Abstract
Purpose of review
To assess the role of serotonin and its control in the manifestations and treatment of lower functional gastrointestinal disorders (FGID). Recent literature has explored several novel concepts in the association of serotonin and symptoms, alterations in tissue levels of serotonin and its reuptake protein (SERT), aspects of the genetic determinants of serotonergic function (particularly 5-HTTLPR) and its relationship to gastrointestinal motor and sensory functions, and novel serotonergic agents used in therapy of lower FGID. The most consistent findings are the increase in plasma 5-HT in diarrheal diseases and reduction in constipation. The SERT in platelets impacts on the circulating level of 5-HT. Meta-analysis shows that 5-HTTLPR genotype is not significantly associated with IBS in Caucasians or Asians. New 5-HT3 antagonists and 5-HT4 agonists are efficacious and promise to provide relief for patients if they can pass regulatory hurdles.
Summary
While the most relevant implication for clinical practice remains the evidence that serotonergic agents are efficacious in the treatment of chronic constipation, chronic diarrhea and irritable bowel syndrome, the role of genetic control of 5-HT and its receptors is the subject of ongoing research, and is likely to enhance understanding of the mechanisms and treatment of these diseases.
Keywords: serotonin-transporter, reuptake, genotype, irritable bowel syndrome, prucalopride, ATI-7505, TD-5108, 5-HT3 antagonist
INTRODUCTION
This brief review of the role of serotonin in the gastrointestinal tract focuses on the literature pertaining to the lower functional gastrointestinal diseases: irritable bowel syndrome, constipation and diarrhea. Nine questions are addressed in the review: Is serotonin a pivotal mechanism in IBS? Is there increased or decreased expression of serotonin in the gastrointestinal tract? Is there a difference in brain 5-HT or responses to 5-HT receptor modulation in IBS? What is the effect of increased serotonin on gastrointestinal motor and secretory functions? Is Intestinal serotonin reuptake abnormal in functional GI disorders? Are tissue SERT levels abnormal in IBS? What are recent advances in the genetics of serotonin receptors and SERT in IBS or affective disorders in IBS? Are there examples of pharmacogenetics related to serotonergic agents? Are there new treatments involving novel serotonergic agents?
1. Is serotonin a pivotal mechanism in IBS?
Since 95% of the serotonin in the body is located in the gut, one would reasonably assume that postprandial plasma levels of 5-HT are derived from the gut. Reports of plasma 5-HT concentrations in different functional GI disorders suggest that there may be a role for circulating serotonin in determining predominant bowel function: serotonin is increased in diarrhea and celiac disease, decreased in constipation. For example, Dunlop et al. reported that platelet-poor plasma 5-HT from 0 to 180 minutes postprandially was significantly lower in C-IBS and higher in PI-IBS patients, and suggested that abnormalities in postprandial 5-HT release may be related to the different IBS symptoms (1).
Is this increase specific to D-IBS? It is possible that increased plasma 5-HT is a non-specific of diarrhea, since patients with celiac disease also have increased plasma 5-HT (2). Houghton et al. documented increased circulating 5-HT postprandially in D-IBS (3). However, the timing of symptoms did not coincide with the increase in plasma serotonin which would be expected to coincide if there was an association between serotonin and symptoms. Thus, the peak serotonin concentration in plasma was reached 2–3 hours after the meal in all study groups and appears to coincide with the arrival of nutrients in the more distal intestine. In contrast, the timing of postprandial symptoms in patients with IBS is earlier and may be mediated by other mechanisms initiated by gastric mechanoreceptors or upper intestinal mediators that stimulate the colonic response to feeding. Candidate mediators released within the foregut or midgut, such as gastrin, cholecystokinin, secretin, pancreatic polypeptide, motilin, the vasoactive intestinal polypeptide family (including PHI/PHM), and neurotensin may be evoking the colonic response and the associated postprandial symptoms, such as urgency or diarrhea which are common in patients with D-IBS (4).
The same discordance between timing of symptoms after ingestion of cold water in D-IBS and elevation of plasma serotonin is noted in another paper, which reported a significant correlation between symptoms and area under the curve of the plasma 5-HT (5).
Studies of plasma levels of 5-HT are complicated by the presence of an avid serotonin transporter (SERT) in platelets. Thus, while the postprandial circulating serotonin likely originates from the gut, interpretation of circulating levels is more complex, and recent data suggest that there may be differences in platelet SERT function in different IBS subgroups.
SERT expressed on platelet membranes of D-IBS patients is characterized by low density and binding affinity. One report suggested a possible correlation between the reduced capacity of serotonin reuptake and the severity of D-IBS symptoms (6). More recently, two groups provided functional evidence in support of this concept by measuring mucosal 5-HT turnover as assessed by mucosal 5-HIAA (metabolite produced after reuptake) to 5-HT ratio. The Nottingham group showed that mucosal 5-HIAA/5-HT ratio was decreased in both C-IBS and post-infectious (PI)-IBS patients, compared with healthy controls (1).
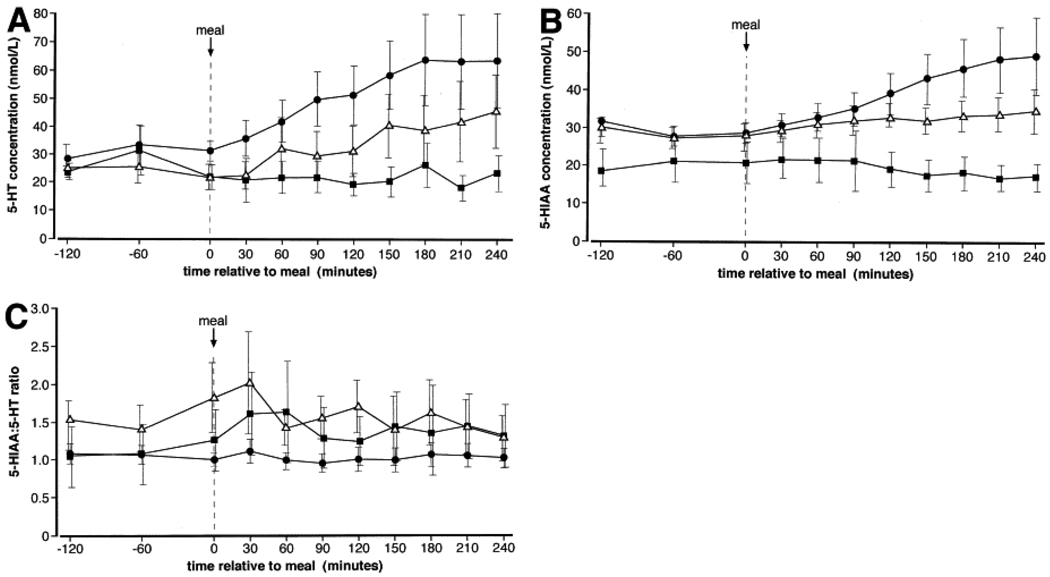
Atkinson et al. (7) showed that D-IBS patients had raised platelet-depleted plasma 5-HT concentrations under fasting and fed conditions (Figure 1) and C-IBS patients failed to show an increase in plasma 5-HT with meal ingestion, compared with controls. C-IBS was associated with decreased plasma 5-HIAA (P<0.01), normal 5-HIAA:5-HT ratio, and increased platelet 5-HT. In contrast, D-IBS with normal plasma 5-HIAA reduced 5-HIAA:5-HT ratio and normal platelet 5-HT. These results support the concept that D-IBS is characterized by reduced 5-HT reuptake, whereas impaired release of 5-HT from platelets may be a feature of C-IBS. These data are consistent with the observation by Bellini et al. (6) that reduced SERT expression in platelets in D-IBS correlates with symptom severity.
Figure 1.
Profiles of 5-hydroxytryptamine (5-HT) (A) and 5-hydroxyindoleacetic acid (5-HIAA) (B) concentrations and ratio of 5-HIAA:5-HT (C) with respect to meal ingestion (t = 0) in patients with constipation(■)- and diarrhea(•)-predominant IBS and healthy controls (Δ). Data are geometric mean and 95% confidence interval. Reproduced from ref. 7, Atkinson W, et al. Gastroenterology 2006;130:34–43.
2. Is there increased or decreased expression of serotonin in the gastrointestinal tract?
This field is controversial, with evidence arguing for increased expression of 5-HT or the rate-limiting enzyme of its synthesis (tryptophan hydroxylase-1, TpH-1) in PI-IBS (8), in patients with constipation (1,9), and in Crohn’s disease patients in remission who experience IBS-like symptoms (10). These patients have increased mucosal TpH-1 levels in the colon, suggesting that increased serotonin biosynthesis in the colon plays a role in the generation of the symptoms in these patients (10). Increased duodenal mucosal 5-HT has also been reported in constipation, though the significance is unclear (11).
Other studies identified decreased 5-HT in jejunal mucosa in IBS (12) or no difference in gastric mucosal 5-HT during fasting or after a water “meal” in IBS (5).
Summarizing this literature, it appears that there is no consistent message or clear mechanistic interpretation that can be gleaned from these findings. Further research is necessary in large numbers of patients with clearly defined phenotype and genotype, given the potential genetic variations in rates of synthesis and re-uptake of serotonin.
3. Is there a difference in brain 5-HT or responses to 5-HT receptor modulation in IBS?
Using an indirect measure to assess central 5-HT responses in IBS, there was an exaggerated release of prolactin in response to buspirone, the 5-HT1A agonist (13). The clinical significance of this finding is unclear.
In studies of brain imaging conducted in association with rectal stimulation, there were significantly greater decreases in activity of the amygdala, ventral striatum, hypothalamus and infragenual cingulate gyrus in 52 non-constipated irritable bowel syndrome patients after 3 weeks of treatment with a 5-HT3-receptor antagonist, alosetron, compared to placebo. The decreases in brain activity were observed in response to unanticipated, anticipated and delivered aversive rectal stimuli. These data suggest that alosetron decreased activation in structures of the emotional motor system, and this is associated with a decrease in gastrointestinal symptoms (14). Subsequent studies showed that greater symptom improvement was predicted by less activity in bilateral orbitofrontal cortex (OFC) and medial temporal gyrus during pre-treatment scans. Lower levels of interpersonal sensitivity predicted greater symptom improvement and were positively related to activity in left OFC (15).
The role of brain 5-HT and its receptors in IBS requires further study.
4. What is the effect of increased serotonin on gastrointestinal motor and secretory functions?
Over 50 years ago, it was demonstrated that 5-HT affects intestinal motility in humans, though initial studies suggested it stimulated small bowel motility and inhibited gastric and colonic phasic contractions (16,17). Sigmoid colonic motility appears to be increased in patients with IBS. Involvement of 5-HT in the dysmotility observed in IBS remains unclear, although data show a possible relationship between endogenous concentrations of 5-HT and sigmoid colonic motility recorded in both IBS and healthy subjects. There were significant correlations of fed sigmoid colonic motor activity index with platelet-depleted plasma 5-HT concentration in IBS patients and healthy volunteers; the R values suggest that the 5-HT level explains less than 20% of the variance in the postprandial colonic motility (18).
There are, however, other human data suggesting a role of 5-HT mechanisms in colonic motility in disease states and in pharmacological models. First, carcinoid diarrhea (the disease associated with highest endogenous 5-HT levels) is associated with exaggerated colonic tone, particularly postprandially (19). Second, the 5-HT4 agonist, tegaserod, increases colonic tone and phasic contractility (20). Third, 5-HT3 antagonists inhibit the increase in colonic tone after a meal in health and in patients with carcinoid diarrhea (21,22).
Serotonin causes secretion; carcinoid diarrhea is a classical example of a secretory diarrhea (23). The secretory effects of serotonin are mediated through different receptors: 5-HT induces secretion across human ileal mucosa via a receptor of the 5-HT4 subtype, whereas a receptor of the 5-HT2A subtype appears to mediate the effect in human sigmoid colon (24).
5. Is intestinal serotonin reuptake abnormal in functional GI disorders?
Serotonin is released diffusely into the lamina propria and its action is rapidly terminated by reuptake by nerve terminals, enterocytes and vascular endothelial cells. Reuptake of 5-HT requires active co-transport with Na+ by the serotonin transporter (SERT), the driving force for uptake being the sodium gradient generated by the basolaterally located Na+, K+ATPase. The functional activity of SERT depends on its membrane expression.
A recent report fully characterized SERT in human intestinal mucosa; expression is localized predominantly to the apical and intracellular compartments and is distributed throughout the crypt-villus axis. There is higher SERT mRNA expression in the human small intestine compared with colon (ileum >> duodenum >> jejunum). These data show there is a functional SERT in human intestinal epithelial cells, capable of removing intraluminal serotonin. This is relevant since serotonin is released by nutrients and serves as a “taste” mechanism, activating local and long reflexes through activation of intrinsic afferents (25). However, the presence of SERT is important for homeostatic control, to prevent an excessive stimulation of secretion or motility by the serotonin (26).
At the present time, several serotonin reuptake inhibitors are available to alter the biological function of 5-HT. While these medications may alter small bowel transit or colonic tone (27,28), they have limited effects on visceral sensation in health or IBS (29). In the future, the level of intestinal serotonin may be more directly influenced by novel specific inhibitors of type 1 tryptophan hydroxylase 1, the rate-limiting enzyme for 5-HT synthesis (30).
6. Are tissue SERT levels abnormal in IBS?
SERT expressed on platelet membranes of D-IBS patients is reduced (6). SERT platelet function may influence levels of circulating 5-HT. However, a controversial question in the literature is: Are rectal mucosal SERT levels abnormal in IBS? Coates et al. reported that both D-IBS and C-IBS had exceedingly low expression of SERT mRNA in rectal mucosa (31). These results were not replicated in a study that included several additional levels of validation in the assays performed, including measurements at multiple sites in the left colon and at two time points separated by an average of three months (32). Moreover, a preliminary report of SERT in mucosal biopsies from gastric antrum, duodenum, and rectum in IBS and functional dyspepsia also failed to show any difference between C-IBS and healthy controls (33). Finally, levels in duodenal biopsies of 5-HT and SERT, but not of TpH-1 were increased in C-IBS compared to controls (11).
In summary, the much heralded finding of markedly decreased SERT in rectal mucosa of patients with IBS has not been replicated. Although a commentary suggested, “There is no obvious explanation for these conflicting findings other than patient heterogeneity” (34), it appears that different assay methodologies, including the selection of the control protein for comparison of expression of SERT mRNA, as well as the greater validation steps conducted in the more recent studies (32,33) suggest that the expression of SERT in rectal mucosa is not altered in IBS. It is also still unclear why D-IBS and C-IBS showed the same expression level (31), and the mechanisms whereby the reduced SERT in RNA was associated with the different phenotypes were not mechanistically investigated in patients.
7. What are recent advances in the genetics of serotonin receptors and SERT in IBS or affective disorders in IBS?
This topic has been extensively reviewed elsewhere (35). After several individual studies of SERT (SLC6A4) genetics and IBS, a meta-analysis (36) concluded that, in combined or separated groups of Caucasian and Asian cohorts, there was not a significant odds ratio for any specific genotype of 5-HTTLPR (the polymorphism is the promoter for the gene for SLC6A4) and IBS, confirming the original and largest individual studies in Caucasian (37) and Asian patients (38).
On the other hand, there is some evidence that 5-HTTLPR genotype modifies the risk for depressive episodes in patients with IBS (39), and another study suggested that 5-HTTLPR genotype in combination with α2C-Del (322–325), which is a deletion in the α2-adrenergic receptor gene, is associated with high somatic symptom scores in IBS (37). The significance of these findings is unclear. It is worth noting that the prevalence of the α2C-Del (322–325) genotype was low (<10%) in Caucasians, and this finding has not yet been replicated.
Studies of the association between motor and sensory functions and carrying the 5-HTTLPR genotype variation have been recently explored in work from our laboratory based on studies conducted in healthy controls and patients with functional gastrointestinal disorders. Specifically, small bowel and colonic transit did not differ significantly when subjects were stratified for LL genotype versus the combined group that carries the short allele [LS or SS genotype (40)]. On the other hand, there was a significant association between 5-HTTLPR LS/SS genotype and increased rectal compliance and increased pain ratings, particularly at sub-noxious levels of distensions. These data suggest that the endophenotype of visceral hypersensitivity in IBS may be partly related to 5-HTTLPR. Interestingly, the increased pain sensation is not attributable to abnormal rectal compliance in association with the genotype, since rectal compliance was actually higher with the LS/SS genotype (41).
A first report documents the association of D-IBS with a functional variant in the microRNA-510 (miR-510) target site of the 5-HT receptor type 3E gene. The authors investigated the untranslated regions (UTRs) of the 5-HT receptor type 3 subunit genes, HTR3A and HTR3E. The novel HTR3E 3'UTR variant c.*76G>A (rs62625044) was associated with female D-IBS compared to controls in patients from United Kingdom, and the finding was replicated in patients from Germany. This genetic variation appears to be functionally relevant since, in a reporter assay, c.*76G>A affected binding of miR-510 to the HTR3E 3'UTR and caused elevated luciferase expression. HTR3E and miR-510 co-localize in enterocytes of the gut epithelium. This suggests that microRNA related expression regulates a 5-HT receptor gene with a cis-regulatory variant affecting this regulation and it appears to be associated with female D-IBS (42). These highly interesting findings are worthy of further study.
8. Are there examples of pharmacogenetics related to serotonergic agents?
Two complementary studies have been published that suggest 5-HTTLPR genetic polymorphisms influence the efficacy of serotonergic treatments. Thus, efficacy of alosetron in D-IBS patients with L/L genotype was associated with greater effect on colonic transit than S/S and S/L genotypes (43).
The 5-HTTLPR genetic polymorphisms also influenced the efficacy of tegaserod treatment in C-IBS patients on clinical efficacy with the L/L genotype being associated with poorer response than S/S and S/L genotypes (44). Since the short allele results in reduced SERT synthesis, the allele reduces reuptake of serotonin, and this could hypothetically result in more competition (and less efficacy) for alosetron and more 5-HT stimulation of post-synaptic 5-HT3 or 5-HT4 receptors (e.g., on cholinergic neurons) resulting in greater efficacy with the 5-HT4 agonist, tegaserod.
9. Are there new treatments involving novel serotonergic agents?
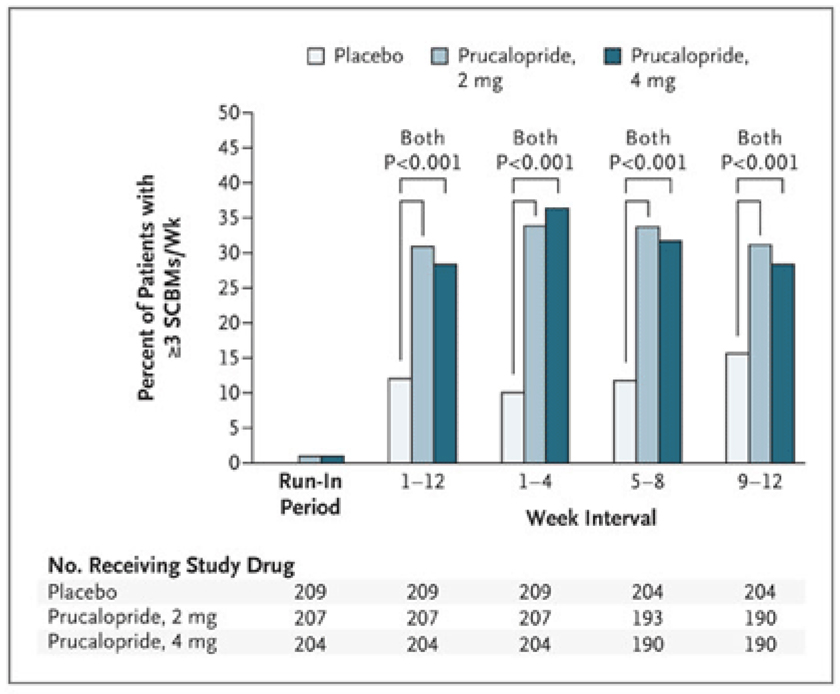
Several new 5-HT4 agonists that are prokinetic, especially in the colon, deserve further study. The attraction to these agents is that their action on 5-HT4 receptors is more specific than with older agents. These agents include prucalopride (Figure 2), ATI-7505 and TD-5108 (45–47). An in vitro study of peristaltic responses suggested that effects of 5-HT4 agonists could be enhanced by combination with an opiate antagonist (48); however, a human study showed that oral naltrexone (μ and δ antagonist) does not enhance tegaserod (5-HT4 agonist)-induced acceleration of colonic transit (49).
Figure 2.
Effect of prucalopride on the proportion of patients having an average of three or more spontaneous, complete bowel movements (SCBMs) per week. Reproduced from ref. 45, Camilleri M, et al. N Engl J Med 2008;358:2344–2354.
5-HT3 antagonists are efficacious in the treatment of D-IBS. A recent meta-analysis demonstrated that both agents, alosetron and cilansetron, were efficacious in D-IBS patients and in both genders (50). Another meta-analysis updated the information about alosetron and reached the same conclusions (51). A new 5-HT3 antagonist, ramosetron, is in development and demonstrates the typical efficacy over placebo at the 5 and 10 µg doses, but not at the 1 µg dose (52).
The big question is whether these 5-HT3 antagonists and 5-HT4 agonists will be approved for more widespread use, given the restricted access approval for alosetron, lack of approval of cilansetron, and withdrawal of tegaserod. It is still unclear whether 5-HT3 antagonists really cause ischemic colitis, given the lack of support for the mechanisms proposed for the development of ischemic colitis (53) and the lack of effect of alosetron on mesenteric flow in an experimental animal model (54). On the other hand, there is little doubt that the dose of 5-HT3 antagonist has to be carefully titrated and monitored to avoid development of constipation, since these agents have a significant benefit on diarrhea and urgency in IBS and the retardation of colonic transit demonstrated with alosetron (55).
CONCLUSION
Serotonin and serotonergic agents will continue to be topical and are likely to play a pivotal role in the mechanisms and treatment of gastrointestinal diseases, particularly the lower functional gastrointestinal disorders. In the future, it is anticipated that the interaction with other neurotransmitters and inflammation will be a subject of research as the role of 5-HT in the biology and potential for treatment of these and other diseases is clarified.
Acknowledgments
Dr. Camilleri is funded in part by grants RO1 DK-54681, RO1 DK-67071 and K24 DK-02638 from National Institutes of Health. I thank Mrs. Cindy Stanislav for excellent secretarial assistance.
REFERENCES
- 1.Dunlop SP, Coleman NS, Blackshaw PE, Perkins AC, Singh G, Marsden CA, Spiller RC. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–357. doi: 10.1016/s1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 2.Coleman NS, Foley S, Dunlop SP, Wheatcroft J, Blackshaw E, Perkins AC, Singh G, Marsden CA, Holmes GK, Spiller RC. Abnormalities of serotonin metabolism and their relation to symptoms in untreated celiac disease. Clin Gastroenterol Hepatol. 2006;4:874–881. doi: 10.1016/j.cgh.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Houghton LA, Atkinson W, Whitaker RP, Whorwell PJ, Rimmer MJ. Increased platelet depleted plasma 5-hydroxytryptamine concentration following meal ingestion in symptomatic female subjects with diarrhoea predominant irritable bowel syndrome. Gut. 2003;52:663–670. doi: 10.1136/gut.52.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cremonini F, Camilleri M. Of actors, bolting horses, and drops in oceans! Gut. 2003;52:619–621. doi: 10.1136/gut.52.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuo XL, Li YQ, Yang XZ, Guo M, Guo YT, Lu XF, Li JM, Desmond PV. Plasma and gastric mucosal 5-hydroxytryptamine concentrations following cold water intake in patients with diarrhea-predominant irritable bowel syndrome. J Gastroenterol Hepatol. 2007;22:2330–2337. doi: 10.1111/j.1440-1746.2006.04772.x. [DOI] [PubMed] [Google Scholar]
- 6.Bellini M, Rappelli L, Blandizzi C, Costa F, Stasi C, Colucci R, Giannaccini G, Marazziti D, Betti L, Baroni S, Mumolo MG, Marchi S, Del Tacca M. Platelet serotonin transporter in patients with diarrhea-predominant irritable bowel syndrome both before and after treatment with alosetron. Am J Gastroenterol. 2003;98:2705–2711. doi: 10.1111/j.1572-0241.2003.08669.x. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2006;130:34–43. doi: 10.1053/j.gastro.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 8.Spiller RC, Jenkins D, Thornley JP, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miwa J, Echizen H, Matsueda K, Umeda N. Patients with constipation-predominant irritable bowel syndrome (ibs) may have elevated serotonin concentrations in colonic mucosa as compared with diarrhea-predominant patients and subjects with normal bowel habits. Digestion. 2001;63:188–194. doi: 10.1159/000051888. [DOI] [PubMed] [Google Scholar]
- 10.Minderhoud IM, Oldenburg B, Schipper ME, ter Linde JJ, Samsom M. Serotonin synthesis and uptake in symptomatic patients with Crohn's disease in remission. Clin Gastroenterol Hepatol. 2007;5:714–720. doi: 10.1016/j.cgh.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Kerckhoffs AP, ter Linde JJ, Akkermans LM, Samsom M, Trypsinogen IV. serotonin transporter transcript levels and serotonin content are increased in small intestine of irritable bowel syndrome patients. Neurogastroenterol Motil. 2008;20:900–907. doi: 10.1111/j.1365-2982.2008.01100.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang SH, Dong L, Luo JY, Gong J, Li L, Lu XL, Han SP. Decreased expression of serotonin in the jejunum and increased numbers of mast cells in the terminal ileum in patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:6041–6047. doi: 10.3748/wjg.v13.45.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Mahony S, Chua AS, Quigley EM, Clarke G, Shanahan F, Keeling PW, Dinan TG. Evidence of an enhanced central 5HT response in irritable bowel syndrome and in the rat maternal separation model. Neurogastroenterol Motil. 2008;20:680–688. doi: 10.1111/j.1365-2982.2007.01065.x. [DOI] [PubMed] [Google Scholar]
- 14.Mayer EA, Berman S, Derbyshire SW, Suyenobu B, Chang L, Fitzgerald L, Mandelkern M, Hamm L, Vogt B, Naliboff BD. The effect of the 5-HT3 receptor antagonist, alosetron, on brain responses to visceral stimulation in irritable bowel syndrome patients. Aliment Pharmacol Ther. 2002;16:1357–1366. doi: 10.1046/j.1365-2036.2002.01287.x. [DOI] [PubMed] [Google Scholar]
- 15.Jarcho JM, Chang L, Berman SM, Suyenobu B, Naliboff BD, Lieberman MD, Ameen VZ, Mandelkern MA, Mayer EA. Neural and psychological predictors of treatment response in irritable bowel syndrome patients with a 5-HT(3) receptor antagonist - a pilot study. Aliment Pharmacol Ther. 2008;28:344–352. doi: 10.1111/j.1365-2036.2008.03721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendrix TR, Atkinson M, Clifton JA, Ingelfinger FJ. The effect of 5-hydroxytryptamine on intestinal motor function in man. Am J Med. 1957;23:886–893. doi: 10.1016/0002-9343(57)90298-x. [DOI] [PubMed] [Google Scholar]
- 17.Misiewicz JJ, Waller SL, Eisner M. Motor responses of human gastrointestinal tract to 5-hydroxytryptamine in vivo and in vitro. Gut. 1966;7:208–216. doi: 10.1136/gut.7.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houghton LA, Atkinson W, Lockhart C, Whorwell PJ, Keevil B. Sigmoid-colonic motility in health and irritable bowel syndrome: a role for 5-hydroxytryptamine. Neurogastroenterol Motil. 2007;19:724–731. doi: 10.1111/j.1365-2982.2007.00943.x. [DOI] [PubMed] [Google Scholar]
- 19.von der Ohe MR, Camilleri M, Kvols LK, Thomforde GM. Motor dysfunction of the small bowel and colon in patients with the carcinoid syndrome and diarrhea. N Engl J Med. 1993;329:1073–1078. doi: 10.1056/NEJM199310073291503. [DOI] [PubMed] [Google Scholar]
- 20.Di Stefano M, Miceli E, Mazzocchi S, Tana P, Missanelli A, Corazza GR. Effect of tegaserod on recto-sigmoid tonic and phasic activity in constipation-predominant irritable bowel syndrome. Am J Gastroenterol. 2007;102:1720–1726. doi: 10.1111/j.1572-0241.2007.01336.x. [DOI] [PubMed] [Google Scholar]
- 21.von der Ohe MR, Camilleri M, Kvols LK. A 5HT3 antagonist corrects the postprandial colonic hypertonic response in carcinoid diarrhea. Gastroenterology. 1994;106:1184–1189. doi: 10.1016/0016-5085(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 22.von der Ohe MR, Hanson RB, Camilleri M. Serotonergic mediation of postprandial colonic tonic and phasic responses in humans. Gut. 1994;35:536–541. doi: 10.1136/gut.35.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donowitz M, Binder HJ. Jejunal fluid and electrolyte secretion in carcinoid syndrome. Am J Dig Dis. 1975;20:1115–1122. doi: 10.1007/BF01070754. [DOI] [PubMed] [Google Scholar]
- 24.Borman RA, Burleigh DE. Heterogeneity of 5-HT receptors mediating secretion in the human intestine. Ann NY Acad Sci. 1997;812:224–225. doi: 10.1111/j.1749-6632.1997.tb48183.x. [DOI] [PubMed] [Google Scholar]
- 25.Grundy D. Signalling the state of the digestive tract. Auton Neurosci. 2006;125:76–80. doi: 10.1016/j.autneu.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Gill RK, Pant N, Saksena S, Singla A, Nazir TM, Vohwinkel L, Turner JR, Goldstein J, Alrefai WA, Dudeja PK. Function, expression, and characterization of the serotonin transporter in the native human intestine. Am J Physiol. 2008;294:G254–G262. doi: 10.1152/ajpgi.00354.2007.Landmark article characterizing SERT in human intestinal and colonic epithelial cells.
- 27.Tack J, Broekaert D, Corsetti M, Fischler B, Janssens J. Influence of acute serotonin reuptake inhibition on colonic sensorimotor function in man. Aliment Pharmacol Ther. 2006;23:265–274. doi: 10.1111/j.1365-2036.2006.02724.x. [DOI] [PubMed] [Google Scholar]
- 28.Chial HJ, Camilleri M, Burton D, Thomforde G, Olden KW, Stephens D. Selective effects of serotonergic psychoactive agents on gastrointestinal functions in health. Am J Physiol. 2003;284:G130–G137. doi: 10.1152/ajpgi.00266.2002. [DOI] [PubMed] [Google Scholar]
- 29.Kuiken SD, Tytgat GN, Boeckxstaens GE. The selective serotonin reuptake inhibitor fluoxetine does not change rectal sensitivity and symptoms in patients with irritable bowel syndrome: a double blind, randomized, placebo-controlled study. Clin Gastroenterol Hepatol. 2003;1:219–228. doi: 10.1053/cgh.2003.50032. [DOI] [PubMed] [Google Scholar]
- 30.Liu Q, Yang Q, Sun W, Vogel P, Heydorn W, Yu XQ, Hu Z, Yu W, Jonas B, Pineda R, Calderon-Gay V, Germann M, O'Neill E, Brommage R, Cullinan E, Platt K, Wilson A, Powell D, Sands A, Zambrowicz B, Shi ZC. Discovery and characterization of novel tryptophan hydroxylase inhibitors that selectively inhibit serotonin synthesis in the gastrointestinal tract. J Pharmacol Exp Ther. 2008;325:47–55. doi: 10.1124/jpet.107.132670.Novel class of compounds with potential to impact on 5-HT synthesis.
- 31.Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, Crowell MD, Sharkey KA, Gershon MD, Mawe GM, Moses PL. Molecular defects in mucosal serotonin content and decreased serotonin re-uptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–1664. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 32.Camilleri M, Andrews CN, Bharucha AE, Carlson PJ, Ferber I, Stephens D, Smyrk TC, Urrutia R, Aerssens J, Thielemans L, Göhlmann H, van den Wyngaert I, Coulie B. Alterations in expression of p11 and SERT in mucosal biopsy specimens of patients with irritable bowel syndrome. Gastroenterology. 2007;132:17–25. doi: 10.1053/j.gastro.2006.11.020.Robust appraisal of mucosal SERT in irritable bowel syndrome, questioning prior observations.
- 33.Foxx-Orenstein A, Camilleri M, Gershon MD, Linden DR, Mawe G, Lewis JT, Jensen KL, Talley N, Szurszewski JH, Zinsmeister A. Alterations in intestinal serotonin expression in dyspepsia and irritable bowel syndrome. Gastroenterology. 2007;132:A72. [Google Scholar]
- 34.Spiller R. Serotonin and GI clinical disorders. Neuropharmacology. 2008 Jul 19; doi: 10.1016/j.neuropharm.2008.07.016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Colucci R, Blandizzi C, Bellini M, Ghisu N, Tonini M, Del Tacca M. The genetics of the serotonin transporter and irritable bowel syndrome. Trends Mol Med. 2008;14:295–304. doi: 10.1016/j.molmed.2008.05.001.Outstanding scholarly review of genetics of SERT.
- 36.Van Kerkhoven LA, Laheij RJ, Jansen JB. Meta-analysis: a functional polymorphism in the gene encoding for activity of the serotonin transporter protein is not associated with the irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:979–986. doi: 10.1111/j.1365-2036.2007.03453.x.Excellent meta-analysis of the 5HTTLPR genetics in IBS.
- 37.Kim HJ, Camilleri M, Carlson PJ, Cremonini F, Ferber I, Stephens D, McKinzie S, Zinsmeister AR, Urrutia R. Association of distinct alpha(2) adrenoceptor and serotonin transporter polymorphisms with constipation and somatic symptoms in functional gastrointestinal disorders. Gut. 2004;53:829–837. doi: 10.1136/gut.2003.030882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park JM, Choi MG, Park JA, Oh JH, Cho YK, Lee IS, Kim SW, Choi KY, Chung IS. Serotonin transporter gene polymorphism and irritable bowel syndrome. Neurogastroenterol Motil. 2006;18:995–1000. doi: 10.1111/j.1365-2982.2006.00829.x. [DOI] [PubMed] [Google Scholar]
- 39.Jarrett ME, Kohen R, Cain KC, Burr RL, Poppe A, Navaja GP, Heitkemper MM. Relationship of SERT polymorphisms to depressive and anxiety symptoms in irritable bowel syndrome. Biol Res Nurs. 2007;9:161–169. doi: 10.1177/1099800407307822. [DOI] [PubMed] [Google Scholar]
- 40.Grudell AB, Camilleri M, Carlson P, Gorman H, Ryks M, Burton D, Baxter K, Zinsmeister AR. An exploratory study of the association of adrenergic and serotonergic genotype and gastrointestinal motor functions. Neurogastroenterol Motil. 2008;20:213–219. doi: 10.1111/j.1365-2982.2007.01026.x. [DOI] [PubMed] [Google Scholar]
- 41.Camilleri M, Busciglio I, Carlson P, McKinzie S, Burton D, Baxter K, Ryks M, Zinsmeister AR. Candidate genes and sensory functions in health and irritable bowel syndrome. Am J Physiol. 2008;295:G219–G225. doi: 10.1152/ajpgi.90202.2008.Novel observation of association of 5HTTLPR genetics and visceral pain.
- 42.Kapeller J, Houghton LA, Mönnikes H, Walstab J, Möller D, Bönisch H, Burwinkel B, Autschbach F, Funke B, Lasitschka F, Gassler N, Fischer C, Whorwell PJ, Atkinson W, Fell C, Büchner KJ, Schmidtmann M, van der Voort I, Wisser AS, Berg T, Rappold G, Niesler B. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet. 2008;17:2967–2977. doi: 10.1093/hmg/ddn195.Novel observation of potential role of 5-HT3e receptor genetics and IBS.
- 43.Camilleri M, Atanasova E, Carlson PJ, Ahmad U, Kim HJ, Viramontes BE, McKinzie S, Urrutia R. Serotonin-transporter polymorphism pharmacogenetics in diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2002;123:425–432. doi: 10.1053/gast.2002.34780. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Nie Y, Xie J, Tang W, Liang P, Sha W, Yang H, Zhou Y. The association of serotonin transporter genetic polymorphisms and irritable bowel syndrome and its influence on tegaserod treatment in Chinese patients. Dig Dis Sci. 2007;52:2942–2949. doi: 10.1007/s10620-006-9679-y.Second pharmacogenetic study documenting relevance of 5-HTTLPR (gene for SERT) in response to 5-HT4 agonist.
- 45.Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008;358:2344–2354. doi: 10.1056/NEJMoa0800670.Robust clinical trial with a novel selective 5-HT4 agonist.
- 46.Camilleri M, Vazquez-Roque MI, Burton D, Ford T, McKinzie S, Zinsmeister AR, Druzgala P. Pharmacodynamic effects of a novel prokinetic 5-HT receptor agonist, ATI-7505, in humans. Neurogastroenterol Motil. 2007;19:30–38. doi: 10.1111/j.1365-2982.2006.00865.x.Selective 5-HT4 agonist and its effects on GI transit.
- 47.Camilleri M, Manini M, McKinzie S, Sweetser S, Grudell A, Ryks MD, Baxter K, Burton DD, Goldberg MR, Kitt MM, Li Y, Zinsmeister AR. Dose-related effects of TD-5108, a selective 5-HT4 receptor agonist with high intrinsic activity, on gastrointestinal (GI) and colonic transit in healthy volunteers. Neurogastroenterol Motil. 2008;20 Suppl.2:6. [Google Scholar]
- 48.Grider JR, Foxx-Orenstein AE, Jin JG. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology. 1998;115:370–380. doi: 10.1016/s0016-5085(98)70203-3. [DOI] [PubMed] [Google Scholar]
- 49.Foxx-Orenstein AE, Camilleri M, Szarka LA, McKinzie S, Burton D, Thomforde G, Baxter K, Zinsmeister AR. Does co-administration of a non-selective opiate antagonist enhance acceleration of transit by a 5-HT4 agonist in constipation-predominant irritable bowel syndrome? A randomized controlled trial. Neurogastroenterol Motil. 2007;19:821–830. doi: 10.1111/j.1365-2982.2007.00944.x. [DOI] [PubMed] [Google Scholar]
- 50.Andresen V, Montori VM, Keller J, West CP, Layer P, Camilleri M. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2008;6:545–555. doi: 10.1016/j.cgh.2007.12.015.Comprehensive meta-analysis demonstrating efficacy of 5-HT3 antagonist class of drugs.
- 51.Rahimi R, Nikfar S, Abdollahi M. Efficacy and tolerability of alosetron for the treatment of irritable bowel syndrome in women and men: a meta-analysis of eight randomized, placebo-controlled, 12-week trials. Clin Ther. 2008;30:884–901. doi: 10.1016/j.clinthera.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Matsueda K, Harasawa S, Hongo M, Hiwatashi N, Sasaki D. A Phase II trial of the novel serotonin type 3 receptor antagonist ramosetron in Japanese male and female patients with diarrhea-predominant irritable bowel syndrome. Digestion. 2008;77:225–235. doi: 10.1159/000150632. [DOI] [PubMed] [Google Scholar]
- 53.Camilleri M. Is there an experimental basis for the development of ischaemic colitis as a result of 5-HT3 antagonist treatment? Neurogastroenterol Motil. 2007;19:77–84. doi: 10.1111/j.1365-2982.2006.00861.x. [DOI] [PubMed] [Google Scholar]
- 54.Grundy D, McLean P, Stead R. Impact of 5-HT3 receptor blockade on colonic haemodynamic responses to ischaemia and reperfusion in the rat. Neurogastroenterol Motil. 2007;19:607–616. doi: 10.1111/j.1365-2982.2007.00938.x.Experimental study exploring a prototype 5-HT3 antagonist and effects of baseline and post-occlusion mesenteric flow.
- 55.Viramontes BE, Camilleri M, McKinzie S, Pardi DS, Burton D, Thomforde GM. Gender-related differences in slowing colonic transit by a 5-HT3 antagonist in subjects with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2001;96:2671–2676. doi: 10.1111/j.1572-0241.2001.04138.x. [DOI] [PubMed] [Google Scholar]