Abstract
Housing laboratory animals under lighting conditions that more closely mimic the natural environment may improve their wellbeing. This study examined the effects of dim light or a long-night photocycle on resting heart rate (HR) of rats and their HR responses to acute procedures. Male and female Sprague–Dawley (SD) and spontaneously hypertensive (SHR) rats, instrumented with radiotelemetry transmitters and housed individually under a 12:12-h light:dark photocycle with 10 lx illumination (dim light) or under an 8:16-h light:dark photocycle with 200 lx illumination (long nights), were compared with control rats individually housed under a 12:12-h light:dark photocycle with 200 lx illumination. Dim light and long nights significantly reduced the HR of undisturbed SD and SHR male and SHR female rats during the day and at night; however, the HR of undisturbed SD females was not affected. When rats were subjected acutely to husbandry, experimental, or stressful procedures, dim light or long nights (or both) reduced HR responses to some procedures, did not alter responses to others, and increased responses to yet other procedures. The pattern of effects varied between strains and between male and female rats. Because basal HR was reduced when rats were housed under 10 lx illumination or an 8:16-h light:dark photocycle, we concluded that housing rats under 12:12-h light:dark, 200 lx ambient light conditions was potentially stressful, We also concluded that dim light or long nights did not uniformly reduce the increased HR responses induced by acute procedures.
Abbreviations: HR, heart rate; SD, Sprague–Dawley; SHR, spontaneously hypertensive
Stress can pose serious problems for experimental animals, depending on the type, intensity, and duration of the stressor and the capacity of the animals to adapt. Chronic or severe stressors can result in distress, which can induce pathologic changes and eventually death if an animal cannot respond adequately to the alteration in its homeostasis. Even stressors to which animals readily adapt can produce responses that seriously confound experimental results if the stressor is not recognized and controlled for or eliminated by the experimental design.
Responses produced by stressors in animals include: (a) enhanced secretion of hormones of the hypothalamic–pituitary–adrenal axis,15,24,55,59 (b) increased or suppressed secretion of other hypothalamic or pituitary hormones (for example, oxytocin,34 prolactin,21,22,27,29,30,43,48,62 growth hormone14,29,48), (c) enhanced activity of the autonomic nervous system,39,45,66 (d) alterations in systems controlled by the autonomic nervous system (for example, cardiovascular,17,18,33,36,39,42,49,51,53,54,60 gastrointestinal,44,46,64 thermoregulatory19,26), (e) suppressed immune function,3,56 and (f) modified behavior.8,17,60 These responses vary with the nature, magnitude, duration, and frequency of exposure of the stressor but also with the sex, strain, and physiologic state of the animal.4,6,9,16,20,30,31,35,38,42,61,63 For example, some strains are more responsive to stressors than are other strains,16,32,44 and female rats generally have greater stress responses than do male rats,2,4,12,20,31,41,61,63 except during lactation or with aging, when some stress responses are blunted.27,30,62
Numerous experimental manipulations produce stress responses in experimental rodents and include physical restraint,22,25,40,42,43 foot or tail shock,1,19 forced swimming,5,28,34,47 adverse social interactions with conspecifics,1,7,8,50 and exposure to noxious agents.23,62 In addition, even routine husbandry and experimental manipulations, some of which are considered benign from a human perspective (for example, transfer to a clean cage, gentle handling), increase blood pressure and heart rate in rats.4,51,53,54
Because stress potentially can degrade the health and wellbeing of research animals and compromise the quality of experimental data, many procedures to reduce or eliminate this variable have been and are being studied. For rodents, these efforts include group housing in standard caging51,53 or in colonies,10 inclusion of enrichment devices in the animal's cage,49,37 and increasing cage size.52 Extending the dark phase of the photocycle has recently been shown to decrease the blood pressure and heart rate of rats.65 Likewise, decreasing ambient illumination may mimic the lighting conditions of the rat's natural burrow environment.
The primary objectives of the present study were to (1) test the hypothesis that housing rats in a dimly illuminated environment would result in decreased heart rate under basal conditions and decreased heart rate responses after various manipulations and (2) confirm and extend previous findings indicating that long nights reduce heart rate. A secondary objective was to determine whether strain or sex influence the effects of the 2 lighting schemes.
Materials and Methods
Adult male and female Holtzman Sprague–Dawley (SD) and spontaneously hypertensive (SHR) rats were purchased from Harlan Sprague-Dawley (Indianapolis, IN) at 175 to 250 g body weight. All rats were obtained from colonies reported to be free from adventitious viruses, Mycoplasma, respiratory and enteric bacteria (except several strains of Helicobacter), and ecto- and endoparasites (except a nonpathogenic commensal protozoa) by the vendor. Potential animal room exposure to infectious agents during the study was evaluated by standard serology screening of blood samples collected at the end of the study from sentinel rats housed in the same room. The results of these assessments showed no differences from the screening results provided by the vendor.
Husbandry during experiment.
The rats were allowed to acclimate to the animal room lighting conditions and husbandry procedures for 2 wk prior to surgical implantation of a radiotelemetry transmitter. Environmental conditions in the animal room were: temperature, 22 to 26 °C; relative humidity, 30% to 60%; lighting, 200 lx at cage level with lights on from 0700 to 1900 (control group) or 10 lx at cage level with lights on 0700 to 1900 (dim light group) or 200 lx at cage level with lights on 0700 to 1500 (long nights group). Temperature, relative humidity, and light level data were recorded at 10-min intervals using an environmental data logging device (HOBO model U12, Onset Computer Corporation, Bourne, MA). Animals were housed individually in conventional solid-bottom polycarbonate cages (nominal floor area, 930 cm2) with standard stainless-steel lids and hardwood chip bedding (depth, 4 to 5 cm; Sanichip, PJ Murphy Forest Products, Montville, NJ). Cages were changed once weekly (Mondays). Pelleted rat chow (Purina 5001, Purina Mills International, Richmond, IN) was provided ad libitum, and tap water was provided in a water bottle with a sipper tube. Rats of both strains were housed in the animal room at the same time, but male and female rats were studied separately.
Surgical procedures.
In preparation for the abdominal and femoral incisions necessary for implantation of the radiotelemetry transmitter, these areas were clipped free of hair, scrubbed with a 7.5% povidone–iodine solution (Betadine Surgical Scrub, Purdue-Frederick Company, Norwalk, CT), and rinsed with sterile 0.9% NaCl. Each transmitter (model TA11PA-C40; Data Sciences International Corporation, St Paul, MN) was implanted aseptically in the abdominal cavity of each rat via a 5- to 6-cm ventral midline incision while the animal was under ketamine (Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (Rompun, Bayer Corporation, Shawnee Mission, KS) anesthesia (80 mg/kg and 7 mg/kg, IP respectively). The catheter attached to the telemetry transmitter was tunneled through abdominal muscle and then subcutaneously to the skin incision in the left femoral triangle and inserted centrally into the femoral artery to a depth of 3.0 cm. The catheter was secured in the artery with sutures and the femoral incision closed. Just prior to closure of the abdominal incision, 20 ml of sterile saline containing 20 mg cefazolin (West-Ward Pharmaceutical, Eatontown, NJ) was flushed into the peritoneal cavity for fluid replacement and to prevent postsurgical infection. We have found that this procedure also eliminates intraabdominal adhesions, which were common before introduction of this peritoneal flush. The telemetry implantation procedure was completed in 30 to 45 min.
The male rats also were given 5 cc of sterile 5% dextrose containing ketoprofen (Ketofen, Fort Dodge Animal Health, Fort Dodge, IA) at 16 mg/kg SC immediately after surgery for analgesia and to provide a short-term glucose supplement. Rats gave no indications that additional doses of ketoprofen were needed. Two groups of female rats were given ketoprofen 30 to 45 min preoperatively at the same dose as male rats, and 1 group was given meloxicam (2 mg/kg SC, Metacam, Vetmedica, St Joseph, MO) preoperatively. The various analgesic protocols were part of a separate study on analgesics and recovery. Rats were placed on clean paper toweling in their home cages immediately after surgery, and the cages were placed on circulating warm water pads until the animals were moving in the cage (approximately 1 to 2 h after surgery), at which point they were placed on telemetry receiver pads on the cage rack.
Postsurgical recovery was monitored by daily visual examination of the incisions and overall condition of the rats, including monitoring signs of pain. Water intake (measured daily), body weight gain (determined every other day), and blood pressure, heart rate, and movement in the cage (measured every 5 min by telemetry beginning about 2 h postsurgery) were used also to assess recovery. Water intake and body weight gain returned to presurgical values by 3 to 5 d after surgery whereas HR, systolic blood pressure, and activity were stable by 7 d.
Animal procedures.
Beginning 11 to 14 d after surgery (25 to 28 d after being placed in the respective lighting conditions), heart rate and blood pressure were monitored at times when no humans were present in the room (for example, 0800 to 0900) and during and for 3 h after exposure to each of several acute procedures. These procedures were selected to be representative of the following functional categories: (1) husbandry procedures (cage change, handling, introduction of a conspecific intruder, removal of a familiar conspecific cage mate); (2) experimental procedures (SC injection, transport and SC injection, IP injection, tail vein injection); and (3) stressful procedures (odors of urine and feces from stressed male or female rats, odor of dried rat blood, prolonged restraint). Six singly housed rats of each strain were subjected to each of the acute procedures at 1000 h on alternate days (Monday, Wednesday, and Friday). The schedule was established to reduce any carryover effects that may exist from one procedure to the next. There were no indications that the rats became conditioned to procedures being conducted on this schedule (that is, no changes in HR at 1000 h on intervening nonexperimental days in the current study, and no differences in HR responses to the same procedure applied on the Monday to Wednesday to Friday schedule for 2 consecutive weeks in a separate study). Over the course of the current study, each rat was subjected to every procedure. Because of the large number of comparisons, only HR data are presented in the Results section.
All procedures were approved by the Wayne State University Institutional Animal Care and Use Committee.
Collection of radiotelemetry data.
Output from telemetry transmitters was collected by using hardware and software from Data Sciences International Corporation (St Paul, MN). Data were sampled for 10 s at 1- or 5-min intervals after the various acute procedures and during undisturbed periods, respectively. These data were saved to the hard drive of a desktop computer and subsequently transferred as spreadsheet files by using Excel (Microsoft Corporation, Redmond, WA) to other computers for summarization and statistical analyses.
Statistical analysis.
The HR data collected during undisturbed periods are expressed as mean ± SEM calculated across the respective time periods (morning, afternoon, and night). The HR responses to the various acute procedures are expressed as the mean ± SEM of the area under the response curve. These data were computed as the sum of changes in HR, relative to the mean value obtained during the 0800 to 0900 undisturbed control period, from the onset of the procedure to the point when the response returned to the mean value obtained from 0800 to 0900. Three-factor analysis of variance followed by the Tukey post hoc test was done (SigmaStat statistical software, Systat Corporation, Point Richmond, CA) to determine whether significant main effects of lighting treatments, strain, or sex and significant interactions between the lighting treatments, strain, and sex (Table 1) were present. This evaluation was followed by comparisons of the lighting treatments within each strain and sex by using 1-actor analysis of variance followed by the Dunnett post hoc test. In addition, comparisons of males and females (within strains and lighting treatments) and comparisons of SD and SHR strains (within sex and lighting treatments) were made by using 1-factor analysis. Means were declared statistically significant at a P value of less than or equal to 0.05.
Table 1.
Results of 3-factor analysis of variance and Tukey post-hoc testing for heart rate of undisturbed SD and SHR rats and their heart rate responses to acute procedures
| Main effects | Interactions | |||||||
| Parameter | Procedure | light | strain | sex | light × strain | light × sex | strain × sex | light × strain × sex |
| Heart rate | ||||||||
| Undisturbed (morning) | L < C | SD > SHR | F > M | SHR: L < C | F: L < C | |||
| D < C | D < C | D < C | ||||||
| D = L | D = L | D = L | ||||||
| SD: L = D = C | M: L < C | |||||||
| D = C | ||||||||
| D = L | ||||||||
| Undisturbed (afternoon) | L < C | SD > SHR | F > M | SHR: L < C | F: L < C | |||
| D < C | D < C | D < C | ||||||
| D = L | D = L | D < L | ||||||
| SD: L < C | M: L < C | |||||||
| D < C | D < C | |||||||
| D = L | L < D | |||||||
| Undisturbed (night) | H | SD > SHR | F > M | F: L < C | H | |||
| D < C | ||||||||
| L = D | ||||||||
| M: L < C | ||||||||
| D < C | ||||||||
| L < D | ||||||||
| Heart rate responses | ||||||||
| Cage change | L = C | SD > SHR | SD: M > F | |||||
| D = C | SHR: M = F | |||||||
| D > L | ||||||||
| Handling | H | H | H | |||||
| Insert cagemate | L = C | SD > SHR | M > F | |||||
| D < C | ||||||||
| D > L | ||||||||
| Remove cagemate | L = C | SD > SHR | F > M | F: L < C | ||||
| D < C | D < C | |||||||
| D = L | D = L | |||||||
| M: L = D = C | ||||||||
| SC injection | L < C | SD > SHR | SD: M > F | |||||
| D = C | SHR: M = F | |||||||
| D > L | ||||||||
| Transport and SC injection | H | H | H | H | H | H | ||
| Tail vein injection | ||||||||
| IP injection | SD > SHR | |||||||
| Odors of male urine and feces | H | SD > SHR | F > M | H | ||||
| Odors of female urine and feces | L < C | SD > SHR | F > M | F: L < C | ||||
| D = C | D < C | |||||||
| D = L | D = L | |||||||
| M: L = D = C | ||||||||
| Odor of dried blood | SD > SHR | F > M | F: L < C | |||||
| D = C | ||||||||
| D = L | ||||||||
| M: L = D = C | ||||||||
| 60 min restraint | L > C | SD > SHR | M > F | F: L = D = C | ||||
| D = C | M: L > C | |||||||
| D < L | D = C | |||||||
| D < L | ||||||||
C, control (12:12-h light:dark photoperiod with 200 lx illumination); D, dim (10 lx) light; F, female; H, a significant light x strain x sex interaction prevented an unambiguous conclusion about indicated main effects and interactions; L, long nights (8:16-h light:dark photoperiod); M, male
Blank cells indicate nonsignificant effects.
Results
Heart rate under undisturbed conditions.
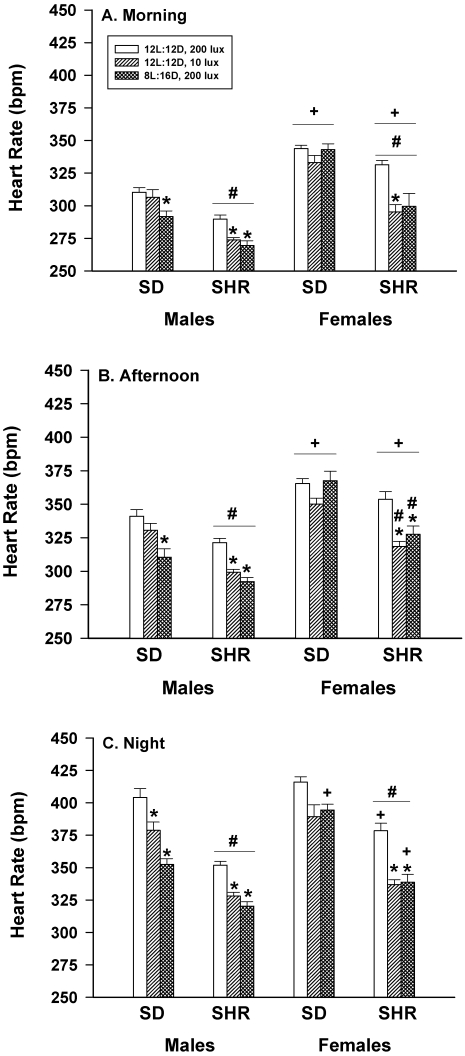
Figure 1 shows that the HR of undisturbed SHR male rats housed under dim lights or long nights were significantly (P < 0.05) lower in the morning (panel A), afternoon (panel B), and night (panel C) compared with those rats housed under control lighting (12:12 h light:dark, 200 lx). The HR of SD male rats during the nocturnal period also was lower during both dim light and long nights, but only long nights reduced HR during the morning and afternoon periods.
Figure 1.
Effect of dim light (10 lx) or long nights (8:16-h light:dark) on the heart rate (beats/min; bpm) of male Sprague–Dawley (SD) and spontaneously hypertensive rats (SHR) during undisturbed periods in the morning (0800 to 0900, panel A), the afternoon (1300 lights off, panel B), and at night (0700 lights off, panel C). *, Significantly (P < 0.05) different from value for 12:12-h light:dark, 200 lx; #, SHR significantly (P < 0.05) different from SD; +, females significantly (P < 0.05) different from males. Each group contained 6 rats.
The HR of undisturbed SD female rats was not significantly affected by housing in dim light or long nights at any of the time periods examined (Figure 1). However, the HR of undisturbed SHR female rats housed in dim light or long nights were significantly (P < 0.05) lower during the afternoon and night compared with those housed under control lighting conditions. The HR of undisturbed SHR female rats housed in dim light, but not in long nights, also were significantly (P < 0.05) lower during the morning compared with that of controls.
Within each lighting treatment, SHR male and female rats had significantly lower HR in the morning and at night than did SD rats (Figure 1). This difference also was observed in the afternoon in the experimental lighting groups but not in the control group. In the morning and afternoon periods, female rats of each strain had significantly higher HR than did their respective male counterparts. This difference also was observed at night in 3 of the 6 groups.
Heart rate responses to acute husbandry procedures.
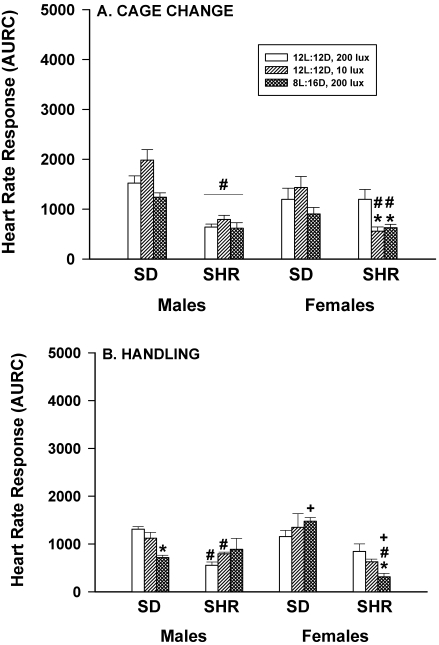
When SD and SHR male and female rats were exposed to 2 common husbandry procedures (cage change and handling; Figure 2 A, B) their HR responses were either unaffected or significantly (P < 0.05) reduced by housing in dim light or long nights, depending on sex and strain. For example, the HR responses of SHR female rats to routine cage change (Figure 2 A) were significantly (P < 0.05) lower under both dim light and long night conditions, compared with controls. However, HR responses of SD female and SD and SHR male rats were not significantly affected by lighting treatments.
Figure 2.
Effect of dim light (10 lx) or long nights (8:16-h light:dark) on heart rate responses (area under response curve; AURC) of male and female Sprague–Dawley (SD) and spontaneously hypertensive rats (SHR) after routine cage change (panel A) or gentle handling for 1 min (panel B). *, Significantly (P < 0.05) different from value for 12:12-h light:dark, 200 lx; #, SHR significantly (P < 0.05) different from SD; +, females significantly (P < 0.05) different from males. Each group contained 6 rats.
Gentle handling (Figure 2 B) was associated with significantly (P < 0.05) lower HR responses in SD males and SHR females housed under dim light, but gentle handling of rats housed under long nights had no effect. In addition, neither dim light nor long nights had any significant effects on the HR responses of SHR males or SD females.
Within all lighting treatments, the HR responses of SHR male rats to cage change were significantly (P < 0.05) less than those of SD males. HR responses of SHR females housed under dim light or long nights were also less than those of SD females. Similar differences between SHR and SD strains were noted in the HR responses to handling. In addition, HR responses of SD female rats to handling were significantly (P < 0.05) greater than those of SD males when rats were housed under long night conditions; whereas, HR responses of SHR female rats to handling were significantly (P < 0.05) smaller than those of SHR males.
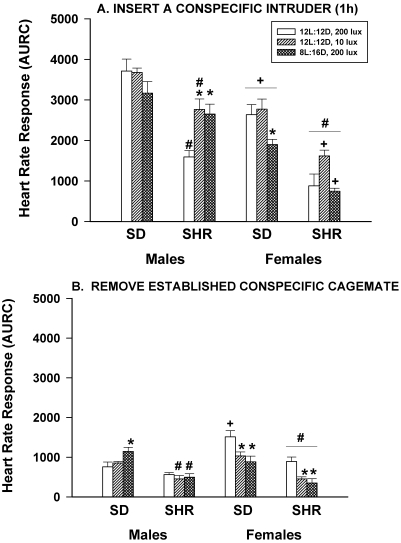
When rats were subjected to husbandry procedures that were potentially more stressful than a routine cage change or gentle handling (for example, insertion of a conspecific intruder and removal of an established conspecific cage mate), HR responses were significantly (P < 0.05) reduced, not significantly (P < 0.05) affected, or significantly (P < 0.05) enhanced by dim light or long nights, depending on the sex and strain. For example, the HR increases of SD males induced by the introduction of an intruder rat were not affected by altering the ambient light intensity or schedule, whereas the HR responses of SHR male rats were significantly (P < 0.05) greater when they were housed in dim light or long nights (Figure 3 A). The HR response of SD female rats was significantly (P < 0.05) reduced by long night but not affected by dim light, and neither lighting scheme altered the HR response of SHR females compared with controls. The HR responses of SHR male and female rats to an intruder were significantly (P < 0.05) less than those of SD rats of the same sex, and female rats of both strains had significantly (P < 0.05) smaller HR responses than did male rats.
Figure 3.
Effect of dim light (10 lx) or long nights (8:16-h light:dark) on heart rate responses (area under response curve; AURC) of male and female Sprague–Dawley (SD) and spontaneously hypertensive rats (SHR) after insertion of a conspecific intruder rat for 1 h (panel A) or removal of an established conspecific cagemate (panel B). *, Significantly (P < 0.05) different from value for 12:12-h light:dark, 200 lx; #, SHR significantly (P < 0.05) different from SD; +, females significantly (P < 0.05) different from males. Each group contained 6 rats.
The removal of an established cage mate induced HR responses in SD male rats that were enhanced by long nights but not affected by dim light (Figure 3 B). In contrast, the HR responses of SHR male rats were not affected by either alteration in lighting. In contrast, the HR responses of both SD and SHR female rats were significantly (P < 0.05) reduced by both dim light and long nights. The HR responses of SHR male and female rats were generally lower than those of SD males and females, respectively.
Heart rate responses to acute experimental procedures.
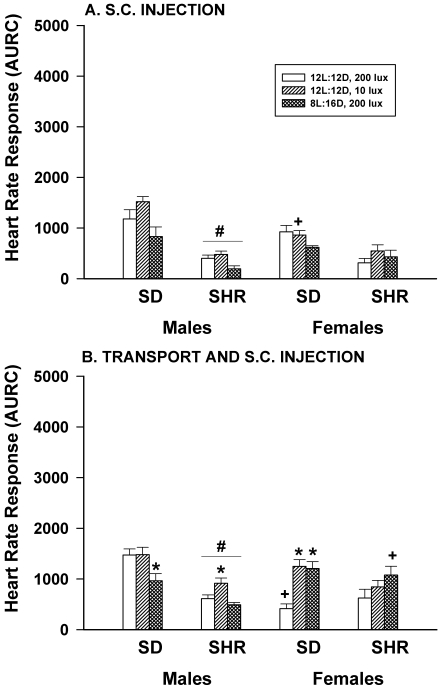
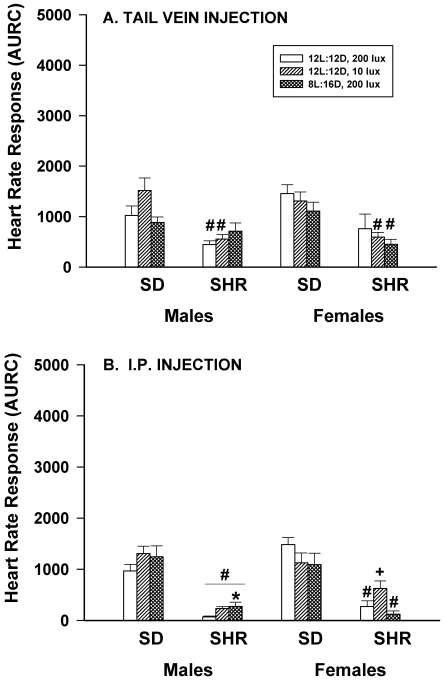
Figure 4 A shows that neither dim light nor long nights affected the HR responses to hand restraint and SC injection of saline performed in the animal room in any of the rats studied. SHR male rats had significantly (P < 0.05) smaller responses than did SD male rats, but this strain effect was not observed in female rats. Male–female differences existed only for SD rats housed in dim light, where female rats had significantly (P < 0.05) smaller responses than did males.
Figure 4.
Effect of dim light (10 lx) or long nights (8:16-h light:dark) on heart rate responses (area under response curve; AURC) of male and female Sprague–Dawley (SD) and spontaneously hypertensive rats (SHR) after hand restraint and SC injection of saline (panel A) or transport in the home cage 100 ft on a cart between the animal housing room and a procedure room plus hand restraint and SC injection of saline (panel B). *, Significantly (P < 0.05) different from value for 12:12-h light:dark, 200 lx; #, SHR significantly (P < 0.05) different from SD; +, females significantly (P < 0.05) different from males. Each group contained 6 rats.
Figure 4 B shows that altered lighting significantly (P < 0.05) affected HR responses to hand restraint and SC injection of saline when the manipulation was performed in a procedure room after transport in the home cage on a cart approximately 100 ft from the animal room and transport back to the animal room. Dim light enhanced the HR responses of SHR male rats but had no significant effect on the responses of SD males. In contrast, dim light enhanced the HR responses of SD female rats but had no effect in SHR females. Long nights significantly (P < 0.05) reduced the HR response of SD male rats but significantly (P < 0.05) enhanced the response in SD females. In contrast, long nights had no significant (P < 0.05) effects on the HR responses of SHR male or female rats. A significant (P < 0.05) strain effect was present in males (SHR < SD) but not in females, and significant (P < 0.05) male–female differences were present only for SD rats housed under control conditions and SHR rats housed under long night conditions.
HR responses to hand restraint and tail vein injection of saline performed in the animal room were not significantly (P < 0.05) affected by altered lighting (Figure 5 A), and only long nights significantly (P < 0.05) reduced the HR response of SHR male rats to IP injection of saline performed in the animal room (Figure 5 B). In general, SHR male and female rats had significantly (P < 0.05) lower responses to both procedures than did SD males and females. There were no significant effects of sex on the responses to tail vein injection (Figure 5A) and the only significant sex effect for responses to i.p. injection was for SHR rats housed under dim light conditions where females had significantly higher HR than males (Figure 5 B).
Figure 5.
Effect of dim light (10 lx) or long nights (8:16-h light:dark) on heart rate responses (area under response curve; AURC) of male and female Sprague–Dawley (SD) and spontaneously hypertensive rats (SHR) after hand restraint and tail vein injection of saline (panel A) or hand restraint and IP injection of saline (panel B). *, Significantly (P < 0.05) different from value for 12:12-h light:dark, 200 lx; #, SHR significantly (P < 0.05) different from SD; +, females significantly (P < 0.05) different from males. Each group contained 6 rats.
Heart rate responses to acute stressful procedures.
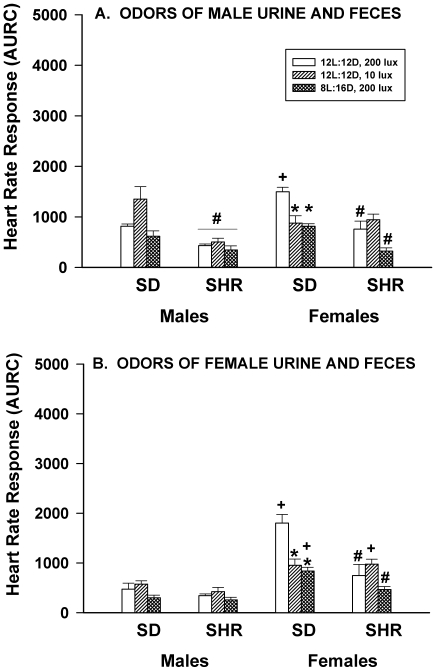
When rats were exposed to the odors of urine and feces from male or female rats stressed by being subjected to a novel environment for 5 minutes (a paper towel covered with dried urine and feces placed on the cage lid for 15 min), the HR responses of SD and SHR male rats were not altered by dim light or long nights. In contrast, the HR responses of SD, but not SHR, female rats were significantly (P < 0.05) reduced by both dim light and long nights (Figure 6 A, B). In response to male urine and feces, SHR male rats had significantly (P < 0.05) lower responses than did SD males. A similar strain difference was noted among female rats. Female SD rats housed under control lighting conditions showed a greater response to the odors of male urine and feces than did SD males housed under similar lighting conditions. When exposed to the odors of female urine and feces, male SD and SHR rats showed similar HR responses, but those of SHR females were significantly (P < 0.05) greater than those of SHR males housed under control and long night conditions. Sex-associated differences occurred in the SD strain, with female rats housed in control and long night conditions having greater HR responses than males. Female SHR rats also had significantly (P < 0.05) greater HR responses than did males when housed in dim light conditions.
Figure 6.
Effect of dim light (10 lx) or long nights (8:16-h light:dark) on heart rate responses (area under response curve; AURC) of male and female Sprague–Dawley (SD) and spontaneously hypertensive rats (SHR) after exposure to a paper towel coated with dried urine and feces from stressed male rats (panel A) or female rats (panel B) that was placed on the cage lid for 15 min. *, Significantly (P < 0.05) different from value for 12:12-h light:dark, 200 lx; #, SHR significantly (P < 0.05) different from SD; +, females significantly (P < 0.05) different from males. Each group contained 6 rats.
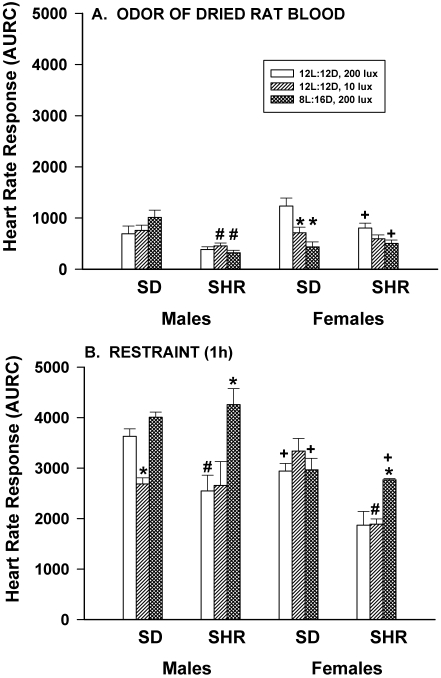
Figure 7 A shows HR responses to the odor of dried rat blood (a paper towel covered with rat blood placed on the cage lid for 15 min). Lighting conditions did not alter the HR responses of SD or SHR male rats, but dim light significantly (P < 0.05) reduced the HR responses of SD, but not SHR, female rats. The responses of SHR male rats housed in dim light and long nights were significantly (P < 0.05) less than those of SD males housed similarly, but there were no significant strain-associated differences in the responses of female rats. No sex-associated differences were present in SD rats, but SHR females housed under control or long night conditions had significantly (P < 0.05) greater HR responses than did SHR males.
Figure 7.
Effect of dim light (10 lx) or long nights (8:16-h light:dark) on heart rate responses (area under response curve; AURC) of male and female Sprague–Dawley (SD) and spontaneously hypertensive rats (SHR) after exposure to a paper towel coated with dried rat blood that was placed on the cage lid for 15 min (panel A) or 1 h of confinement in a clear acrylic rat restrainer placed in the home cage (panel B). *, Significantly (P < 0.05) different from value for 12:12-h light:dark, 200 lx; #, SHR significantly (P < 0.05) different from SD; +, females significantly (P < 0.05) different from males. Each group contained 6 rats.
The HR responses to 1 h of physical restraint are shown in Figure 7 B. Male SD rats housed in dim light, but not long nights, had significantly (P < 0.05) lower HR responses than did those housed under control lighting conditions. In contrast, the HR responses of SHR male rats were not changed by dim light but were enhanced by long night conditions, relative to control values. This same pattern was present in SHR female rats. The HR responses of SD female rats were not affected by dim light or long nights. Minimal strain effects were observed with restraint; SHR male rats housed under control conditions and SHR female rats housed in dim light had significantly (P < 0.05) lower HR responses than did SD rats housed in comparable lighting conditions. Female rats had significantly (P < 0.05) lower HR responses compared with those of male rats when animals were housed under control, dim light (SD), or long night (SHR) conditions.
Discussion
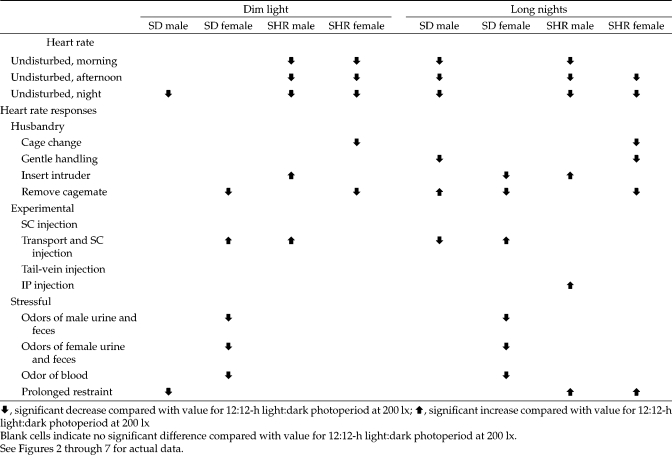
As summarized in Table 2, the current results show that housing rats under dim light or long night conditions significantly (P < 0.05) reduced HR of SD male and SHR male and female rats during the day and at night when they were undisturbed, compared with rats housed under 12:12-h light:dark, 200 lx conditions. In contrast, HR of undisturbed SD female rats was not affected by these altered lighting schemes. The current results also show that when rats housed under dim light or long nights were acutely subjected to husbandry, experimental, or stressful procedures, HR responses to only some of these procedures were reduced. Responses to other procedures were not affected by alterations in ambient lighting, and the responses to still others were significantly (P < 0.05) enhanced. Both HR during undisturbed conditions and HR responses after acute procedures varied with the strain and sex of the rats. Male rats had lower HR than did females, and SHR rats had lower HR than did SD rats when animals were undisturbed. Male and female rats of both strains generally had equivalent HR responses to acute procedures—the exceptions being the introduction of an intruder and restraint, after which males had greater HR responses than did females, and odors of female rat urine and feces and odor of rat blood, after which female rats had greater responses than did males. SHR rats generally had lower HR responses than did SD rats.
Table 2.
Summary of effects of dim light (10 lx) or long nights (8:16-h light:dark photoperiod) on undisturbed heart rate during the morning, afternoon, and night and on heart rate responses to husbandry, experimental, and stressful procedures performed in the morning
 |
That long nights reduced HR in both SD and SHR males and SHR females during periods of the day and night when they were undisturbed is in agreement with a previous report.63 However, the current data are the first to our knowledge that demonstrate that low-level illumination suppresses HR, although anxiety scores after elevated plus-maze testing were reduced by housing rats in dim light.13
The decreasing effect of altered lighting on HR in undisturbed animals is consistent with the possibility that rats housed in 12:12-h light:dark at 200 lx are stressed to some degree. The HR effect of altered lighting in undisturbed animals is consistent with altered lighting causing an increase in parasympathetic and/or a decrease in sympathetic tonus to the heart. However, these possibilities were not directly tested in the current study. The pharmacologic determination of parasympathetic and sympathetic tonus to the heart of rats housed in 12-h light:dark at 200 lx, dim light or 8:16-h light:dark as described previously11 would help resolve these questions.
The significant interactions between the light treatments and strain during undisturbed periods (Table 1) indicate that the HR of SHR rats was reduced more by altered light than was that of SD rats. This difference is interesting in view of the fact that SHR rats had significantly lower HR than did SD in the morning and at night when animals were housed under control lighting conditions (Figure 1 A and C). That altered lighting reduced HR in rats with an already decreased HR suggests that SHR rats may be more sensitive to alterations in light intensity or schedule than are SD rats or that SHR rats increase parasympathetic tone or reduce sympathetic tone to the heart to a greater degree than do SD rats. Although these possibilities were not tested directly in the current study, others11 have shown that after acute exercise, HR in male and female SHR rats is reduced, with exercise reducing both sympathetic and parasympathetic tone to the heart but having a greater effect on sympathetic tone.
The current results clearly indicate that alterations in environmental lighting did not universally reduce HR responses (Figures 2 through 7, Table 2). Instead, the effects appeared to be related to the type of procedure to which rats were exposed as well as to the strain and sex of the rats.
The variable effects of altered lighting across procedures may have been due in part to differences in the sensory inputs initiated by the different procedures. For example, responses to odors of urine and feces from stressed rats and the odor of dried rat blood, all of which activate the olfactory system, were decreased by altered light, but only in SD female rats. In contrast, the injection procedures, which activate somatosensory pathways, were minimally affected by altered lighting, but when effects were present they were enhanced. Responses to some of the husbandry procedures (cage change and handling) that involved novelty or somatic sensory stimuli were reduced, but primarily in SHR female rats. Responses to procedures that involve social stimuli (introduction of conspecific intruder and removal of an established conspecific cagemate) were increased in male rats but decreased or ineffective in female rats’ HR responses to prolonged physical restraint and were affected very little by the altered lighting schemes, but in the few instances when responses was altered, they usually were enhanced. This pattern suggests 2 possibilities: (1) responses to definitive stressors are not reduced by dim light or long nights and (2) the HR responses to prolonged restraint are so robust that they cannot be easily reduced by environmental changes.
The significant interaction between lighting treatments and sex with regard to HR responses to acute procedures (Table 1) suggests that female rats were affected more by altered light than were male rats. Further, reduction of HR responses by the lighting schemes occurred more often in female rats than in males, whereas light-related enhancements occurred more frequently in male rats than in females (Table 2). The mechanisms involved in these sex-related differences are unknown, but one can speculate that gonadal hormones play a role. Additional studies using gonadectomized and gonadectomized, steroid-replaced rats are needed to appropriately address the sex-associated differences in HR response.
Despite differences in the nature of the 2 ambient lighting schemes, the effects of dim light and long nights were similar. The pattern of effects (Table 2) indicates that long nights produced a few more changes than did dim light but that the effects, when present, were generally in the same direction. At the onset of the study, we hypothesized that both lighting treatments would reduce HR and HR responses because these light levels mimic the natural burrow environment of the species in the wild or because they are light conditions that a nocturnal species might prefer over the typical animal room conditions of a 12:12-h light:dark schedule with 200 lx illumination. This hypothesis is supported by other work,13,63 which shows that similar alterations in light reduced HR and blood pressure or anxiety scores. Although the dim light treatment (12 h of late dusk or early dawn and 12 h of total darkness) may mimic the lighting condition of a rat burrow, the animals may perceive a 8:16-h light:dark schedule with 200 lx illumination as a seasonal change because this light condition mimics a bright day in the middle of winter but without the cold temperatures. Although the current results generally support our original hypothesis, additional studies are required to examine the effects of different light intensities and of other, more natural, lighting schedules on HR responses. In addition, the effects of light should be studied at different ambient temperatures, because recent studies have shown that ambient temperature has a significant effect on HR and blood pressure in rats58 and on the balance of parasympathetic and sympathetic nerve activity in mice.57
Finally, cardiovascular or other responses to experimental manipulations may be affected by reduced lighting or altered photocycle in other rat strains or in other rodent species, and these possibilities should be investigated.
References
- 1.Adams N, Lins MD, Blizard DA. 1987. Contrasting effects of social stress and foot-shock on acute cardiovascular response in salt-sensitive rats. Behav Neural Biol 48:368–381 [DOI] [PubMed] [Google Scholar]
- 2.Aloisi AM, Steenbergen HL, van de Poll NE, Farabollini F. 1994. Sex-dependent effects of restraint on nociception and pituitary–adrenal hormones in the rat. Physiol Behav 55:789–793 [DOI] [PubMed] [Google Scholar]
- 3.Amat J, Torres AR, Lechin F. 1993. Differential effect of footshock stress on humoral and cellular immune responses of the rat. Life Sci 53:315–322 [DOI] [PubMed] [Google Scholar]
- 4.Azar T, Sharp J, Lawson D. 2005. Stress-like cardiovascular responses to common procedures in male versus female spontaneously hypertensive rats. Contemp Top Lab Anim Sci 44:25–30 [PubMed] [Google Scholar]
- 5.Bargiel Z, Nowicka H, Wojcikowska J. 1981. Swim-stress–induced changes of rat adrenal catecholamine level depending on metabolic state and different ambient temperature. Folia Histochem Cytochem (Krakow) 19:31–37 [PubMed] [Google Scholar]
- 6.Beck KD, Luine VN. 2002. Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiol Behav 75:661–673 [DOI] [PubMed] [Google Scholar]
- 7.Berton O, Aguerre S, Sarrieau A, Mormede P, Chaouloff F. 1998. Differential effects of social stress on central serotonergic activity and emotional reactivity in Lewis and spontaneously hypertensive rats. Neuroscience 82:147–159 [DOI] [PubMed] [Google Scholar]
- 8.Blanchard RJ, McKittrick CR, Blanchard DC. 2001. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol Behav 73:261–271 [DOI] [PubMed] [Google Scholar]
- 9.Brown KJ, Grunberg NE. 1995. Effects of housing on male and female rats: crowding stresses males but calm females. Physiol Behav 58:1085–1089 [DOI] [PubMed] [Google Scholar]
- 10.Caplea A, Seachrist D, Dunphy G, Ely D. 2000. SHR Y chromosome enhances the nocturnal blood pressure in socially interacting rats. Am J Physiol Heart Circ Physiol 279:H58–H66 [DOI] [PubMed] [Google Scholar]
- 11.Chandler MP, DiCarlo SE. 1998. Acute exercise and gender alter cardiac autonomic tonus differently in hypertensive and normotensive rats. Am J Physiol 274:R510–R516 [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Chandler MP, DiCarlo SE. 1997. Daily exercise and gender influence postexercise cardiac autonomic responses in hypertensive rats. Am J Physiol 272:H1412–H1418 [DOI] [PubMed] [Google Scholar]
- 13.Cosquer B, Galani R, Kuster N, Cassel JC. 2005. Whole-body exposure to 2.45-GHz electromagnetic fields does not alter anxiety responses in rats: a plus-maze study including test validation. Behav Brain Res 156:65–74 [DOI] [PubMed] [Google Scholar]
- 14.Day TA, West MJ, Willoughby JO. 1983. Stress suppression of growth hormone secretion in the rat: effects of disruption of inhibitory noradrenergic afferents to the median eminence. Aust J Biol Sci 36:525–530 [DOI] [PubMed] [Google Scholar]
- 15.De Boer SF, Koopmans SJ, Slangen JL, Van der Gugten J. 1990. Plasma catecholamine, corticosterone, and glucose responses to repeated stress in rats: effect of interstressor interval length. Physiol Behav 47:1117–1124 [DOI] [PubMed] [Google Scholar]
- 16.Dhabhar FS, McEwen BS, Spencer RL. 1993. Stress response, adrenal steroid receptor levels, and corticosteroid-binding globulin levels: a comparison between Sprague–Dawley, Fischer 344, and Lewis rats. Brain Res 616:89–98 [DOI] [PubMed] [Google Scholar]
- 17.Dielenberg RA, Carrive P, McGregor IS. 2001. The cardiovascular and behavioral response to cat odor in rats: unconditioned and conditioned effects. Brain Res 897:228–237 [DOI] [PubMed] [Google Scholar]
- 18.Ely DL, Friberg P, Nilsson H, Folkow B. 1985. Blood pressure and heart rate responses to mental stress in spontaneously hypertensive (SHB) and normotensive (WKY) rats on various sodium diets. Acta Physiol Scand 123:159–169 [DOI] [PubMed] [Google Scholar]
- 19.Endo Y, Shiraki K. 2000. Behavior and body temperature in rats following chronic foot shock or psychological stress exposure. Physiol Behav 71:263–268 [DOI] [PubMed] [Google Scholar]
- 20.Faraday MM, Blakeman KH, Grunberg NE. 2005. Strain and sex alter effects of stress and nicotine on feeding, body weight, and HPA axis hormones. Pharmacol Biochem Behav 80:577–589 [DOI] [PubMed] [Google Scholar]
- 21.Gala RR. 1990. The physiology and mechanisms of the stress-induced changes in prolactin secretion in the rat. Life Sci 46:1407–1420 [DOI] [PubMed] [Google Scholar]
- 22.Gala RR, Haisenleder DJ. 1986. Restraint stress decreases afternoon plasma prolactin levels in female rats. Influence of neural antagonists and agonists on restraint-induced changes in plasma prolactin and corticosterone. Neuroendocrinology 43:115–123 [DOI] [PubMed] [Google Scholar]
- 23.Goodman GT, Lawson DM. 1977. The influence of long-term estrogen treatment on plasma prolactin levels induced by ether anesthesia in ovariectomized rats. Experientia 33:536–537 [DOI] [PubMed] [Google Scholar]
- 24.Guo AL, Petraglia F, Criscuolo M, Ficarra G, Nappi RE, Palumbo M, Valentini A, Genazzani AR. 1994. Acute stress- or lipopolysaccharide-induced corticosterone secretion in female rats is independent of the oestrous cycle. Eur J Endocrinol 131:535–539 [DOI] [PubMed] [Google Scholar]
- 25.Guth PH, Mendick R. 1964. The effect of chronic restraint stress on gastric ulceration in the rat. Gastroenterology 46:285–286 [PubMed] [Google Scholar]
- 26.Harkin A, Connor TJ, O'Donnell JM, Kelly JP. 2002. Physiological and behavioral responses to stress: what does a rat find stressful? Lab Anim (NY) 31:42–50 [DOI] [PubMed] [Google Scholar]
- 27.Higuchi T, Negoro H, Arita J. 1989. Reduced responses of prolactin and catecholamine to stress in the lactating rat. J Endocrinol 122:495–498 [DOI] [PubMed] [Google Scholar]
- 28.Jodar L, Takahashi M, Kaneto H. 1995. Effects of footshock, psychological, and forced-swimming stress on the learning and memory processes: involvement of opioidergic pathways. Jpn J Pharmacol 67:143–147 [DOI] [PubMed] [Google Scholar]
- 29.Kant GJ, Lenox RH, Bunnell BN, Mougey EH, Pennington LL, Meyerhoff JL. 1983. Comparison of stress response in male and female rats: pituitary cyclic AMP and plasma prolactin, growth hormone, and corticosterone. Psychoneuroendocrinology 8:421–428 [DOI] [PubMed] [Google Scholar]
- 30.Kehoe L, Janik J, Callahan P. 1992. Effects of immobilization stress on tuberoinfundibular dopaminergic (TIDA) neuronal activity and prolactin levels in lactating and nonlactating female rats. Life Sci 50:55–63 [DOI] [PubMed] [Google Scholar]
- 31.Klein SL, Lambert KG, Durr D, Schaefer T, Waring RE. 1994. Influence of environmental enrichment and sex on predator stress response in rats. Physiol Behav 56:291–297 [DOI] [PubMed] [Google Scholar]
- 32.Knardahl S, Hendley ED. 1990. Association between cardiovascular reactivity to stress and hypertension or behavior. Am J Physiol 259:H248–H257 [DOI] [PubMed] [Google Scholar]
- 33.Kvetnansky R, McCarty R, Thoa NB, Lake CR, Kopin IJ. 1979. Sympathoadrenal responses of spontaneously hypertensive rats to immobilization stress. Am J Physiol 236:H457–H462 [DOI] [PubMed] [Google Scholar]
- 34.Lang RE, Heil JW, Ganten D, Hermann K, Unger T, Rascher W. 1983. Oxytocin, unlike vasopressin, is a stress hormone in the rat. Neuroendocrinology 37:314–316 [DOI] [PubMed] [Google Scholar]
- 35.Lawler JE, Abel MM, Naylor SK. 1993. Effects of salt intake on blood pressure and heart rate responses to footshock stress in SHR, BHR, and WKY rats. Physiol Behav 53:97–102 [DOI] [PubMed] [Google Scholar]
- 36.Lawler JE, Cox RH, Hubbard JW, Mitchell VP, Barker GF, Trainor WP, Sanders BJ. 1985. Blood pressure and heart rate responses to environmental stress in the spontaneously hypertensive rat. Physiol Behav 34:973–976 [DOI] [PubMed] [Google Scholar]
- 37.Lawson DM, Churchill M, Churchill PC. 2000. The effects of housing enrichment on cardiovascular parameters in spontaneously hypertensive rats. Contemp Top Lab Anim Sci 39:9–13 [PubMed] [Google Scholar]
- 38.Lemaire V, Mormede P. 1995. Telemetered recording of blood pressure and heart rate in different strains of rats during chronic social stress. Physiol Behav 58:1181–1188 [DOI] [PubMed] [Google Scholar]
- 39.Li SG, Lawler JE, Randall DC, Brown DR. 1997. Sympathetic nervous activity and arterial pressure responses during rest and acute behavioral stress in SHR versus WKY rats. J Auton Nerv Syst 62:147–154 [DOI] [PubMed] [Google Scholar]
- 40.Ling S, Jamali F. 2003. Effect of cannulation surgery and restraint stress on the plasma corticosterone concentration in the rat: application of an improved corticosterone HPLC assay. J Pharm Pharm Sci 6:246–251 [PubMed] [Google Scholar]
- 41.Livezey GT, Miller JM, Vogel WH. 1985. Plasma norepinephrine, epinephrine and corticosterone stress responses to restraint in individual male and female rats and their correlations. Neurosci Lett 62:51–56 [DOI] [PubMed] [Google Scholar]
- 42.McDougall SJ, Paull JR, Widdop RE, Lawrence AJ. 2000. Restraint stress: differential cardiovascular responses in Wistar-Kyoto and spontaneously hypertensive rats. Hypertension 35:126–129 [DOI] [PubMed] [Google Scholar]
- 43.Morehead MH, Gala RR. 1987. Restraint stress depresses prolactin surges in pseudopregnant rats and adrenalectomy does not alter the response. Life Sci 41:1491–1498 [DOI] [PubMed] [Google Scholar]
- 44.Pare WP. 1989. Stress ulcer and open-field behavior of spontaneously hypertensive, normotensive, and Wistar rats. Pavlov J Biol Sci 24:54–57 [DOI] [PubMed] [Google Scholar]
- 45.Randall DC, Brown DR, Brown LV, Kilgore JM. 1994. Sympathetic nervous activity and arterial blood pressure control in conscious rat during rest and behavioral stress. Am J Physiol 267:R1241–R1249 [DOI] [PubMed] [Google Scholar]
- 46.Ray A, Sullivan RM, Henke PG. 1987. Adrenergic modulation of gastric stress pathology in rats: a cholinergic link. J Auton Nerv Syst 20:265–268 [DOI] [PubMed] [Google Scholar]
- 47.Salman H, Bergman M, Weizman A, Bessler H, Weiss J, Straussberg R, Djaldetti M. 2000. Effect of diazepam on the immune response of rats exposed to acute and chronic swim stress. Biomed Pharmacother 54:311–315 [DOI] [PubMed] [Google Scholar]
- 48.Seggie JA, Brown GM. 1975. Stress response patterns of plasma corticosterone, prolactin, and growth hormone in the rat following handling or exposure to novel environment. Can J Physiol Pharmacol 53:629–637 [DOI] [PubMed] [Google Scholar]
- 49.Sharp J, Azar T, Lawson D. 2005. Effects of a cage-enrichment program on heart rate, blood pressure, and activity of male Sprague–Dawley and spontaneously hypertensive rats monitored by radiotelemetry. Contemp Top Lab Anim Sci 44:32–40 [PubMed] [Google Scholar]
- 50.Sharp J, Azar T, Lawson D. 2005. Selective adaptation of male rats to repeated social encounters and experimental manipulations. Contemp Top Lab Anim Sci 44:28–31 [PubMed] [Google Scholar]
- 51.Sharp J, Zammit T, Azar T, Lawson D. 2003. Stress-like responses to common procedures in individually and group-housed female rats. Contemp Top Lab Anim Sci 42:9–18 [PubMed] [Google Scholar]
- 52.Sharp JL, Azar TA, Lawson DM. 2005. Does cage size affect heart rate and blood pressure of male rats at rest or after procedures that induce stress-like responses? Contemp Top Lab Anim Sci 42:8–12 [PubMed] [Google Scholar]
- 53.Sharp JL, Zammit TG, Azar TA, Lawson DM. 2002. Stress-like responses to common procedures in male rats housed alone or with other rats. Contemp Top Lab Anim Sci 41:8–14 [PubMed] [Google Scholar]
- 54.Sharp JL, Zammit TG, Lawson DM. 2002. Stress-like responses to common procedures in rats: effect of the estrous cycle. Contemp Top Lab Anim Sci 41:15–22 [PubMed] [Google Scholar]
- 55.Smith SW, Gala RR. 1977. Influence of restraint on plasma prolactin and corticosterone in female rats. J Endocrinol 74:303–314 [DOI] [PubMed] [Google Scholar]
- 56.Stefanski V, Engler H. 1998. Effects of acute and chronic social stress on blood cellular immunity in rats. Physiol Behav 64:733–741 [DOI] [PubMed] [Google Scholar]
- 57.Swoap SJ, Li C, Wess J, Parsons AD, Williams TD, Overton JM. 2008. Vagal tone dominates autonomic control of mouse heart rate at thermoneutrality. Am J Physiol Heart Circ Physiol 294:H1581–H1588 [DOI] [PubMed] [Google Scholar]
- 58.Swoap SJ, Overton JM, Garber G. 2004. Effect of ambient temperature on cardiovascular parameters in rats and mice: a comparative approach. Am J Physiol Regul Integr Comp Physiol 287:R391–R396 [DOI] [PubMed] [Google Scholar]
- 59.Vachon P, Moreau JP. 2001. Serum corticosterone and blood glucose in rats after two jugular vein blood sampling methods: comparison of the stress response. Contemp Top Lab Anim Sci 40:22–24 [PubMed] [Google Scholar]
- 60.van den Buuse M, Van Acker SA, Fluttert M, De Kloet ER. 2001. Blood pressure, heart rate, and behavioral responses to psychological ‘novelty’ stress in freely moving rats. Psychophysiology 38:490–499 [DOI] [PubMed] [Google Scholar]
- 61.Weinstock M, Razin M, Schorer-Apelbaum D, Men D, McCarty R. 1998. Gender differences in sympathoadrenal activity in rats at rest and in response to footshock stress. Int J Dev Neurosci 16:289–295 [DOI] [PubMed] [Google Scholar]
- 62.Wiggins C, Ratner A, Wise PM. 1983. Differences in the stress response of prolactin in young and aged female rats. Life Sci 32:1911–1917 [DOI] [PubMed] [Google Scholar]
- 63.Williams TD, Carter DA, Lightman SL. 1985. Sexual dimorphism in the posterior pituitary response to stress in the rat. Endocrinology 116:738–740 [DOI] [PubMed] [Google Scholar]
- 64.Xie YF, Jiao Q, Guo S, Wang FZ, Cao JM, Zhang ZG. 2005. Role of parasympathetic overactivity in water-immersion stress-induced gastric mucosal lesion in rat. J Appl Physiol 99:2416–2422 [DOI] [PubMed] [Google Scholar]
- 65.Zhang BL, Zannou E, Sannajust F. 2000. Effects of photoperiod reduction on rat circadian rhythms of BP, heart rate, and locomotor activity. Am J Physiol Regul Integr Comp Physiol 279:R169–R178 [DOI] [PubMed] [Google Scholar]
- 66.Zhang W, Thoren P. 1998. Hyper-responsiveness of adrenal sympathetic nerve activity in spontaneously hypertensive rats to ganglionic blockade, mental stress, and neuronglucopenia. Pflugers Arch 437:56–60 [DOI] [PubMed] [Google Scholar]