Abstract
The use of analgesics to prevent or treat postprocedural pain in rodents is increasingly encouraged by the laboratory animal community and federal funding agencies. However, the effects of analgesics on experimental outcomes are not well-documented. In this study, we incorporated ketoprofen into a well-established experimental protocol. Of the 44 Sprague–Dawley (SD) rats obtained from vendor A that were given either ketoprofen (10 mg/kg SC) or saline and underwent ovariectomy, 19 that received ketoprofen died or were euthanized due to clinical illness within 3 to 7 d after surgery. Necropsy revealed gastrointestinal ulceration consistent with toxicity from nonsteroidal antiinflammatory drug. In an attempt to identify factors responsible for this unanticipated outcome, SD rats from vendors A and B were subjected to the same protocol, but no clinical signs or pathologic lesions were observed in any of these rats, regardless of source. A third experiment with rats obtained from vendor A and housed in barriers 1 and 2 was done to clarify the conflicting results and to determine whether response to ketoprofen differed at the barrier level. Three of the 6 rats from barrier 2 that received ketoprofen in the third study had gastrointestinal lesions similar to those observed in the first study, whereas none of the rats from barrier 1 had any lesions. These results suggest that the adverse effects seen after administration of ketoprofen were due to differences between barriers.
Abbreviation: Cox, cyclooxygenase; NSAID, nonsteroidal antiinflammatory drug; SD, Sprague–Dawley
For many decades, pain management in animals was poorly understood and therefore rarely done. Recently, efforts have been made to study and improve pain management to enhance animal welfare. Studies from the 1970s explored the use of various analgesics and their effects on animals' response to painful stimuli.6,25,28 The use of analgesics is more common in the current literature, but consideration must be given to drugs that may affect research results. Analgesics are incorporated into new research protocols less often when past research did not involve their use. The possibility of altering the results of new research as well as limited data on the effectiveness and safety of analgesics has limited their incorporation.
The use of analgesics to prevent or treat postprocedural pain in rodents is increasingly encouraged by laboratory animal veterinarians, federal funding agencies, and animal use committees. Analgesics can be beneficial in limiting pain during recovery and may help animals recover more quickly by decreasing pain-associated behaviors,22 decreasing postoperative weight loss,24 and limiting the release of stress hormones secondary to the pain response. However, analgesics have a myriad of potential side effects that may harm, rather than help, animals and confound research outcomes. The present study focuses on ketoprofen, which is used frequently as an analgesic in rodent surgery.21 Ketoprofen is not labeled for use in rodents; however, dosages have been published in veterinary drug compendia18,27 and are reported in the current literature.1,2,10,19,21,22 Adverse effects are commonly documented when ketoprofen is given at higher-than-recommended doses3,11,13 or given long-term at published doses10 but are rarely reported after a single dose within acceptable dose ranges.7
Ketoprofen [(RS)2-(3-benzoylphenyl)-propionic acid] is a nonsteroidal antiinflammatory drug (NSAID) with analgesic, antiinflammatory, and antipyretic effects.10,14,18,27 Ketoprofen inhibits the cyclooxygenase catalysis of arachadonic acid and prostaglandin precursors thereby inhibiting the synthesis of prostaglandin production in tissue.5,8,10,12 Cyclooxygenase (Cox) has 2 isoenzymes (Cox1 and Cox2), which differ in function. Cox1 is a protein that acts as an enzyme to produce prostaglandins in the endothelium and has a protective effect on the mucosa of the gastrointestinal tract, kidneys, and various tissues throughout the body.21 Cox2 is induced by inflammation and acts as an enzyme to produce prostaglandins during tissue damage.21 After the discovery of the 2 isoenzymes of cyclooxygenase, specific NSAIDs were produced to attempt to block the inflammatory pathway and the induction of Cox2 but spare the protective effects of Cox1.21 Most NSAIDs, including ketoprofen, cannot selectively inhibit Cox-2, leading to undesirable side effects. When administered long-term or at high doses,17 NSAID use has been implicated in gastric mucosal damage, gastrointestinal ulceration, renal necrosis, and hepatitis.1,4,12,18,20
Our laboratory incorporated ketoprofen into our collaborator's existing NIH study examining the effects of estrogen on brain inflammation. The dose of ketoprofen was selected based on a previous study,22 in which the analgesic effects of 3 doses of ketoprofen were evaluated. We arbitrarily selected the middle dose (10 mg/kg) from that study. No indications of safety issues were noted at the 10- and 15-mg/kg doses in that study. By incorporating analgesics into an existing study, we were able to compare the data obtained with analgesic use with those obtained previously. The initial hypothesis was that incorporating ketoprofen into the study would be beneficial to the rats' surgical recovery but would not affect the study outcome.
Materials and Methods
Animals.
Female Sprague–Dawley (SD) rats, each weighing approximately 250 g, were obtained from 2 commercial vendors (A and B). The rats obtained from vendor A were from 3 separate barriers in different geographic locations. All barriers were free of known pathogens including sialodacryoadenitis virus, RPV, Sendai virus, pneumonia virus of mice, reovirus 3, lymphocytic choriomeningitis virus, Hantaan virus, Mycoplasma pulmonis, Clostridium piliforme, cilia-associated respiratory bacillus, Helicobacter spp., and enteric protozoa. Microbiologic status was confirmed via monthly or quarterly serology, bacteriology, PCR assay, and parasitology. The rats obtained from vendor B were obtained from a single barrier that was free of known pathogens including pneumonia virus of mice, reovirus, Sendai virus, sialodacryoadenitis/rat coronavirus, Kilham rat virus, rat parvovirus, rat minute virus, cilia-associated respiratory bacillus, Helicobacter spp., and enteric protozoa. Microbiologic status was confirmed quarterly with serology, PCR, and parasitology. No serologic monitoring was done on the rats after they arrived at the research facility, but the animal room was confirmed to be free of known pathogens via quarterly sentinel monitoring.
The study protocol was approved by the Institutional Animal Care and Use Committee of Texas A and M University. Animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals15 in an AAALAC-accredited facility. All rats were pair-housed in standard polycarbonate caging on a filtered, suspended shelf rack with aspen hardwood chip bedding. Animals were given ad libitum access to 4% rodent diet (8604, Harlan Teklad, Madison, WI ) and filtered tap water provided via water bottles. Cages and water bottles were changed twice weekly. The room was maintained at 21.9 °C on a 12:12-h light:dark cycle.
Surgical and postoperative procedures.
The animals were obtained in groups of 12 and allowed to acclimate for at least 1 wk prior to surgery. Surgical procedures were performed on groups of 4. Procedures were scheduled every other day for each group of 12 rats. A standard ovariectomy was performed according to the protocol used by our collaborators.9 All rats were anesthetized with 87 mg/kg ketamine (Fort Dodge Animal Health, Fort Dodge, IA) and 13 mg/kg xylazine (Phoenix Scientific, St Joseph, MO) IP. The rats were allowed to recover under a heat lamp and were given 3 ml of warm saline subcutaneously. Animals were monitored closely by a veterinarian and a licensed veterinary technician for the entire recovery period. Animals were removed from the heat and returned to the animal room once they were awake and moving around the cage. Animals were provided food and water once they returned to the animal room. After surgery, all animals were monitored 4 times daily by the veterinarian, technician, or animal care staff, and any abnormalities were reported to the veterinarian immediately. Any animal that developed clinical signs was treated with warm saline and monitored every few hours for improvement. Clinical signs included lethargy, anorexia, diarrhea, hunched posture, ruffled hair coat, and porphyrin staining around eyes and nose. All sick animals that did not improve with supportive care and all moribund animals were euthanized by CO2 inhalation and underwent complete necropsy. Animals were euthanized by CO2 inhalation at the end of each study and a complete necropsy was performed. Histopathology was performed on all animals that had clinical signs or that had developed lesions and on 10 animals from each study that did not develop clinical signs or gross lesions. Histopathology was performed on similar sections of the stomach, jejunum, duodenum, and ileum.
Study design.
First experiment.
Forty-four SD rats obtained from vendor A (barriers 1 & 2) were obtained for ovariectomy (Table 1); 32 of these rats were given ketoprofen (10 mg/kg SC) postoperatively. The remaining 12 rats received saline and served as controls for analgesic use.
Table 1.
Clinical signs among rats from vendor A
| Rats | Barrier | Analgesic or saline? | No. of rats with clinical signs/no. of rats in group |
| 1–6 | 1 | Saline | 0/6 |
| 7–12 | 1 | Analgesic | 1/6 |
| 13 & 14 | 2 | Analgesic | 1/2 |
| 15 & 16 | 2 | Saline | 0/2 |
| 17–21 | 2 | Analgesic | 2/5 |
| 22–25 | 2 | Saline | 1/4 |
| 26–44 | 2 | Analgesic | 14/19 |
Clinical signs among the 19 rats that showed them included: sudden death, 3 rats (1 death attributed to anesthesia); lethargy, 11 rats; diarrhea, 3 rats; hunched posture, 9 rats; porphyrin staining around eyes and nose, 8 rats.
Second experiment.
Twelve SD rats each were obtained from vendors A (barrier 3) and B. Rats from each vendor were divided into 2 groups. Six rats from each source were given ketoprofen (10 mg/kg SC) immediately after surgery, and the other 6 rats from each source received saline (0.3 ml SC). All rats received 3 ml warm saline subcutaneously postoperatively. All rats were monitored for 10 d after surgery and were euthanized with CO2.
Third experiment.
The third experiment involved SD rats from barriers 1 and 2 of vendor A. Six rats from each barrier were given ketoprofen (10 mg/kg SC) immediately after surgery. The other 6 rats from each barrier received saline (0.3 ml SC). Blood and tissues were collected and submitted for histopathology when the animal exhibited clinical signs or at the end of 10 d.
Results
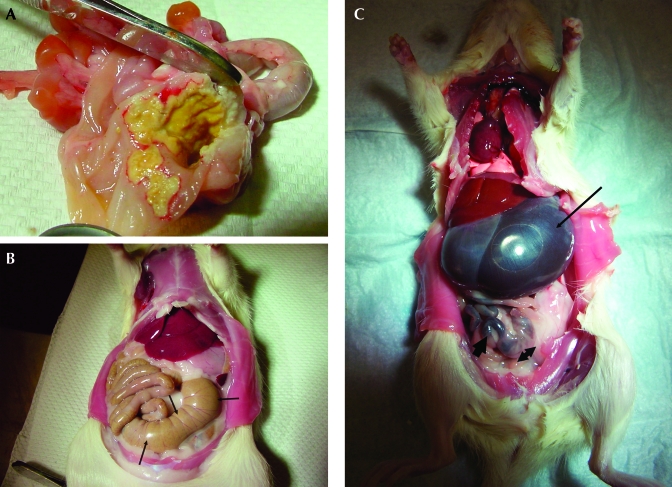
In the first experiment, 32 SD rats each received a single, preoperative dose of ketoprofen. Nineteen of the 32 SD rats that received ketoprofen exhibited lethargy, anorexia, diarrhea, hunched posture, porphyrin staining around eyes and nose, and a ruffled hair coat within 7 d. Necropsy revealed gastric ulceration (Figure 1 A) and peritonitis, which was confirmed with histopathology. The 12 rats that received saline had no pathology on necropsy (Figure 1 B), as compared with the rats that received ketoprofen (Figure 1 C). Because these clinical signs were similar to those of ketoprofen toxicity associated with an overdose, a thorough evaluation of the use of ketoprofen ensued to determine the cause of the signs and lesions. The dose administered was within the acceptable reference range found in veterinary drug compendia18 and current literature.22 Improper administration of the analgesic, possible drug interactions, and contamination of the bottles were ruled out. The subsequent hypothesis was that the unexpected result was attributable to a phenotype specific to SD rats obtained from a particular vendor. To test this hypothesis, a second experiment was designed to incorporate SD rats from 2 different vendors and subject them to the same procedures and treatments as in the first experiment.
Figure 1.
(A) Mucosal ulceration in the stomach found on gross necropsy of a rat that received ketoprofen perioperatively. A yellow, fibrinous plaque extensively covers the lesion. (B) Gross necropsy of a rat that received saline preoperatively. No abnormalities are noted. Cecum is large and well-differentiated (arrows). The small intestine is distended with digested food. The stomach is small and a normal, light-pink color. (C) Gross necropsy of a rat that received ketoprofen preoperatively. Stomach is grossly distended with fluid (thin arrow). Cecum is very small with a minimal amount of dark ingesta (arrowhead). A minimal amount of dark feces is noted in the intestines (thick arrow).
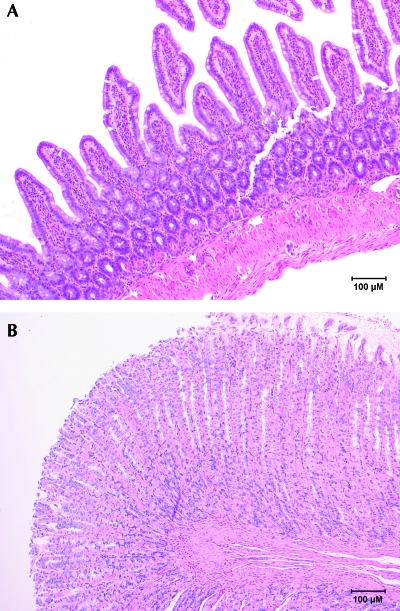
In the second experiment, 12 rats (6 from each of 2 vendors) received ketoprofen, and 12 rats received saline. All rats underwent the same surgical procedure (ovariectomy) and received the same postoperative care. All rats were closely monitored for 10 d after surgery, and none of the rats had any clinical signs regardless of the vendor from which they were obtained. No gross lesions were noted at necropsy, and no abnormalities were noted on histopathology (Figure 2 A, B). This outcome was unanticipated in light of the results of the first experiment. Further investigation revealed that rats used in the first experiment had originated in two barriers of Vendor A. A third experiment was designed to test animals from both of the barriers represented in the first experiment.
Figure 2.
Normal histology of (A) small intestine and (B) stomach from a rat that received saline preoperatively. Hematoxylin and eosin stain.
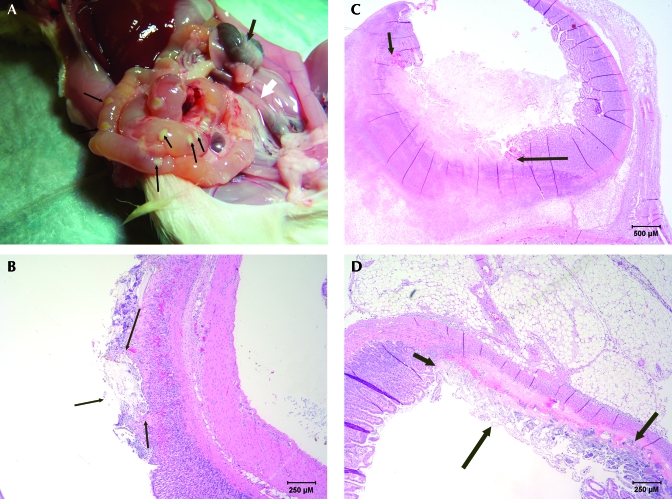
In the third experiment, 12 rats (6 from each barrier) received ketoprofen, and 12 rats (6 from each barrier) received saline. Three of the 12 rats that received ketoprofen had gastrointestinal lesions (Figure 3 A) similar to those observed in the first study. Histopathology (Figure 3 B through D) of the affected rats revealed inflammatory gastroenteritis, ulcerative and necrotizing gastroenteritis with bacterial septicemia, and acute hepatic necrosis. The 3 affected rats were from barrier 2, the same barrier from which the affected rats in the first experiment had originated. The 6 rats from barrier 1 that received ketoprofen had no clinical signs or lesions on gross necropsy. No pathology on necropsy or histopathology was noted in the rats from barrier 1 or in the control rats from either barrier.
Figure 3.
(A) Gross necropsy of a rat that received ketoprofen preoperatively. Abscesses were located throughout the small intestine (thin arrows). Intestines are distended with fluid. Cecum is very small, and contents are very dark (thick, dark arrow). A lack of feces is noted throughout the gastrointestinal tract (open arrow). (B) Histopathology of a stomach from a rat that received ketoprofen preoperatively. Section demonstrates superficial ulceration of mucosal layer (arrows outline lesion). Hematoxylin and eosin stain; magnification, ×40. (C) Histopathology of the small intestine of a rat that received ketoprofen preoperatively. Section demonstrates severe transmural necrosis with inflammation extending into adjacent mesenteric attachment (arrows outline lesion). Hematoxylin and eosin stain. (D) Histopathology of the small intestine from a rat that received ketoprofen preoperatively. Section demonstrates superficial to transmural necrosis at the mesenteric attachment (arrows outline lesion). Hematoxylin and eosin stain.
Discussion
Analgesics generally are considered safe and beneficial when used correctly. Current scientific literature documents the benefits of analgesia.9,18,23 The dosages of ketoprofen used in the present study were those that have been reported in the literature and veterinary drug compendia.16,22,26 The illness and death of the rats that received ketoprofen prompted an investigation of possible causes. Potential causes involving the ketoprofen included bacterial contamination of the ketoprofen stock, incorrect dose calculation or preparation, incorrect administration, and possible drug interactions.
The bottle of ketoprofen was cultured for aerobic bacteria and was negative. The other drugs (ketamine and xylazine) used were not cultured because deaths occurred only among rats that received ketoprofen. The ketoprofen stock was not diluted prior to administration because the dose could be measured accurately by using a tuberculin syringe. After the unexpected deaths, the stock was diluted prior to administration to minimize the potential for a dosage error.
To rule out incorrect dose calculation and preparation, the dose was calculated independently by the authors and the veterinary technician administering the drug. All calculated dosages were identical and correlated with the dosage administered. After each dose was prepared, a veterinarian and a veterinary technician verified that the correct amount was in the syringe prior to administration. All of these practices ruled out dosage and dose administration as causes of the unexpected deaths.
One resource suggested that ketoprofen should be administered postoperatively because of its potent nonselective cyclooxygenase inhibition and that surgery could increase the intensity of the side effects.27 In addition, decreased blood pressure secondary to anesthesia was hypothesized to have contributed to an increase in toxicity despite use of the correct dose. Our results did not change when ketoprofen was administered postoperatively.
Obvious factors surrounding dose and administration were addressed and ruled out. Why one particular subset of rats expressed increased sensitivity to ketoprofen remains unknown. Factors to consider include environmental variability between the barriers, environmental differences during the dates of the study, and facility variability during study dates. Genetic variability between barriers was considered, but the possibility is incredibly remote given the stringent breeding records and protocols. The barrier in question may have had a specific microbiologic variability that was not tested for but that would cause increased sensitivity to NSAIDs. All known pathogens were tested for and results were negative at the specific dates animals were used from that barrier.
The findings of these experiments may lead to the discovery of a new model to develop more specific and less toxic NSAIDs. Because NSAIDs are known to be beneficial in assisting people to live productive lives and yet have so many serious side effects, these rats may be a model for understanding genetic susceptibility to various NSAIDs and the key to developing better NSAIDs in the future.
Acknowledgments
We sincerely thank Andrea Taylor from the Comparative Medicine Department for all technical assistance with the project, Dr Christine Sivula from the Pathobiology Department for assistance with the project, and Vanessa Nordell from Dr Sohrabji's laboratory for assistance with surgical training. We also thank Dr Lynn Cassone from Texas Veterinary Medical Diagnostic Laboratory for the histopathology and Dr Brian Porter from the Pathobiology Department for the histopathology pictures.
We wish to thank the ACLAM Foundation for funding this project.
References
- 1.Blandizzi C, Fornai M, Colucci R, Natale G, Lubrano V, Vassalle C, Antonioli L, Lazzeri G, Del Tacca M. 2005. Lansoprazole prevents experimental gastric injury induced by nonsteroidal antiinflammatory drugs through a reduction of mucosal oxidative damage. World J Gastroenterol 11:4052–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi JS, Jin M, Han H. 2006. Intestinal absorption characteristics of ketoprofen in rats. Biopharm Drug Dispos 27:17–21 [DOI] [PubMed] [Google Scholar]
- 3.de la Lastra CA, Nieto A, Motilva V, Martin MJ, Herrerias JM, Cabre F, Mauleon D. 2000. Intestinal toxicity of ketoprofen–trometamol vs its enantiomers in rat. Role of oxidative stress. Inflamm Res 49:627–632 [DOI] [PubMed] [Google Scholar]
- 4.Fiorucci S, Distrutti E, de Lima O, Romano M, Mencarelli A, Barbanti M, Palazzini E, Morelli A, Wallace J. 2003. Relative contribution of acetylated cyclooxygenase (COX)-2 and 5-lipooxygenase (LOX) in regulating gastric mucosal integrity and adaptation to aspirin. FASEB J 17:1171–1191 [DOI] [PubMed] [Google Scholar]
- 5.Fornai M, Natale G, Colucci R, Tuccori M, Carazzina G, Antonioli L, Baldi S, Lubrano V, Abramo A, Blandizzi C, Del Tacca M. 2005. Mechanisms of protection by pantoprazole against NSAID-induced gastric mucosal damage. Naunyn Schmiedebergs Arch Pharmocol 372:79–87 [DOI] [PubMed] [Google Scholar]
- 6.Harada T, Takahashi H, Kaya H, Inoki R. 1979. A test for analgesics as an indicator of locomotor activity in writhing mice. Arch Int Pharmacodyn Ther 242:273–284 [PubMed] [Google Scholar]
- 7.Holzer P, Jocic M, Cabre F, Mauleon D. 2001. Estimation of acute flurbiprofen and ketoprofen toxicity in rat gastric mucosa at therapy-relevant doses. Inflamm Res 50:602–608 [DOI] [PubMed] [Google Scholar]
- 8.Humes JL, Winter CA, Sadowski SJ, Kuehl FA. 1981. Multiple sites on prostaglandin cyclooxygenase are determinants in the action of nonsteroidal antiinflammatory agents. Proc Natl Acad Sci USA 78:2053–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jezierski MK, Sohrabji F. 2001. Neurotropin expression in the reproductively senescent forebrain is refractory to estrogen stimulation. Neurobiol Aging 22:309–319 [DOI] [PubMed] [Google Scholar]
- 10.Kido H, Murakami N, Ito A, Kimura K, Kodera N, Doi T, Naruse T. 1998. Antiinflammatory, analgesic, and antipyretic effects of d-2[4-(3-methyl-2-thienyl)phenyl] propionic acid (M5011), a new nonsteroidal antiinflammatory drug, in rats and guinea pigs. Jpn J Pharmacol 76:75–86 [DOI] [PubMed] [Google Scholar]
- 11.Laudanno OM, Cesolari JA, Esnarriaga J, San Miguel P, Bedini OA. 2000. In vivo selectivity of nonsteroidal antiinflammatory drugs and gastrointestinal ulcers in rats. Dig Dis Sci 45:1359–1365 [DOI] [PubMed] [Google Scholar]
- 12.Legen I, Kristl A. 2002. Ketoprofen-induced intestinal permeability changes studied in side-by-side diffusion cells. J Pharm Pharmacol 54:1419–1422 [DOI] [PubMed] [Google Scholar]
- 13.Librowski T, Czarnecki R, Czekaj T, Marona H. 2005. New xanthone derivatives as potent antiinflammatory agents. Medicina (Kaunas) 41:54–58 [PubMed] [Google Scholar]
- 14.Lu WL, Zhang Q, Zheng L, Wang H, Li R, Zhang L, Shen W, Tu X. 2004. Antipyretic, analgesic, and antiinflammatory activities of ketoprofen β-cyclodextrin inclusion complexes in animals. Biol Pharm Bull 27:1515–1520 [DOI] [PubMed] [Google Scholar]
- 15.National Research Council 1996. Guide for the care and use of laboratory animals Washington (DC): National Academy Press [Google Scholar]
- 16.Nieto AI, Cabre F, Moreno F, De La Lastre C. 2002. Mechanisms involved in the attenuation of intestinal toxicity induced by (S)-(+)- ketoprofen in re-fed rats. Dig Dis Sci 47:905–913 [DOI] [PubMed] [Google Scholar]
- 17.Oh YH, Han H. 2006. Altered pharmacokinetics of zalcitabine by concurrent use of NSAIDs in rats. Acta Pharmacol Sin 27:119–122 [DOI] [PubMed] [Google Scholar]
- 18.Plumb DC. 2002. Plumb's veterinary drug handbook, 4th ed Hoboken (NJ): Wiley–Blackwell [Google Scholar]
- 19.Prado WA, Pontes RMC. 2002. Presurgical ketoprofen, but not morphine, dipyrone, diclofenac, or tenoxicam, preempts postincisional mechanical allodynia in rats. Braz J Med Biol Res 35:111–119 [DOI] [PubMed] [Google Scholar]
- 20.Rainsford KD, Stetsko PI, Sirko SP, Debski S. 2003. Gastrointestinal mucosal injury following repeated daily oral administration of conventional formulations of indometacin and other nonsteroidal antiinflammatory drugs to pigs: a model for human gastrointestinal disease. J Pharm Pharmacol 55:661–668 [DOI] [PubMed] [Google Scholar]
- 21.Roughan JV, Flecknell PA. 2000. Effects of surgery and analgesic administration on spontaneous behaviour in singly housed rats. Res Vet Sci 69:283–288 [DOI] [PubMed] [Google Scholar]
- 22.Roughan JV, Flecknell PA. 2001. Behavioural effects of laparotomy and analgesic effects of ketoprofen and carprofen in rats. Pain 90:65–74 [DOI] [PubMed] [Google Scholar]
- 23.Sharp J, Azar T, Lawson D. 2005. Selective adaptation of male rats to repeated social encounters and experimental manipulations. Contemp Top Lab Anim Sci 44:28–31 [PubMed] [Google Scholar]
- 24.Sharp J, Zammit T, Azar T, Lawson D. 2003. Recovery of male rats from major abdominal surgery after treatment with various analgesics. Contemp Top Lab Anim Sci 42:22–27 [PubMed] [Google Scholar]
- 25.Sofia RD, Vassar HB, Knobloch LC. 1975. Comparative analgesic activity of various naturally occurring cannabinoids in mice and rats. Psychopharmacologia 40:285–295 [DOI] [PubMed] [Google Scholar]
- 26.Takagi-Matsumoto H, Ng B, Tsukimi Y, Tajimi M. 2004. Effects of NSAIDs on bladder function in normal and cystitis rats: a comparison study of aspirin, indomethacin, and ketoprofen. J Pharmacol Sci 95:458–465 [DOI] [PubMed] [Google Scholar]
- 27.USP Veterinary Pharmaceutical Information Monographs- Anti-inflammatories 2004. J Vet Pharmacol Ther 27Suppl 1:75–85 [DOI] [PubMed] [Google Scholar]
- 28.Winter CA, Kling PJ, Tocco DJ, Tanabe K. 1979. Analgesic activity of diflunisal [MK-647; 5-(2,4-difluorophenyl) salicylic acid] in rats with hyperalgesia induced by Freund's adjuvant. J Pharmacol Exp Ther 211:678–685 [PubMed] [Google Scholar]