Abstract
Institutions worldwide have experienced a rapid growth in the use of zebrafish as a research model for a variety of molecular and genetic studies of vertebrate development. This expansion in zebrafish research essentially has outpaced the establishment of specific recommendations for the care and use of fish in research. In some cases, this situation has created a dilemma where an Institutional Animal Care and Use Committee, which is responsible for oversight of vertebrate animal research, is not fully prepared to undertake this role for a decentralized zebrafish facility. IACUC inspectors will be more equipped to ask pertinent questions by understanding the basic principles of zebrafish health and facility management. Concurrently, zebrafish facility managers can contribute to the progress of a semiannual facility inspection by maintaining fully accessible operating records. In the context of presenting a well-established and useful model of zebrafish management and recordkeeping to the zebrafish facility operator, the information we present here also prepares a potential IACUC inspector to conduct a constructive and positive inspection.
Abbreviation: GAC, granular activated carbon; IACUC, Institutional Animal Care and Use Committee; MS222, 3-aminobenzoic acid ethyl ester (tricaine); RO, reverse osmosis; SOP, standard operating procedures; SPF, specific pathogen-free
The zebrafish (Danio rerio) has gained in popularity as a uniquely important animal model for the study of vertebrate development and genetics. The species typically is used to analyze how the vertebrate nervous system is regulated at the cellular, genetic, and molecular levels.25 The rapid expansion of zebrafish research in North America necessitates better education and resources on the part of the Institutional Animal Care and Use Committees (IACUCs), whose role it is to assure that animal research meets the minimum standards set forth in the Guide for the Care and Use of Laboratory Animals (the Guide).18 Within the animal use program, centralized and satellite animal facilities must be inspected semiannually. The Guide, however, does not provide criteria specific for establishing or evaluating fish programs, and therefore each IACUC must construct standards of its own.13
Performing inspections of investigator-managed satellite facilities that use any number of different housing styles can be particularly challenging for IACUC inspectors, especially those with little background in aquaculture. IACUC inspectors will be more equipped to ask pertinent questions by obtaining a good understanding of the principles of zebrafish facility operations. In addition, zebrafish facility managers can be better prepared for inspections by establishing standard operating procedures (SOPs) and complete facility operating records, thereby enabling inspectors to easily evaluate facility function, preparedness, and zebrafish health. By introducing a potential IACUC inspector to the basics of fish husbandry and by sharing with fish facility operators 1 paradigm of recordkeeping that was central to a well-organized zebrafish facility with an excellent inspection history, the hope is that this review will assist both parties in managing their assignments cooperatively.
The Guidelines for the Use of Fishes in Research24 was intended to be used as a supplement to the Guide in addressing the differences between fish and other vertebrate laboratory animals. Although these guidelines give specific attention to the roles, responsibilities, and information needs of the IACUCs in regard to protocol review, facility inspection is not addressed specifically.
The Canadian Council on Animal Care (CCAC) Guidelines on the Care and Use of Fish in Research, Teaching, and Testing10 is a comprehensive document with a lengthy set of guidelines enumerating all aspects of fish care and welfare. Although this document has not officially been adopted by a US regulatory agency, the concepts harmonize well with the Guide, and we highly recommend inspectors and facility managers review the CCAC document.
Zebrafish Facility Model
For the purposes of this review, we are using a Washington University zebrafish facility as the model for an overview of basic principles widely applicable to other zebrafish facilities. Although the tables and figures in this article specifically refer to the Washington University model, they serve as useful examples of facility recordkeeping that can be adapted to fit any system.
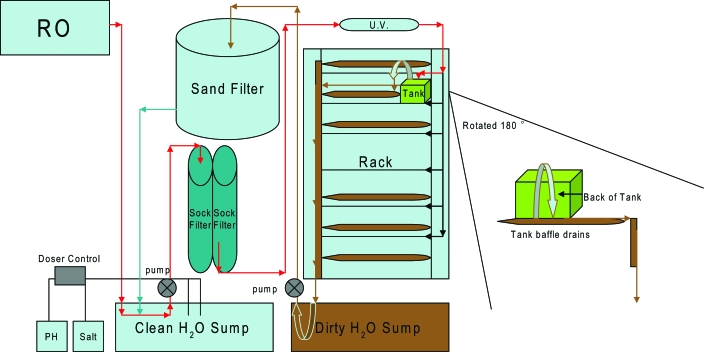
Adult zebrafish tanks are maintained on a recirculating filtration system using water treated by reverse osmosis (RO). The system was designed with a combination of equipment (Aquatic Habitats, Apopka, FL, and Aquaneering, San Diego, CA), including 2 double-sided and 2 single-sided stainless steel racks. Space exists for 384 3-L autoclavable polycarbonate plastic tanks suitable for housing 15 zebrafish or fewer. The principal biological filter for the system is a fluidized bed filter, specifically a sand filter. In addition, two 50-μm sock filters and 2 carbon cartridges aid in pre-filtration before exposure to UV light. The 2 double-sided racks share a single UV germicidal bulb, and each single-sided rack has 1 UV germicidal bulb. The UV dose rate is 30,000 μW·s/cm2 at a flow rate of 88 gallons per minute (Aquatic Habitats, Apopka, FL). The entire system has a single point of entry for new, clean water and a single point of discharge for dirty water. A pH–salt doser is inline with the system's clean water sump. The water is under pressure through use of 2 pumps. One pump keeps the fluidized bed in suspension, whereas the other forces the water to the top of the racks. A valve is in place for entry of new water into the system to replace some of the dirty water that exits through the overflow pipe. The amount of water replaced varies daily depending on measured water chemistries, specifically nitrate and salt (conductivity). Figure 1 illustrates the system's water flow and filtration.
Figure 1.
Recirculating system (intermediate type). Reverse osmosis (RO) water flows from a holding tank into a reserve clean water sump tank (adjusted by a pH–salt doser), through 2- to 50-μm sock filters, is pumped to the top of the tank racks, exposed to UV light, into the fish tanks, out the tank baffle drains, through prefilter pads into the dirty water sump, from the dirty water sump into the fluidized sand filter, and finally back to the clean water sump. Engaging the flow-through switch or opening the valve allows some of the water to exit the filtration cycle at the dirty water sump through the overflow drain, where the water runs into the main sanitary sewer drain.
Quarantine tanks are located in the same room as the general colony tanks, but use filtration equipment that is separate from the main system. Static tanks with sponge filters and air stones are used for housing quarantined fish. Standard operating procedures are designed to prevent cross-contamination between quarantine tanks and those on the main system. Quarantine tanks are handled last or by caretaker designated for quarantine work only.
Static nursery tanks used for raising juveniles to adulthood are located in temperature-controlled (28 °C) incubators in a designated area of the facility. Standard water chemistry parameters for this system are: pH, 6.8 to 7.0; NH3, 0 ppm; nitrites, 0 ppm; nitrates, less than or equal to 10 ppm; dissolved oxygen, 8 ppm; temperature, 28 °C; and conductivity, 450 μS. Adult zebrafish are fed live Artemia spp. (Brine Shrimp Direct, Ogden, UT) twice daily and supplemented with a blended dry diet (Hikari Micro Pellets, Hayward, CA; Tetra Min Flakes, Atlanta, GA), vitamin C (Sigma, St Louis, MO), and dried krill (Aquatic Ecosystems) once daily. Fry (larvae and juvenile fish) are fed an ad libitum diet of live rotifers hatched inhouse. All zebrafish are housed and cared for under protocols approved by the Washington University Animal Studies Committee.
This system is not, by any means, the only way to maintain a zebrafish facility. Although the same general principles of fish biology and aquaculture apply to the management of any zebrafish facility, different systems necessitate diverse approaches to conform to these basic principles. Discussion with other members of the fish community provides a collective understanding of husbandry techniques and a broader exposure to different methods of fish care suitable to the specific research needs. Fish hobbyist books and reputable websites are also good references for general aquatic animal husbandry. The general goal is to achieve the most efficient system possible by way of steady water quality, good nutrition, good system maintenance, and timely identification and remedy of problems, all contributing to optimal zebrafish health and high-quality research.
We will discuss filtration systems, water systems, environment, biosecurity, recordkeeping, and other miscellaneous topics briefly. IACUC inspectors should make note of the suggested items to be evaluated on a semiannual inspection. Items will differ depending on the design of the facility and the goal of the research. Using these and other pertinent topics, specific checklists can be designed by the IACUC for site visits to each facility.
Filtration Systems
Mechanical, chemical, and biologic filtration.
Achieving appropriate water quality is paramount and depends on the use of filtration devices for the elimination of wastes. Three major categories of filtration work together: mechanical, chemical, and biological. In general, solid wastes are removed by mechanical filtration, nitrogenous wastes are removed by biological filtration, and organic and inorganic compounds are removed by chemical filtration.
The amount of time spent on maintenance for each system depends on the quality of the components and its inhabitants, with bioload (fish population, uneaten feed and fish waste) playing an integral role.6 At each inspection written SOPs that describe the optimum care for system maintenance should be readily available.
Mechanical filtration.
The first objective of any recirculating system is the removal of solid wastes, which originate from uneaten feed, excrement, and bacteria. One way to remove suspended solids is through the use of screens (for large particles), porous media filters (such as sponges, paper cartridges, and polystrand),31 and granular media filters. Granular media filters can control the widest range of solids by the passage of water through a bed of granular material (media) and deposition of solids onto the media.27
Chemical filtration.
Chemical filtration removes dissolved waste molecules from the water. Filtering water through granular activated carbon (GAC) is the most common type of chemical filtration. Microscopic pores present in GAC trap waste particles by adsorption and ion exchange at the molecular level. This process also removes heavy metals and organic molecules, therefore improving water clarity and odor.22,31 Carbon can also be considered a mechanical filter because of its sieve-like texture, giving it the ability to trap solid particles. Higher-quality GAC with fewer phosphates is less likely to promote algae growth than lower-grade material. Regardless of whether carbon is used in a system, some carbon should always be available in the fish facility for clean-up of suspected chemical toxicity or heavy-metal contamination.
Biological filtration.
Biological filtration can be an effective means of controlling ammonia. The process of ammonia removal by a biological filter is called nitrification and consists of the successive oxidation of ammonia to nitrite via Nitrosomonas bacteria and then to nitrate via Nitrobacter bacteria.27 Ammonia is toxic to fish at levels greater than 0.02 ppm, and nitrite is toxic to fish at levels greater than 1 ppm.9,20 The temperature, pH, and salinity of the water all play important roles in maintaining healthy populations of nitrifying bacteria and subsequent acceptable levels of all forms of inorganic ammonia-nitrogen compounds, also called total ammonia nitrogen (TAN).
Nitrifying bacteria grow either attached to a surface (fixed films) or suspended in the water column.30 Almost all recirculating systems use fixed-film bioreactors where nitrifying bacteria grow on a wetted or submerged media surface.27 Typical media used in aquaculture biofilters are sand, crushed rock or river gravel, and some form of plastic or ceramic material shaped as small beads or large spheres, rings, or saddles.27 Glass beads and porcelain are good alternatives.31 The larger the surface area of a chosen substrate, the greater the bioload supported by the system. Several types of biofilters are available for recirculating aquaculture systems, each with its own strengths and weaknesses. Submerged biofilters, trickling biofilters, granular filters (fluidized beds and floating bead beds), and dynamic bead biofilters are just a few of the choices. The basic function of all biofilters is maximal exposure of healthy nitrifying bacteria to water for completion of the nitrification cycle. Due to its large surface area, sand in fluid suspension makes an ideal bacterial substrate. A fluidized bed with sand media requires a minimal amount of maintenance and tends to offer good water quality predictability. As an added benefit, the contents of a fluidized sand filter generally do not need to be changed. The IACUC inspector will review the system components and obtain an understanding of the filtration sources used in the system for ease of facility record evaluation.
Water systems.
Role of oxygen.
Water plays an essential role in promoting an oxygen and nutrient-rich environment critical for health of fish and function of the nitrification system. When the system is adjusted correctly and good quality water is flowing at the appropriate rate, oxygen will not drop to dangerously low levels. But loss of either water or flow will result in loss of fish.27
In most fish species, water oxygen saturation levels should be above 90%,10 or around 6 to 8 ppm (mg/L), at 28 °C.21,27 The availability of dissolved oxygen in the water is determined by water temperature, atmospheric pressure and salinity.5,10 In addition, the bacterial media must remain exposed to constantly moving and well-aerated water. Achieving constantly moving water can be as simple as using an air stone to generate ripples in a static tank or as involved as the airlifts required for fluidized bed filters. The bacteria can live for short periods without water flow but will quickly perish if air is removed. Rapidly increasing or decreasing the bioload on a system also will jeopardize a biological filter. Loading and unloading of the fish system should be moderated to make gradual changes.
Covering sumps will reduce evaporation, prevent splashing and spillage of potential contaminants, and deter escaped zebrafish and vermin from entering the system. Escaped fish that do manage to enter the sump have become exposed to water from the entire rack and may be useful as sentinels. The IACUC inspector will check the system for constantly moving and well aerated water and fresh water input. He will also verify that overflow water is not being obstructed and sumps are covered.
Water flow.
Aquatic systems are either static or flowing.6 Flowing systems can be either flow-through or recirculating systems. In recirculating systems, a proportion of the water exiting the system is recycled.
Static water system.
A static water system is one that does not receive a continuous supply of new or recirculated water. It has very low inherent biological carrying capacity and relies mostly on partial-volume water changes. Water quality can be improved with the addition of devices that create water movement, such as pumps or air stones.10 A sponge filter can aid in mechanical collection of debris and is gentle enough to be used in static tanks when rapid growth of fry is desired. These filters also provide a suitable substance and increased surface area for nitrifying bacteria to grow. Algae will grow in static tanks and should be kept under control but not eliminated. A minimum of algae should be removed regularly to keep the nitrogen cycle moving. Water chemistry testing is recommended as often as necessary to determine the proper tank cleaning and water change schedule for this type of system. Water changes of 75% or more of the volume usually are required once or twice weekly. Ammonia toxicity is an important concern in static tanks and must be measured daily. Although the goal is to keep ammonia levels below 0.02 ppm and nitrite levels below 1 ppm,9 zebrafish can acclimate and survive in suboptimal water conditions, but this may impact negatively on growth, gamete production and immune function.20 The IACUC inspector will observe static water systems and review SOPs for care and check maintenance and water quality records. Algae growth should not prevent visibility of the fish when looking through the side of the tank.
Flowing water systems.
A flow-through system requires a continuous supply of water either from a natural source (river, lake or ocean), a municipal source (tap water) or a well. Water is pumped separately into individual tanks, while the runoff water exits the tanks into a drain without recirculation. Because no holding tank is present typically, the incoming source must be good-quality water that is pretreated or filtered before it enters the tanks. This goal can be accomplished by mechanical, chemical, or biological filtration or some combination thereof. Fluctuations in water condition at the source will be reflected in the system water quality. Flow-through systems are not commonly used in zebrafish facilities, but may be desirable in quarantine areas where it is preferable to separate each tank. In a waterfront facility with access to an unlimited supply of natural water, a flow-through system may be the system of choice. In such situations, zebrafish facility operators will have to deal with the difficulty of stabilizing water chemistries due to the changes in the natural environment. The IACUC inspector will observe quarantine tanks that use flow-through systems and check maintenance and water quality records.
The components of a recirculating system accomplish 4 main objectives: the mechanical removal of solids, sterilization, biofiltration, and oxygenation. Mechanical, chemical, and biological filtration methods together allow reuse of the ‘dirty water.’ To maximize energy efficiency and use the fewest number of pumps, components are placed in series so that water can move by gravity whenever possible.12 The runoff water from each tank is collected in a sump, where it is pumped through the filter mechanism before reentering the tanks. Filtration systems can be integrated under each rack (1 to 5 rack systems) or centralized to service a larger facility. An intermediate system within the recirculating system can be created for ease of repeated water changes by introducing a switch that allows some of the water to be sent down the overflow pipe, while the majority of water is captured in the ‘dirty’ sump and pumped back through the filters.10 Water quality can be optimized by allowing a sufficient supply of clean water to enter the system. A recirculating water system is a common feature in zebrafish facilities and can be used to operate quarantine racks if maintained properly.
In an intermediate type of recirculating system like that we have described, the flow can be described as shown in Figure 1. All facilities should maintain current SOPs for all of their water systems.
Macroenvironment
Facility maintenance SOPs.
Written SOPs and preventative maintenance schedules must be developed specifically for each zebrafish facility, based on a thorough knowledge of the requirements for care and use of equipment as determined by the manufacturer and for proper maintenance of the physical structure. Completed maintenance duties should be documented for easy inspection.10
Heating, ventilation, and air conditioning.
Airflow in animal rooms and procedure rooms should be sufficient to allow surfaces to dry properly10 and to permit an adequate oxygen supply and carbon dioxide removal.21 A general guideline is 10 to 15 fresh air changes hourly.18
Room humidity is targeted to acceptable levels for personnel in the facility and should not be uncomfortably high. Humidity of 30% to 60% is recommended as a suitable range comfort range for humans. Higher humidity levels can contribute to structural damage and encourage the growth of bacteria and fungi through aerosol transfer.10 The IACUC inspector will note temperature and humidity in the room and review logs (if available).
Electrical system.
Ground fault circuit interrupters should be present on all circuits. Electrical outlets should be water-resistant or fitted with waterproof covers. Extension cords, power strips, and surge protectors should be used safely and must be kept away from water exposure to help prevent electrical shock. Electrical components and equipment should be placed outside the splash zone and located in a moisture-proof enclosure.10 If access to emergency power outlets is not available within the facility, standby generators may be necessary in case of a power outage. The IACUC inspector will check for electrical safety and inquire about emergency electricity supply.
Noise and vibration.
Machinery within the zebrafish housing areas such as pumps and blowers should not be creating excessive noise or vibration that can disturb the fish.21 Machinery should be easily accessible for servicing. The IACUC inspector will consider vibration and noise level of machinery in the proximity of fish. If questionable, it is the responsibility of the zebrafish facility staff to assure that the equipment is operating properly.
Light.
Light influences physiologic and behavioral processes including growth, development, and reproduction in fish.26 If windows are present, they should be covered adequately to prevent light from passing through. A 14:10-h light:dark cycle typically is recommended because it nearly mimics the natural zebrafish environment and can be programmed with room timers.21,29 Recommended light levels are between 5 and 30 fc or 54 to 324 lx at the surface of the water.21 Fluorescent lights usually are mounted in the ceiling. Optimally, lights should be phased on and off to minimize the startle reflex of fish upon activation or deactivation. The IACUC inspector will review the light cycle program and logs (if available) and confirm that windows are covered (if present).
Temperature.
Water temperature will equilibrate to room temperature and should be monitored continuously. The common housing temperature for zebrafish is 28 °C.29 A comfortable range is 22 to 30 °C,21 however zebrafish have been shown to tolerate a wide temperature range.11,20 Rapid water temperature changes can cause unnecessary stress and increase susceptibility to pathogens.4 Temperature should not change more than 5 °C in a 24-h period22,31. Handheld thermometers should be used to check the accuracy of electronic monitors. The IACUC inspector will review the temperature logs and question any temperatures out of optimal range.
Housing.
Rooms where fish are housed should be clean and orderly and separated from personnel areas. Food and beverage intended for human consumption should not enter the animal rooms. Fish should be separated from each other by species, age, and disease status. Protocol numbers and the names of principal investigators should be posted in each room (preferably on each tank), with contact information readily available.
Walls, doors, ceilings, and floors should all be clean and well-maintained. There should be little evidence of corrosion or water damage. Surfaces should be made of impervious, smooth, material that is sealed and easily sanitized. Floors should be made of nonskid material and be clear of standing water. Unless it is well-sealed and impermeable to water, wood should not be present in areas of constant dampness.
Main floor drains should be oversized to handle a transient large flow of water and should be kept clear of debris. Floors should be sloped toward the drains. Mesh of various sizes can be incorporated somewhere in the drainage system to prevent escaped fish from entering. Managers must be familiar with the regulations of the city, state, and federal agencies with regard to the discharge of live animals in the municipal sewer system. The IACUC inspector will note area cleanliness, facility structural maintenance, and check that contact information is available for each principal investigator.
Food storage.
Zebrafish typically are fed a mixture of live prey and commercially prepared diets. Storage areas for commercially packaged, dry feed (such as pelleted diet and fish flakes) should be cool [less than 20 ºC (68 °F)], dry (<75% humidity), away from direct sunlight, and free of vermin. When received, original packaging should be labeled with the date of receipt and expiration date (sometimes stated as ‘best used by’). When opened, contents remaining in the package should be sealed in waterproof containers protected from heat, humidity, and light and labeled with the product name and expiration date.21 Shelf life of zebrafish feed varies, and each manufacturer should be consulted for appropriate use and storage guidelines. Pelleted feed can last 2 to 3 y when stored correctly, whereas fish flakes have a shorter shelf life (up to 12 mo). Opened and unopened packages of artemia cysts (brine shrimp eggs) will last 3 to 4 wk if refrigerated at or below 4 ºC (40 °F). Packages must be resealed tightly to prevent moisture from entering. Freezing will increase the shelf life of Artemia cysts markedly.8 Other live prey such as rotifers and paramecium used to feed fry must be propagated continually and cannot be stored.
Chemical storage.
Drugs, biologics, and hazardous materials must be identified, and stored separately from feed, according to the manufacturer's specification. The IACUC inspector will check feed storage areas, shelf life of feed, labels on storage containers, and storage of other materials.
Waste.
Waste material must be discarded appropriately. After euthanasia, zebrafish carcasses should be frozen until they are disposed of according to facility SOPs. Biohazard waste must be discarded according to local, state, and federal regulations. The IACUC inspector will inquire about the handling of waste material.
Microenvironment
Identification.
According to the Guide, “means of animal identification include room, rack, pen, stall, and cage cards with written or bar coded information …. Identification cards should include the source of the animal, the strain or stock, names and locations of the responsible investigators, pertinent dates and protocol number when applicable.”18 This goal can be accomplished with individual tank or rack cards, room signs, or a coding system where information regarding each tank or rack can be viewed in a database easily accessible in each fish housing room. The IACUC inspector will check tank and rack identification cards. Identification methods should be consistent throughout the colony.
Primary enclosures.
Zebrafish usually are kept in rectangular tanks or aquaria made of transparent glass, acrylic, or polycarbonate, permitting easy observation of the animals.10,21 The interior surface of the tank should be smooth, sealed, and inert. Tanks should be equipped with a covering that prevents fish from jumping out.6 The height between the water surface and lid should minimize the risk of damage to the fish if they jump. Space requirements are affected by water quality, size, age, and feeding regimen and therefore may require optimization in each facility.21
Water quality.
Regular water-quality monitoring is essential for fish and aquatic system health. Every facility must design and use specific SOPs for the testing, measuring, monitoring, recording and adjustment of water quality parameters. Monitoring of water quality is one aspect of management where the choices made with regard to system design will have a large effect on allocation of time. With the proper maintenance, the nitrification process can be optimized to reduce toxic effects of ammonia, nitrites, and nitrates. The accumulation of nitrate in the water, although not directly responsible for acute mortality, can be stressful to fish.3,7,31 Nitrates eventually will accumulate in a closed system and must be eliminated through water replacement. The maximal level of nitrates considered acceptable for a healthy system has been reported to be between 500 and 800 ppm in striped bass.3,23 In juvenile fish, best results are expected when nitrates do not exceed 200 ppm.3,23 Completely eliminating nitrates from the water is ideal but difficult to achieve.
Monitoring water-quality parameters with chemical test kits is more labor-intensive than using electronic monitoring devices. Various electronic devices are available for continuously tracking a variety of water parameters. When using electronic devices to measure water quality parameters, the sensor probes may require routine calibration or adjustments according to the manufacturer's recommendations. A chemical test can be used as a method of checking accuracy of the electronic system.
The use of an automatic pH and salt dosing unit is beneficial to any automated fish system. The doser housing is calibrated and programmed according to the system's needs. The buffer and concentrated salt water are stored in nearby holding tanks, with hoses or pipes connecting them to the system sump tank. Without an automatic pH–salt dosing system, additional time will be required to adjust the system's water quality for these 2 parameters. Furthermore, conductivity and pH will need to be checked and recorded once or twice daily. Although automatic, the salt and pH dosing unit requires monitoring to ensure correct functioning.
Time allocated to monitoring water quality applies to each system in place. Setting up a single system decreases the overall time required for water quality management but increases the risk of heavy losses if that system fails or becomes contaminated. Dedicating additional resources to operating a redundant system likely will save time in the long run by preserving fish. The IACUC inspector will inquire about water quality monitoring systems and reliability.
Algae.
Algae growth is expected in zebrafish tanks.2 The conditions that contribute to the growth of algae are the accumulation of nitrates and phosphates in the water and the presence of light.2 Phosphates can originate as a byproduct of the mineralization of decomposing dead matter14 or enter the system from the fish food or water (including RO water). Nitrate is the aerobic endproduct of the biological nitrification process, without which algae would not be able to survive. Nitrate accumulates until it reaches equilibrium with processes that allow elimination such as water changes, plant or algae growth and removal, and chemical filtration.14 A moderate amount of algae growth demonstrates evidence of nitrogen cycle completion. Excessive growth of algae can be indicative of increased nitrate levels, which can be detrimental to the fish. Because algae are photosynthetic eukaryotes, they produce oxygen during the light period but use oxygen at night and when decaying. When more algae decay than grow, the result is a net decrease in dissolved oxygen, which is stressful for fish.14 Improving tank visibility is another benefit of minimizing algae load in the tank. The amount of algae that can be removed from the tank will depend on the system design. Tanks on systems with a separate biological filter substrate that is loaded with beneficial bacteria can be wiped completely clean. Cleaning the pipes and reservoirs of large systems is best done in a staggered manner a few times a year to prevent excessive bacterial death. Removing algae from a small, independent tank with a separate biological filter located adjacent to the tank (that is a self-contained 30-gallon tank typical of home aquariums) is also safe. To avoid removing the nitrifying bacteria living among the algae, the sides of static tanks should not be wiped down thoroughly with water changes. The amount and frequency of water changes in static tanks are based upon stable water chemistries.
Cyanobacteria (blue-green algae) are photosynthetic organisms that can be mistaken for true algae, but in reality they are toxin-producing prokaryotic bacteria virtually unrelated to algae. Cyanobacteria usually grow more quickly than algae, have a slimy appearance, and grow in ‘sheets’ or like a ‘carpet.’ Cyanobacteria are much easier to remove than algae but harder to control and very difficult to eliminate.2 The IACUC inspector should observe the amount of algae in the system. Algae load will vary, but is generally a sign of a healthy system. Control of algae and cyanobacteria overgrowth will enable better surveillance of diseased fish and smooth operation of system components.
Stocking density.
Stocking density is defined as number of fish per volume of water. The published guidelines for zebrafish stocking densities vary depending on the effort and expertise in optimizing production. The stocking density for each age group should be optimized and then incorporated into the facility SOPs. Guidelines from the Zebrafish International Resource Center are as follows: Starting with 20 eggs (1 to 10 h old) or embryos (10 to 72 h old) per 100 ml water, 20 fish can be kept in 400 mL as young larvae (3 to 30 d old); this volume is increased to 3 L as they approach juvenile stage (1 to 4 mo old).21 Recommended density for growing juvenile fish and holding adults is 5 fish per liter of water.21 The goal is to achieve 80% to 95% survival with the length of the fish measuring 1.0 to 1.5 cm by 21 d post fertilization. Overcrowding increases stress, susceptibility to disease, and injury as well as the amount of oxygen required in the system for both fish and biological filters20,27,28 Laboratories that maintain higher than recommended densities of zebrafish should increase the frequency of water-quality monitoring to verify that the system can handle this additional load. In addition, fish health should be monitored more closely. The IACUC inspector will observe the tanks for signs of overcrowding and compare the number of fish in each tank with what is stated in the SOPs.
Biosecurity.
Four points of control are relevant to biosecurity: people, fish, equipment, and water. The level of biosecurity for any facility should correlate with the requirements and functions of the laboratory and be determined in discussions with the principal investigator, facility manager, and veterinary or fish health personnel. SOPs should be developed to address all aspects of biosecurity in a specific facility.
People.
Only people with a need to enter should be allowed into a zebrafish facility. The room should be closed when the facility is unoccupied during working hours and locked at the end of each day. A facility-use orientation should be provided for anyone entering the zebrafish facility unescorted to avoid compromising the health of the system and the fish.
Fish.
Only the facility manager should be authorized to approve the introduction of fish to the colony. The facility should never house any fish that have not completed the established quarantine program. Adult fish for quarantine should be obtained only from a reliable source with an active sentinel-testing program. Our experience suggests that interest in specific pathogen-free fish facilities is increasing, but currently very few exist. Eggs from all sources should be decontaminated by bleaching prior to being introduced to nursery tanks; a protocol for bleaching embryos can be found in the Zebrafish Book.29 Once viable offspring are obtained, the imported adult fish in quarantine should be euthanized.22
Equipment.
The use of only clean, adequately disinfected equipment is essential. After the removal of gross organic material, equipment can be sanitized with hot water, bleach, or other disinfectants. Care must be taken to ensure that the chemical disinfectants have been completely eliminated from the equipment by rinsing, chemical neutralization, or air-drying before reuse. Nets or other equipment coming into contact with unclean surfaces should never be placed back into the system without disinfection. Any equipment used in quarantine spaces should be dedicated for that area and not allowed back into general use for the main facility.
Aerosols can pose a risk to fish health and should be kept to a minimum within animal housing areas. “Aerosols may be liquid or solid and are comprised of matter finely divided and suspended in the atmosphere for an appreciable time.”32 If a contaminated tank exists, droplet spray and aerosolization of the system water poses a risk to fish health from the transfer of potential pathogens. This risk can be minimized by adjusting the ventilation systems to limit spray or spread of aerosol plume, keeping tanks covered, and creating a physical barrier between quarantine and colony tanks.4
Fumes from bleach, lotions, and perfumes should be minimized. Water is a universal solvent and will pick up any fumes in the room, posing a potential health risk to the fish. Toxic chemicals, especially after opened, should be stored in a cabinet in a separate room.22
Water.
Common sources of water include unprocessed water (lake, river, and ocean), protected water (wells and aquifers), and municipal tap water that is processed to remove chlorine and chloramines (reverse osmosis or distilled). Recirculated, processed (RO) municipal tap water is desirable due to its ease of stabilization.
Record keeping.
Database.
A practical database is a priority for raising healthy, fecund zebrafish. A variety of data collection methods are available, and SOPs should be created and used for the maintenance of an accurate form of recordkeeping. Computer software is useful for detailed recordkeeping. The program we use (File Maker Pro, Filemaker, Santa Clara, CA) allows searching advantages unavailable through a more standard spreadsheet program and offers the ability to create custom templates. Records should include location, assigned stock numbers, tank numbers, matings, deaths, illnesses, fertilization dates, and genetic information (genetic composition, parental stock names and number). Unique observations and health concerns are noted in the records also. In addition to establishing general records, it is prudent to maintain information about all lines of fish, especially those unique to the lab. Complete descriptions and pictures of mutants, transgenics, chimeras as well as a catalog of plasmids would aid in efficiency of colony management.
Action log.
The wide variety of numerous tasks to be completed on time in a fully functional zebrafish facility can be managed through use of an ‘action log’ customized for the facility. When used appropriately, an action log will keep a lab running smoothly, and in our case has been instrumental in facilitating the successful management and inspection of a healthy zebrafish research facility. An example of an action log specific to the system described in this article is displayed in Table 1 and organized by frequency. This log includes a list of actions, the suggested frequency associated with that action, and the date the action was completed; we describe the various time categories in following sections. If several people are involved in tasks listed on the action log, each person should initial the task that he has completed. This action log can be presented to the IACUC inspector for quick review and will demonstrate that duties are performed in a timely manner.
Table 1.
An example of an action log specific to the system described in this paper and organized by frequency
| Date |
||||||||||
| Action | Frequency | 1-Mar | 2 | 3 | 4 | 5 | 6 | 7 | … | |
| Refill 50-gal salt carboy | 2XM | |||||||||
| Refill rotifer concentrated saltwater | 2XM | |||||||||
| Change rotifer water | 2XW | |||||||||
| Change RO filters | 2XY | |||||||||
| Water-test rotifers | 2XW | |||||||||
| Send email of laid eggs | 4XW | |||||||||
| Clean harvested eggs | as n | |||||||||
| Fill pH–salt doser | as n | |||||||||
| Harvest eggs | as n | |||||||||
| Harvest rotifers | as n | |||||||||
| Make stock solutions | as n | |||||||||
| Move fish | as n | |||||||||
| Move new fish into nursery | as n | |||||||||
| Refill nursery water carboy | as n | |||||||||
| Receive new fish–set up tank–enter database | as n | |||||||||
| Record screened fish on paper/database | as n | |||||||||
| Screen fish | as n | |||||||||
| Set up new matings | as n | |||||||||
| Euthanize unwanted fish | as n | |||||||||
| Feed three feedings | D | |||||||||
| Check dropped chambers | D | |||||||||
| Check for dead fish | D | |||||||||
| Check system pH and temperature and correct | D | |||||||||
| Harvest shrimp | D | |||||||||
| Record matings and eggs laid in database | D | |||||||||
| Record matings and eggs laid in lab book | D | |||||||||
| Record moves, deaths, etc. in database | D | |||||||||
| Set up brine shrimp | D | |||||||||
| Top off RO water | D | |||||||||
| Aliquot algae | e 3-4 M | |||||||||
| Change sock filters | e/o W | |||||||||
| Make fish food | M | |||||||||
| Inventory/census | M | |||||||||
| Wash floor | M | |||||||||
| Check water chemistry graphs for fish room | M | |||||||||
| Bleach wares | W | |||||||||
| Change prefilters | W | |||||||||
| Change system water | W | |||||||||
| Clean baffles | W | |||||||||
| Clean baffles | W | |||||||||
| Clean tanks | W | |||||||||
| Change quarantine water change | W | |||||||||
| Record water chemistries in database | W | |||||||||
| Email next week's experiments, injections, and matings | W | |||||||||
| Set up screening database for week | W | |||||||||
| Check water chemistries | W | |||||||||
| Change UV bulb | Y | |||||||||
as n, as needed; D, daily; e, every; e/o, every other; M, monthly; W, weekly; X, times; Y, yearly
Daily (D).
Check that all systems have appropriate water flow.
Remove dead and sick fish. Sick fish can be isolated for treatment or euthanized with an overdose of MS222 (M-aminobenzoic acid ethyl ester; tricaine methanesulfonate; Sigma, St Louis, MO);1,29 the use of MS222 is discussed in more detail later in the current article. Describe and record associated clinical signs of affected fish and note disposition (treated, euthanized, submitted for diagnostic testing, and so forth) Records of data regarding dead and sick fish will be very helpful to both the facility manager and the veterinary staff when evaluating system-wide problems. For rapid assessment of mortality trends, keeping a separate mortality log in addition to the general fish inventory database is beneficial.
The temperature and pH of water can fluctuate rapidly and must be checked daily. Small changes in these values can jeopardize fish health and reproduction. If an electronic probe is used for measuring the pH, a manual water chemistry test or secondary electronic device should be used weekly to confirm the accuracy of the primary testing instrument. Electronic probes used to measure water quality parameters should be calibrated on a regular basis, as stipulated in the manufacturer's recommendations, and a separate log of those calibration events (including dates and type of calibration) should be kept up to date. Records of data from measured water-quality parameters will be helpful to both the facility manager and veterinary staff when evaluating system-wide problems.
Adult zebrafish in our facility are offered food as often as 3 times daily to maximize breeding. Fry are fed 4 or more times daily to maximize growth. Facility specific growth rate and survival tests should be used to optimize results.21 Adjusting feeding schedules requires assuring that the fish are getting enough to eat while avoiding excess food that pollutes the water. Zebrafish get enough to eat when they are fed ad libitum for approximately 5 minutes with little to no remaining food.29 Basing feed amounts on body weight is probably more accurate.20
Scientific needs may require the use of special diets or alternative feeding schedules. Any experiments consisting of extreme feedings need to be fully described in an IACUC-approved protocol, and feeding must correlate with experimental design. Tanks using special food should be marked appropriately.
The importance of maintaining a healthy environment for the live prey is as important as maintaining a healthy fish environment. Zebrafish in our facility were offered rotifers (Brachionus plicatilis) and brine shrimp (Artemia spp.) cultured in system water. Attention to hatch rate is important to ensure adequate amount of food available for feeding fish. Live food being raised in a lab should be maintained according to the manufacturer's instructions; the Zebrafish Book also provides instructions.29 Brine shrimp are set up and harvested daily.
The RO system filters out impurities by using pressure to force water through a semipermeable membrane. The collection of RO water is time-consuming and should be prepared in advance. If a holding tank is used to store excess or reserve RO water, the tank should be filled daily to ensure maximal availability. Other sources of water may be used provided there is adequate prefiltration to remove solutes and dissolved gasses, followed by carbon filtration to remove sediment, chlorine, chloramines, and volatile organic compounds.
Records of system parameters, matings, transfers, deaths, and general activities in the fish room should be entered into their appropriate databases daily. The IACUC inspector will check sick animal and treatment records, inspect mortality log and trends over time, and check daily pH and temperature records. Fish should be fed according to the protocol, and live food should be healthy and readily available if needed. If treated water is used, a source should be readily available.
Semiweekly (2XW).
System water should be changed either weekly or semiweekly.
Static tanks containing live food such as rotifers should have water changes weekly to semiweekly. Static tanks may be used to saturate the fish environment with food to maximize growth or to grow up large quantities of live food. Fish in static tanks need to be observed more closely for stress behaviors, such as rapid breathing or abnormal swimming and abnormal eating habits. The water should be changed, but the algae layer does not need to be removed completely (see Water quality).
When growing rotifers in static tanks, the water-change frequency will vary depending on conditions. Testing the ammonia level in the rotifer water will help determine the need for water replacement. A water sample can be filtered through a sieve for capture of rotifers prior to testing, in an effort to avoid subjecting rotifers to chemicals. The IACUC inspector will check water quality records for fish and live food. Water in static tanks must be changed according to protocol.
Weekly (W).
Zebrafish larvae can be reared in a variety of ways. A recirculating system set at a slow drip is ideal. However, larvae were reared until as late as 18 d after fertilization ‘off system’ in static tanks in our facility; the tanks were cleared of dead fish and excess food weekly. Surface skimming, or removing the top layer of water, helps to expunge proteins and oils that may impede oxygen diffusion. The frequency and extent of this type of cleaning may increase with peak production. Mating chambers, nets, beakers, and other utensils should be cleaned with hot water, bleached, or autoclaved at least once each week. When using bleach or other chemical disinfectants, caution should be exercised to avoid exposure of a fish tank or water in the system. Equipment cleaned in this manner must be rinsed sufficiently and dried.
Many systems require the weekly replacement of prefilters or disposable filtration components. The weekly maintenance requirements of the system should be followed and recorded on the system log.
Water in static tanks containing fish should be changed at least once each week. The proportion of water to be changed is determined by the specific purpose of the static tank (maintenance or increased growth).
Tank lids should be wiped clean with water (no detergent or disinfectants) once weekly, primarily to remove any debris left behind during feeding. Removing food remnants from the vicinity minimizes potential pest problems. Alcohol can be used to disinfect surfaces, but care should be taken to avoid spillage inside the tanks.
Tank baffles and rack return manifolds should be checked for clogs and cleaned accordingly.
Tank cleaning is performed weekly on a rotating basis to reduce algae and improve water clarity. In the intermediate type of recirculating system described here, the removal of algae does not make a negative contribution to water quality, due to the biological filtration occurring in the fluidized bed. Once fish are relocated to a clean tank, the contents of the dirty tank can be discarded. The tank can then be cleaned by simply removing the gross debris and then reusing the tank on the same system. Alternatively, the tank can be removed from the system and scrubbed with an algae mitt and water. The tank can then be bleached, rinsed well, and reused.
Water chemistries should be recorded weekly on a task action log as well as in a database. Task action logs and databases should include the normal ranges for all water chemistries for a quick reference. Any values that are out of range should be reported and addressed as needed. Creating a graph from water chemistry data is beneficial. Basic water assessment should include temperature, conductivity, pH, ammonia, nitrite, and nitrate. Other compounds can be added to this list at the discretion of each facility. Parameters such as hardness, alkalinity, and dissolved gasses are helpful additions.
Floors in fish room should be cleaned and swept weekly. The IACUC inspector will confirm that fish in nursery tanks are fed to satiation. Be familiar with the optimum water level for the tanks and check that tanks are being maintained in the optimum range. Baffles should be operating properly so that tanks are not overflowing. Weekly chemistries should be recorded
Monthly (M).
Each system has a different requirement for monthly maintenance. The guidelines for the specific system should be followed. Back-up pumps on the system should be tested at monthly intervals.
The floors of fish rooms can be cleaned by sweeping and mopping with hot water and a dilute bleach solution (1%). Care should be taken not to splash any of the bleach into the system.
Conducting a census or genetic inventory of the colony and culling unwanted fish on a monthly basis will help to maximize available space and reduce per diem charges. A census can help determine fish loss while being used to update records. In facilities with a large number of fish, a detailed inventory may be unrealistic.
Semiannually and annually (2XY and Y).
Tasks undertaken semiannually and annually are usually dependent on the requirements of the system. Examples would be changing UV bulbs, wiping quartz sleeves clean, and changing RO system filters according to the manufacturer's recommendations.
As needed (AsN).
This category is by far the largest. Many duties in the fish facility are done on an irregular basis. Refilling stocks of water and food, changing out clogged sock filters that result in pressure buildup, harvesting food and eggs, moving fish to clean tanks, receiving new fish, and culling unwanted fish are all examples of duties that occur at irregular times. When organizing an action log, think about how often the ‘as needed’ tasks may need to be performed. Do not leave the ‘frequency’ column blank; customize it according to specific needs of the lab, and readjust the frequency when appropriate. For example, 1 lab may need to make food once a week, whereas another may need to make food every other week.
Clean lids and baffles periodically to improve tank visibility. Regularly dislodging debris from baffles will allow water to flow freely and prevent overflow of water and subsequent loss of fish. Baffles should be cleaned when the water of the tank is level with the overflow holes in the baffle.
All electronic meters should be calibrated periodically, and records of calibrations should be available. The IACUC inspector will review SOPs and log sheets for tasks done as needed. He will also inquire about the performance of duties and management of the colony in the event of personnel absence.
Table 2 compares the live food care, fish care, and cleaning duties involved for each of 3 types of systems (A, B, and C). System A represents a recirculating system, as modeled in this paper. System B represents a static system like what might be used for a nursery or quarantine area. System C is a true flow-through system similar to what might be used in a facility with an unlimited water source.
Table 2.
This time chart lists the suggested minimal frequency for each task based on the type of system (A, B, or C)
| Live Food Care System | Fish Care System | Cleaning and Maintenance System | |
| Daily | Harvest Shrimp A, B, & C | Feedings A, B, & C | Check System pH and Temperature and Correct If Needed A,B, & C |
| Set Up Shrimp A, B, & C | Check For Dead/Sick Fish A, B, & C | ||
| Record Mating, Moves | Check System Parameters A & C | ||
| Enter Deaths In Database A, B, & C | |||
| Semiweekly | Change Rotifer Water A, B, & C | ||
| Water-test Rotifers A, B, & C | |||
| Weekly | Bleach Wares A, B, & C | ||
| Change Pre-Filters A | |||
| Change System Water A, B, & C | |||
| Clean Baffles A & C | |||
| Clean Tanks A, B, & C | |||
| Quarantine Water Change A, B, & C | |||
| Record Water Chemistries Into Database A, B, & C | |||
| Every other week | Change Sock Filters A | ||
| Semimonthly | Refill Rotifer Water Carboy A | Concentrated Salt Water Carboy Refilling A | |
| Monthly | Inventory/Census A, B, & C | Wash Floor A, B, & C | |
| Test Back-up Pumps A | |||
| Semiannually | Change RO Filters A | ||
| Yearly | Change UV Bulbs A & C | ||
| As needed | Harvest Rotifers A, B, & C | Harvest Eggs A, B, & C | Aliquot Algae A, B, & C |
| Refill Rotifer Water Carboy B & C | Clean Harvested Eggs A, B, & C | Change Sock Filters C | |
| Move Fish A, B, & C | Fill pH/Salt Doser A, & C | ||
| New Fish Into Nursery A, B, & C | Make Stock Solutions A, B, & C | ||
| Receive New Fish, Set Up Tank, and Record In Database A, B, & C | Nursery Water Carboy Refilling A, B, & C | ||
| Make Fish Food A, B, & C | Refill Concentrated Saltwater Carboy B & C | ||
| Euthanize Unwanted Fish A, B, & C | |||
| Screen Fish A, B, & C | Change Prefilters C | ||
| Change RO Filters B & C | |||
| Calibrate pH Meter A & C |
A, a specific recirculating/modified flow-through system; B, a static system; C, a true flow-through system
Miscellaneous Items
Mating.
Mating fish is a highly variable activity. The fish can either be mated ‘on system’ by using mating chambers or ‘off system’ with breeding traps. The use of ‘squeezing’ (expression of eggs by applying gentle pressure to the sides of the fish) as a mating technique is usually done via off-system breeding traps.29 On the action log, all duties involved in mating are considered as needed activities.
All mating procedures should be accomplished with fish well-being in mind and according to protocol description. When mating on-system or by natural breeding, precautions should be taken to minimize fish distress due to netting, transfer, and habitat disturbance. Precautions include using slow, fluid movements when netting fish and minimizing the time fish are exposed to air.31 Any fish kept off-system should be returned to the system within 5 d, because the water quality of these tanks will deteriorate rapidly. Daily water changes are recommended for off-system fish. Keep a copy of the mating protocol in the fish facility, and thoroughly train all personnel who will be handling the fish.
Chemicals.
All chemicals should be stored according to Environmental Health and Safety guidelines in regards to location, disposal and labeling. Chemicals should be kept away from the general vicinity of fish housing areas. Two chemicals frequently used in fish facilities are bleach and MS222 (tricaine). A Tris-buffered (pH 7.0) tricaine solution in water can be used at a concentration of 100 to 200 mg/L as an anesthetic for procedures such as squeezing (egg collection) and caudal fin biopsy.21,29 Euthanasia can be accomplished by increasing the concentration to 200 to 500 mg/L.21,29 All bottles should be labeled with chemical name, concentration, and date of dilution. The person making the solution should initial the label, and the solution should be stored properly and discarded when aged. Avoid using expired anesthetics and other pharmaceuticals on live animals.
Emergency.
Redundancy in critical systems, filters, pumps, and other essential life support components will ensure that equipment failures cause only minimal interruptions in operation. In-house stocking of replacement parts may be indicated. Alarm systems must remain operational. A written plan should be readily available to address any potential emergencies associated with system malfunction. The emergency program should be integrated with the institution's emergency disaster management plan to coordinate the movement of tanks and emergency euthanasia. General protocols will address all other types of emergencies. The emergency protocols should be kept in a separate and preferably different colored binder.
All personnel with access to the fish rooms should be trained to respond to an emergency. An emergency contact list with names and phone numbers should be posted in a prominent location in the fish facility. In most cases, if an emergency is detected, fish should not be fed until the problem is resolved. This practice will reduce food contamination of the water and decrease ammonia excretion by the fish. Adult fish can survive without food for at least a week if necessary.15 During emergencies, pumps should be turned off, and the flow of water into the tanks should be interrupted. The IACUC inspector will review emergency animal care plan, inquire about back-up systems in case of equipment failure and about the outcome of past equipment failures.
Animal health.
Healthy fish should swim in a relaxed motion orientated parallel to the surface of the water. Normal behavior includes effortless breathing (movement of the operculum) and an interest in activity such as swimming and eating. Signs of illness can include poor body condition (low ratio of body weight in grams to the cube of the body length in cm)33 rapid breathing, obvious lesions (masses, ulcers, wounds), and changes in color, shape, or locomotion.19,21 Sharp turns, spinning, listing (leaning to one side) or bobbing (fish oriented perpendicular to surface) are also signs of illness.19,21 Some of the clinical signs may be expected due to random genetic mutations perpetuated by the research or more specifically a targeted transgene. Unexpected clinical signs of illness should be reported to veterinary services such that sick zebrafish can be evaluated by appropriate diagnostic testing including necropsy and histopathology to determine etiology whenever feasible. Treatment of individual zebrafish may be challenging, unproductive, and financially prohibitive; therefore sick zebrafish typically are euthanized.
Routine health monitoring through sentinel testing at least twice a year16 is beneficial for detecting pathogens at early stages. If fish necropsy and diagnosis of sentinels or affected animals cannot be performed on site, submitting specimens to an outside fish diagnostic laboratory (for example, Cornell, the Zebrafish International Resource Center) is suggested.4,34 Although describing the details of setting up a sentinel program is beyond the scope of this article, an expert can design a sentinel program specifically for the needs of the facility so that it is worthwhile and cost-effective in preventing devastating outbreaks.
On occasion, zebrafish suspected of disease and sentinels pulled from the sumps have been sent to the Zebrafish International Resource Center34 for complete analysis, but a regular sentinel program has not been in place for our facility. The IACUC inspector will look around various areas of the facility to get an overall idea of fish health. Observe body condition, population, and overt signs of illness. Assure that fish are being euthanized humanely according to protocol.
Protocol compliance.
Personnel involved in the handling of fish on the approved protocol should understand the procedures and techniques that are described within. Only those procedures approved in the protocol should be performed. Amendments to the protocol must be approved before the application of new procedures. The program description of facility management and equipment use is best provided to the IACUC in advance of the inspection. The SOPs should be reviewed so inspectors can assure practices observed during inspection are consistent with planned IACUC approved facilities, procedures, and practices. The protocols and SOPs used in each facility should be readily available to the inspector either through the IACUC or from the individual labs. Protocol procedures should be followed as described.
Discussion
An IACUC inspector must approach a zebrafish facility semiannual inspection with the same confidence, ability and care that have long been the standards for assessing rodent and large animal facilities. To promote these goals, it is beneficial to begin to create some general guidelines that are applicable to a variety of zebrafish housing paradigms. With a better understanding of the various housing systems and aspects of a high-quality environment and factors affecting fish health and reproduction, an IACUC member will be well prepared to fulfill the role of a zebrafish facility inspector.
A zebrafish facility operator must have an understanding of the purpose of an inspection and be familiar with the criteria that inspectors use to assess the quality of a zebrafish program. With that understanding, details of animal care and facility maintenance can be presented to the inspector in a well organized fashion. Anecdotal information gathered from various zebrafish facility operators has led to the conclusion that the IACUCs are not inspecting the facilities uniformly, are using standards adapted from mammalian research animal inspection guidelines, and are ill-prepared to ask pertinent questions reflecting an adequate knowledge of aquaculture or aquatic animal facilities.
In the interest of good research, ease of inspection, compliance with Public Health Service regulations and the success of zebrafish colony health, zebrafish facility operators, lab animal veterinarians, and IACUC site visitors must come together with the goal of educating others through our own experiences and knowledge to better prepare all sides for inspections. This article provides a starting point for open communication between the parties and advances the idea that good recordkeeping is essential to successful zebrafish research, with the added benefit of contributing to a smooth and cooperative IACUC inspection.
Acknowledgments
We thank David G White (Research Coordinator, University of Washington, Seattle, Washington), Mary A Ellenberger, DACLAM (Washington University, St Louis, Missouri), and Rachel Wong (Professor of Biological Structure, University of Washington, Seattle, Washington).
References
- 1.American Veterinary Medical Association 2007. AVMA guidelines on euthanasia, 2007 update. [Cited Aug 2008]. Available at http://www.avma.org/issues/animal_welfare/euthanasia.pdf
- 2.Aquariumpros.com 2008. Nuisance algae. [Cited Aug 2008]. Available at http://www.aquariumpros.com/articles/algae.shtml
- 3.Bonn EW, Bailey WM, Bayless JD, Erickson KE, Stevens RE. 1976. Guidelines for striped bass culture, p 69. Sponsored by the Striped Bass Committee of the Southern Division, AFS [Google Scholar]
- 4.Bowser P. 2008. Professor of Aquatic Animal Medicine, Cornell University, College of Veterinary Medicine. Personal correspondence dated 2 May 2008 [Google Scholar]
- 5.Boyd CE. 1979. Water quality in warm water fish ponds, p 359. Alabama Agricultural Experiment Station. Alabama Univ., Alabama. [Google Scholar]
- 6.Brand M, Granato M, Nusslein-Volhard C. 2002. Keeping and raising zebrafish. In: Nusslein-Volhard C, Dahm R.Zebrafish, a practical approach. p 7–37 [Google Scholar]
- 7.Bromage NR, Shepherd CJ, Roberts J. 1988. Farming systems and husbandry practice. In: Shepherd CJ, Bromage NR. Intensive fish farming. Oxford: BSp Professional, p 94–95 [Google Scholar]
- 8.Brine Shrimp Direct. 2008 [Cited Aug 2008]. Available at http://www.hikariusa.com/
- 9.Buttner JK, Soderberg RW, Terlizzi DE. 1993. An introduction to water chemistry in freshwater aquaculture. Northeastern Regional Aquaculture Center (NRAC) Fact Sheet No. 170 [Google Scholar]
- 10.Canadian Council on Animal Care (CCAC) 2005. Guidelines on: the care and use of fish in research, teaching and testing. [Cited Aug 2008]. Available at http://www.ccac.ca
- 11.Cortemeglia C, Beitinger TL. 2005. Temperature tolerances of wild-type and red transgenic zebra Danios. Trans Am Fish Soc 134:1431–1437 [Google Scholar]
- 12.Courtland S. 2002. Recalculating systems for zebrafish. Lab Anim (NY) 31:53–56 [DOI] [PubMed] [Google Scholar]
- 13.DeTolla LJ, Srinivas S, Whitaker BR, Andrews C, Hecker B, Kane AS, Reimschuessel R. 1995. Guidelines for the care and use of fish in research. ILAR J 37:159–173 [DOI] [PubMed] [Google Scholar]
- 14.Detrich WH, 3rd, Westerfield M, Zon LI. 2004. The zebrafish: genetics, genomics, and informatics, methodes in cell biology (77). Elsevier Academic Press, Amsterdam: 2nd Ed. p 686 [Google Scholar]
- 15.Goldsmith MI, Iovine MK, O'Reilly-Pol T, Johnson SL. 2006. A developmental transition in growth control during zebrafish caudal fin development. Dev Biol 296:450–457 [DOI] [PubMed] [Google Scholar]
- 16.Gourdon J. 2004. Zebrafish health monitoring program. Cornell University. [Cited Aug 2008]. Available at http://research.cornell.edu/care/documents/SOPs/CARE623.pdf
- 17.Hikari USA. 2008 [Cited Aug 2008]. Available at http://www.hikariusa.com/
- 18.Institute of Laboratory Animal Resources, Commission of Life Sciences, National Research Council 1996. Guide to the care and use of laboratory animals. Washington D.C.; National Academy Press [Google Scholar]
- 19.Lawler A. 2006. Some signs of a sick fish. Aquarticles. [Google Scholar]
- 20.Lawrence C. 2007. The husbandry of zebrafish (Danio rerio): a review. Aquaculture 269:1–20 [Google Scholar]
- 21.Matthews M, Trevarrow B, Ph D, Matthews JDVM, Ph D. 2002. A virtual tour of the Guide for zebrafish users. Lab Anim (NY) 3:34–40 [DOI] [PubMed] [Google Scholar]
- 22. Mount Desert Island Biological Lab (MDIBL). 2007. Health management of laboratory fish course book. [Google Scholar]
- 23.Nicholson LC, Woods LC, Woiwode JG. 1990. Intensive culture techniques for the striped bass and its hybrids. In: Harrell RM, Kerby JH, Minton RV. Culture and Propagation of Striped Bass and its Hybrids, AFS p 141-158 [Google Scholar]
- 24.Nickum JG, Bart HL, Jr, Bowser PR. Guidelines for the use of fishes in research. American Fisheries Society, Bethesda, MD 2004. [Cited Aug 2008]. Available at http://www.fisheries.org/test/publicpolicy/guidelines2004.pdf
- 25.Overstreet RM, Barnes SS, Manning CS, Hawkins WE. 2000. Facilities and husbandry (small fish models). In: Ostrander GK. The laboratory fish Ch.2. Academic Press, San Diego. p 43 [Google Scholar]
- 26.Stickney RR. 1994. Principles of aquaculture. p 502. John Wiley & Sons, New York [Google Scholar]
- 27.Timmons MB, Ebeling JM, Wheaton FW, Summerfelt ST, Vinci BJ. 2002. [Google Scholar]
- 28.Wedemeyer GA. 1996. Physiology of fish in intensive culture systems. Chapman & Hall / International Thompson Publishing New York, NY, USA [Google Scholar]
- 29.Westerfield M. 2007. The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio), Eugene (OR): University of Oregon Press [Google Scholar]
- 30.Wheaton FW, Hochheimer JN, Kaiser GE. 1991. Principles of biological filtration, p 1–31. In: Timmons MB. Engineering aspects of intensive aquaculture. Ithaca (NY): Northeast Regional Agricultural Engineering Service, Cooperative Extension [Google Scholar]
- 31.White D. 2006. University of Washington, Seattle. HSB zebrafish facility SOPs. [Cited Aug 2008]. Available at http://staff.washington.edu/dgw5079
- 32.Wooster GA, Bowser PR. 1996. The aerobiological pathway of fish pathogen: survival and dissemination of Aeromonas salmonicida in aerosols and its implications in fish health management. J World Aquac Soc 27:7–14 [Google Scholar]
- 33.Yang H, Carmichael C, Varga ZM, Tiersch TR. 2007. Development of a simplified and standardized protocol with potential for high-throughput for sperm cryopreservation in zebrafish Danio rerio. Theriogenology 68:128–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zebrafish International Resource Center (ZIRC) Eugene, OR. [Cited Aug 2008]. Available at http://zebrafish.org.