Abstract
A novel surgical method for collecting oocytes from unique and irreplaceable mice is described. This method, surgical oocyte retrieval (SOR), facilitates the collection of ovulated oocytes, does not require euthanasia, and preserves reproductive potential. The surgery involves a small incision in the ampulla region of the oviduct, through which the cumulus oocyte mass is removed with a gel-loading pipette. The incision then is closed by using a tissue adhesive, which is required to ensure healing of the incision and containment of any oocytes ovulated after SOR. Two anesthetics, isoflurane and tribromoethanol, were compared for oocyte toxicity during SOR. More dead oocytes were recovered when tribromoethanol was used than when isoflurane was used. Combining SOR and traditional oocyte collection methods yielded more oocytes per BALB/cByJ than did traditional methods alone (41 versus 28 oocytes, respectively). Oocytes collected by using SOR were fertilized and subsequent embryos developed to term comparable to controls. This technique provides an alternative method for oocyte collection and will be valuable for maximizing the number of oocytes from irreplaceable mice.
Abbreviation: SOR, surgical oocyte retrieval; COM, cumulus oocyte mass; hCG, human chorionic gonadotropin
The traditional method for obtaining mature ovulated mouse oocytes9 has not changed since it was first described in 1957.6 The technique requires euthanizing the female mouse, removing the oviducts, and dissecting cumulus oocyte masses from the ampullae. Although this method is a terminal procedure, it is widely accepted and used because, in general, mice are readily available. In other species such as cattle,1,4 humans,7 goats and sheep,5 laparoscopic surgical or nonsurgical transvaginal oocyte retrieval methods that do not require euthanasia are typical. A similar method would be advantageous for some assisted reproductive applications in mice, but laparoscopic surgery and transcervical methods for oocyte collection currently are not practical methods for use in small rodents. A nonterminal method to collect mature oocytes would allow more oocytes to be collected from an individual female mouse and would preserve her reproductive ability. Here we describe a novel surgical method, surgical oocyte retrieval (SOR), for collecting mature ovulated oocytes that maintains the reproductive potential of the female mouse, allowing her to breed and produce litters. The purpose for developing this procedure was to provide an alternative method for collecting mature oocytes from genotypically rare mice while maintaining their reproductive potential and maximizing the number of oocytes collected.
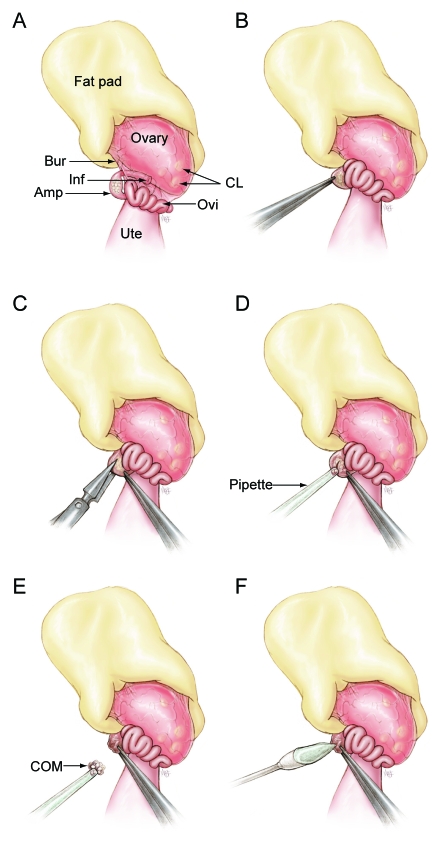
In developing this method, we have taken advantage of the anatomy of the female mouse reproductive tract. In the mouse, the ovary and proximal portion of the oviduct including the infundibulum is surrounded by a thin bursal membrane (Figure 1 A). During ovulation, the bursa helps guide the cumulus oocyte complexes to the infundibulum, where they enter the oviduct. Once in the oviduct, these complexes travel to the ampulla, a specialized region of the oviduct, where they are held until fertilization occurs. Once in the ampulla, cumulus oocyte complexes clump together and become a cumulus oocyte mass (COM). The ampulla wall stretches to accommodate the COM, and it is through this wall that COMs are collected during the SOR procedure. The procedure involves anesthetizing a superovulated mouse 12 to 15 h after injection of human chorionic gonadotropin (hCG), surgically exposing the ovary and oviduct, making an incision in the ampulla wall, removing the COM, sealing the ampulla incision with a tissue adhesive, and returning the oviduct to the body cavity. We also discuss the choice of anesthetic and closure of the ampulla incision.
Figure 1.
Structures of the female anterior reproductive tract and the steps of the surgical oocyte retrieval (SOR) process are illustrated. (A) The ovarian fat pad, ovary, and oviduct are exteriorized and positioned as shown. (B) The ampulla is grasped by using Dumont forceps and held in place. (C) A 1- to 2-mm incision is made in the ampulla wall by using Vannas microdissecting scissors. (D) The cumulus oocyte mass (COM) is suctioned gently from the ampulla by using a gel-loading pipette attached to a mouth pipette. (E) The COM is not aspirated into the bore of the pipette tip but adheres to the tip once freed from the ampulla. (F) A swab is used to apply tissue adhesive to the ampulla incision. Amp, ampulla; Bur, bursa membrane; CL, corpus luteum; Inf, infundibulum; Ovi, oviduct; Ute, uterine horn.
After SOR, the female mouse can be superovulated again, thus maximizing the number of oocytes produced, or she can be mated to produce litters naturally. We demonstrate the utility of this technique by using an inbred strain.
Materials and Methods
Animals.
The Institutional Animal Care and Use Committee of The Jackson Laboratory approved all procedures used in this study, and all mice were maintained at The Jackson Laboratory (Bar Harbor, ME) in accordance with institutional protocols and the Guide for the Care and Use of Laboratory Animals.10 The room was maintained on a 14:10-h photoperiod (lights on, 05:00 AM). All mice were housed in a low barrier facility in duplex polycarbonate mouse cages containing pine shavings as bedding (depth, 16 to 26 mm). Sterilized 6% fat rodent chow (LabDiet 5K52, PMI International, St Louis, MO) and acidified water were provided ad libitum. CByB6F1/J (JAX stock number, 100009) and inbred BALB/cByJ (JAX stock number, 001026) mice were used.
Superovulation.
Superovulation was induced in 4- to 7-wk-old female mice by intraperitoneal injection of 2.5 IU (for F1) or 5.0 IU (for inbred) pregnant mare serum gonadotropin (Calbiochem, La Jolla, CA) between 17:30 and 18:30, followed by intraperitoneal injection of 5.0 IU hCG (Sigma, St Louis, MO) 48 to 50 h later.
Removing cumulus for oocyte counting.
Cumulus oocyte masses were collected into a culture dish containing PBS with 4 mg/mL bovine serum albumin. Cumulus cells were dissociated from the oocytes with hyaluronidase (0.3 mg/mL) and gentle pipetting at ambient temperature. If COMs were used for in vitro fertilization, the cumulus cells were not removed. The numbers of live, dead, and fragmented oocytes collected from the left and right oviduct of each mouse were counted. The percentage of live oocytes was calculated as: no. live oocytes / total no. oocytes × 100%.
In vitro fertilization and embryo transfer.
In vitro fertilization was performed as previously described,3 except Mouse Vitro Fert (MVF, Cook, Australia) was used as the fertilization and sperm collection medium. Oocytes obtained from BALB/cByJ mice and freshly collected BALB/cByJ sperm were used. Fertilization rate, the proportion of oocytes that cleave to the 2-cell stage, was calculated as: no. 2-cell embryos / total no. oocytes ×100%. Ten 2-cell embryos were surgically transferred into the left oviduct of each 0.5-d pseudopregnant CByB6F1/J recipient. Embryo recipients were euthanized at day 17 of pregnancy, and the uterine contents were examined. The numbers of live fetuses, resorbed fetuses, and implantation sites that did not develop were counted. Embryo transfer success, the proportion of live fetuses, was calculated as: no. live fetuses / no. embryos transferred × 100%.
Description of surgical procedure.
The surgical approach used for SOR was similar to those previously described for embryo transfer8, 9 and ovary transplantation.2, 9 Mice were prepared for surgery according to institutional protocols and were anesthetized 14 to 15 h after injection of hCG by intraperitoneal injection of 2% tribromoethanol (Fisher Scientific, Pittsburgh, PA, 0.2mL/10g body weight) or inhalation of isoflurane (3%, Baxter Healthcare, Deerfield, IL) in oxygen (2 L/min). Fur was removed by shaving the hair from the surgical area. The surgical site was cleaned with 70% alcohol, followed by povidone iodine solution (Betadine, Purdue Pharma, Stamford, CT), and finished with 70% alcohol. Veterinary opthalmic ointment (Pharmaderm, Melville, NY) was applied to the eyes to prevent drying, and an analgesic (0.1 mL/g; Carprofen, 5 mg/kg, Pfizer, New York, NY) was administered subcutaneously under the loose skin on the back of the neck. The mouse was placed on a pad in a ventrolateral position.
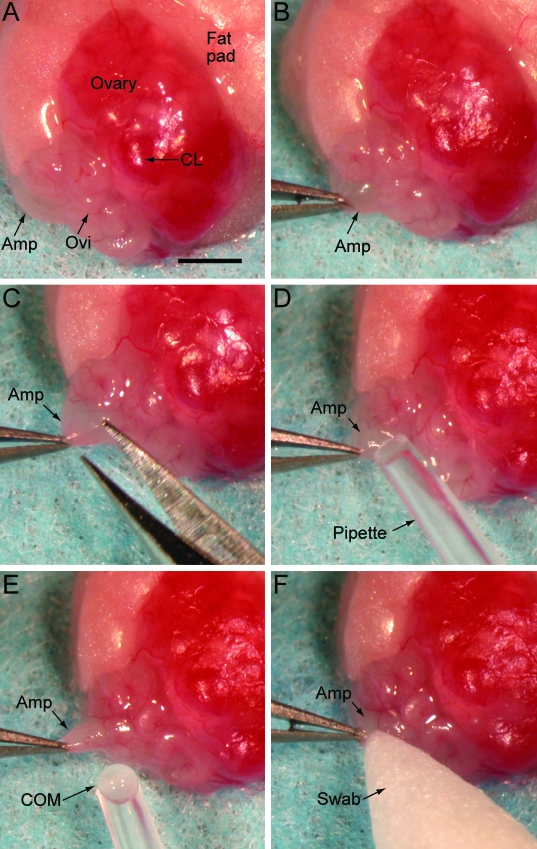
Surgery was performed by using a stereomicroscope. A 3- to 5-mm skin incision was made midway between the last rib and the femur, directly over the ovary. The peritoneal cavity was entered through a second incision in the abdominal muscles, while avoiding blood vessels. The ovarian fat pad was exteriorized and placed on a sterile drape, with the ovary and oviduct exposed and positioned as shown (Figures 1 A and 2 A). The distended ampulla was identified and grasped with a pair of Dumont (#5) forceps to immobilize it (Figures 1 B and 2 B). A 0.1- to 0.2-mm incision was made on the mid to proximal end of the ampulla with Vannas microscissors (Roboz, Rockville, MD; Figures 1 C and 2 C). The COM was suctioned gently from the ampulla by using a gel-loading tip (0.25 mm outside diameter, Fisher Scientific, Pittsburgh, PA) attached to a mouth pipette with an inline 0.22-µm filter (Figures 1 D and 2 D). The COM was not aspirated into the bore of the pipette tip because of its size, but once freed from the ampulla, it adhered to the pipette tip (Figures 1 E and 2 E) and was transferred to a 500-µL drop of M2 medium.9 The pipette tip was submerged in the drop of medium, and (if needed) a pair of Dumont forceps was used gently to free the COM from the pipette tip. The incision in the ampulla was closed with synthetic absorbable tissue adhesive (Tissumend II, Veterinary Products Laboratories, Phoenix, AZ), which was applied in a thin layer to the ampulla incision by using a swab (Qosina, Edgewood, NY; Figures 1 F and 2 F). After the tissue adhesive dried (15 to 30 s), the ovarian fat pad, ovary, and oviduct were returned to the abdominal cavity. The abdominal muscle incision was closed with 5-0 suture material (Dexon S, Syneture, Norwalk, CT), and the skin incision was closed with a 7-mm wound clip. The procedure was repeated on the opposite oviduct unless that oviduct was used as a control. The mouse received an ear punch for later identification and was placed in a clean mouse box on a slide warmer (37 °C) until it became ambulatory.
Figure 2.
Photographs of the surgical oocyte retrieval (SOR) process. (A) The ovarian fat pad, ovary, and oviduct are exteriorized and positioned as shown. (B) The ampulla is grasped by using Dumont forceps and held in place. (C) A 0.1- to 0.2-mm incision is made in the ampulla wall by using Vannas microdissecting scissors. (D) The cumulus oocyte mass (COM) is suctioned gently from the ampulla by using a gel-loading pipette attached to a mouth pipette. (E) The COM is not aspirated into the bore of the pipette tip but adheres to the tip once freed from the ampulla. (F) A swab is used to apply tissue adhesive to the ampulla incision. Amp, ampulla; CL, corpus Luteum; Ovi, oviduct; COM, cumulus oocyte mass. Bar, 1.0 mm.
Design of experiments.
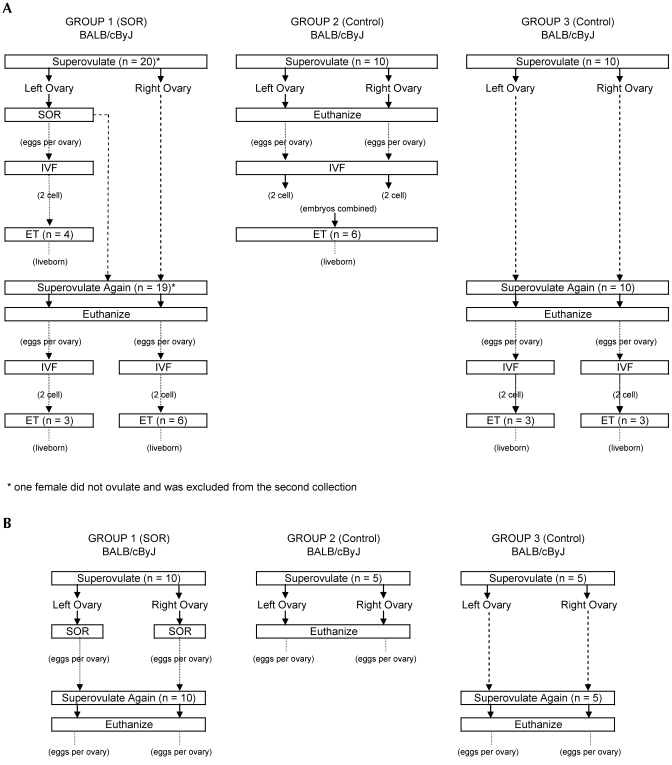
The objective of experiment I (Figure 3 A)was to demonstrate the utility of SOR in an inbred strain by collecting oocytes by using SOR, fertilizing them by in vitro fertilization, and producing live pups by using embryo transfer. An inbred strain, BALB/cByJ, was used for this experiment because in general, inbred strains ovulate fewer oocytes and are more likely to benefit from SOR than are hardy F1 hybrid strains that ovulate well. Mice were randomized between the SOR and control groups, and those in group 1 were superovulated twice. After the first superovulation (14 to 15 h after hCG injection), SOR was done on the left oviduct only and the right oviduct was left intact and served as a control for the SOR procedure. Two weeks after the SOR procedure, the mice were superovulated again and euthanized 14 to 15 h after hCG injection, and oocytes were collected from the left and right oviducts. The control group females were superovulated once (group 2) or twice (group 3), and oocytes were collected from the left and right oviducts after euthanasia. Oocytes recovered were fertilized in vitro, and some of the resulting 2-cell embryos were transferred subsequently to observe any differences in the number of fetuses developing between groups. A separate experiment was done to evaluate the total number of oocytes that could be collected per mouse when SOR was used on both the left and right oviducts followed by a second posteuthanasia collection. The experimental design with the number of animals in each group is shown in Figure 3 B. The number of oocytes collected per mouse by using SOR and a second collection after euthanasia was compared with the number collected by using just the traditional posteuthanasia method.
Figure 3.
Experimental design for experiment I. Group 1 is the surgical oocyte retrieval (SOR) group. Groups 2 and 3 are control groups and were superovulated but did not undergo surgery. (A) The experimental design to compare fertilization and embryo transfer successes is shown. In this experiment, SOR was not performed on the right oviduct. (B) The experimental design to determine the maximal number of oocytes that can be collected from a mouse is shown. SOR was performed on the left and right oviducts. *, One mouse did not ovulate and was excluded from the second collection. IVF, in vitro fertilization; ET, embryo transfer.
The objective of experiment II was to determine whether female mice could breed and produce litters after SOR. Three groups were tested (n = 5 CByB6F1/J per group): mice that underwent SOR on both oviducts; superovulated mice with no surgery; and female mice that received no treatment. The female mice each were housed with a CByB6F1/J male beginning 2 wk after surgery or superovulation and were monitored for 16 wk. Each mouse bore 3 or 4 litters, and the number of live pups was recorded. The average number of live pups produced per mouse was compared between groups.
Injectable anesthetics administered intraperitoneally are used frequently in rodent surgery. However, any agent administered by intraperitoneal injection will come in direct contact with the cumulus oocyte mass during the SOR procedure and could have toxic effects on the oocytes. The objective of experiment III was to test 2 anesthetics, isoflurane and tribromoethanol, determine whether the choice and delivery method of the anesthetic adversely affected the oocytes. Superovulated F1 female mice were randomly assigned to 3 groups (n = 5 per group) to receive tribromoethanol, isoflurane, or no anesthetic (control). Control animals were not anesthetized, but were euthanized by cervical dislocation for COM collection by the SOR method. Oocytes were counted as live, dead, or fragmented. The proportion of live oocytes was calculated as: no. live oocytes / (no. live + no. dead + no. fragmented) × 100%.
The objective of experiment IV was to determine whether closing the ampulla incision was necessary during the SOR procedure. Superovulated F1 mice were anesthetized, and the COM was removed from the left ampulla only. Mice were randomly assigned to 3 groups: SOR with tissue adhesive to close the ampulla incision (n = 15); SOR with no tissue adhesive (n = 5); and 3) sham surgery (n = 5). For the sham surgery, the entire surgery except the oviduct incision was performed. No surgery was performed on the right oviduct, which served as a control to verify ovulation for the second oocyte collection. At 2 and 6 wk after surgery, mice from each group were superovulated again and euthanized by cervical dislocation; COMs from each ampulla were removed, and oocytes were counted as live, dead, or fragmented.
Statistics.
Oocyte counts were analyzed by using 1-way ANOVA, and means were compared by using the Tukey–Kramer test (JMP version 6.0.3, SAS Institute, Cary, NC). An arc–sine transformation was performed on the proportion of live oocytes in the anesthetic experiment, the proportion of fertilized oocyte data after in vitro fertilization, and the proportion of live pups after embryo transfer. The transformed data were analyzed by 1-way ANOVA, and means were compared by using the Tukey–Kramer test.
Results
Experiment I: Application of SOR with an inbred strain.
In practice, oocytes collected by SOR would be fertilized and the resulting embryos transferred to produce live animals. To demonstrate that oocytes collected by SOR are capable of these processes, we collected oocytes by SOR, created embryos by using in vitro fertilization, and transferred the embryos to recipient mice. Fertilization rate (mean ± SEM) did not differ between SOR and control groups (group 1: first collection by SOR from left oviduct, 69% ± 9%; second collection from left oviduct, 71% ± 12%; second collection from right oviduct 74% ± 5%; group 2: control from left oviduct, 90% ± 5%; control from right oviduct, 89% ± 5%; and group 3: control from left oviduct, 89% ±3%; control from right oviduct, 86% ± 3%). The proportion of embryos that developed to day 17 fetuses did not differ among groups (group 1: first collection by SOR, 65% ± 6%; left oviduct second collection, 75% ± 12%; right oviduct, 65% ± 9%; group 2: control, 62 ± 11%; group 3: left oviduct, 60% ± 6%; right oviduct, 80% ± 6%).
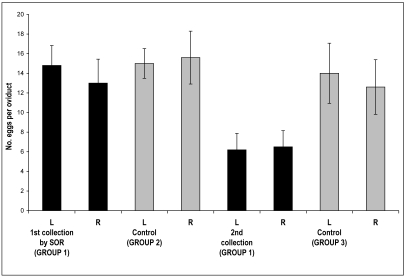
SOR followed by traditional oocyte collection after euthanasia yielded more oocytes than a single collection (Figure 4). On average, 28 oocytes were harvested by SOR and an additional 13 oocytes were collected from the same mice after euthanasia (Figure 3B, group 1). The number of oocytes collected by SOR (mean, 28; range, 10 to 47) is comparable to that from a single collection by traditional methods (mean, 31; range, 23 to 40; Figure 3B, groups 1 and 2). When SOR was followed by the traditional oocyte collection method, the mean increased to 41 (range, 18 to 58) oocytes per mouse (Figure 3B, group 1 first and second collection) compared with 31 oocytes by using the traditional method alone. These data represent a 32% increase in the average number of oocytes collected per mouse compared with using traditional methods alone.
Figure 4.
The utility of SOR for oocyte collection and embryo production with an inbred strain is shown. Group numbers correspond to those in Figure 3 B. Oocytes were collected from BALB/cByJ mice by using SOR for the first collection and then after euthanasia for the second collection (group 1). Control oocytes were collected after euthanasia from mice that had been superovulated once (group 2) or twice (group 3). L, left ovary; R, right ovary. Bars represent mean ± SEM; differences between groups were not significant.
Experiment II: Reproductive performance after SOR.
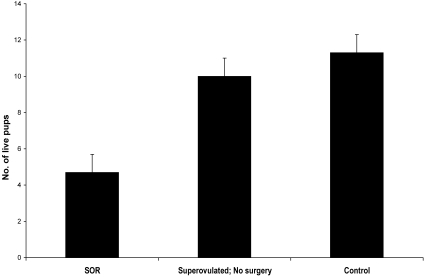
In experiment II, we determined whether litters could be produced through natural mating after SOR. The average litter size produced by mice that previously had undergone SOR is shown in Figure 5. The average number of pups per litter produced by the SOR group (5 ± 1 pups) was smaller than that from superovulated control group (10 ± 1 pups) and the untreated controls (11 ± 1 pups; P < 0.05 for both comparisons) mice. Of the 5 SOR treated females mated, only 1 did not produce any litters within 16 wk.
Figure 5.
The reproductive potential of mice is maintained after SOR. CByB6F1/J mice that had undergone SOR were mated to CByB6F1/J male mice to assess fertility. Mice that were superovulated, but did not have surgery served as a control for hormone stimulation effects. Mice that were not manipulated surgically served as a control for SOR procedures. Bars represent mean ± SEM, n = 5 mice per group. Mice that had undergone SOR produced fewer (*, P < 0.05) liveborn pups than did other groups.
Experiment III: Comparison of anesthetics.
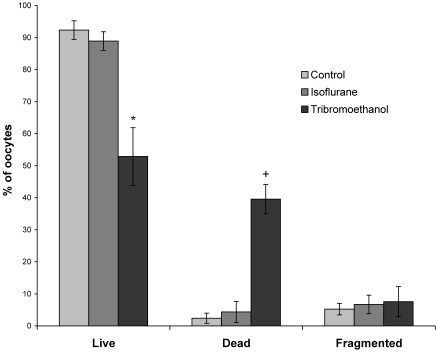
Two anesthetics were evaluated for effects on oocyte survival when used during SOR. Mice anesthetized with tribromoethanol for SOR had a greater proportion of dead oocytes (40% ± 5%) compared with mice anesthetized with isoflurane for SOR (4% ± 3%) or the control (2% ± 2%) group euthanized prior to oocyte collection (P < 0.05 for both comparisons; Figure 6). The groups did not differ significantly in the proportion of fragmented oocytes.
Figure 6.
The anesthetic used during SOR affects CByB6F1/J oocytes. The percentages of live, dead, and fragmented oocytes after SOR using tribromoethanol or isoflurane anesthesia are shown. Mice were superovulated and anesthetized 14 to 15 h after hCG injection. Cumulus oocyte masses were collected, and oocytes were dissociated for counting using hyaluronidase. Values are mean ± SEM, n = 5 per group. Using tribromoethanol as an anesthetic resulted in fewer (*, P < 0.05) live oocytes and more (+, P < 0.05) dead oocytes than those from the isoflurane and control groups.
Experiment IV: Closure of ampulla incision.
If a tissue adhesive was not used to close the ampulla incision (n = 5 mice), no oocytes were found in the oviduct after superovulation at 2 wk (n=3) or 6 wk (n=2) after surgery (Table 1). However, if tissue adhesive was used to close the ampulla incision and mice were superovulated 2 wk later (n = 8), oocytes were found in the oviduct (mean, 4; unmanipulated oviduct, 10). At 6 wk, an average of 1 oocyte (unmanipulated oviduct, 9) was found per oviduct that had undergone SOR (n = 7). At 2 and 6 wk after surgery, the tissue adhesive was still in place. The number of oocytes recovered at the 2 and 6 wk collections were not significantly different, therefore these data were combined for data analysis, and the averages are shown in Table 1. After 14 wk, the tissue adhesive (Tissumend II, Veterinary Products Laboratories) was not grossly visible, and the ampulla was adhered to the adjacent ovarian fat pad. When this small adhesion was teased apart, the incision in the ampulla was completely healed in 4 of 8 oviducts examined and had partially healed in the remaining oviducts.
Table 1.
Using tissue adhesive to seal the ampulla incision increases the number of oocytes retrieved at the second collection
| No. of oocytes after SOR | No. of oocytes at 2nd collection |
|||
| No. of mice | Left ovary | Right ovary | ||
| No tissue adhesive | 5 | 9 ± 1 | 0b | 10 ± 2a |
| Tissue adhesive | 15 | 10 ± 2 | 3 ± 1a,b | 10 ± 1a |
| Sham surgery | 5 | not done | 6 ± 1a | 4 ± 1b |
CByB6F1/J mice were superovulated, and oocytes were collected by SOR from the left oviduct only. At 2 to 6 wk after SOR, oocytes were collected from the left and right oviducts after euthanasia. Results did not differ between the week 2 and 6 collections (data not shown), therefore these data were combined. The ampulla incision was either closed with tissue adhesive or not closed. Sham surgery was identical to SOR except that no ampulla incision was made. Data are presented as mean ± SEM.
Values with different superscripts within the same column are different (P < 0.05).
Discussion
A novel method for collecting mature ovulated oocytes from the ampulla of superovulated mice is described. The method was developed to maximize the number of oocytes that can be collected from a single genetically important mouse and to allow eggs to be collected from a mouse while maintaining her ability to breed afterward. Two important concerns were identified that affected the quantity and quality of oocytes retrieved by SOR: the type of anesthetic used and closure of the ampulla incision.
Tribromoethanol, a liquid anesthetic, is administered by intraperitoneal injection. The volume of anesthetic required for surgical procedures (0.4 to 0.6 mL) is enough to fill the peritoneal cavity of an adult mouse and as a result bathes the oviduct. In contrast, isoflurane, a gas anesthetic, is administered through inhalation and therefore does not come into direct contact with the oviduct. A large proportion of oocytes were dead immediately after SOR when tribromoethanol was used but not when isoflurane was used. Tribromoethanol is commonly used as an anesthetic for embryo transfer surgeries9 and does not have any reported adverse effects on the number of preimplantation embryos that develop to term.13 Why oocytes may be more sensitive to tribromoethanol than are embryos is unknown, but a similar effect has been reported previously with propofol.12 Propofol, a short-acting anesthetic given intravenously to humans, perturbs the development of mouse oocytes in vitro but not of embryos at the 2-cell stage, showing that developmental stages have different tolerance levels for chemical stress or routes of anesthetic delivery. For this reason, the anesthetic used for SOR should be evaluated carefully to prevent toxic effects on the oocytes.
We also demonstrated that closure of the ampulla incision is a critical step in SOR to ensure that oocytes produced in subsequent ovulations will be contained within the oviduct. In the absence of a tissue adhesive, the ampulla did not heal spontaneously [even by 5 mo after surgery (data not shown)], and oocytes were not present in the manipulated oviduct even though they were present in the control oviduct. During the initial development of SOR, a nonabsorbable tissue adhesive (Nexaband S/C, Closure Medical Corporation, Raleigh, NC) was used for closure of the ampullar incision. To minimize the risk of a foreign body reaction, we switched to a tissue adhesive that is formulated for internal use and is absorbed in 60 to 90 d (Tissumend II) and found it was as effective as nonabsorbable adhesive for closure of the ampullar incision. We conclude that a tissue adhesive or a comparable method must be used to close the ampulla incision to ensure that future oocytes will be contained in the oviduct.
The utility of SOR was shown by using an inbred strain, BALB/cByJ. When used in an in vitro fertilization, oocytes collected by using SOR developed to the 2-cell stage, comparable to control oocytes. When the resulting embryos subsequently were transferred, there was no difference between SOR and control groups in the proportion of embryos that developed to day 17 fetuses. When SOR was used in conjunction with traditional methods, the number of oocytes collected increased by 32% compared to a single oocyte collection after euthanasia. Therefore, the overall result is in an increase in the number of fetuses produced per donor female mouse.
The fertility of mice after SOR further supported the utility of the method. Female mice successfully produced normal live pups after undergoing SOR, although the average litter size after SOR was approximately 50% smaller than that for controls. Further improvements to the described method may increase the number of live pups produced. We consider that incomplete healing of the ampulla incision is the main contributor to reduced litter size.
SOR can be useful in mouse colony management because the technique maximizes the number of oocytes that can be collected from a single mouse while preserving her fertility. The technique likely will be most useful for genetically rare and valuable female mice. For example, the Genetic Stability Program11 established at The Jackson Laboratory aims to reduce genetic drift over time in the foundation stock colonies of inbred strains. This goal is achieved by cryopreserving embryos from a small pool of foundation stock animals and using them to replenish the colony every 5 generations.11 The ability to collect more oocytes from an individual mouse likely will increase the number of embryos produced from each animal, further extending the initial stock of cryopreserved embryos and limiting genetic drift over a longer time span. For some strains that are particularly difficult to breed, using SOR may be the only way to introduce them into such a program.
The SOR technique, in combination with other assisted reproductive technologies, can be used as an alternative method for producing embryos for cryopreservation, rederivation, and other purposes. Many complex genetically modified mouse strains are maintained as small homozygous colonies. One barrier to cryopreservation of these strains is the inability to produce enough egg donors. SOR is a method by which to maximize the utility of each female mouse. Another potential benefit of using SOR is shortening the generation interval by 3 to 4 wk. Oocytes could be collected from 3-wk-old mice fertilized in vitro and transferred to a recipient female mouse instead of waiting until the donor mouse reached breeding age. The donor mouse can continue to breed and serve as a backup if embryo production fails.
Using SOR, multiple oocyte collections can be made from a single mouse, with the first collection by SOR and a second collection after euthanasia of the mouse. Multiple collections maximize the number of oocytes collected from a single animal. The SOR technique could potentially be used more than once to make several oocyte collections from a single mouse, thereby allowing the study of oocytes from an individual animal over time. However, the ability of the ampulla to recover and be functional after multiple SORs has not been determined.
In summary, the SOR technique increases the number of eggs collected per animal but does not decrease measures of fertilization or live fetus outcome. In addition, mice that have undergone SOR can still breed and produce litters.
Acknowledgments
The authors thank Dr Bonnie Lyons and Dr Mary Ann Handel for reviewing the manuscript and Megan E Foldenauer for the medical illustrations. This project was funded in part by the Ellison Medical Foundation and Howard Hughes Medical Institute.
References
- 1.Baker AA, Jillella D. 1978. Techniques of surgical and nonsurgical ova collection of superovulated cows. Vet Rec 103:558–562 [DOI] [PubMed] [Google Scholar]
- 2.Boot LM, Muhlbock O. 1953. Transplantations of ova in mice. Acta Physiol Pharmacol Neerl 3:133–136 [PubMed] [Google Scholar]
- 3.Byers SL, Payson SJ, Taft RA. 2006. Performance of ten inbred mouse strains following assisted reproductive technologies (ARTs). Theriogenology 65:1716–1726 [DOI] [PubMed] [Google Scholar]
- 4.Callesen H. 1987. Ultrasonically guided aspiration of bovine follicular oocytes. Theriogenology 27:217 [Google Scholar]
- 5.Cox JF, Alfaro V. 2007. In vitro fertilization and development of OPU-derived goat and sheep oocytes. Reprod Domest Anim 42:83–87 [DOI] [PubMed] [Google Scholar]
- 6.Fowler RE, Edwards RG. 1957. Induction of superovulation and pregnancy in mature mice by gonadotrophins. J Endocrinol 15:374–384 [DOI] [PubMed] [Google Scholar]
- 7.Lenz S, Lauritsen JG. 1982. Ultrasonically guided percutaneous aspiration of human follicles under local anesthesia: a new method of collecting oocytes for in vitro fertilization. Fertil Steril 38:673–677 [DOI] [PubMed] [Google Scholar]
- 8.McLaren A, Michie D. 1956. Studies on the transfer of fertilized mouse eggs to uterine foster-mothers: I. Factors affecting the implantation and survival of native and transferred eggs. J Exp Biol 33:394–416 [Google Scholar]
- 9.Nagy A, Gertsenstein M, Vintersten K, Behringer R. 2003. Manipulating the mouse embryo: a laboratory manual, 3rd ed Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press [Google Scholar]
- 10.National Research Council 1996Guide for the care and use of laboratory animals. Washington (DC): National Academy Press [Google Scholar]
- 11.Taft RA, Davisson M, Wiles MV. 2006. Know thy mouse. Trends Genet 22:649–653 [DOI] [PubMed] [Google Scholar]
- 12.Tatone C, Francione A, Marinangeli F, Lottan M, Varrassi G, Colonna R. 1998. An evaluation of propofol toxicity on mouse oocytes and preimplantation embryos. Hum Reprod 13:430–435 [DOI] [PubMed] [Google Scholar]
- 13.Zeller W, Meier G, Burki K, Panoussis B. 1998. Adverse effects of tribromoethanol as used in the production of transgenic mice. Lab Anim 32:407–413 [DOI] [PubMed] [Google Scholar]