Abstract
The sudden onset of unilateral blepharospasm and hyphema, without evidence of corneal damage, initiated a thorough diagnostic work-up of an 11-wk-old purpose-bred intact male domestic shorthair kitten. Secondary acute anterior uveitis and hyphema were most likely due to trauma within the primary enclosure.
Anterior uveitis occurs frequently in random-source cats, but tends not to be seen as often in purpose-bred cats unexposed to common feline pathogens and living in an environment protected from outdoor physical dangers. Clinical signs associated with this type of ocular inflammation are variable and range from visible exudates in the anterior chamber to a reddened and painful eye.12,23,28 Causes of uveal tract inflammation can be infectious or noninfectious and may arise directly from the eye or by way of a systemic mechanism.10,23 The following report describes the clinical signs in a vendor-raised kitten that developed a sudden case of anterior uveitis, the prescribed treatments, and diagnostics used to attempt to uncover an etiology. A presumptive traumatic cause remains the most likely explanation for this animal's diagnosis of secondary acute anterior uveitis with hyphema.
Case Report
Twenty-eight 7-wk-old domestic shorthair kittens were obtained from a commercial feline vendor for an IACUC-approved study of feline immunodeficiency virus pathogenesis. Vendor health summaries indicated that cat colonies were seronegative for feline immunodeficiency virus, feline infectious peritonitis, feline leukemia virus, calicivirus, herpesvirus, coronavirus, panleukopenia virus, syncytical virus, Toxoplasma gondii, and Chlamydia psittaci. At the request of the principal investigator, animals were not vaccinated. Kittens were assigned to pens of 7 each. Covered pens were constructed of galvanized fencing measuring 58 × 127 × 76 in., with solid stainless steel panels on the bottom half of the side panels and a sliding latched gate for entrance. Food and water bowls and litter boxes were placed on the concrete floor, and airline crates were used to provide both hiding places and elevated resting surfaces. Kittens were given a physical exam on arrival, and no abnormalities were noted. Research technicians played with the kittens every day for socialization and acclimation purposes, but did not detect problems with any of the kittens’ eyes.
Four weeks into the acclimation period (prior to initiation of the research protocol), one 1.3-kg energetic intact male kitten was reported to have hyphema and blepharospasm in the left eye. Marked hemorrhage in the ventral anterior chamber nearly obscured the miotic pupil, and the iris was a deeper yellow color than the pale yellow-green of the right eye. Ocular discharge was absent. No obvious foreign body or corneal scratch was noted grossly, and there was no fluorescein dye uptake by the cornea. The right eye appeared normal. Facial features were symmetrical, and there was no facial or periorbital swelling or tenderness. The physical exam was normal otherwise.
Biomicroscopic examination of the left eye with a portable slit lamp (SL-15, Kowa Optimed, Torrance, CA) revealed diffuse hemorrhage and a fibrin clot in the anterior chamber. Lens position and pupillary light responses were normal, and the withdrawal response to bright light directed into the eye (that is, the dazzle reflex) was present in both eyes. The menace response was present in the right eye but absent in the left eye. After a couple of hours, the hyphema appeared to clot in the anterior chamber and then bleed again during restraint. Because of the spasm of the iris muscle, as evidence by the miotic pupil, the clinical diagnosis was secondary acute anterior uveitis with hyphema.
Trauma was the suspected etiology in this case. However, given the nature of the research and potential health implications for the colony, diagnostics were performed to rule out other causes, such as immune-mediated, coagulopathy, infection, systemic hypertension, trauma, congenital ciliary muscle defect, toxicity, and idiopathic.
Blood was drawn for a hemogram, serum chemistry panel, and coagulation profile. After the 25-gauge butterfly needle was removed, the blood clotted quickly. A clot formed on the side of the sodium citrate tube for the coagulation profile, making the sample unusable but suggesting adequate coagulation function. The decision was made not to stress the kitten further with a repeat blood draw, as coagulopathy was unlikely in this case in light of a normal platelet count and packed cell volume and lack of epistaxis and petechiae.
The results of the initial and follow-up hemogram were compared with those published for kittens and were interpreted to be normal for a kitten at this age with the exception of a stress leukogram and increased alkaline phosphatase due to probable individual elevation of osseous alkaline phosphatase isoenzyme.4,7,13,27
To rule out arterial hypertension-induced hyphema, serial blood pressures were obtained (oscillometric Cardell Veterinary Monitor 9401 BP, Sharn Veterinary, Tampa, FL) and a 3- to 5-cm inflation cuff (6V1, size 1, Sharn Veterinary) wrapped with the sensor placed over the median artery and then over the brachial artery of the forelimb. Because the kitten was uncooperative, the readings were considered to be unreliable. The procedure was repeated 2 weeks later in a quieter environment, with time allowed for the kitten to acclimate to the staff and equipment. The sensor was placed over the median caudal artery of the tail. While the kitten was calm, 4 readings were taken, which averaged 134 mm Hg systolic and 93 mm Hg diastolic, with a mean arterial pressure of 108 mm Hg and heart rate of 156 beats per minute. These values were less than those consistent with hypertension, that is, exceeding 170 mm Hg systolic and 100 mm Hg diastolic or 150 mm Hg systolic and 95 mm Hg diastolic.17,25
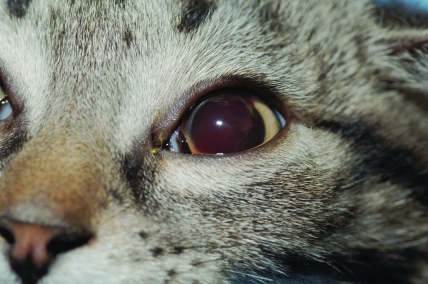
After initial examinations, the affected eye was treated with 1 drop of 1% prednisolone acetate ophthalmic suspension (Pacific Pharma, Irvine, CA) every 6 h and 1% atropine sulfate ophthalmic eyedrops (Fougera, Melville, NY) every 12 h; meloxicam (0.25 mg; Boehringer Ingelheim Vetmedica, St Joseph, MO) was given orally once daily. The next morning, the eye was improved (Figure 1). Much of the hyphema was resorbed and appeared to have created a vertical fibrin clot over the pupil. The pupil could be visualized through the blood-tinged aqueous humor, and the blepharospasm had subsided. There was no uptake of fluorescein dye by the cornea.
Figure 1.
Photograph 1 d after presentation of kitten with hyphema in anterior chamber. Pupil is dilated due to atropine sulfate ophthalmic eye drops given to relieve pain from ciliary spasm. Oral meloxicam and prednisolone acetate ophthalmic suspension were administered also.
Tonometric measurements to rule out glaucoma were made with a handheld tonometer (Tono-Pen Vet Applanation Tonometer, Reichert, Depew, NY) after application of 1 drop of 0.5% proparacaine hydrochloride (Alcon Laboratories, Fort Worth, TX). Intraocular pressures were within normal limits: 22 mm Hg in the left eye and 19 mm Hg in the right eye (reference range, 9 to 31 mm Hg).16 A fundic examination could not be performed on the affected eye due to the remaining hyphema, but examination of the right eye showed the fundus to be within normal limits with no retinal hemorrhage. A direct pupillary light reflex could not be performed in the affected eye due to the mydriasis from the atropine administration (Figure 2), but the reflex was normal in the right eye. When light was flashed in the left eye, a consensual pupillary light response was seen in the right eye. The dazzle reflex was present in both eyes.
Figure 2.
Photograph of affected eye 3 d after start of treatment with topical atropine, topical prednisolone, and oral meloxicam. Pupil remains dilated and blood clot continues to resorb.
The kitten was kept separated from the other cats for several days to prevent further accidental trauma and to reduce the risk of rebleeding. Prednisolone was continued at a frequency of every 6 h, and the atropine was decreased to once every other day. Oral meloxicam was continued once daily. Providing food reward and non-treatment interactions (holding and petting) immediately after treatment of the eye led to the kitten's greeting the technician at treatment time and improved overall socialization.
Ten days after presentation, the hyphema was markedly improved: the remaining blood clot had settled into the medioventral aspect of the anterior chamber, and there was no evidence of blepharospasm or other signs of discomfort. The kitten remained energetic, with a good appetite and no other clinical signs. Intraocular pressure was normal but decreased (12 mm Hg in the left eye and 13 mm Hg in the right eye), and slit-lamp biomicroscopy showed a small area of abnormal pigmentation on the ventromedial lens. The kitten was considered to be visual in both eyes (Figure 3). Atropine and oral meloxicam were discontinued, but conservative treatment with prednisolone was continued. The dose of prednisolone was tapered to twice daily for a week and then once daily for a week.
Figure 3.
Photograph of both eyes at day 16. Affected eye appears normal but is still being treated with prednisolone eye drops to control inflammation and discomfort.
Although the abnormalities were nearly resolved, we remained interested in ruling out other possible causes of the uveitis. Approximately 2 wk after the initial presentation, the kitten was anesthetized lightly with sevoflurane administered by face mask to allow for blood collection. Samples were submitted for a follow up hemogram and serologic titer for Bartonella henselae, feline leukemia virus antigen, and feline immunodeficiency virus antibody. Additional serum was banked for further testing should the case not resolve. Serologic titers for Bartonella henselae and feline leukemia virus antigen and antibody tests for feline immunodeficiency virus were negative. Given the vendor history and progress in this kitten and the fact that other kittens remained clinically normal, no additional serologic testing for other infectious agents was performed. A final follow-up ophthalmic exam was conducted 6 wk after initial presentation. One small area of hyperpigmentation was detected in the left eye, adjacent to the pupil and indicative of a scar. No other abnormalities were noted, and the anterior uveitis and hyphema were considered to be resolved.
The kitten exhibited complete return of its visual status, as evidenced by ocular examinations and excellent peripheral and central vision when watching a red spot from a laser pointer toy moving around the floor and wall of the run.
Discussion
This kitten's miosis was diagnosed as secondary acute anterior uveitis with hyphema. Uveitis is inflammation of the uveal tract, which is comprised of the iris, ciliary body, and choroid. Anterior uveitis affects the anterior uvea (the iris and ciliary body), whereas posterior uveitis involves the choroid.10,28 Inflammatory cell migration to the peripheral anterior vitreous (pars planitis) involves the pars plana of the ciliary body.10,23 All 3 types of uveitis can occur simultaneously.23
Clinical presentation of anterior uveitis is nonspecific and can include any of the following signs: blepharospasm, photophobia, third eyelid protrusion, epiphora, scleral reddening, hyphema, hypopyon, miosis, opacities on the anterior lens capsule, iridial swelling, and hypotony (low ocular pressure) from impaired aqueous humor formation.12,23,28
Causes of feline anterior uveitis can be divided into 3 main categories: primary (ocular), systemic (infectious and noninfectious), and idiopathic. Primary anterior uveitis can arise from blunt trauma to the eye or head, penetrating trauma, neoplasia, or deep keratitis.10 Direct head or ocular injury can lead to uveitis through rupture of the fibrous tunic of the eye, lens luxation, corneal endothelial damage, retinal detachment, or proptosis.26 Spasm of the iris muscle due to trauma is thought to be the reason behind this kitten's miotic pupil.
Anterior uveitis associated with systemic disease can be unilateral or bilateral. Etiologies include Toxoplasma gondii, feline leukemia virus, feline immunodeficiency virus, feline infectious peritonitis, systemic mycoses, parasitic migration, bacteremia and septicemia, and immune-mediated conditions.11,20,22,23,26,28
Anterior uveitis also can be caused by feline herpesvirus 1, although conjunctivitis and keratitis are the most frequent clinical signs.2,15,19 Infected cats may (or may not) have systemic illness and upper respiratory signs, and carriers of feline herpesvirus 1 may be asymptomatic.2,14,26 A recent case report suggested bartonellosis (Bartonella henselae) as a cause of anterior uveitis in a 6-y-old cat.11 In addition, uveitis can be a sign of primary and secondary intraocular neoplasia.26
Idiopathic is the third category for a cause of uveitis, in which no specific etiologic agent is identified. The condition may be immunologically mediated, as lymphocytic–plasmacytic infiltration of the anterior uvea has been found on histopathology in cases lacking etiologic diagnosis.23,28 Some cases, classified as idiopathic, might have actually be due to undiagnosed feline herpesvirus 1 or feline immunodeficiency virus.2,26
Causes of hyphema include congenital malformations, blunt or penetrating trauma, coagulopathies, systemic hypertension, neoplasia, uveal or retinal neovascularization, and iatrogenic causes17,28 and vasculitis associated with infectious disease, for example, Ehrlichia canis.23,28 Prognosis for hyphema depends on the etiology, the amount of bleeding, and whether irreversible damage occurs to the lens, ciliary body, or retina. In addition, if glaucoma develops, surgical intervention may be required to alleviate pain.18
This cat's condition most likely was due to an impact to the head that resulted in a tear of the ciliary body with subsequent hyphema and miosis. Trauma can cause breakdown of the blood–aqueous barrier, allowing inflammatory cells and proteins from the vascular uvea to leak into the aqueous humor resulting in hyphema, hypopyon, or subsequent aqueous flare (or combinations thereof).10 Ophthalmic ultrasonography might have provided more information with respect to the primary site of the bleed, but this technology was not available to the clinical staff at the time of the incident.
The suspected cause of this kitten's presumed direct nonpenetrating ocular damage was activity-related blunt trauma (for example, running into the metal frame of the run while playing with other kittens or miscalculating a jump to the floor after climbing to the top of the enclosure gate). However, because a primary traumatic event was not witnessed and given the nature of the research and implications for the rest of the colony, further assurance was desired to rule out various infectious causes of anterior uveitis. The kitten was seronegative on ELISA for feline leukemia virus antigen and lacked characteristic changes in blood work, including nonregenerative anemia, azotemia, increased liver enzymes, and increased serum bilirubin.21 Similarly, the kitten was seronegative for feline immunodeficiency virus and lacked hematologic abnormalities associated with acute-stage disease, including leukopenia and neutropenia and characteristic gingivitis or stomatitis.9
The normal serum globulin in this kitten was not consistent with feline infectious peritonitis, and further testing through serum protein gamma-electrophoresis to identify a polyclonal gammopathy, was considered unnecessary. Anterior uveitis can be caused by feline herpesvirus 1, but the clinical presentation and progression in this case were not consistent with that etiology.2 Given the lack of characteristic signs in any of the 28 kittens and the negative status of the original colony, conjunctival biopsy for diagnostic PCR was not performed. In addition, the vendor colony was considered free of Toxoplasma gondii, and intermediate hosts were not present in the housing area; therefore, additional testing was not performed.
Immunofluorescence assay for antibodies against Bartonella henselae was done, because the vendor does not test for this disease and because the agent has only recently been identified as a differential diagnosis for anterior uveitis.11 The titer in this kitten was negative (less than 1:16). The vector for feline bartonellosis is the cat flea (Ctencephalides felis), which may have managed to access this kitten either at the vendor location, during packing for transport, or while housed in our facility.5 Neither fleas nor flea dirt were seen on this kitten or its cagemates, and we unaware of any documentation of vertical or direct transmission of Bartonella species.1 Anterior chamber paracentesis was not performed because the kitten was responding well to the treatments, but checking the antibody levels in the aqueous humor and comparing them to serum antibodies would be another piece of information to ascertain a definitive diagnosis for bartonellosis.6
Uveitis can be seen with melanoma and lymphosarcoma.26 If the cat had been older or had other clinical signs been present, imaging of the thoracic cavity would have been considered to help identify tumors or fungal infections. Resolution of this kitten's uveitis was rapid and did not require antimicrobial therapy, which further supports our conclusion that the uveitis was of a noninfectious etiology.
The hyphema in this case likely would have resorbed without treatment, but treatment was administered to alleviate pain and inflammation and to reduce the likelihood of complications (secondary glaucoma, synechiae, cataract formation, and blindness).24,26,28
Topical atropine sulfate produces cycloplegia and mydriasis, thus reducing pain from the ciliary spasm, and helps to restore a compromised blood–aqueous barrier.26 However, because the intraocular pressure of the affected eye was slightly higher than that of the normal eye, the frequency of atropine application was quickly reduced and then discontinued to decrease the potential for glaucoma. In the absence of corneal ulceration or keratitis, topical prednisolone acetate can be used as an antiinflammatory agent to decrease iridial inflammation and reduce discomfort.24 Meloxicam is a nonsteroidal antiinflammatory drug that was used to decrease general inflammation and reduce the frequency of steroid use. In the clinical judgment of the attending veterinarian, providing analgesia outweighed the minimal risk of adverse effects of meloxicam on platelet function.3,8 The response to therapy supports the diagnosis of secondary acute anterior uveitis due to trauma.
Acknowledgments
We extend special thanks to Sarah Wall for her technical assistance, midnight treatments, and care for the patient. The authors are also grateful for the time spent and expertise provided by veterinary ophthalmology resident, Dr Richard McMullen. Thank you also to the North Carolina State University Tick Borne Disease Laboratory for their assistance and to veterinary clinical pathologist, Dr Carol Grindem, for her help with the hemogram and serum chemistry interpretations.
References
- 1.Abbott RC, Chomel BB, Kasten RW, Floyd-Hawkins KA, Kikuchi Y, Koehler JE, Pedersen NC. 1997. Experimental and natural infection with Bartonella henselae in domestic cats. Comp Immunol Microbiol Infect Dis 20:41–51 [DOI] [PubMed] [Google Scholar]
- 2.Andrew SE. 2001. Ocular manifestations of feline herpesvirus. J Feline Med Surg 3:9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brainard BM, Meredith CP, Callan MB, Budsberg SC, Shofer FS, Driessen B, Otto CM. 2007. Changes in platelet function, hemostasis, and prostaglandin expression after treatment with nonsteroidal antiinflammatory drugs with various cyclooxygenase selectivities in dogs. Am J Vet Res 68:251–257 [DOI] [PubMed] [Google Scholar]
- 4.Chandler ML. 1992. Pediatric normal blood values. In: Kirk RW, Bonagura JD. Kirk's current veterinary therapy, vol. XI: small animal practice Philadelphia: WB Saunders Company; p. 981–984 [Google Scholar]
- 5.Chomel BB, Boulouis HJ, Breitschwerdt EB. 2004. Cat scratch disease and other zoonotic Bartonella infections. J Am Vet Med Assoc 224:1270–1279 [DOI] [PubMed] [Google Scholar]
- 6.Crystal MA. 2003. Bartonellosis (cat scratch disease). In: Norsworthy GD, Crystal MA, Grace SF, Tilley LP. The feline patient, 2nd ed Baltimore: Lippincott, Williams, and Wilkins; p. 136–138 [Google Scholar]
- 7.Everett RM. 1977. Alkaline phosphatases in tissues and sera of cats. Am J Vet Res 38:1533–1538 [PubMed] [Google Scholar]
- 8.Fresno L, Moll J, Penalba B, Espada Y, Andaluz A, Prandi D, Ruiz de Gopegui R, Garcia F. 2005. Effects of preoperative administration of meloxicam on whole blood platelet aggregation, buccal muscosal bleeding time, and haematological indices in dogs undergoing elective ovariohysterectomy. Vet J 170:138–140 [DOI] [PubMed] [Google Scholar]
- 9.Grace SF. 2003. Feline immunodeficiency virus infection. In: Norsworthy GD, Crystal MA, Grace SF, Tilley LP. The feline patient, 2nd ed. Baltimore (MD): Lippincott, Williams, and Wilkins; p. 211–214 [Google Scholar]
- 10.Hopper C, Crispin S. 1997. Differential diagnosis of uveitis. InBoden E, Melling M. Feline practice 2. London: WB Saunders Company; p 55–80 [Google Scholar]
- 11.Lappin MR, Black JC. 1999. Bartonella spp. infection as a possible cause of uveitis in a cat. J Am Vet Med Assoc 214:1205–1207 [PubMed] [Google Scholar]
- 12.Lappin MR, Kordick DL, Breitschwerdt EB. 2000. Bartonella spp. antibodies and DNA in aqueous humour of cats. J Feline Med Surg 2:61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy JK. 2006. Effect of age on reference intervals of serum biochemical values in kittens. J Am Vet Med Assoc 228:1033–1037 [DOI] [PubMed] [Google Scholar]
- 14.Low HC, Powell CC, Veir JK, Hawley JR, Lappin MR. 2007. Prevalence of feline herpesvirus 1, Chlamydophila felis, and Mycoplasma spp. DNA in conjunctival cells collected frorm cats with and without conjunctivitis. Am J Vet Res 68:643–648 [DOI] [PubMed] [Google Scholar]
- 15.Maggs DJ, Lappin MR, Reif JS, Collins JK, Carman J, Dawson DA, Bruns C. 1999. Evaluation of serologic and viral detection methods for diagnosing feline herpesvirus 1 infection in cats with acute respiratory tract or chronic ocular disease. J Am Vet Med Assoc 214:502–507 [PubMed] [Google Scholar]
- 16.Miller PE, Pickett JP, Majors LJ, Kurzman ID. 1991. Evaluation of two applanation tonometers in cats. Am J Vet Res 52:1917–1921 [PubMed] [Google Scholar]
- 17.Morgan RV. 1986. Systemic hypertension in four cats: ocular and medical findings. J Am Anim Hosp Assoc 22:615–621 [Google Scholar]
- 18.Nasisse MP. 2006. Hyphema. In: Miller PE, Tilley LP, Smith FWK. The 5-minute veterinary consult canine and feline specialty handbook: ophthalmology. Baltimore (MD): Lippincott Williams & Wilkins; p. 148–155 [Google Scholar]
- 19.Nasisse MP, Weigler BJ. 1997. The diagnosis of ocular feline herpesvirus infection. Vet Comp Ophthalmol 7:44–51 [Google Scholar]
- 20.Norsworthy GD. 2003. Toxoplasmosis. In: Norsworthy GD, Crystal MA, Grace SF, Tilley LP. The feline patient, 2nd ed. Baltimore (MD): Lippincott, Williams, and Wilkins; p. 481–484 [Google Scholar]
- 21.Norsworthy GD. 2003. Feline leukemia virus diseases. In: Norsworthy GD, Crystal MA, Grace SF, Tilley LP. The feline patient, 2nd ed. Baltimore (MD): Lippincott, Williams, and Wilkins; p. 220–224 [Google Scholar]
- 22.Norsworthy GD. 2003. Feline infectious peritonitis. In: Norsworthy GD, Crystal MA, Grace SF, Tilley LP. The feline patient, 2nd ed. Baltimore (MD): Lippincott, Williams, Wilkins; p. 215–219 [Google Scholar]
- 23.Powell CC, Lappin MR. 2001. Causes of feline uveitis. Comp Cont Ed Prac Vet 23:128–140 [Google Scholar]
- 24.Powell CC, Lappin MR. 2001. Diagnosis and treatment of feline uveitis. Comp Cont Ed Prac Vet 23:258–266 [Google Scholar]
- 25.Ungemach FR. 2003. Hypertension. In: Egner B, Carr A, Brown S. Essential facts of blood pressure in dogs and cats, 3rd ed. Babenhausen (Germany): BE VetVerlag; p. 143–158 [Google Scholar]
- 26.Wilkie DA. 1999. Advances in feline ophthalmology. In: Proceedings of the 23rd Waltham–OSU symposium: advances in feline medicine. Vernon (CA): Waltham USA; p. 57–63 [Google Scholar]
- 27.Willard MD. 1989. Gastrointestinal, pancreatic, and hepatic disorders. In: Willard MD, Tvedten H, Turnwald GH. Small animal clinical diagnosis by laboratory methods. Philadelphia: WB Saunders Company; p. 189–228 [Google Scholar]
- 28.Willis AM. 2003. Diseases of the anterior uveal tract. In: Morgan RV, Bright RM, Swartout MS. Handbook of small animal practice, 4th ed. Philadelphia: Saunders; p. 978–987 [Google Scholar]