, as well as a 341 bp sequence from the Syt IV 3′UTR. Zebra finch Syt I and Syt IV coding sequences contain regions that correspond to the transmembrane and two C2 calcium-binding domains described for all members of the synaptotagmin family. The deduced amino acid identities between the functional domains of zebra finch Syt I and Syt IV and those of several orthologs are shown in Figure 2. As expected, the putative C2A domain of zebra finch Syt IV contains a single amino acid substitution (Asp → Ser; position 244) that has thus far been observed in animals as diverse as flies and nematodes (Littleton et al., 1999), zebra fish (Woods et al., 2005), and humans (Ferguson et al., 2000b).
Figure 2.
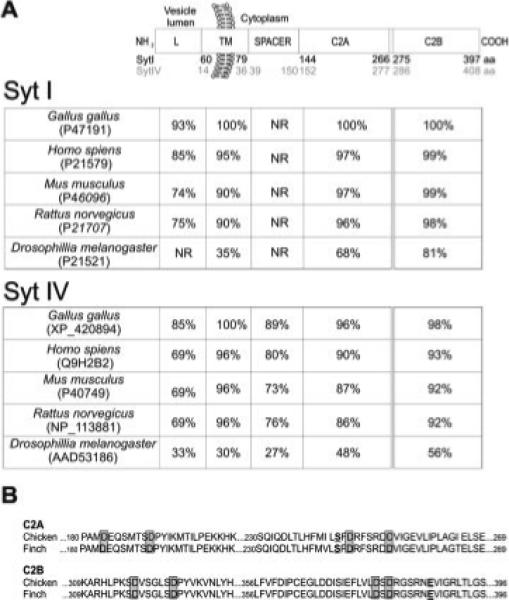
Domain structure and percent identity of zebra finch Syt I and Syt IV orthologs. (A) Top schematic highlights the Syt I and Syt IV functional domains. Approximate amino acid (aa) boundaries in the deduced zebra finch sequences are indicated for the luminal (L), transmembrane region (TM), spacer region (SPACER) and the two C2 calcium-binding domains (C2A and C2B) of Syt I (black) and Syt IV (gray). Beneath, two tables show the percent amino acid identity of zebra finch Syt I or Syt IV to corresponding sequences of chicken, mammals and fruit fly with GenBank accession numbers indicated. The range of residues that comprise the spacer region of Syt I has not been reported for mammalian and fly orthologs (NR). (B) Alignment of portions of the avian C2A and C2B domains highlights the conserved aspartate residues (gray boxes) thought to coordinate calcium binding. The amino acid substitution (Asp → Ser) characteristic of the Syt IV C2A domain is bolded and underlined. Within the C2B domain, a conservative substitution (Glu → Asp) occurs at position 386 that is thus far unique to avian sequences.
The full length zebra finch Syt I amino acid sequence was 93, 95, and 63% identical to Syt I in rat, human, and fly, respectively. The zebra finch Syt IV amino acid sequence was highly similar to mammalian orthologs: 85% identical to rat and 88% identical to human Syt IV. However, zebra finch Syt IV was only 48% identical to fly Syt IV. Dai et al. (2004) have suggested that there may be functional differences between Drosophila and mammalian Syt IV proteins. The low sequence conservation between the zebra finch and fly Syt IV orthologs (48%) suggests that zebra finch Syt IV might also be functionally distinct from fly Syt IV. In comparison, Syt I is thought to share the same function across species, supported here by the greater sequence conservation between zebra finch and fly Syt I (63%) These observations, together with the high sequence conservation between mammalian and zebra finch Syt IV orthologs (85−88%), suggest that zebra finch Syt IV resembles mammalian, more than Drosophila, Syt IV.
As expected, the zebra finch Syt I and Syt IV sequences were highly similar to those of the chicken (Fig. 2); zebra finch Syt I sequence was 99% identical to chicken Syt I and zebra finch Syt IV was 95% identical to chicken Syt IV. Zebra finch and chicken Syt IV shared identical amino acid substitutions at the putative calcium-binding sites within the C2A and C2B domains. Within the Syt IV C2B domain, there was a conservative avian-specific substitution (Glu → Asp, position 386) in one of the calcium-binding sites [Fig. 2(B)].
In situ hybridizations with either the Syt I or Syt IV full length riboprobes with brain sections of unstimulated (non-singing or saline-injected) birds revealed broad, robust expression of both synaptotagmins throughout the zebra finch telencephalon, with differences in their patterns. For example, the Syt I ribop-robe strongly hybridized within the ventral mesopallium (M; Supplementary Fig. 1) while the Syt IV probe produced more moderate signals (M; Fig. 3). These observations suggest that the riboprobes were specific for their respective sequences, as expected based on the low level of nucleotide identity (57%) between their entire coding regions. The difference in the inducibility of the two genes following depolarization (see below) further suggests that each probe was specific for its target. We confirmed this for Syt IV by comparing expression patterns obtained with the full length versus the 3′UTR Syt IV riboprobe. Indeed, the hybridization patterns were identical (Supplementary Fig. 2), indicating specific detection of Syt IV.
Figure 3.
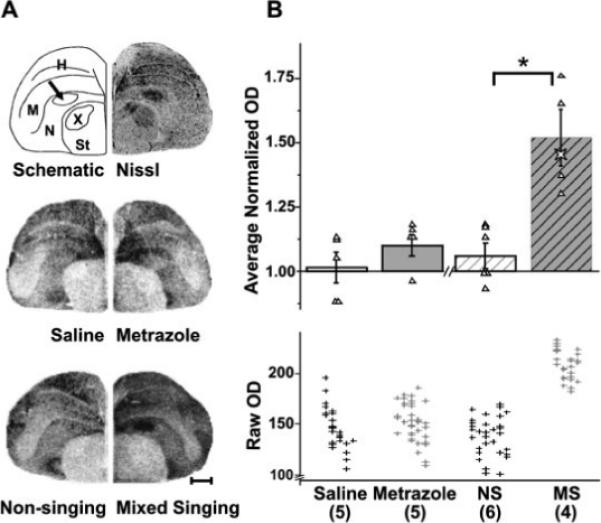
Singing, but not seizure, induces Syt IV in the song control nucleus LMAN. Syt IV expression in LMAN. (A) Top row shows a drawing (left) of the hemicoronal Nissl-stained section (right) at the level of LMAN (arrow) and indicates neuroanatomical boundaries used in gene expression analysis. Middle and bottom rows show representative photomicrographs of film autoradiograms from brain sections hybridized with an antisense probe for Syt IV. Scale bar, 1 mm. (B) The bar graph compares average normalized Syt IV mRNA levels in LMAN across treatment groups. ODs of Syt IV signals from saline-injected and metrazole-injected birds show similar intensities. In contrast, mixed singing birds show induced Syt IV mRNA expression relative to non-singing birds (*p = 0.010). Triangles indicate average values obtained for each subject, used to create the group means. The star symbol in the mixed singing group is from a singing bird used for a different study, but that was collected and processed identically to the singing birds presented here. Although not included in the statistical analyses, its Syt IV levels fit within those of the mixed singers, reinforcing the reliability of these results. The bottom scatter plot shows nonnormalized OD measurements that were used to generate the average normalized OD values shown above. Within each group cluster, measurements for an individual bird are aligned vertically. Data are expressed as means ± SEM. Numbers within parentheses indicate sample size. H, hyperpallium, M, mesopallium; N, nidopallium; NS, non-singing; MS, mixed singing; St, striatum; X, song nucleus Area X of the medial striatum.
Effects of Generalized Seizure Activity on ZENK and Synaptotagmin Expression
To determine whether Syt IV is inducible by seizure in birds, as in rodents (Vician et al., 1995), we administered the GABAA receptor antagonist, metrazole, or saline to male zebra finches, generally following the procedures of Mello and Clayton, 1995 (Fig. 1), and compared resultant brain levels of ZENK, Syt I, and Syt IV mRNAs. The IEG ZENK was selected as a positive control of general depolarization (in addition to the seizures themselves) because its robust induction following metrazole administration in zebra finches is well-characterized (Mello and Clayton, 1995). Syt I was chosen as a negative control because it is not an IEG; therefore its expression should not be modulated by neuronal depolarization (Mahata et al., 1992; Vician et al., 1995). ZENK, Syt I, and Syt IV mRNA expression levels were evaluated 30 min after seizure, the time at which ZENK induction is detectable following metrazole treatment (Mello and Clayton, 1995), and peaks following the onset of singing behavior (Jarvis and Nottebohm, 1997). Expression of all three transcripts was also assessed at 2 and 4 h post-seizure, consistent with the time-course for Syt IV induction in rats (Vician et al., 1995).
As expected, ZENK mRNA increased markedly in the telencephalon of metrazole-injected birds sacrificed 30 min after seizure onset, relative to saline-injected controls. This observation, coupled with the seizures themselves, confirmed the efficacy of our metrazole injection paradigm in depolarizing regions of the zebra finch brain (Supplementary Fig. 3) including song nuclei. Our ZENK data largely confirm those previously reported (Mello and Clayton, 1995). For example, using quantitative measures similar to those of Mello and Clayton (1995) we found a ∼2-fold increase in ZENK mRNA levels within song nucleus HVC at 30 min following seizure relative to levels in saline-injected controls (nonnormalized OD ± SEM: saline = 56 ± 2.6, n = 3; metrazole = 100 ± 20.0, n 3; p = 0.049).
Unlike ZENK, Syt I transcripts did not noticeably increase following metrazole-induced seizures, in any brain area, at any time-point (Supplementary Fig. 1). Syt I mRNA was broadly distributed in the zebra finch brain. In all treatment groups, Syt I expression was robust throughout the cortical-like pallium, with lower levels observed in the striatum. Among the song control regions, Syt I expression was high in HVC, but relatively low in LMAN, Area X and RA. Syt I exhibited regionalized expression within thalamic and brainstem structures and showed strong expression in cell layers of the optic tectum and cerebellum (Supplementary Fig. 1).
Although changes in Syt IV expression were observed at 30 min following metrazole-induced seizures, they were not robust (data not shown). By 2 h, however, increases in Syt IV mRNA levels were obvious in the brains of metrazole-injected birds relative to those of saline-injected birds (Fig. 4), and these changes largely persisted at 4 h (data not shown). Consequently, we quantified Syt IV signals at the 2-h time-point. Intriguingly, Syt IV induction by seizure appeared limited to non-song control areas (Fig. 4). For example, Syt IV expression in the striatum (St), outside of Area X, was ∼20% higher in metrazole-injected birds than that in saline-injected birds (saline = 0.83 ± 0.04, n = 5; metrazole = 0.99 ± 0.04, n = 5; p = 0.043). Of note, within Area X, the striatal region of songbirds that is dedicated to song (Sohrabji et al., 1990; Scharff and Nottebohm, 1991), there was no Syt IV induction (saline = 0.62 ± 0.04; n = 5; metrazole = 0.62 ± 0.03; n = 5; n.s., Fig. 4). The lack of Syt IV induction in Area X by seizure was in sharp contrast to the robust increase in ZENK levels within Area X that we and others observe at 30 min following metrazole administration (data not shown and Mello and Clayton, 1995). The nonsignificant statistics for other song control regions that lacked seizure-driven Syt IV induction are reported below in the section on singing, to aid comparison with the significant differences observed when birds sing.
Figure 4.
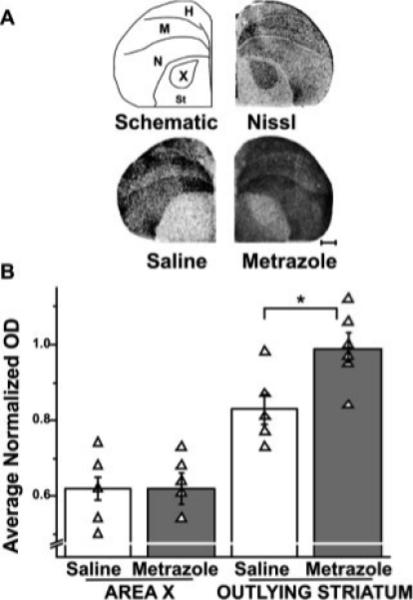
Syt IV induction by seizure is limited to non-song control regions. Syt IV induction in the striatum of metrazole-injected birds. (A) Top drawing (left) indicates neuroanatomical boundaries used in gene expression analysis. The drawing was made from the photomicrograph (right) showing a hemicoronal Nissl-stained section at the level of Area X. Beneath, photomicrographs of autoradio-grams from brain sections at the level of the striatum, hybridized with a Syt IV antisense probe. Scale bar, 1 mm. (B) Bar graphs compare normalized Syt IV mRNA levels in Area X and outlying striatum across treatment groups. Optical densities of Syt IV signals in Area X were low relative to the surrounding striatum in saline-injected birds (n = 5) and did not differ from those in metrazole-injected birds (n = 5). In contrast, Syt IV levels increase in the striatum outlying Area X following metrazole injection (*p 0.045). H, hyperpallium; M, mesopallium; N, nidopallium; St, striatum; X, song nucleus Area X of the medial striatum.
Syt IV null mutant mice are impaired on the rotorod test for motor skill (Ferguson et al., 2000a,b), a task that depends in part on cerebellar control (Lalonde et al., 1995). In normal rodents, Syt IV expression in the cerebellum is constitutively high (Vician et al., 1995; Berton et al., 1997; Ferguson et al., 2000a,b). Therefore, we examined Syt IV expression in the cerebellum of control birds and compared them with birds that had undergone convulsions. In both groups, Syt IV was robustly expressed within the Purkinje and granule cell layers, as indicated by silver grain signals that overlaid these regions on emulsion-dipped slides from all groups (Fig. 5). As described for mammals, these high basal levels were not affected by seizure (saline = 1.99 ± 0.10, n = 3; metrazole = 1.68 ± 0.14, n = 6, n.s.).
Figure 5.
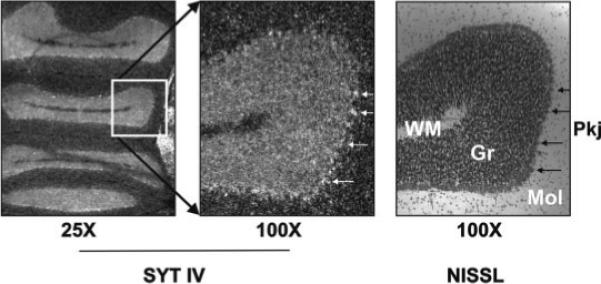
Syt IV is robustly expressed in cerebellar granule and Purkinje cell layers. Photomicro-graphs show darkfield images of emulsion dipped coronal section that was hybridized to the full length Syt IV riboprobe (left, middle) or of an adjacent Nissl-stained section (right) within the cerebellum of a mixed singing bird. Silver grains in the dark-field images correspond to the Nissl-dense Purkinje cell bodies (arrows) at left. Gr, granule cell layer; Mol, molecular layer; Pkj, Purkinje cell bodies; WM, white matter.
Summarizing these data, metrazole-driven seizure activity induced Syt IV mRNA in areas of the avian telencephalon at 2 h following seizure. However, Syt IV induction did not occur in major song control regions in response to this pharmacological activation.
Effects of Singing on ZENK and Synaptotagmin Gene Expression
To determine whether singing behavior induces Syt IV in the zebra finch brain, gene expression was analyzed at 2 h after ‘lights on’ (for non-singing males) or song onset (for singing males - generally within 2.5 h of ‘lights on’). The 2-h time-point was chosen because this was the earliest time at which robust Syt IV induction following metrazole-induced seizure was reliably observed. Analysis of the ZENK expression levels revealed that, although housed with females, the singing birds sang some portion of undirected songs (Supplementary Fig. 4). This was evident by high levels of ZENK expression within Area X, as previously demonstrated for undirected singers (Jarvis et al., 1998). Therefore, we refer to this group of birds as mixed-singers to indicate that they sang both directed and undirected songs.
As with seizure-driven neural activity, singing-driven activity failed to alter Syt I expression levels or patterns. Examination of film autoradiograms suggested that Syt I mRNA levels of singing birds did not differ from those of non-singing birds (Supplementary Fig. 1).
Unlike seizure, mixed singing resulted in dramatic Syt IV induction in the song control regions LMAN, HVC, and RA (Figs. 3 and 6) and decreased Syt IV in Area X (Table 1). In both saline-injected and metrazole-injected birds, Syt IV mRNA levels in LMAN were comparable to those in the surrounding nidopallium used for normalization (saline = 1.01 ± 0.06, n = 5; metrazole = 1.10 ± 0.04 n = 5; n.s., Fig. 3). When birds sang, Syt IV was induced by ∼50% within LMAN relative to levels of outlying nidopallium. No such induction was observed in any of the non-singing birds (non-singing = 1.06 ± 0.05, n = 6; mixed singing = 1.52 ± 0.11, n = 4; p 0.010). Of note, the almost complete lack of overlap between the raw Syt IV optical density values in LMAN of singing birds and the lower values from the other treatment groups [Fig. 3(B)] emphasizes the robustness of this finding.
Figure 6.
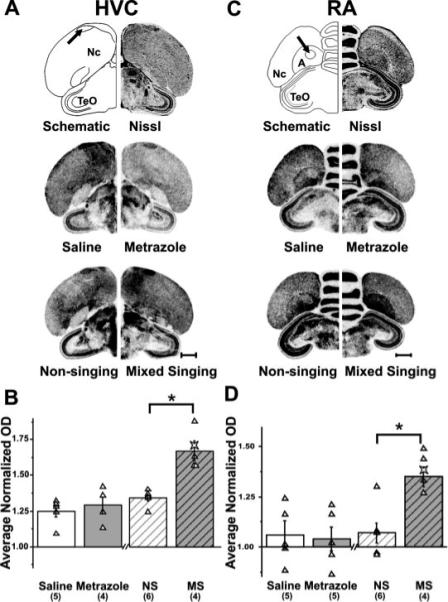
Singing, but not seizure, induces Syt IV in HVC and RA. Syt IV expression in HVC and RA. (A) Top drawing (left) is of the hemi-coronal Nissl-stained section (right) at the level of HVC (arrow) and indicates neuroanatomical boundaries used in gene expression analysis. Middle and bottom photomicrographs of film autoradiograms show representative brain sections hybridized with the Syt IV antisense probe. These reveal Syt IV induction in HVC of mixed singing birds, but no induction in the other groups. (B) Bar graph compares average normalized Syt IV mRNA levels in HVC across treatment groups. Syt IV expression levels are higher in mixed singing than in non-singing birds, while Syt IV signals from saline, metrazole-injected and non-singing birds show similar intensities (*p = 0.010). (C) Top drawing (left) is of a hemi-coronal Nissl-stained section (right) at the level of RA (arrow). Middle and bottom panels show photomicrographs of brain sections hybridized with the Syt IV antisense probe. These reveal Syt IV induction in RA of mixed singing birds, but no induction in the other groups. D) Bar graphs show that Syt IV expression levels are higher in mixed singing than in non-singing birds, while Syt IV signals from saline-injected, metrazole–injected, and non-singing birds show similar intensities (*p = 0.018). The star symbol in the mixed singing group is from a singing bird used for a different study, but that was collected and processed identically to the singing birds presented here. Although not included in the statistical analyses, its Syt IV levels in both HVC and RA fit within those of the mixed singers, reinforcing the reliability of these results. A, arcopallium; HVC, HVC (used as the proper name); Nc, caudal nidopallium; NS, non-singing; MS, mixed singing; RA, robust nucleus of the arco-pallium; TeO, optic tectum. Scale bar, 1 mm. Numbers within parentheses indicates sample size.
Table 1.
Summary of p-Values for Syt IV Normalized OD Measurements Across Major Treatment Groups
| Region of interest | Metrazole vs. Saline | Mixed singing vs. Non-singing |
|---|---|---|
| LMAN | n.s. (0.18) | |
| HVC | n.s. (0.62) | |
| RA | n.s. (0.72) | |
| AREA X | n.s. (0.75) | |
| STRIATUM | ||
| ARCOPALLIUM | n.s. (0.27) | n.s. (1.00) |
| CEREBELLUM | n.s. (0.30) | n.s. (0.39) |
Significance levels for regions of interest, including the song control nuclei which are highlighted in the gray box. Nonsignificant values are indicated by n.s. and corresponding p-values are shown in parenthesis. A p-value within an upward arrow indicates Syt IV induction in the experimental groups (metrazole, singing) relative to the control groups (saline, non-singing). A p-value within a downward arrow indicates Syt IV expression decreased in the experimental group relative to the control group.
In HVC, Syt IV levels were ∼25% higher than those in the surrounding nidopallium but were unaffected by seizure (saline = 1.25 ± 0.04, n = 6; metrazole = 1.29 ± 0.06, n = 4; n.s.; Fig. 6). In contrast to seizure, singing induced Syt IV levels in HVC relative to levels from non-singing birds (non-singing = 1.34 ± 0.02, n = 6; mixed singing = 1.67 ± 0.07, n = 4; p = 0.010). Results from RA were similar to those obtained in HVC. Syt IV levels in RA did not change as a result of seizure activity (saline = 1.06 ± 0.06, n = 5; metrazole = 1.04 ± 0.06, n = 5; n.s.) but were induced as a result of singing (non-singing = 1.07 ± 0.05, n = 6; mixed singing = 1.35 ± 0.05, n = 5; p = 0.018). Intriguingly, when birds sang, Syt IV expression within the striatum, including in Area X, was slightly, but significantly, lower than that of non-singing birds (St: non-singing 0.80 ± 0.01, n = 4; mixed singing = 0.72 ± 0.02, n = 6; p = 0.033) (Area X: non-singing = 0.66 ± 0.02, n = 6; mixed singing = 0.60 ± 0.01, n 4; p 0.033).
A summary of Syt IV induction in the zebra finch brain, both for metrazole-induced seizures and for mixed singing, is presented in Table 1. The table shows whether changes in Syt IV expression occurred and the statistical p-values obtained for each region.
To discover whether the social context of singing influences Syt IV induction, as has been shown previously for ZENK (Jarvis et al., 1998), we examined Syt IV expression levels in a small cohort of birds that sang either 100% directed or 100% undirected songs. Surprisingly, when birds directed their songs entirely to females, Syt IV levels in song nuclei remained stable (Fig. 7) in that they resembled levels obtained from non-singing birds in the prior experiments (Figs. 3 and 6). In contrast, when birds sang purely undirected songs, Syt IV levels were induced in LMAN relative to levels of directed-singers (Fig. 7; undirected = 1.23 ± 0.05, n = 3; directed = 1.04 ± 0.03, n = 3; p = 0.049). In fact, the range of values for the two groups did not overlap (range normalized OD, undirected = 1.14−1.29, n = 3; versus directed = 0.98−1.07, n = 3). similar results were obtained for HVC and RA (Fig. 7), although levels of Syt IV induction were not significant in these areas.
Figure 7.
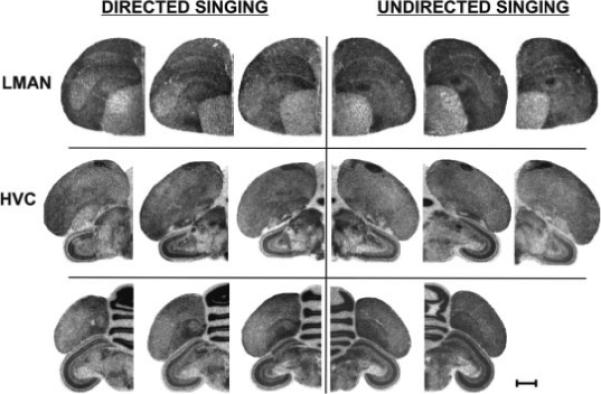
Syt IV mRNA accumulation in directed versus undirected singers. Syt IV is induced by singing in the LMAN (top) of undirected singers (right), but not of directed singers (left). Similar trends occur in HVC (middle) and RA (bottom). Images of film autoradiograms from hemicoronal sections of each bird are provided to illustrate the nonsignificant trends in HVC and RA. Scale bar, 1 mm.
Levels of Syt IV Induction Correlate with Amount of Singing
Syt IV induction in song control nuclei by singing likely results from song-related neural activity in these regions. If this is the case, then greater amounts of singing, driven by increased neural activity, should produce more Syt IV message, as has been shown for ZENK (Jarvis and Nottebohm, 1997). To test this hypothesis, we counted the number of bouts that each mixed-singer sang [Fig. 1(C)]. The number of song bouts was indeed strongly and positively correlated with Syt IV mRNA levels in LMAN, HVC and RA (Fig. 8), with the strongest correlation observed for HVC (r = 0.96, p < 0.0001). In contrast, there was no correlation between the decreased Syt IV expression in Area X and the number of song bouts (r = 0.14, p > 0.05).
Figure 8.
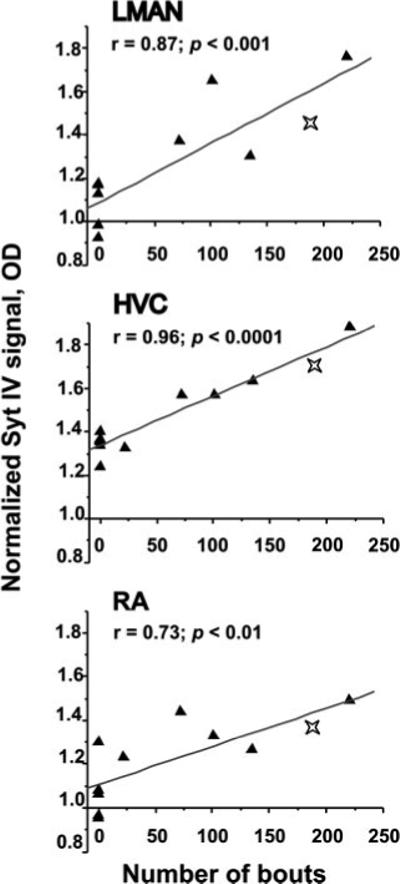
Syt IV mRNA expression correlates positively with the amount of singing. Syt IV signals in LMAN (top), HVC (middle) and RA (bottom) correlate with the number of bouts sung by each bird. Lines indicate simple linear regressions. Data for both mixed singing and non-singing (0 points on the X-axis) birds are plotted for comparison. Stars indicate a bird used for a separate study but that was acquired and processed as the mixed singers. Although not included in the statistical analyses, its Syt IV levels fit within those of the mixed singers, reinforcing the reliability of these results.
DISCUSSION
Syt IV is unique within the synaptotagmin family of membrane-trafficking proteins; it is the only synaptotagmin that is inducible by neural depolarization (reviewed in Sudhof, 2002). However, no natural stimulus for Syt IV induction in vivo has been identified previously. Here, using the zebra finch, we show that neural activity associated with singing provides a natural source of depolarization that drives Syt IV expression specifically within song circuit neurons.
We isolated and sequenced zebra finch Syt IV and Syt I complete coding sequences for use in measuring mRNA expression levels. The predicted Syt IV amino acid sequence in birds contains the single amino acid substitution in the C2A domain that is conserved across species from fly to human (Fig. 2). In vitro, this substitution decreases the calcium affinity (Ullrich et al., 1994; von Poser et al., 1997) and alters the lipid-binding capacity of the molecule (Fukuda et al., 1996). In unstimulated birds, Syt IV and Syt I mRNAs are broadly distributed throughout the telencephalon. On top of this constitutive expression, we find that Syt IV mRNA is inducible in birds, as it is in mammals (Fig. 4; Ferguson et al., 2004). However, Syt IV mRNA accumulation following metrazole-driven seizures appears limited to non-song control areas. In contrast, three pallial song circuit nuclei, LMAN, HVC and RA, exhibit striking Syt IV induction when birds sing (Figs. 3 and 6; Table 1). Thus, the neural activity associated with singing appears to drive Syt IV expression, whereas generalized seizure activity fails to do so - even though these song nuclei contain GABAA receptors (Schmidt and Perkel, 1998; Spiro et al., 1999; Rosen and Mooney, 2000), the pharmacological target of metrazole. These data suggest that Syt IV induction in a circuit for procedural, sensorimotor learning requires neural activity that is precisely patterned, rather than generally elevated.
In zebra finches, the transcription factor and IEG ZENK is induced in song nuclei by neural activity associated with singing (Jarvis and Nottebohm, 1997). ZENK is also induced by metrazole-driven seizures in these same regions (our data and Mello and Clayton, 1995). Indeed, lack of ZENK expression in neurons following seizure has been taken as evidence that such neurons are incapable of expressing this IEG (Burmeister et al., 2005). To our knowledge, Syt IV is the first gene to show induction only by select patterns of depolarization in the song circuit, namely the firing patterns associated with the production of learned song. The activity-regulated cytoskeletal-associated (Arc) gene is another nontranscription factor that is induced by singing in song nuclei and also in auditory processing areas of the songbird brain (Velho et al., 2005). The role of Arc as an early effector of synaptic plasticity has been well described in mammalian systems where its mRNA is rapidly induced by seizures and by learning (reviewed in Steward and Worley, 2001). Whether induction of Arc, like Syt IV, is selective for patterned activity over seizure activity within songbird auditory areas or song nuclei is not known. Distinct activation patterns are likely to activate distinct sets of kinases and transcription factors, including those involved in learning and memory. For example, in hippocampal dendrites, spaced stimuli are effective in stabilizing an activated MAP kinase pathway, leading to protrusion of new dendritic filopodia, whereas a single prolonged stimulus does not (Wu et al., 2001). Responses of T lymphocytes to different environmental stimulants can result in different frequencies of calcium oscillations which, in turn, activate differential subsets of transcription factors (Dolmetsch et al., 1997). Patterned neural activity associated with singing may similarly drive a transcriptional cascade distinct from seizure-related pathways, and include factors that activate Syt IV mRNA transcription.
Alternatively, Syt IV induction may be gated by neuromodulation associated with singing. In contrast to pharmacological seizures, courting behavior is associated with a rise in testosterone and other neuro-chemicals (reviewed in Ball and Balthazart, 2004). In our initial experiments with singing birds, males (mixed-singers) were housed with females to whom they could direct their courtship songs. However, analysis of ZENK expression revealed high levels in Area X (Supplementary Fig. 4) indicating that, despite the female's presence, not all songs were directed to her (Jarvis et al., 1998). We thus tested Syt IV induction by singing in a cohort of males that sang either 100% directed or 100% undirected songs. We found that, in LMAN, induction occurred during undirected, but not directed, singing (Fig. 7). A similar trend was observed in both HVC and RA, but differences in these regions were not significant, potentially due to the following: (1) in the experiment using mixed singers, Syt IV induction was most robust in LMAN (∼50%; Fig. 3) with a smaller effect in HVC and RA (∼25%; Fig. 6), indicating that changes in LMAN are more readily observable; (2) the 100% undirected-singers sang fewer songs (range = 107−116 bouts) than the mixed singers (range = 73−220 bouts; Fig. 8), and the level of Syt IV induction in LMAN, HVC and RA depends on the amount of singing (Fig. 8); (3) our sample size was small. Future studies designed to probe the biological basis of the effect of social context on Syt IV induction in LMAN, using larger sample sizes, will provide more evidence as to whether such effects also obtain in HVC and RA.
In zebra finches, neuromodulators such as nor-adrenaline ‘gate’ the song-selective auditory responses of song circuit neurons (Dave et al., 1998; Cardin and Schmidt, 2004). Moreover, pharmacological depletion of noradrenergic inputs to Area X resulted in elevated ZENK expression in directed singers, similar to levels observed in undirected singers (Castelino and Ball, 2005). This suggests that noradrenergic drive to Area X may normally be high in directed singers, and may suppress ZENK induction during performance of courtship songs to females. If so, noradrenaline and/or other neuromodulators may similarly gate the effect of social context for multiple gene transcripts (Teramitsu and White, 2006), including Syt IV, within song nuclei.
In the striatum, including song nucleus Area X, Syt IV levels were slightly reduced in singing birds. This reduction may indicate that, within the avian basal ganglia, Syt IV is expressed in cells that are inhibited during singing. For example, Area X contains both striatal and pallidal-like cell types which are both inhibitory (Farries and Perkel, 2002). One scenario is that, during singing, HVC inputs to Area X excite striatal cell types which, in turn, inhibit pallidal ones. If these pallidal cells express Syt IV, such inhibition could, hypothetically, result in lower Syt IV expression in Area X during singing. Future studies will examine the cellular identity of song circuit neurons that exhibit Syt IV induction.
Our studies do not indicate whether Syt IV regulation by singing is due to the motor act itself, or to auditory feedback of the bird's own song. Singing-related neural activity and ZENK induction persists in the song circuit of deafened birds (McCasland and Konishi, 1981; Jarvis and Nottebohm, 1997; Hessler and Doupe, 1999a,b) and this activity might induce Syt IV. Future studies in deafened singing birds will clarify whether this is so, and whether deterioration of song quality (Nordeen and Nordeen, 1992; Brainard and Doupe, 2001) is associated with altered Syt IV induction.
What is the significance of Syt IV induction in zebra finch song circuitry? Among songbirds, zebra finches are known as “closed-ended learners” meaning that once they are mature, they sing the same song relatively unchanged throughout life. Open-ended learners such as mockingbirds are continuously capable of imitating new sounds. Never-the-less, the entire zebra finch song circuit remains active throughout life and contributes to maintaining song quality (Jarvis and Nottebohm, 1997; Hessler and Doupe, 1999a,b; Williams and Mehta, 1999; Brainard and Doupe, 2000). Syt IV induction by singing in adult birds may be part of the mechanism for maintenance of adult song quality. Testing young birds whose songs are still undergoing sensorimotor learning will clarify whether behavioral plasticity or behavioral stability is more strongly correlated with modulation of Syt IV levels.
In mammals, Syt IV is proposed to act presynaptically as a negative or homeostatic regulator of neuro-transmitter release following its induction by prior depolarization (Vician et al., 1995). This hypothesis is partly based on Syt IV's C2A domain which has reduced calcium affinity relative to Syt I (von Poser et al., 1997). Syt IV can heterodimerize with Syt I in vitro (Osborne et al., 1999). Such heterodimers would presumably be less sensitive to calcium influx than Syt I homomers. Induced Syt IV levels might, therefore, act in a dominant negative fashion to decrease vesicular release in vivo, including at song circuit synapses. Indeed, in PC12 cells, Syt IV over-expression inhibits depolarization-induced vesicular exocytosis (Machado et al., 2004). However, recent studies in hippocampal cell cultures failed to find any effect of acute Syt IV over-expression on excitatory fast synaptic transmission, release probability, or fusion pore kinetics (Ting et al., 2006). These findings provoke a rethinking of the dominant hypothesis of Syt IV function but may not accurately depict endogenous Syt IV function in vivo since nonphysiological over-expression of a poor calcium sensor may compensate for the reduced calcium affinity and thus suffice for normal synaptic transmission. Further, in vitro culture conditions might omit other regulatory factors that interact with Syt IV in vivo.
Syt IV null mutant mice have both a behavioral and a synaptic phenotype: behaviorally, they are impaired in the social transmission of food preference and exhibit deficits in associative passive avoidance memory (Ferguson et al., 2000a,b). In adult song-birds, loss of Syt IV might similarly disrupt the maintenance of socially-learned song. At the synaptic level, Syt IV null mutant mice demonstrate enhanced short-term plasticity in the CA1 region of the hippo-campus (Ferguson et al., 2004). The latter observation includes enhanced paired pulse facilitation, which is thought to occur when the first pulse fails to exhaust the readily releasable pool of vesicles but primes the nerve terminal for even greater release at the second pulse. Whether the enhanced paired pulse facilitation at Syt IV−/− synapses can be interpreted as evidence that wild type Syt IV levels support neurotransmitter release is not clear. Since Syt IV−/− mice lack Syt IV throughout development, levels of other Syt family members may be altered developmentally in compensation for Syt IV loss. Indeed, Syt IV function may depend critically on levels of other Syt family members. To date, no temporally regulated constructs have been used to test the real-time effects of altering Syt IV levels in vivo.
The localization of Syt IV to presynaptic nerve terminals of mammals has been questioned (Berton et al., 2000; Ibata et al., 2000; Fukuda et al., 2001; Zhang et al., 2004). Moreover, the putative Syt IV ortholog in Drosophila has been localized to the post-synaptic compartment (Yoshihara et al., 2005). Some of these discrepancies may reflect species differences (Dai et al., 2004). Because of its greater sequence similarity, the zebra finch Syt IV is likely to function more like its mammalian, than its insect, ortholog (Fig. 2). Over-expression of Syt IV in mammalian central nervous system cell cultures indicates that at least a majority of the protein is localized to presynaptic nerve terminals and can indeed be found on synaptic vesicles (Ting et al., 2006).
In contrast to Syt IV, Syt I is not induced in the zebra finch brain either by seizure or by singing (Supplementary Fig. 1). This result is expected; in other systems Syt I is not an IEG. In zebra finches, we find robust constitutive levels of Syt I mRNA broadly distributed throughout the telencephalon with a few exceptions: in the song nuclei LMAN and RA, Syt I is low relative to the surrounding nidopallium and arcopallium, respectively. Syt I levels are also low in the medial striatum, including within the song nucleus Area X. The pallial HVC area is the only song nucleus we examined in whichweobservedhigh Syt I levels, even more robust than outlying regions. It is upon this varied background of song circuit Syt I expression (low in LMAN and RA, but high in HVC) that Syt IV induction occurs during singing. The ratio of Syt I to Syt IV protein may determine the synaptic consequences of Syt IV induction. During singing, the zebra finch song circuit thus contains at least two distinct Syt IV:Syt I ratios - a high ratio in LMAN where Syt IV is induced on a background of low Syt I expression, versus a lower ratio in HVC where Syt IV induction is balanced by high constitutive Syt I expression. Future electrophysiological experiments could examine the synaptic effects of naturally-driven induction on a background of high Syt I levels in HVC versus a background of low Syt I levels in LMAN.
In summary, studies with zebra finches, a songbird species, have identified a naturally learned behavior that induces unique Syt IV neural expression patterns that correlate with the behavior. The use of natural behaviors to induce Syt IV in songbirds and in other systems may illuminate the synaptic function of Syt IV in the underlying circuitry. The failure of generalized depolarization to induce Syt IV in the song circuit indicates that either neuromodulation or patterned neural activity associated with singing is required for Syt IV modulation. Moreover, induction in LMAN by undirected, but not directed, singing may indicate that both neuromodulation and patterned activity are required. As Syt IV is known to be an IEG in other systems, it is tempting to speculate that its induction by patterned neural activity reflects the temporal nature of information coding in neural circuits. The dynamic regulation of Syt IV observed in the song circuit, a specialized cortico-basal ganglia-thalamo-cortical pathway, may extend more generally to other procedurally learned sensorimotor behaviors.
Supplementary Material
Acknowledgments
We thank Dr. Arthur Arnold for the zebra finch cDNA library and the use of imaging equipment, and Dr. Barney Schlinger for sharing resources. Dr. David Clayton provided the clone for canary ZENK. Ms. Linh Bui provided double-blinded quantification of Syt IV expression. Dr. Felix Schweizer, two anonymous reviewers, and members of the White laboratory provided helpful comments on the manuscript.
Contract grant sponsor: NIH; contract grant number: NS28660.
Contract grant sponsor: Sloan Foundation Fellowship; contract grant number: MH070712.
Footnotes
Note Added in Proof
During publication of this manuscript, another study (Wada et al., 2006) that examined multiple genes regulated by singing also found Syt IV to be one of them.
REFERENCES
- Ball GF, Balthazart J. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiol Behav. 2004;83:329–346. doi: 10.1016/j.physbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Berton F, Cornet V, Iborra C, Garrido J, Dargent B, Fukuda M, et al. Synaptotagmin I and IV define distinct populations of neuronal transport vesicles. Eur J Neurosci. 2000;12:1294–1302. doi: 10.1046/j.1460-9568.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- Berton F, Iborra C, Boudier JA, Seagar MJ, Marqueze B. Developmental regulation of synaptotagmin I, II, III, and IV mRNAs in the rat CNS. J Neurosci. 1997;17:1206–1216. doi: 10.1523/JNEUROSCI.17-04-01206.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer SW, Arnold AP. Developmental plasticity in neural circuits for a learned behavior. Ann Rev Neurosci. 1997;20:459–481. doi: 10.1146/annurev.neuro.20.1.459. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature. 2000;404:762–766. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Postlearning consolidation of birdsong: Stabilizing effects of age and anterior forebrain lesions. J Neurosci. 2001;21:2501–2517. doi: 10.1523/JNEUROSCI.21-07-02501.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister SS, Jarvis ED, Fernald RD. Rapid behavioral and genomic responses to social opportunity. PLo S Biol. 2005;3:e363. doi: 10.1371/journal.pbio.0030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Noradrenergic inputs mediate state dependence of auditory responses in the avian song system. J Neurosci. 2004;24:7745–7753. doi: 10.1523/JNEUROSCI.1951-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelino CB, Ball GF. A role for norepinephrine in the regulation of context-dependent ZENK expression in male zebra finches (Taeniopygia guttata). Eur J Neurosci. 2005;21:1962–1972. doi: 10.1111/j.1460-9568.2005.04028.x. [DOI] [PubMed] [Google Scholar]
- Dai H, Shin OH, Machius M, Tomchick DR, Sudhof TC, Rizo J. Structural basis for the evolutionary inactivation of Ca2+ binding to synaptotagmin 4. Nat Struct Mol Biol. 2004;11:844–849. doi: 10.1038/nsmb817. [DOI] [PubMed] [Google Scholar]
- Dave AS, Yu AC, Margoliash D. Behavioral state modulation of auditory activity in a vocal motor system. Science. 1998;282:2250–2254. doi: 10.1126/science.282.5397.2250. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- Farries MA. The oscine song system considered in the context of the avian brain: Lessons learned from comparative neurobiology. Brain Behav Evol. 2001;58:80–100. doi: 10.1159/000047263. [DOI] [PubMed] [Google Scholar]
- Farries MA, Perkel DJ. A telencephalic nucleus essential for song learning contains neurons with physiological characteristics of both striatum and globus pallidus. J Neurosci. 2002;22:3776–3787. doi: 10.1523/JNEUROSCI.22-09-03776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson GD, Anagnostaras SG, Silva AJ, Herschman HR. Deficits in memory and motor performance in synaptotagmin IV mutant mice. Proc Nat Acad Sci USA. 2000a;10:5598–5603. doi: 10.1073/pnas.100104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson GD, Chen XN, Korenberg JR, Herschman HR. The human synaptotagmin IV gene defines an evolutionary break point between syntenic mouse and human chromosome regions but retains ligand inducibility and tissue specificity. J Biol Chem. 2000b;275:36920–36926. doi: 10.1074/jbc.M005801200. [DOI] [PubMed] [Google Scholar]
- Ferguson GD, Thomas DM, Elferink LA, Herschman HR. Synthesis degradation, and subcellular localization of synaptotagmin IV, a neuronal immediate early gene product. J Neurochem. 1999;72:1821–1831. doi: 10.1046/j.1471-4159.1999.0721821.x. [DOI] [PubMed] [Google Scholar]
- Ferguson GD, Wang H, Herschman HR, Storm DR. Altered hippocampal short-term plasticity and associative memory in synaptotagmin IV (–/–) mice. Hippocampus. 2004;14:964–974. doi: 10.1002/hipo.20013. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Ibata K, Mikoshiba K. A unique r domain of synaptotagmin IV is essential for Golgi localization. J Neurochem. 2001;77:730–740. doi: 10.1046/j.1471-4159.2001.00266.x. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Kojima T, Mikoshiba K. Phospholipid composition dependence of Ca2+-dependent phospholipid binding to the C2A domain of synaptotagmin IV. J Biol Chem. 1996;271:8430–8434. doi: 10.1074/jbc.271.14.8430. [DOI] [PubMed] [Google Scholar]
- Hahnloser RHR, Kozhevnikov AA, Fee MS. An ultrasparse code underlies the generation of neural sequences in a songbird. Nature. 2002;419:65–70. doi: 10.1038/nature00974. [DOI] [PubMed] [Google Scholar]
- Hessler NA, Doupe AJ. Singing-related neural activity in a dorsal forebrain-basal ganglia circuit of adult zebra finches. J Neurosci. 1999a;19:10461–10481. doi: 10.1523/JNEUROSCI.19-23-10461.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler NA, Doupe AJ. Social context modulates singing in the songbird forebrain. Nat Neurosci. 1999b;2:209–211. doi: 10.1038/6306. [DOI] [PubMed] [Google Scholar]
- Huang X, Miller W. A time-efficient, linear-space local similarity algorithm. Adv Appl Mathe. 1991;12:337–357. [Google Scholar]
- Ibata K, Fukuda M, Hamada T, Kabayama H, Mikoshiba K. Synaptotagmin IV is present at the Golgi and distal parts of neurites. J Neurochem. 2000;74:518–526. doi: 10.1046/j.1471-4159.2000.740518.x. [DOI] [PubMed] [Google Scholar]
- Ibata K, Hashikawa T, Tsuboi T, Terakawa S, Liang F, Mizutani A, Fukuda M, et al. Non-polarized distribution of synaptotagmin IV in neurons: Evidence that synaptotagmin IV is not a synaptic vesicle protein. Neurosci Res. 2002;43:401–406. doi: 10.1016/s0168-0102(02)00066-4. [DOI] [PubMed] [Google Scholar]
- Jarvis ED. Learned birdsong and the neurobiology of human language. Ann N Y Acad Sci. 2004;1016:749–777. doi: 10.1196/annals.1298.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Güntürkün O, Bruce L, Csillag A, Karten HJ, Kuenzel W, Medina L, et al. Avian brains and a new understanding of vertebrate evolution. Nat Rev Neurosci. 2005;6:151–159. doi: 10.1038/nrn1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Nottebohm F. Motor-driven gene expression. Proc Nat Acad Sci USA. 1997;94:4097–4102. doi: 10.1073/pnas.94.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the bird sings: Context-dependent gene expression. Neuron. 1998;21:775–788. doi: 10.1016/s0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature. 2005;433:638–643. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Bensoula AN, Filali M. Rotorod sensorimotor learning in cerebellar mutant mice. Neurosci Res. 1995;22:423–426. doi: 10.1016/0168-0102(95)00916-h. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Serano TL, Rubin GM, Ganetzky B, Chapman ER. Synaptic function modulated by changes in the ratio of synaptotagmin I and IV. Nature. 1999;400:757–760. doi: 10.1038/23462. [DOI] [PubMed] [Google Scholar]
- Livingston FS, White SA, Mooney R. Slow NMDAEPSCs at synapses critical for song development are not required for song learning in zebra finches. Nat Neurosci. 2000;3:482–488. doi: 10.1038/74857. [DOI] [PubMed] [Google Scholar]
- Machado HB, Liu W, Vician LJ, Herschman HR. Synaptotagmin IV overexpression inhibits depolarization-induced exocytosis in PC12 cells. J Neurosci Res. 2004;76:334–341. doi: 10.1002/jnr.20072. [DOI] [PubMed] [Google Scholar]
- Mahata SK, Marksteiner J, Sperk G, Mahata M, Gruber B, Fischer-Colbrie R, Winkler H. Temporal lobe epilepsy of the rat: Differential expression of mRNAs of chromogranin B, secretogranin II, synaptin/synaptophysin and p65 in subfield of the hippocampus. Brain Res Mol Brain Res. 1992;16:1–12. doi: 10.1016/0169-328x(92)90187-g. [DOI] [PubMed] [Google Scholar]
- McCasland JS, Konishi M. Interaction between auditory and motor activities in an avian song control nucleus. Proc Nat Acad Sci USA. 1981;78:7815–7819. doi: 10.1073/pnas.78.12.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Clayton DF. Differential induction of the ZENK gene in the avian forebrain and song control circuit after metrazole-induced depolarization. J Neurobiol. 1995;26:145–161. doi: 10.1002/neu.480260112. [DOI] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Nat Acad Sci USA. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ. Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches. Behav Neur Biol. 1992;57:58–66. doi: 10.1016/0163-1047(92)90757-u. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194:211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Kelley DB, Paton JA. Connections of vocal control nuclei in the canary telencephalon. J Comp Neurol. 1982;207:344–357. doi: 10.1002/cne.902070406. [DOI] [PubMed] [Google Scholar]
- Olveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLo S Biol. 2005;3:e153. doi: 10.1371/journal.pbio.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne SL, Herreros J, Bastiaens PI, Schiavo G. Calcium-dependent oligomerization of synaptotagmins I and II. Synaptotagmins I and II are localized on the same synaptic vesicle and heterodimerize in the presence of calcium. J Biol Chem. 1999;274:59–66. doi: 10.1074/jbc.274.1.59. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Mello CV, Jarvis ED. Songbirds and the revised avian brain nomenclature. Ann N Y Acad Sci. 2004;1016:77–108. doi: 10.1196/annals.1298.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson IM, Ranjan R, Schwarz TL. Synaptotagmins I and IV promote transmitter release independently of Ca(2+) binding in the C(2)A domain. Nature. 2002;418:336–340. doi: 10.1038/nature00915. [DOI] [PubMed] [Google Scholar]
- Rosen MJ, Mooney R. Intrinsic and extrinsic contributions to auditory selectivity in a song nucleus critical for vocal plasticity. J Neurosci. 2000;20:5437–5448. doi: 10.1523/JNEUROSCI.20-14-05437.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: Implications for vocal learning. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MF, Perkel DJ. Slow synaptic inhibition in nucleus HVc of the adult zebra finch. J Neurosci. 1998;18:895–904. doi: 10.1523/JNEUROSCI.18-03-00895.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neur Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- Spiro JE, Dalva MB, Mooney R. Long-range inhibition within the zebra finch song nucleus RA can coordinate the firing of multiple projection neurons. J Neurophysiol. 1999;81:3007–3020. doi: 10.1152/jn.1999.81.6.3007. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF. A cellular mechanism for targeting newly synthesized mRNAs to synaptic sites on denddrites. Proc Natl Acad Sci USA. 2001;98:7062–7068. doi: 10.1073/pnas.131146398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes TM, Leonard CM, Nottebohm F. The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. J Comp Neurol. 1974;156:337–374. doi: 10.1002/cne.901560305. [DOI] [PubMed] [Google Scholar]
- Sturdy CB, Wild JM, Mooney R. Respiratory and telencephalic modulation of vocal motor neurons in the zebra finch. J Neurosci. 2003;23:1072–1086. doi: 10.1523/JNEUROSCI.23-03-01072.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. Synaptotagmins: Why so many? J Biol Chem. 2002;277:7629–7632. doi: 10.1074/jbc.R100052200. [DOI] [PubMed] [Google Scholar]
- Teramitsu I, Kudo LC, London SE, Geschwind DH, White SA. Parallel FoxP1 and FoxP2 expression in songbird and human brain predicts functional interaction. J Neurosci. 2004;24:3152–3163. doi: 10.1523/JNEUROSCI.5589-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramitsu I, White SA. FoxP2 Regulation during undirected singing in adult songbirds. J Neurosci. 2006;26:7390–7394. doi: 10.1523/JNEUROSCI.1662-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JT, Kelley BG, Sullivan JM. Synaptotagmin IV does not alter excitatory fast synaptic transmission or fusion pore kinetics in mammalian CNS neurons. J Neurosci. 2006;26:372–380. doi: 10.1523/JNEUROSCI.3997-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolivia J, Tolivia D. A new technique for differential and simultaneous staining of nerve cells and fibers. J Neurosci Methods. 1985;13:305–311. doi: 10.1016/0165-0270(85)90078-0. [DOI] [PubMed] [Google Scholar]
- Ullrich B, Li C, Zhang JZ, McMahon H, Anderson RG, Geppert M, Sudhof TC. Functional properties of multiple synaptotagmins in brain. Neuron. 1994;13:1281–1291. doi: 10.1016/0896-6273(94)90415-4. [DOI] [PubMed] [Google Scholar]
- Velho TAF, Pinaud R, Rodrigues PV, Mello CV. Co-induction of activity-dependent genes in songbirds. Eur J Neurosci. 2005;22:1667–1678. doi: 10.1111/j.1460-9568.2005.04369.x. [DOI] [PubMed] [Google Scholar]
- Vician L, Lim IK, Ferguson G, Tocco G, Baudry M, Herschman HR. Synaptotagmin IV is an immediate early gene induced by depolarization in PC12 cells and in brain. Proc Natl Acad Sci USA. 1995;92:2164–2168. doi: 10.1073/pnas.92.6.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Poser C, Ichtchenko K, Shao X, Rizo J, Sudhof TC. The evolutionary pressure to inactivate. A subclass of synaptotagmins with an amino acid substitution that abolishes Ca2+ binding. J Biol Chem. 1997;272:14314–14319. doi: 10.1074/jbc.272.22.14314. [DOI] [PubMed] [Google Scholar]
- Wada K, Howard JT, McConnell P, Whitney O, Lints T, Rivas MV, Horita H, Patterson MA, White SA, Scharff C, Haesler S, Zhao S, Sakaguchi H, Hagiwara M, Shiraki T, Hirozane-Kishikawa T, Skene P, Hayashizaki Y, Carninci P, Jarvis ED. A molecular neuroethological approach for identifying and characterizing a cascade of behaviorally regulated genes. Proc Nat Acad Sci USA. 2006 doi: 10.1073/pnas.0607098103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CT, Grishanin R, Earles CA, Chang PY, Martin TF, Chapman ER, Jackson MB. Synaptotagmin modulation of fusion pore kinetics in regulated exocytosis of dense-core vesicles. Science. 2001;294:1111–1115. doi: 10.1126/science.1064002. [DOI] [PubMed] [Google Scholar]
- Wild JM. The avian nucleus retroambigualis: A nucleus for breathing, singing and calling. Brain Res. 1993;606:319–324. doi: 10.1016/0006-8993(93)91001-9. [DOI] [PubMed] [Google Scholar]
- Williams H, Mehta N. Changes in adult zebra finch song require a forebrain nucleus that is not necessary for song production. J Neurobiol. 1999;39:14–28. [PubMed] [Google Scholar]
- Woods IG, Wilson C, Friedlander B, Chang P, Reyes DK, Nix R, Kelly PD, et al. The zebrafish gene map defines ancestral vertebrate chromosomes. Genome Res. 2005;15:1307–1314. doi: 10.1101/gr.4134305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GY, Deisseroth K, Tsien RW. Spaced stimuli stabilize MAPK pathway activation and its effects on dendritic morphology. Nat Neurosci. 2001;4:151–158. doi: 10.1038/83976. [DOI] [PubMed] [Google Scholar]
- Yoshihara M, Adolfsen B, Galle KT, Littleton JT. Retrograde signaling by Syt 4 induces presynaptic release and synapse-specific growth. Science. 2005;310:858–863. doi: 10.1126/science.1117541. [DOI] [PubMed] [Google Scholar]
- Yu AC, Margoliash D. Temporal hierarchical control of singing in birds. Science. 1996;273:1871–1875. doi: 10.1126/science.273.5283.1871. [DOI] [PubMed] [Google Scholar]
- Zann RA. The Zebra Finch: A Synthesis of Field and Laboratory Studies. Oxford; New York: 1996. pp. 157–246. [Google Scholar]
- Zhang Q, Fukuda M, Van Bockstaele E, Pascual O, Haydon PG. Synaptotagmin IV regulates glial glutamate release. Proc Natl Acad Sci USA. 2004;101:9441–9446. doi: 10.1073/pnas.0401960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


