Abstract
Exposure to CO2 is a common method used to euthanize rodents in biomedical research and rodent production. The purpose of this study was to determine the length of CO2 exposure required to euthanize neonatal rats (0 to 10 d old). Multiple groups of rats were exposed to 100% CO2 for 5 to 60 min. After CO2 exposure, rats were placed in room air for 20 min to allow for possible recovery. No difference was found in comparing 1 inbred strain and 1 outbred stock of rats. Time to death varied inversely with the age of the animals, requiring as long as 35 min on the day of birth. The time to death decreased steadily with increasing age, with 100% of the rats euthanized after 5 min of CO2 exposure at 10 d of age. The time required for 100% mortality decreased by 3 min for every 1 d increase in age between days 0 and 10.
Because laboratory rodents are the most common animals used in research today, optimizing the methods used for euthanasia of these animals is important to researchers, veterinarians, and care staff. Unlike many other laboratory animals, rodents often are bred in research facilities. Breeding may require euthanasia of very young animals, most frequently because of undesired phenotype or genotype or the production of surplus animals. Although many studies have evaluated the ethical and physiologic considerations of various euthanasia methods on adult laboratory rodents, the literature is relatively sparse on the euthanasia of neonatal rodents.
Because most research institutions do not have to euthanize large numbers of neonates at one time, the limited research on the euthanasia of neonatal rodents is unsurprising. However, this paucity leaves rodent producers and others who must euthanize groups of neonatal rodents at a loss for best practice guidelines. The American Veterinary Medical Association (AVMA) Panel on Euthanasia defines “acceptable” methods of euthanasia for rodents and other small mammals as: barbiturates, inhalant anesthetics, carbon monoxide, potassium chloride in conjunction with general anesthesia, microwave irradiation, and CO2.2 For some of these methods, their use to ensure the humane death of large numbers of neonatal rats would present several challenges. Ideally, the method chosen would minimize distress to the animals, would have a minimal health impact on personnel performing the euthanasia, be easy to administer, lack complicated record-keeping or disposal requirements, not be distressing to personnel performing or witnessing euthanasia, and be cost-effective to employ for large numbers of animals. In addition, an ideal euthanasia method would leave the euthanized animals suitable for other uses, whether for research or as food for rodent-eating animals. None of the methods approved by the AVMA meet all these criteria, but CO2 is the closest. European Union recommendations for the euthanasia of neonatal rodents include decapitation, concussion, rapid freezing, and hypothermia;7 of these methods, only rapid freezing could meet the criteria just listed, but hazards are associated with the use of liquid nitrogen. In addition, immersion in liquid nitrogen is not recommended as an euthanasia method in the United States, unless the animals are first rendered insensible.2,15 In many cases, the method used prior to freezing or hypothermia is CO2 inhalation. Carbon dioxide is widely accepted as an euthanasia agent due to the quick onset of effects, including unconsciousness,4,5,16,29 minimal tissue artifact production,12,15 and ease of use.14
The use of CO2 for euthanasia of adult rodents is the subject of much opinion and debate.3,20,22 The current study was not intended to evaluate the merits of using CO2 to euthanize laboratory animals, but rather to determine the parameters of its use in the euthanasia of neonatal rats. The aim was to determine the length of exposure to 100% CO2 necessary to euthanize 100% of rats 0 to 10 d of age.
Materials and Methods
Animals.
Pregnant Crl:CD(SD) and F344/DuCrl female rats (Rattus norvegicus) were obtained from Charles River Kingston (Kingston, NY) and Charles River Raleigh (Raleigh, NC), respectively. The strain and stock chosen for testing were based on the numbers sold by Charles River and was thought to represent a reasonable proportion of animals in use in the United States. All experiments were conducted at Charles River's Wilmington, MA, facility. Animals arrived at the Wilmington facility at approximately day 12 of gestation and were housed in 1 area until the termination of the experiment. All experiments were approved by Charles River's Institutional Animal Care and Use Committee. All work was conducted according to standards set forth in the Guide for the Care and Use of Laboratory Animals.17
Animals were housed in solid-bottomed plastic cages, by using paper-based contact bedding (CareFresh, Midwest Filtration, Cincinnati, OH). Cages were sanitized once weekly. Environmental conditions were maintained at 21° ± 2 °C (70° ± 3 °F) with 50% ± 20% relative humidity and 15 air changes hourly. Animals were kept on a 12:12-h light:dark cycle and provided ad libitum access to water and feed (Lab Diet 5K52 or Lab Diet 5L79, Purina Mills, Richmond, IN). All animals were from colonies that tested negative for the following viral agents: Sendai virus, pneumonia virus of mice, sialodacryoadenitis virus, Kilham rat virus, H1 virus, rat minute virus, reovirus, rat theilovirus, lymphocytic choriomeningitis virus, hantavirus, mouse adenovirus, rat respiratory virus, and rat parvovirus. In addition, the colonies were free of the following bacterial and fungal agents: Bordetella bronchiseptica, CAR bacillus, Clostridium piliforme, Corynebacterium kutscheri, Encephalitozoon cuniculi, Helicobacter hepaticus, Helicobacter bilis, and other Helicobacter spp., Klebsiella oxytoca, Klebsiella pneumoniae, Mycoplasma pulmonis, Pasteurella pneumotropica, Pseudomonas aeruginosa, Salmonella spp., Streptobacillus moniliformis, Streptococcus pneumoniae, and beta-hemolytic Streptococcus spp. Animals were also free of endoparasites and ectoparasites. Pregnant female rats were allowed to litter naturally, and the day of birth was recorded as day 0 of the pup's life. Pups used for experiments were selected arbitrarily from litters at the desired age for experimentation and combined into experimental groups, regardless of sex.
Procedures.
The total number of animals tested was 791, comprising 71 inbred animals at 0 to 10 d of age that were tested in a preliminary study and 720 outbred animals 0 to 10 d of age that were tested in the main study. Each experimental group included 10 rats of mixed sex of the same age. Groups were placed in a clear plastic bag [17 × 7.5 × 39 cm (6 × 3 × 15 in.), low-density polyethylene, Polymer Packaging, North Canton, OH] containing an unmeasured small quantity of room air that entered the bag when it was opened to insert the animals. The bag was filled to 4 L in volume with 100% CO2 from a compressed gas cylinder, delivered at a rate of 0.5 L/s, and then placed upright in a bedded cage.
A pilot exposure study at 0 d of age was performed with limited numbers (10 per time point) of inbred rat pups to determine the maximal exposure time at that age that would be required. Forty minutes was chosen for the upper limit of CO2 exposure, Because all animals in the pilot experiment died within 35 min of exposure and because only 1 group of animals was tested in the pilot, the next exposure time (40 min) was chosen as the maximal exposure time for the actual study, to afford a margin of safety. Five minutes was chosen as the lower limit of exposure, based both on the policies at many facilities, which require a 5-min exposure to CO2 for adult rodents, and the AVMA Panel's recommendations. For each group of animals except day 0, the first exposure time was based on the successful euthanasia time (that is, the exposure leading to death of all animals in the group) of the animals a day younger. CO2 exposure time was measured starting from the initial introduction of gas into the bag. During the exposure, animals were observed for movement, apparent consciousness, and respiratory pattern for the first 30 s after CO2 exposure and then at 1-min intervals until 5 min. After 5 min of exposure, animals were monitored at 5-min intervals until the end of the exposure period. At the end of the CO2 exposure period, animals were removed from the plastic bag, placed in a bedded cage, and observed continuously for 20 min for recovery. Recovery was considered to have occurred if any CO2-exposed animal took a single breath during the recovery period. If any animal from a group recovered, the CO2 exposure period was lengthened by 5 min, and another group of that age was tested. Animals were not palpated or instrumented to detect cessation of heartbeat; failure to recover when exposed to room air was considered death. Neonatal rats that recovered were euthanized by using a physical method (decapitation).
Unconsciousness was determined by loss of righting reflex, cessation of spontaneous respiration, and failure to respond to nocioceptive stimuli (for example, a gentle toe or tail pinch applied without opening the plastic bag). Animals were not instrumented to determine conscious state. All potential stimulation of the rats was avoided during the recovery period. Therefore, recovering rats were not palpated for a heartbeat. Rats were kept at room temperature during the recovery period to simulate conditions under which animals might recover from an euthanasia attempt. All rats were confirmed to be dead through observation of cessation of respiration and palpation for a lack of heartbeat before being placed in a holding freezer.
Statistical evaluation.
This study was designed to make recommendations for the euthanasia of neonatal rats with inhaled 100% CO2. The study did not attempt to determine the exact time required to kill 100% of the animals at each day of age. A pilot study used a limited number of inbred animals to determine approximate times around which to evaluate initial exposure. Then 2 separate groups of 10 neonates were exposed to CO2 at each time point. Recovery from CO2 exposure was an unacceptable result and indicated an inadequate exposure time. Because the recovery of any animal was considered an undesirable outcome, that exposure time was discarded and a longer exposure time was tested. Once the 100% mortality time point was reached, another 2 groups of 10 animals was tested at a yet longer exposure to determine whether the 100% mortality found at the prior time point was an anomaly. Regression analysis (Minitab, State College, PA) was used to relate the time to reach 100% mortality to the age of the pups.
Results
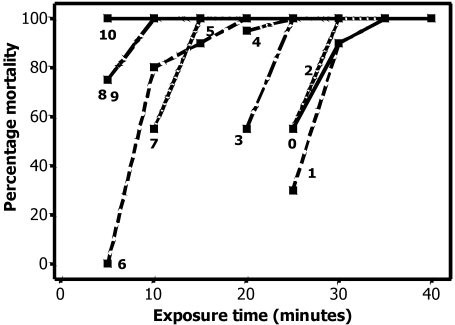
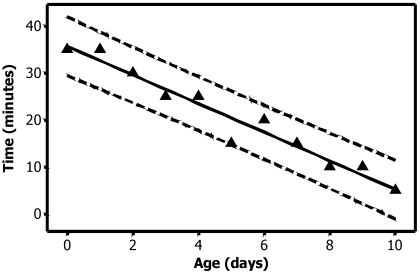
Time to death did not differ between inbred and outbred animals on day 0 (Table 1). After evaluation of these results, I tested only outbred rats thereafter (Table 1, Figure 1). Rats of both sexes were equally susceptible to CO2 and regardless of age appeared to be unconscious within 60 s of exposure to CO2. Animals differed in their susceptibility to CO2 by age, with younger animals being less susceptible than older. The strong linear negative relationship between the time to reach 100% mortality and the age of the pups is shown in Figure 2. The regression equation was time = 35.7 – (3.0 × age). The coefficient of multiple correlation (R2) was 95.1. Although euthanasia of day 0 animals required at least 35 min exposure to CO2, a 5-min CO2 exposure was sufficient for euthanasia at 10 d of age. When animals recovered after CO2 exposure, the pups that recovered appeared dead at the beginning of the recovery period, with no respiration or heartbeat noted. However, some pups did recover, even after CO2 exposures of 30 min or longer. Recovery occurred as long as 10 min after removal from CO2 exposure in 0- to 4-d-old rats. Whenever recovery occurred in a group, multiple animals (which were in different bags) recovered, indicating that the exposure time was insufficient and that the recovery was not due to failure of the euthanasia chamber.
Table 1.
Numbers of neonatal and adult rats tested.
| Age (d) | CO2 exposure (min) | No. of pups exposed | No. of pups euthanized |
| Pilot study | |||
| 0 | 20 | 11 | 1 |
| 0 | 25 | 10 | 1 |
| 0 | 30 | 10 | 8 |
| 0 | 35 | 10 | 10 |
| 0 | 40 | 10 | 10 |
| 0 | 50 | 10 | 10 |
| 0 | 60 | 10 | 10 |
| Main study | |||
| 0 | 25 | 20 | 11 |
| 0 | 30 | 20 | 18 |
| 0 | 35 | 20 | 20 |
| 0 | 40 | 20 | 20 |
| 1 | 25 | 20 | 6 |
| 1 | 30 | 20 | 18 |
| 1 | 35 | 20 | 20 |
| 2 | 25 | 20 | 11 |
| 2 | 30 | 20 | 20 |
| 2 | 35 | 20 | 20 |
| 3 | 20 | 20 | 11 |
| 3 | 25 | 20 | 20 |
| 3 | 30 | 20 | 20 |
| 4 | 20 | 20 | 19 |
| 4 | 25 | 20 | 20 |
| 4 | 30 | 20 | 20 |
| 5 | 15 | 20 | 20 |
| 5 | 20 | 20 | 20 |
| 5 | 25 | 20 | 20 |
| 6 | 5 | 20 | 0 |
| 6 | 10 | 20 | 16 |
| 6 | 15 | 20 | 18 |
| 6 | 20 | 20 | 20 |
| 7 | 10 | 20 | 11 |
| 7 | 15 | 20 | 20 |
| 7 | 20 | 20 | 20 |
| 8 | 5 | 20 | 15 |
| 8 | 10 | 20 | 20 |
| 8 | 15 | 20 | 20 |
| 8 | 20 | 20 | 20 |
| 9 | 5 | 20 | 15 |
| 9 | 10 | 20 | 20 |
| 9 | 15 | 20 | 20 |
| 10 | 5 | 20 | 20 |
| 10 | 10 | 20 | 20 |
| 10 | 15 | 20 | 20 |
The pilot study was performed by using the inbred strain F344/DuCrl, and the main experiment was done by using the outbred stock Crl:CD(SD). Pups were considered to be euthanized if they did not recover within the allotted recovery time.
Figure 1.
Mortality as a function of exposure time for rats of each age group. Each point is the percentage mortality in a group of 20 animals.
Figure 2.
Time to 100% mortality as a function of age (days). Dashed lines represent the 95% prediction intervals. The regression equation is time = 35.7 – (3.0 × age), R2 = 95.1. The regression coefficient is 3.0, indicating that the time required for 100% mortality decreased by 3 min for every 1-d increase in age between days 0 and 10.
Discussion
This work confirms that neonatal rats euthanized with CO2 take substantially longer to die than the euthanasia times reported and recommended in the literature for adult rats. The necessity of a prolonged CO2 exposure time for neonatal rats can be explained by the mechanisms of action of CO2 and the innate resistance of the neonate to hypercarbia and hypoxia. Because embryonic hemoglobin is replaced by adult forms of hemoglobin by day 18 of gestation,18 the increased affinity of fetal hemoglobin for oxygen is not a mechanism of resistance to CO2 in the neonatal rat. Blood has a buffering capacity for CO2, because CO2 is also a byproduct of normal cellular metabolic processes. If the capacity of this buffering system is exceeded, an excess of CO2 in the blood leads to acidosis, or a lowering of the pH of the blood and associated fluids. Mild respiratory acidosis leads to a compensatory increase in depth and rate of respiration, in an effort to ‘blow off’ the excess CO2. Profound respiratory acidosis quickly suppresses the respiratory centers of the brain, leading to a slow, gasping respiratory pattern.13,23 The cerebrospinal fluid lacks the buffering capacity of the blood, such that during acute respiratory acidosis, the pH of the cerebrospinal fluid will drop precipitously.25 This lowering of the pH of the cerebrospinal fluid is directly associated with anesthetic depth and insensibility to pain and results in a quick onset of stupor and coma in humans,21 in addition to the respiratory effects noted above. Acidosis leads to depression of myocardial contractility; in addition, CO2 has a direct effect on the myocardium, inducing hyperkalemia, precipitating arrhythmias, and slowing the heart rate.24,26 Therefore, hypoxia is not the primary means by which CO2 exposure causes death, but rather it is the direct action of CO2 on vital systems that produces unconsciousness and death.
Although hypoxia is not the means by which CO2 produces death, the resistance of neonates to both hypoxia and hypercarbia appears to explain why altricial neonates show prolonged survival times when exposed to CO2. Acute protective responses in the neonate to severe hypoxia and hypercarbia include hypothermia, reduction of heart and respiration rates (the diving reflex), and reduction of blood pH.30 All the mechanisms of action of CO2 on animals are compensated for by the newborn's normal responses to hypoxic or hypercarbic stress and therefore are better tolerated by newborns than adults.30 This hypoxic hypometabolism seen in newborns also may explain why euthanasia agents that appear to act solely through hypoxia, such as N2, have extended neonatal survival times when compared with CO2.1,10,11,19
Although adult rats are often the subjects of CO2 euthanasia studies, I found only 2 published references to the euthanasia of young rats with CO2. In 1 study, rats at 1, 2, 3, 4, 5, 10, and 12 d of age were exposed to 100% CO2; recorded survival times were 23, 28, 13, 13, 7, and 5 min, with survival times generally decreasing as the animals aged.11 The authors of that study listed 7 ages but only 6 survival times, and graphs were not provided to aid in interpretation of the listed results.11 In the other published study, the responses of young Wistar rats with an average weight of 88 g were compared with those of more mature Wistar rats with an average weight of 220 g.4 The young rats took longer to “collapse” when exposed to 100% CO2 but had a shorter time to death.4 Although the stock and source of rats were not specified in the study, if a Wistar stock were selected from a commercial breeder, the age based on a typical age–weight correlation would be 29 to 34 d old for the 88-g rats and 49 to 81 d old for the 220-g weight group.6 According to usual weaning practices, these animals were well past the typical weaning age of 21 d, and therefore this study provides no insight into the immediate neonatal period.
Several comments and recommendations may be made with regard to euthanasia of neonatal rats with CO2. I used a simple plastic bag as the euthanasia chamber for several reasons. A plastic bag allowed easy observation and manipulation of the rats without changing the CO2 levels within the chamber. Plastic bags can be sealed completely and provide immediate evidence of failure if they deflate. Because the euthanasia of neonates requires extended CO2 exposure times, the use of a plastic bag allows the concurrent use of more sophisticated euthanasia equipment for the euthanasia of adult animals. Care should be taken to avoid overfilling the bag with animals, however, because doing so could affect time to euthanasia or cause distress. In this experiment, relatively few animals were placed in the plastic bag; all animals could stand or move freely in the bag. In addition, the bag was placed in a bedded cage to provide a stable platform for the animals.
The decision to expose the animals to 100% CO2 was based on research showing that supplementation with oxygen or room air prolongs time to death in adult rats.7,8,29 The rate of delivery of CO2 in this experiment was chosen to minimize potential animal distress due to hypothermia and noise associated with filling of the euthanasia chamber. The small size of the chamber resulted in rapid filling, and the animals may have been chilled by the gas. Chilling neonatal rats lowers their metabolic rate,28 perhaps prolonging the time to death in this experiment. Animals are more likely to remain normothermic when kept in groups and allowed to huddle.28 In this experiment, animals were not prevented from huddling together, which may have mitigated any chilling effects of the gas or being removed from the dam. Heated gas delivery systems may be a way to decrease the time to euthanasia of neonatal rats. Heating the CO2 delivered from 24 °C to 34 °C decreased the time to euthanasia in a previous study.11
The majority of the current work was performed in a single Sprague–Dawley-derived stock of outbred rats due to their popularity in research and their prevalence in commercial breeding situations. Unlike the apparent differences between inbred and outbred mice, regardless of the strain of inbred mouse,27 a pilot experiment with a single strain of inbred rats did not reveal a difference in response from outbred rats, so I did not use inbred rats for further testing. In contrast, in mice, marked differences between inbred and outbred animals occurred with regard to euthanasia with CO2 during the first few days of life, but the time to death for the inbred mice was equivalent to that of outbred mice by 7 d of age.27 No such difference was noted in the current experiment, which could be due to the fact that only 1 strain and 1 stock were tested or to some intrinsic difference in the development of rats compared with mice. An intrinsic difference seems more likely, because the differences between inbred and outbred mice were readily apparent at day 0, regardless of the strains and stocks compared.27 Compared with mice,27 neonatal rats appear to be more sensitive to the effects of 100% CO2.
The CO2 exposure time necessary for euthanasia declined linearly as the age of the neonatal rats increased. Age and attendant development appear to be the critical factors in determining susceptibility to CO2; therefore, developmental cues may be used to determine the age of the animal. If the exact age of animals to be euthanized is unknown, physical development rather than size should serve as an indicator of age. If the age is questionable, one should err on the side of assuming the animal's immaturity and use a longer CO2 exposure. If an error is made in aging pups, animals might be underexposed to CO2 and inadvertently recover, whereas overexposure to CO2 will prevent recovery of the animal. A sample exposure chart is given in Table 2. The divisions chosen were based on easily recognizable physical signs (for example, eyelid opening) associated with specific days of age in the rat. In Table 2, a safety margin is added to each exposure time recommendation to minimize the chance of postexposure recovery. This safety margin is added to accommodate inevitable human error and possible mechanical failure in any recommendations and to aid in recognition of susceptibility to CO2 by physical signs of maturity. Recommendations on exposure times for animals 14 to 21 d of age and adult rats are extrapolated from the data for day 10 animals and common recommendations in place for the euthanasia of adult rats.
Table 2.
Suggested CO2exposure time for euthanasia of rats of various ages
| Appearance of animal | CO2 exposure time (min) |
| Nonhaired pups (0 to 6 d of age) | 40 |
| Haired pups, eyes closed (7 to 13 d of age) | 20 |
| Haired pups, eyes open, preweaning (14 to 20 d of age) | 10 |
| Weanlings and adults (21 d of age and older) | 5 |
The sample exposure chart presented here applies to the exposure chamber and filling parameters used in this study. The use of other chamber types and fill rates will require separate calibration. All euthanasia times recommended in this table should be validated at each facility, using current equipment and animals.
The recommendations made for euthanasia times for neonatal rats in Table 2 fall roughly into week-long divisions in the development of the rat. Alternatively, facilities may want to make exposure time decisions less complicated and categorize animals into 2 age groups to determine necessary times for CO2 exposure: unweaned animals and adults. Exposure times should be validated at each facility, using the intended equipment and gas delivery system. Because the present study only addresses exposure times for F344 and CD rats, facilities may wish to validate exposure times for other stocks or strains in use. All equipment should be calibrated and revalidated regularly to ensure effective euthanasia without the need for backup methods to ensure death.
Acknowledgments
The author would like to acknowledge B Hayes for animal care over the course of this experiment, M Ripperton for editorial assistance, and MFW Festing for statistical consultation.
References
- 1.Adolph EF. 1969. Regulation during survival without oxygen in infant mammals. Respir Physiol 7:356–368 [DOI] [PubMed] [Google Scholar]
- 2.AVMA Panel on Euthanasia 2000. 2000 report of the AVMA panel on euthanasia. J Am Vet Med Assoc 218:669–696 [DOI] [PubMed] [Google Scholar]
- 3.Bennett T. 2005. Letter to the editor. Comp Med 55:11. [PubMed] [Google Scholar]
- 4.Blackshaw JK, Fenwick DC, Beattie AW, Allan DJ. 1988. The behaviour of chickens, mice, and rats during euthanasia with chloroform, carbon dioxide, and ether. Lab Anim 22:67–75 [DOI] [PubMed] [Google Scholar]
- 5.Cartner SC, Barlow SC, Ness TJ. 2007. Loss of cortical function in mice following decapitation, cervical dislocation, potassium chloride injection, and CO2 inhalation. Comp Med 57: 570–573 [PubMed] [Google Scholar]
- 6.Charles River [Internet] Research animal models pricing and literature, the Wistar rat. [cited 5 January 2009]. Available at http://www.criver.com/SiteCollectionDocuments/rm_rm_c_wistar_rats.pdf
- 7.Close B, Banister K, Baumans V, Bernoth EM, Bromage N, Bunyan J, Erhardt W, Flecknell P, Gregory N, Hackbarth H, Morton D, Warwick C. 1997. Recommendations for euthanasia of experimental animals: part 2. DGXI of the European Commission. Lab Anim 31:1–32 [DOI] [PubMed] [Google Scholar]
- 8.Coenen AM, Drinkenburg WH, Hoenderken R, van Luijtelaar EL. 1995. Carbon dioxide euthanasia in rats: oxygen supplementation minimizes signs of agitation and asphyxia. Lab Anim 29:262–268 [DOI] [PubMed] [Google Scholar]
- 9.Danneman PJ, Stein S, Walshaw SO. 1997. Humane and practical implications of using carbon dioxide mixed with oxygen for anesthesia or euthanasia of rats. Lab Anim Sci 47:376–385 [PubMed] [Google Scholar]
- 10.Duffy TE, Kohle SJ, Vannucci RC. 1975. Carbohydrate and energy metabolism in perinatal rat brain: relation to survival in anoxia. J Neurochem 24:271–276 [DOI] [PubMed] [Google Scholar]
- 11.Fazekas JF, Alexander FAD, Himwich HE. 1941. Tolerance of the newborn to anoxia. Am J Physiol 134:281–287 [Google Scholar]
- 12.Feldman DB, Gupta BN. 1976. Histopathologic changes in laboratory animals resulting from various methods of euthanasia. Lab Anim Sci 26:218–221 [PubMed] [Google Scholar]
- 13.Forslid A, Augustinsson O. 1988. Acidosis, hypoxia, and stress hormone release in response to 1-minute inhalation of 80% CO2 in swine. Acta Physiol Scand 132:223–231 [DOI] [PubMed] [Google Scholar]
- 14.Hackbarth H, Kuppers N, Bohnet W. 2000. Euthanasia of rats with carbon dioxide—animal welfare aspects. Lab Anim 34:91–96 [DOI] [PubMed] [Google Scholar]
- 15.Hauser R, Jankowski Z, Gos T, Krzyzanowski M. 2001. Haemorrhages in head tissues during the asphyxiation process. Forensic Sci Int 124:235–236 [DOI] [PubMed] [Google Scholar]
- 16.Hewett TA, Kovacs MS, Artwohl JE, Bennett BT. 1993. A comparison of euthanasia methods in rats, using carbon dioxide in prefilled and fixed flow-rate–filled chambers. Lab Anim Sci 43:579–582 [PubMed] [Google Scholar]
- 17.Institute of Laboratory Animal Resources (ILAR), National Research Council 1996. Guide for the Care and Use of Laboratory Animals. Washington, D.C.: National Academy Press [Google Scholar]
- 18.Iwahara SI, Abe Y, Okazaki T. 1996. Identification of five embryonic hemoglobins of rat and ontogeny of their constituent globins during fetal development. J Biochem 119:360–366 [DOI] [PubMed] [Google Scholar]
- 19.Kabat H. 1940. The greater resistance of very young animals to arrest of the brain circulation. Am J Physiol 130:588–599 [Google Scholar]
- 20.Marris E. 2006. Bioethics: an easy way out? Nature 441:570–571 [DOI] [PubMed] [Google Scholar]
- 21.Meyer JS, Gotoh F, Tazaki Y. 1961. CO2 narcosis. An experimental study. Neurology 11:524–537 [DOI] [PubMed] [Google Scholar]
- 22.Morton DB. 2005. Letter to the editor. Comp Med 55:1115803621 [Google Scholar]
- 23.Petty WC, Sulkowski TS. 1971. CO2 narcosis in the rat. I. Effects on respiration and blood parameters. Aerosp Med 42:547–552 [PubMed] [Google Scholar]
- 24.Petty WC, Sulkowski TS. 1971. CO2 narcosis in the rat. II. Effects on the ECG. Aerosp Med 42:553–558 [PubMed] [Google Scholar]
- 25.Pontén U. 1966. Consecutive acid–base changes in blood, brain tissue and cerebrospinal fluid during respiratory acidosis and baseosis. Acta Neurol Scand 42:455–471 [DOI] [PubMed] [Google Scholar]
- 26.Price HL. 1960. Effects of carbon dioxide on the cardiovascular system. Anesthesiology 21:652–663 [DOI] [PubMed] [Google Scholar]
- 27.Pritchett K, Corrow D, Stockwell J, Smith A. 2005. Euthanasia of neonatal mice with carbon dioxide. Comp Med 55:275–281 [PubMed] [Google Scholar]
- 28.Saiki C, Mortola JP. 1996. Effect of CO2 on the metabolic and ventilatory responses to ambient temperature in conscious adult and newborn rats. J Physiol 491:261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharp J, Azar T, Lawson D. 2006. Comparison of carbon dioxide, argon, and nitrogen for inducing unconsciousness or euthanasia of rats. J Am Assoc Lab Anim Sci 45:21–25 [PubMed] [Google Scholar]
- 30.Singer D. 2004. Metabolic adaptation to hypoxia: cost and benefit of being small. Respir Physiol Neurobiol 141:215–228 [DOI] [PubMed] [Google Scholar]