Abstract
The majority of regulatory T cells (Tregs) are believed to be of thymic origin. It has been hypothesized that this may result from unique intrathymic environmental cues, possibly requiring a dedicated antigen-presenting cell (APC). However, T cell-intrinsic developmental regulation of the susceptibility to Treg differentiation remains a mutually non-exclusive scenario. We found that upon exposure of monoclonal T cells of sequential developmental stages to a thymic microenvironment expressing cognate antigen, the efficiency of Treg induction inversely correlated with progressive maturation. This inclination of immature thymocytes toward Treg differentiation was even seen in an APC-free in vitro system, providing only TCR stimulation and IL-2. In support of quantitative but not qualitative features of external cues being critical, thymic epithelial cells as well as different thymic dendritic cell (DC)-subtypes efficiently induced Treg development of immature thymocytes, albeit at strikingly different optimal doses of cognate antigen. We propose that the intrinsically high predisposition of immature thymocytes to Treg development may contribute to the predominantly thymic origin of the Treg repertoire. The underlying instructive stimulus, however, does not require unique features of a dedicated APC and can be delivered by hematopoietic as well as epithelial thymic stromal cells.
Keywords: thymic antigen presenting cell, thymus, tolerance
Regulatory T cells (Tregs) expressing the forkhead/winged helix transcription factor Foxp3 are essential for immune-tolerance and homeostasis (1). A substantial overlap between the TCR sequences of thymic and peripheral Tregs suggests that the majority of Tregs originate from the thymus (2–4). Furthermore, data from superantigen-specific or TCR-transgenic systems strongly support that Treg differentiation is a result of intrathymic self-antigen encounter (5–7). However, when and how this dedicated T cell lineage branches of from “mainstream” thymocyte development remains controversial. We and others have suggested that Tregs arise at the CD4 single-positive (SP) stage through what may be called “altered negative selection” in the thymic medulla (8, 9). Other studies have proposed that Treg differentiation is the consequence of “altered positive selection” of cortical CD4+CD8+ double-positive (DP) thymocytes (10–14). To account for why Tregs or their immediate precursors are not subject to clonal deletion, some investigators have suggested a stochastic/selective mode of Treg development, whereby thymocytes may randomly, that is, in an at least initially antigen-independent manner, commit to a developmental program that subsequently protects developing Tregs from clonal deletion (15). Alternatively, largely unknown instructive signals provided by dedicated niches, for example, particular stromal cell types and/or cytokine and co-stimulatory milieus, may favor Treg development over clonal deletion (16). A variation of an instructive mode of Treg induction assumes a pivotal role of the avidity of self-antigen encounter, thus bearing resemblance to classical models of positive selection (6, 17).
Some of this controversy certainly arises from the fact that prospective identification of Treg precursors remains a significant experimental challenge. Foxp3-reporter mice unable to express the functional Foxp3 protein have been instrumental in delineating its role in the control of late stage Treg differentiation and acquisition of functional competence (18–20). However, because these studies position Foxp3 function relatively far downstream in Treg development, they did not reveal the external cues or the molecular and phenotypic changes that coordinate Treg differentiation upstream of Foxp3. Significant progress in this regard was very recently achieved by the demonstration that the Foxp3−CD25+ subset of polyclonal CD4 SP thymocytes is enriched in Treg precursors (21). These cells represent the penultimate stage before Foxp3 expression, and acquisition of the “mature” Foxp3+ Treg phenotype required only IL-2, but was largely independent of TCR engagement. These findings support a 2-step model whereby Treg development segregates into a TCR-driven “instructive” phase and a cytokine-driven “consolidation” phase. It remained open, however, at which stage of thymocyte development and by which stromal cell type(s) the “instructive” TCR signal can be delivered.
A number of open issues concerning intrathymic Treg differentiation, such as the eventual existence of a T cell-intrinsic developmental “window of opportunity,” the role of antigen dose, and similarly the potential requirement for external cues provided by a dedicated APC, are difficult to address in vivo in the steady state. Here, using intrathymic (i.t) transfer of post-positive selection “naïve” CD4 SP thymocytes of known antigen-specificity, we found that Treg induction by agonist encounter in vivo does not obligatorily require cognate interactions at the CD4+CD8+ DP stage. The progeny of i.t.-injected monoclonal CD4 SP cells segregated into bona fide Treg cells expressing CD25 and Foxp3, vigorously cycling Foxp3− cells, and apoptotic cells. Importantly, this approach faithfully recapitulated hallmarks of steady-state intrathymic Treg development in TCR-transgenic and wild-type (WT) mice and what has been outlined in the 2-step model of Treg development. Furthermore, we found that developmental progression within the CD4 T cell lineage is a critical parameter for the efficacy of Treg induction, whereby the inclination toward Treg differentiation upon exposition to an antigen-expressing thymic microenvironment decreased considerably in the order immature CD4 SP cells → mature CD4 SP cells → peripheral CD4 T cells. This T cell-intrinsic developmental control of Treg differentiation was also seen in in vitro assays, irrespective of whether stromal cells of hematopoietic or epithelial origin were used as APCs, and even in an APC-free system.
Results
Initiation of Treg Differentiation at the CD4 SP Stage.
We have previously described that in the TCR-HA × AIRE-HA double-transgenic model, thymocytes specific for the neo-self antigen influenza hemagglutinin (HA) differentiate into Tregs due to expression of cognate antigen under control of the Autoimmune Regulator (aire) gene locus in medullary thymic epithelial cells (mTECs) (8). Although topological considerations suggested initiation of Treg development at the CD4 SP stage in this model, cognate antigen interactions at earlier developmental stages could not formally be ruled out in this complex steady-state system.
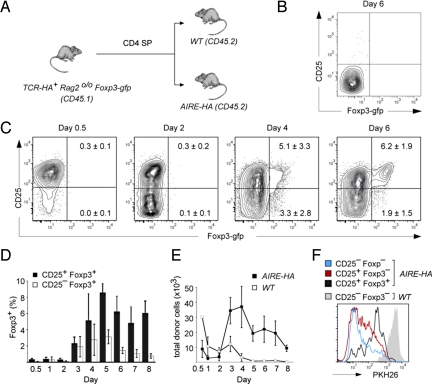
We first addressed whether Treg differentiation in the AIRE-HA thymus can be dissociated from positive selection and CD4 lineage commitment. CD4 SP cells from TCR-HA Foxp3-gfp Rag2o/o mice, that is, truly naïve, monoclonal cells that did not contain any preexisting Foxp3+ cells, were transferred into AIRE-HA thymi (Fig. 1A). In AIRE-HA recipients, but not WT controls (Fig. 1B), this resulted in a homogenous up-regulation of CD25 within 12 h after injection (Fig. 1C). After 48 h, about half of the cells had down-regulated CD25 again. Between days 3 and 4, the first Foxp3+ cells appeared, and these cells segregated into CD25− and CD25+ cells. Subsequently, Foxp3+CD25− cells essentially disappeared, whereas the frequency of Foxp3+CD25+ cells reached a maximum between days 4 and 5 and remained relatively stable thereafter (Fig. 1D). Essentially identical findings were obtained with a second, OVA-specific TCR transgenic system (DO11.10 Foxp3-gfp Rag2o/o) upon i.t. injection into an OVA-expressing host thymus (Fig. S1) (8).
Fig. 1.
Naïve TCR-HA+ cells give rise to Treg after intrathymic injection into AIRE-HA recipient mice. (A) Experimental design: 5 × 105 CD4 SP cells from CD45.1 TCR-HA Rago/o Foxp3-gfp mice were intrathymically transferred into either WT or AIRE-HA recipients (CD45.2). (B) In WT recipients, transferred cells exhibited a stable Foxp3−CD25− phenotype. (C) Kinetics of intrathymic Treg development in AIRE-HA thymi. Injected cells were analyzed for Foxp3-gfp and CD25 expression at different time points as indicated above the plots. Numbers in quadrants indicate the percentage (± SD, n = 3) of cells within the respective quadrant. (D) Emergence of CD25−Foxp3+ and CD25+Foxp3+ cells. The diagram depicts the average percentage (± SD, n = 3) of CD25−Foxp3+ or CD25+Foxp3+ recovered from AIRE-HA recipient mice at the indicated time points. (E) Recovery of injected cells. The diagram shows the average absolute number (± SD., n = 3) of cells recovered from intrathymically injected AIRE-HA or WT mice at the indicated time points. (F) Proliferation upon intrathymic antigen encounter. PKH26 labeled cells were i.t. injected into either WT or AIRE-HA recipient mice. The histogram shows an overlay of the 3 phenotypically distinct donor-derived subpopulations in AIRE-HA recipients and of total donor cells in WT recipient mice 5 days after i.t. injection. All data in Fig. 1 are representative of at least 3 independent experiments.
Early after transfer, the recovery of donor cells in AIRE-HA thymi was considerably lower than in WT controls, most likely indicating clonal deletion of a fraction of the injected cells (Fig. 1E). However, whereas in control WT recipients the number of donor cells continually decreased over time, presumably as a result of thymic egress (22), donor cell numbers in AIRE-HA recipients sharply increased from day 2 onward, reached a maximum around day 4, and gradually declined thereafter. Labeling of donor cells with the vital dye PKH26 confirmed that these dynamic changes resulted from proliferative expansion of the injected cohort of cells. Thus, essentially all donor cells in AIRE-HA hosts, but not in WT controls, displayed reduced PKH26 fluorescence 5 days after transfer, that is, had gone through at least 1 cell cycle (Fig. 1F). Notably, cells that had acquired a Foxp3+CD25+ phenotype retained substantially more PKH26 dye than Foxp3−CD25+ or Foxp3−CD25− cells (Fig. 1F).
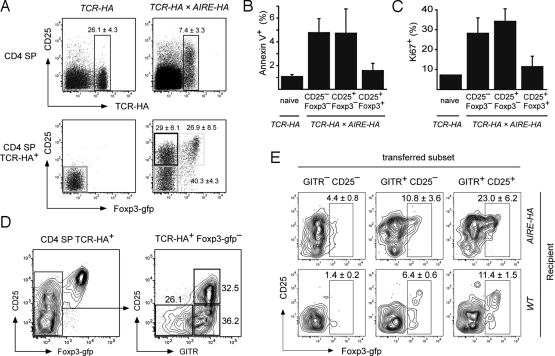
We next asked whether the heterogeneity of phenotypes and cell fate decisions among antigen-specific CD4 SP cells in the adoptive transfer setting, that is, clonal deletion, (abortive?) proliferation, and Treg differentiation, reflected steady-state Treg development in the thymus of AIRE-HA × TCR-HA Rag+/+ mice. Indeed, TCR-HA+ CD4 SP cells in AIRE-HA × TCR-HA thymi also segregated into Foxp3+CD25+, Foxp3−CD25+ and Foxp3−CD25− cells, bearing striking resemblance to the observations after i.t. injection (compare Fig. 1 C and Fig. 2A). Reminiscent of the initial loss of donor cells subsequent to i.t. injection into AIRE-HA hosts, substantial numbers of Annexin V+ apoptotic cells were seen among TCR-HA positive Foxp3−CD25+ and Foxp3−CD25− CD4 SP cells in AIRE-HA × TCR-HA thymi (Fig. 2B), indicating that these phenotypes coincided with a developmental dead-end for a substantial fraction of steady-state cells. At the same time, revealing a further commonality between the i.t. transfer system and steady-state T cell development, Foxp3−CD25+ and Foxp3−CD25− CD4 SP cells in AIRE-HA × TCR-HA mice also contained elevated frequencies of cycling Ki67+ cells (compare Figs. 1F and 2C). Polyclonal Foxp3−CD25−GITR+ and Foxp3−CD25+GITR+ CD4 SP thymocytes were recently reported to contain committed precursors of Foxp3+ Treg that require cytokine signaling, but not TCR stimulation, for further maturation (21). In agreement with this, a substantial fraction of TCR-HA positive Foxp3−CD25−GITR+ and Foxp3−CD25+GITR+ CD4 SP thymocytes from AIRE-HA × TCR-HA thymi progressed toward a Foxp3+CD25+ phenotype upon adoptive transfer into WT thymi, that is, in the absence of continual antigen encounter (Fig. 2 D and E).
Fig. 2.
Phenotype and precursor/progeny relationship of HA-specific CD4 SP thymocytes in TCR-HA × AIRE-HA mice. (A) Staining of CD4 SP cells in 5-week-old TCR-HA or TCR-HA × AIRE-HA mice for TCR-HA and CD25 expression (Upper) or Foxp3-gfp and CD25 expression on gated TCR-HA+ CD4 SP cells (Lower). Numbers indicate the average frequency (± SD) of cells within gates. (n = 5 for TCR-HA mice, n = 35 for TCR-HA × AIRE-HA mice). (B and C) Foxp3-gfp negative TCR-HA+ CD4 SP cells of TCR-HA × AIRE-HA mice show increased rates of apoptosis and proliferation. The percentage of apoptotic (B) or dividing cells (C) as assessed by staining for Ki67 or Annexin V, respectively, is shown. (D and E) Foxp3− CD4 SP subpopulations of TCR-HA × AIRE-HA mice contain Treg precursors. Foxp3− cells of TCR-HA × AIRE-HA CD45.1 mice were sorted into the indicated subpopulations based upon CD25 and GITR expression (D) and i.t. transferred into AIRE-HA or WT mice. After 4 days, mice were killed and donor-derived thymocytes were analyzed for Foxp3-gfp and CD25 expression (E). Representative plots for each subset injected into AIRE-HA or WT mice are shown. Numbers in plots indicate the average frequency (± SD) of donor derived Foxp3-gfp+ cells recovered. Data are representative of 4 independent experiments.
Together, these observations established that self-antigen driven intrathymic Treg differentiation can be initiated in the absence of “nominal” antigen encounter before the CD4 SP stage and does not obligatorily involve a Foxp3+ DP stage. Concomitant to some cells entering the Treg lineage via a transitory Foxp3−CD25+ stage, others were clonally deleted and/or engaged in extensive proliferation whose extent inversely correlated with Treg differentiation.
Progressive Maturation within the CD4 T cell Lineage Inversely Correlates with Treg Differentiation.
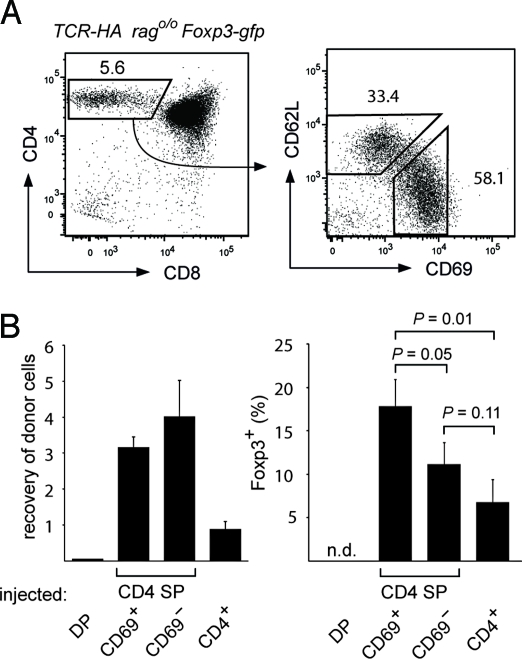
In the AIRE-HA thymus, mTEC-derived HA is not only presented by mTECs themselves, but also transferred to and presented by DCs (8). It was therefore possible that the heterogeneous fate of TCR-HA+ SP thymocytes when injected into an AIRE-HA host thymus was related to antigen recognition on different APCs. Arguing against this, the overall outcome and the efficiency of Treg induction was very similar when TCR-HA Rag2o/o Foxp3-gfp CD4 SP cells were i.t. transferred into chimeras that were either sufficient or deficient in their ability to “cross-present” HA on hematopoietic cells (Fig. S2). We therefore asked whether intrinsic heterogeneity within the starting population, for example the maturation stage within the CD4 SP compartment, may impinge on the outcome of intrathymic antigen encounter. TCR-HA Rag2o/o Foxp3-gfp CD4 SP cells segregated into immature (CD69+CD62L−) and mature (CD69−CD62L+) subsets (Fig. 3A), whereby the levels of HSA correlated with CD69 expression in the expected manner. When these subpopulations were injected into AIRE-HA thymi, Treg cells emerged at a substantially higher frequency among the progeny of immature cells (Fig. 3B). This attenuation of the receptiveness for Treg-inducing stimuli with progressive maturation similarly applied to peripheral CD4 T cells. Thus, only a small fraction of peripheral naïve TCR-HA+ CD4 T cells differentiated into Treg upon i.t. injection, and peripheral CD4 T cells from donors thymectomized 6 weeks earlier, that is, that were free of recent thymic emigrants, did so even less efficiently (Fig. 3B).
Fig. 3.
The maturation stage of CD4 SP cells determines the efficiency of Treg conversion in vivo. (A) Gated TCR-HA Rag2o/o CD4 SP thymocytes (Left) can be subdivided into immature (CD69+CD62L−) and mature (CD69−CD62L+) cells (Right). Numbers indicate the percentage of cells in the respective gates. (B) Intrathymic transfer of thymocyte subpopulations and peripheral T cells. DP, CD69+CD62L− CD4 SP, CD69−CD62L+ CD4 SP, and peripheral CD4+ cells from TCR-HA Rago/o Foxp3-gfp mice (CD45.1) were sorted and mixed with a PKH26 labeled reference population of total CD4 SP cells (CD45.1) at a ratio of 1:2.6 before intrathymic transfer into AIRE-HA recipients. After 5 days, the recovery of the different tester populations was determined as the ratio of the respective cells to reference cells among donor cells (CD45.1+) (Left). The Right diagram shows the percentage of Foxp3-gfp+ cells among tester cells (n.d. = not detectable). Data are representative of 2 independent experiments with n = 3.
Taken together, these findings revealed a sliding scale of T cell-intrinsic responsiveness to identical conditions of antigen encounter as a critical determinant of Treg differentiation.
Treg Differentiation of CD4 SP Cells Does Not Require a Dedicated APC.
The conclusive delineation of thymocyte/stromal cell interactions and ensuing cell fate decisions in vivo upon i.t. transfer is complicated by factors that can only insufficiently be controlled for, such as eventually distinct homing properties and/or preferential retention or egress of a particular subset of cells. A further caveat of in vivo assays concerns the unambiguous definition of the cellular interactions that underlie Treg induction. For example, it has recently been shown that functional MHC/peptide complexes can be transferred from TEC to DCs, so that data obtained in bone marrow chimeric settings need to be interpreted with caution (23). We therefore sought to establish an analogous in vitro system that would minimize migration-related caveats, reduce the complexity of cellular interactions, and, importantly, would also allow manipulating the antigen dose.
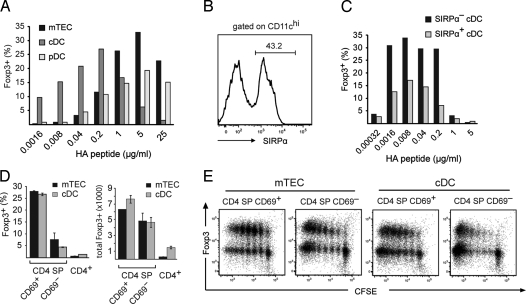
Using a minimal in vitro system consisting only of naïve TCR-HA Rag2o/o Foxp3-gfp CD4 SP responders, thymic stromal APCs, and IL-2, we first confirmed that the recognition of endogenously expressed cognate antigen on mTECs from AIRE-HA mice was sufficient for Treg generation. To address the capacity of different thymic APCs to convert CD4 SP cells into Tregs, and, at the same time, gain insight into the role of the antigen dose, we co-cultured TCR-HA+ CD4 SP responders together with either mTECs, plasmacytoid DCs (pDCs) (CD11cintCD45RA+) or conventional DCs (cDCs) (CD11chiCD45RA−) from WT mice and titrated amounts of HA peptide (Fig. 4A). Somewhat unexpectedly, this revealed that all 3 APC subtypes could efficiently support Treg differentiation of CD4 SP cells, provided that APC-type dependent optimal doses of peptide were available. Thus, whereas Treg induction by mTECs and pDCs not only tolerated, but actually peaked at very high doses of cognate antigen, the maximal efficacy of Treg induction by cDCs was observed at a 2 orders of magnitude lower range of peptide doses (Fig. 4A). The absolute numbers of Tregs followed an essentially identical distribution. Functional in vitro assays confirmed that Foxp3+ cells arising upon co-culture with mTECs or cDCs displayed potent suppressive activity (Fig. S3).
Fig. 4.
Induction of Treg cells by different thymic stromal APCs in vitro. (A) TCR-HA Rag2o/o CD4 SP thymocytes were co-cultured with mTECs, thymic cDCs, and thymic pDCs in the presence of increasing amounts of HA peptide. After 5 days, T cells were analyzed for CD25 and Foxp3-gfp expression. The bar diagram shows the percentage of Foxp3+ cells recovered. (B) Separation of thymic cDC into Sirpα+ and Sirpα− subpopulations. The histogram shows a Sirpα staining of gated CD11chigh thymic dendritic cells (C) Sirpα+ and Sirpα− DC were co-cultured with TCR-HA+ CD4 SP cells in the presence of increasing amounts of HA peptide. The diagram shows the percentage of Foxp3+ cells recovered after 5 days. (D) T cell maturation impinges on the efficiency of Treg induction in vitro. The indicated T cell subpopulations were co-cultured with mTEC or cDC at their respective optimal peptide concentrations. The diagrams show the percentage (Left) or total number (Right) of Foxp3+ cells recovered after 5 days. (E) Inverse correlation of Treg induction and proliferation. CFSE-labeled mature and immature CD4 SP cells from TCR-HA Rag2o/o mice were co-cultured with mTEC or cDC at their respective optimal peptide concentration and analyzed for Foxp3 expression and CFSE dilution after 5 days.
Thymic cDCs can be further subdivided into Sirpα− or Sirpα+ cells (Fig. 4B) (24), of which the latter subset largely consists of migratory DCs that enter the thymus from the periphery and that have recently been implicated in Treg induction (25). When tested for their capacity to induce Treg in vitro, Sirpα− and Sirpα+ cDCs had essentially identical dose optima, whereby the relative yield of Foxp3+ cells was consistently higher with Sirpα− cDCs (Fig. 4C).
In another series of in vitro experiments, we confirmed and extended our in vivo findings concerning the inverse correlation of T cell maturation and the propensity to undergo Treg differentiation. The relative and absolute yield of Foxp3+CD25+ cells with mTECs or cDCs at their respective peptide optimum was consistently higher with immature CD69+ CD4 SP responders compared with mature CD69− cells (Fig. 4D). Along these lines, conversion of peripheral CD4+ responders from TCR-HA Rag2o/o Foxp3-gfp mice was barely detectable.
Whereas the predisposition for Treg differentiation decreased with maturation of CD4 SP cells, the propensity to proliferate upon antigenic stimuli exhibited an inverse behavior (Fig. 4E). Thus, when CFSE-labeled CD69+ or CD69− CD4 SP responder cells from TCR-HA Rag2o/o mice were co-cultured with either mTECs or cDCs at the respective optimal peptide concentration, the progeny of mature cells went through a considerably higher number of cell cycles. This was seen with both types of APCs and irrespective of whether the T cells had acquired a Foxp3+ phenotype or not (Fig. 4E).
Together, these findings not only confirmed the T cell-intrinsic developmental control of Treg development in an in vitro system of minimized complexity, but also revealed a surprising degree of redundancy among thymic stromal APCs in their principle capacity to support Treg differentiation, given that APC-specific optimal doses of agonist ligand are provided.
Treg Differentiation of TCR Transgenic and Polyclonal Thymocytes in an APC-Free System.
The largely redundant capacity of thymic APCs to orchestrate Treg development may indicate that all of these cells similarly provide known and unknown critical co-stimulatory ligands or factors. Alternatively, Treg differentiation of thymocytes may proceed with minimal requirements beyond a matching TCR stimulus and cytokine signaling. We therefore assessed Treg differentiation in an APC-free system in which only signal 1 in the form of plate-bound anti-CD3 together with exogenous IL-2 would be present. Of several conditions tested, anti-CD3 coated at 10 μg/mL was found to induce the differentiation of significant numbers of CD25+Foxp3+ cells within TCR-HA Rag2o/o Foxp3-gfp thymocytes (Fig. 5A). Importantly, this APC-free system again recapitulated the gradual loss of competence for Treg differentiation with progressive maturation within the CD4 lineage (Fig. 5A). Supporting the general relevance of our observations beyond TCR transgenic systems, we observed an analogous behavior for polyclonal CD4 T cells of various developmental stages (Fig. 5B).
Fig. 5.
Treg induction in an APC-free system. (A) Immature and mature CD4 SP T cells from TCR-HA rago/o Foxp3-gfp mice were sorted and cultured in the presence of plate-bound anti-CD3. Cells were analyzed for CD25 and Foxp3-gfp expression after 3 days. The percentage (Left) and absolute numbers (Right) of Foxp3+ cells is depicted. (B) Immature (CD69+CD62L−) and mature (CD69−CD62L+) polyclonal CD25−Foxp3− CD4 SP cells were sorted from Foxp3-gfp mice and cultured and analyzed as in A.
Discussion
The decreasing inclination toward Treg differentiation that accompanies CD4 SP thymocyte maturation bears striking resemblance to a similar developmental switch from susceptibility to resistance for clonal deletion within the CD4 SP compartment (26). It is conceivable that concomitant to gradually losing the susceptibility to being deleted “aging” thymocytes may enter a phase of exquisite inclination toward Treg development. Nevertheless, clear T cell-intrinsic developmental demarcations between these conditions are unlikely to exist, and the 2 “windows of opportunity” might even be largely overlapping. It also remains open at which developmental stage the principle responsiveness toward Treg inducing stimuli is established. Our data do not exclude that this occurs in tight association with positive selection at the DP stage. In fact, we have observed the agonist-driven emergence of Foxp3+ cells in in vitro differentiation assays using postpositive selection CD69+ DP cells, whereby the interpretation of these findings is certainly blurred by the programmed progression of the input cells into the CD4 SP stage during the incubation period. Finally, agonist independent (stochastic?) priming of a given fraction of monoclonal cells to enter the Treg lineage upon receiving adequate TCR stimulation remains a formal possibility; however, we do not have any evidence for this scenario.
Irrespective of these considerations, our findings suggest that T cell-intrinsic developmental control in conjunction with agonist stimulation of matching strength, but not qualitative features of a dedicated thymic stromal APC, are critical parameters of thymic Treg differentiation. Importantly, whereas the comparable competence of epithelial or hematopoietic thymic APCs to induce Tregs applies to cell type-dependent optimal doses of antigen, it remains open whether this principle redundancy translates into a true “qualitative” redundancy at the level of specificities selected into the polyclonal Treg repertoire. Given the evidence that the thymic microenvironment represents a mosaic of stromal niches in which self-antigens may not be homogeneously available (27, 28), we consider it more likely that different stromal APCs induce complementing pools of Tregs. Reports on similarly large Treg compartments irrespective of genetic ablation of particular stromal APCs, expression of MHC class II only in the cortex or pharmacological retention of thymocytes in the cortex are not at odds with this scenario (8, 10, 13, 29), because these findings may reflect cytokine driven homeostatic mechanisms acting downstream of bona fide differentiation processes and may therefore mask shifts in the composition of the Treg repertoire.
Methods
Animals.
Mouse colonies were maintained in individually ventilated cages. TCR-HA, AIRE-HA, and AIRE-HCO mice have been described (8). Foxp3-gfp knock-in mice (30) were kindly provided by Alexander Rudensky. All animal studies were approved by local authorities.
Antibodies and Flow Cytometry.
Biotin-conjugated monoclonal antibodies (mAbs) to CD8 (53–6.7), CD24 (30-F1), CD62L (MEL-14), CD45RA (14.8), and CD69 (H1.2F3), PE-conjugated streptavidin, annexin-V, and mAbs to Ki67 (B56) and GITR (DTA-1), CyChrome-conjugated mAb to CD8 (53–6.7), APC-conjugated CD45.1 (A20), and CD172a/Sirpα (P84), APC-Cy7 conjugated mAb to CD4 (GK1.5), and PE-Cy7 conjugated streptavidin and mAb to CD25 (PC61) were obtained from Becton Dickinson. The mAbs to the TCR-HA (6.5) and DO11.10 (KJ1–26) were purified and conjugated to Alexa647, PE, or biotin in our laboratory.
Purification of CD4 SP Cells and CD4+ Peripheral Cells.
CD4 SP cells or subpopulations of CD4 SP cells were purified by CD8 depletion, staining for the indicated surface markers and sorting with a FACSAria cell sorter (Becton Dickinson). Naïve CD4+ peripheral cells were obtained from pooled spleens and lymph nodes by MACS enrichment of CD4+ cells, staining for the indicated surface markers, and subsequent sorting.
Intrathymic Transfer.
Totals of 4 × 105 CD4 SP thymocytes or 3 × 105 cells of sorted subpopulations of TCR-HA × AIRE-HA donors (CD45.1) were injected in 3 μL PBS into one thymic lobe of WT (CD45.2) or AIRE-HA (CD45.2) recipients. Where indicated, sorted cells were PKH26 (Sigma Aldrich)-labeled according to the manufacturer's instructions. To normalize for variations in injection efficiency and allow for a more accurate comparison of donor cell recovery, sorted “tester” subpopulations were spiked with 1.5 × 105 PKH26 labeled total “reference” CD4 SP cells from TCR-HA Rag2o/o mice (CD45.1). The relative recovery of tester cells was calculated as follows: recovery = [total number of tester derived cells/total number of reference derived cells].
The analysis of injected thymi was carried out at various time points after injection by depletion of CD8+ cells, staining for the indicated surface markers, and analysis by flow cytometry.
Preparation of Thymic Stroma.
Stromal cells were isolated by enzymatic digestion and density fractionation as described elsewhere (8). Subsets were sorted according to CD45, Ly51, EpCAM, CD11c, CD45RA, and Sirpα expression (mTEC = CD45−Ly51−EpCAM+; pDC = CD45+CD11cintCD45RA+; Sirpα+ cDC = CD45+CD11chighCD172a+; Sirpα− cDC = CD45+CD11chighCD172a−).
In Vitro Differentiation Assay.
Sorted thymocytes (5 × 104) from TCR-HA Rag2o/oFoxp3-gfp mice were co-cultured with sorted stromal APCs (1 × 104) in the presence of the indicated amounts of HA (107–119) peptide and 100 U/mL recombinant IL-2 (Preprotech). For APC-free in vitro differentiation assays, flat-bottom 96-well plates were coated with CD3 antibody (145–2C11) in PBS (10 μg/mL) at 37 °C for 3 h. To monitor proliferative expansion, assays were carried out with CFSE (Invitrogen)-labeled T cell populations. Cells were analyzed for Foxp3 expression using an intracellular staining kit (Ebioscience) according to the manufacturer's instructions.
Suppression Assay.
MACS-enriched naive TCR-HA+ CD4+ T cells (2 × 104) from spleen and lymph nodes of TCR-HA Rag2o/o mice were cultured either alone or together with Foxp3-gfp+ cells (2 × 104) sorted from in vitro Treg-differentiation assays in the presence of irradiated (3,000 rads) BALB/c splenocytes (2 × 105) and 10 μg/ml HA (107–119) peptide. Proliferation was measured by scintillation counting after cells were pulsed with 1 μCi [3H]thymidine per well for the last 24 h of a 96-h incubation period.
Statistical Analysis.
Statistical significance was assessed by the 2-tailed Student's t test with unequal variance.
Supplementary Material
Acknowledgments.
We thank Jan Emmerich for discussions and Christian Spona for excellent technical assistance. This work was supported by Deutsche Forschungsgemeinschaft Grant SFB 571 and the Austrian National Science Fund (Sonderforschungsbereich) Grant F023. Research at the Research Institute of Molecular Pathology is sponsored by Boehringer Ingelheim.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901877106/DCSupplemental.
References
- 1.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 2.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 3.Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity. 2006;25:249–259. doi: 10.1016/j.immuni.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Wong J, Mathis D, Benoist C. TCR-based lineage tracing: No evidence for conversion of conventional into regulatory T cells in response to a natural self-antigen in pancreatic islets. J Exp Med. 2007;204:2039–2045. doi: 10.1084/jem.20070822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 6.Jordan MS, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 7.Romagnoli P, Hudrisier D, van Meerwijk JP. Preferential recognition of self antigens despite normal thymic deletion of CD4(+)CD25(+) regulatory T cells. J Immunol. 2002;168:1644–1648. doi: 10.4049/jimmunol.168.4.1644. [DOI] [PubMed] [Google Scholar]
- 8.Aschenbrenner K, et al. Selection of Foxp3(+) regulatory T cells specific for self antigen expressed and presented by Aire(+) medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 9.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bensinger SJ, Bandeira A, Jordan MS, Caton AJ, Laufer TM. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+)25(+) immunoregulatory T cells. J Exp Med. 2001;194:427–438. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cabarrocas J, et al. Foxp3+ CD25+ regulatory T cells specific for a neo-self-antigen develop at the double-positive thymic stage. Proc Natl Acad Sci USA. 2006;103:8453–8458. doi: 10.1073/pnas.0603086103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feuerer M, et al. Enhanced thymic selection of FoxP3+ regulatory T cells in the NOD mouse model of autoimmune diabetes. Proc Natl Acad Sci USA. 2007;104:18181–18186. doi: 10.1073/pnas.0708899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liston A, et al. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proc Natl Acad Sci USA. 2008;105:11903–11908. doi: 10.1073/pnas.0801506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribot J, et al. Shaping of the autoreactive regulatory T cell repertoire by thymic cortical positive selection. J Immunol. 2007;179:6741–6748. doi: 10.4049/jimmunol.179.10.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Santen HM, Benoist C, Mathis D. Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. J Exp Med. 2004;200:1221–1230. doi: 10.1084/jem.20041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu YJ. A unified theory of central tolerance in the thymus. Trends Immunol. 2006;27:215–221. doi: 10.1016/j.it.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Liston A, Rudensky AY. Thymic development and peripheral homeostasis of regulatory T cells. Curr Opin Immunol. 2007;19:176–185. doi: 10.1016/j.coi.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Gavin MA, et al. Foxp3-dependent programme of regulatory T cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 19.Lin W, et al. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- 20.Wan YY, Flavell RA. Regulatory T cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 21.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCaughtry TM, Wilken MS, Hogquist KA. Thymic emigration revisited. J Exp Med. 2007;204:2513–2520. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Millet V, Naquet P, Guinamard RR. Intercellular MHC transfer between thymic epithelial and dendritic cells. Eur J Immunol. 2008;38:1257–1263. doi: 10.1002/eji.200737982. [DOI] [PubMed] [Google Scholar]
- 24.Lahoud MH, et al. Signal regulatory protein molecules are differentially expressed by CD8- dendritic cells. J Immunol. 2006;177:372–382. doi: 10.4049/jimmunol.177.1.372. [DOI] [PubMed] [Google Scholar]
- 25.Proietto AI, et al. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc Natl Acad Sci USA. 2008;105:19869–19874. doi: 10.1073/pnas.0810268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kishimoto H, Sprent J. Negative selection in the thymus includes semimature T cells. J Exp Med. 1997;185:263–271. doi: 10.1084/jem.185.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 28.Mathis D, Benoist C. Back to central tolerance. Immunity. 2004;20:509–516. doi: 10.1016/s1074-7613(04)00111-6. [DOI] [PubMed] [Google Scholar]
- 29.Rossi SW, et al. RANK signals from CD4(+)3(-) inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med. 2007;204:1267–1272. doi: 10.1084/jem.20062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.