Abstract
Objectives
Early testing of aerosolized sargramostim therapy demonstrated anecdotal clinical responses in patients with metastatic melanoma associated with emergence of systemic anti-tumor immunity. In order to improve the clinical and immunological efficacy of therapy without compromising patient safety, we performed a further dose escalation trial in patients with metastatic melanoma.
Methods
We conducted a dose-escalation clinical trial of HLA-A2+ patients with metastatic melanoma to the lung treated with aerosolized GM-CSF (500–2000ug/dose, with increments of 250ug/dose/cohort) twice/day on days 1–7 & 15–21 every 28 days until progression or severe toxicity to find a dose where majority of patients develop anti-tumor immunity. Five patients were treated per each dose level. Clinical, immune, and safety parameters were examined.
Results
The study accrued 40 patients. Toxicity was acceptable. All doses levels were exhausted without identifying a dose of GM-CSF at which majority of patients (≥ 3 of 5) demonstrated significant up-regulation of anti-tumor immunity. Three of 16 patients that were tetramer positive for at least one melanoma antigen (e.g. MART-1) pre-treatment developed an immune response (IR) to different tumor antigens. Two of 9 patients who were tetramer negative to all melanoma antigens pre-treatment developed an IR against gp100. The greatest changes in anti-tumor immunity occurred at the highest dose levels.
Conclusions
A dose of aerosolized GM-CSF capable of inducing anti-tumor immunity in the majority of patients was not reached. All tested doses were well tolerated. The greatest increase in anti-tumor T cell immune responses was achieved at the highest doses of GM-CSF.
Keywords: GM-CSF, melanoma, aerosol, immunotherapy
Introduction
The presence of increased concentrations of sargramostim (granulocyte macrophage colony stimulating factor, GM-CSF) in the tumor/antigen-immune interface appears to result in the generation of effective systemic anti-tumor immunity and tumor regression1,2. Direct intra/peri tumor injections of recombinant GM-CSF have resulted in melanoma regression3–5. GM-CSF appears to function as a classic immune adjuvant potentiating the immunogenicity of co-administered antigen (vaccine) or endogenous malignancy. We previously reported a phase I trial of aerosolized GM-CSF administered twice/day on days 1–7 & 15–21 of a 28 day treatment cycle in patients with carcinomas metastatic to the lung. Due to concerns of inflammatory pulmonary/airway toxicities, the doses of GM-CSF tested in this study were very low: 60ug, 120ug and 240ug. The study identified no significant toxicity or impact on pulmonary function tests in the tested dose ranges6. Both patients with metastatic melanoma treated on this trial experienced dramatic prolongation of progression free survival. In a separate study, prolongation of progression free survival seemed to be associated with the emergence of melanoma specific cytotoxic T lymphocytes in peripheral blood suggesting that the effect of aerosolized GM-CSF may be immune (T cell) mediated 6,7.
Based on these findings, we hypothesized that aerosol delivery of GM-CSF to the immune/tumor interface in the lung could promote tumor-antigen presentation by tumor associated antigen presenting cells leading to systemic, anti-melanoma immunity. Increasing the dose of aerosol GM-CSF further, may lead to more effective autologous anti-tumor immunization and improved clinical outcomes. Thus, we conducted a step-wise dose escalation clinical trial of HLA-A2+ patients with stage IV melanoma involving the lung (and other sites of metastases) that were treated with aerosolized GM-CSF at doses ranging from 500ug to 2000ug (250ug/dose increments) administered twice/day on days 1–7 & 15–21 of a 28 day cycle until intolerable toxicity of tumor progression. All patients were evaluated for safety and immunological efficacy (emergence of melanoma specific T lymphocytes in the peripheral blood). Increased numbers of melanoma specific cytotoxic T lymphocytes (CTL) in peripheral blood would suggest GM-CSF mediated up-regulation of antigen presentation of tumor antigens by the tumor associated antigen-presenting cells leading to systemic anti-tumor immunity. The method to quantitate emergence of tumor-specific CTL immunity was the tetramer assay for HLA-A2 cognant melanoma differentiation antigen specific peptides (MART-127–35, gp100209–217 or tyrosinase368–376). Herein we present the clinical and laboratory results of this study and discuss these observations on future applications of aerosol GM-CSF therapy.
Materials and Methods
The trial enrolled patients who were ≥18 years of age with histologically proven melanoma with radiographic evidence of involvement of the lungs (other sites of metastases in addition to the lung were allowed). Eligibility criteria included: measurable disease, HLA-A2+ status, and FEV1 ≥ 65% of expected and at least 1.5L. Contradictions to study entry included: unsatisfactory hematologic or blood chemistry profiles, known immune deficiency or ongoing immunosuppressive therapy, ECOG performance status of 3 or 4, uncontrolled infection, discontinuation of other cancer therapy <4 weeks pre-registration, central nervous system metastases stable less than 3 months, other malignancies within the last 5 years, and inability to provide informed written consent. All women of child-bearing potential had a serum pregnancy test within 7 days of registration (pregnant or lactating women were ineligible). Patients were assigned to the currently open dose level of GM-CSF. Aerosolized GM-CSF was administered twice daily on days 1–7 and 15–21 of a 28 day cycle. The dose (ug) levels under investigation were: 500, 750, 1000, 1250, 1500, 1750, and 2000. Aerosolized GM-CSF was self-administered via the Pari-LC nebulizer system (Starnberg, Germany).
Each patient underwent a peripheral blood collection, tumor burden assessment (RECIST) and toxicity evaluation using the NCI-CTC version 2.0 criteria prior to the first 2 cycles of treatment and every other cycle thereafter until treatment discontinuation. Patients who developed moderate dyspnea (grade 3) had to hold further study treatment until symptoms improved to ≤grade 2 and reduce GM-CSF dose by 50% for subsequent treatments. If symptoms did not improve within 2 weeks, treatment was discontinued. Patients who developed a grade 4 dyspnea immediately discontinued treatment.
Study design
The goal of this dose escalation trial was to find a dose of aerosolized GM-CSF with an acceptable level of toxicity and evidence of increased frequencies of anti-melanoma differentiation antigen specific cytotoxic T lymphocytes (CTL) in at least 3 of 5 patients treated at the given dose level. A dose level was considered to be excessively toxic if ≥2 (or ≥4) of the first 5 (or 10) patients enrolled onto that dose level developed a dose limiting toxicity where dose limiting toxicities included grade 4 hematologic toxicity for more than 7 days or leukocytosis > 50,000 for more than 7 day, any grade 4 pulmonary toxicity, or any ≥ grade 3 non-hematologic toxicity. A dose level was considered to have a promising level of immuno-stimulatory activity if at least 3 of 5 (or 7 of 10) patients treated at that dose level developed an immune response where an immune response was defined as at least a 5-fold increase in frequency of peripheral blood anti-melanoma tumor-specific CTL from pre-treatment levels within the first 2 cycles of treatment if pre-treatment levels were detectable (tetramer frequencies of ≥ 0.05% of circulating lymphocytes); or detection of anti-melanoma CTL immune responses within the first 2 cycles of treatment if pre-treatment levels were not detectable (<0.05%). The scheme by which dose escalation was carried out is as follows: five patients were entered at a given dose level. If 2 or fewer of these 5 patients developed an immune response, then 5 other patients would be enrolled onto the next higher dose level. If 3 or more patients developed an immune response, 5 additional patients would be enrolled at the current dose level (total of 10 patients). If 7 or more the 10 patients enrolled on the present dose level develops an immune response, enrollment would stop and this would be declared the optimal immunologically effective (IE) dose. If not, 5 other patients would be enrolled on the next higher dose level. Dose escalation would continue until excessive toxicity was encountered, the IE was found, or all 7 dose levels were exhausted.
Sargramostim (GM-CSF, Leukine®)
Yeast derived, glycosylated recombinant GM-CSF (Sargramostim/Leukine, Berlex Labs, Settle WA) was provided by the manufacturer. Each vial contained powdered GM-CSF that was reconstituted with sterile or bacteriostatic water for injection, per package insert. Patients were instructed on dose preparation prior to the first cycle of therapy.
Nebulization
Reconstituted GM-CSF (with sterile or bacteriostatic water) was added into a nebulizer bowl of a PAR LC PLUS nebulizer connected to a standard air compressor (Pulmo-Aide; DeVilbiss) to generate the aerosol mist. When necessary (at the low dose range) Bronchosaline (Blalrex labs, Columbus, OH) was added to the nebulizer bowl to bring up the volume of the GM-CSF to 2mL (minimal volume for nebulization). Patients inhaled the mist through a valved mouthpiece provided with the nebulizer kit. Aerosol treatments were typically completed in 5−15 min. The first aerosol GM-CSF treatment was delivered under medical supervision to be certain that maximal aerosol drug delivery is achieved and no immediate toxicity was experienced. All subsequent treatments were self administered.
Monitoring of pulmonary functions
Patients were instructed on the use of telephonic remote spirometry (Asthmalong, Datalog, Stillwater, MN) at the time of nebulization training. The Asthmalog reliably measures FVC, FEV1, peak flow and FEF25–75. The instrument also provides feedback to the patient indicating poor effort or significant change (>10%) in tested parameters (red/green light on the instrument). Following each set of tests the patients transmitted the information to Datalog and from there the PFTs were faxed to the Mayo Clinic study data center for review.
Immune monitoring
Immune response monitoring was performed using peripheral blood collections obtained prior to immunization and every month while on study. One hundred milliliters of blood were collected into heparinized vacutainer tubes prior to each peptide vaccination. Immediately following collection, peripheral blood mononuclear cells (PBMC) were isolated using a density gradient (Ficol-hypaque, Amersham, Uppsala, Sweden) and frozen in complement-inactivated fetal calf serum with 10% DMSO (Stigma, St. Louis, MO) and then stored in liquid nitrogen. In order to minimize inter-assay variability, all individual patient samples were analyzed at the same time.
Immunophenotyping
Upon completion of sample collection for a given patients, all specimens (peripheral blood mononuclear cells, PBMCs) were batch analyzed for surface expression of the following CD antigens: CD3, CD4, CD8, CD11c, CD14, CD16, CD20, CD22, CD32, CD44, CD45, CD56, CD62, CD69, HLA-DR, CD80, CD83, CD86, and CD123. The stained cells were washed and fixed with 2% paraformaldehyde. Flow-cytometric analysis was performed using a FACSCalibur flow cytometer and analyzed by Cellquest (Becton-Dickinson, Franklin Lakes, NJ). Inter assay variability demonstrates a coefficient of variation (CV) of <10%.
Tetramer staining
For tetramer analysis of tumor antigen specific cytotoxic T lymphocytes (CTL), thawed PBMCs were stained with FITC conjugated anti-CD8, PC5 conjugated anti-human CD4, CD14 and CD19 and PE labeled HLA-A2 tetramers containing melanoma specific differentiation antigen peptides MART-127–35, gp100209–217 or tyrosinase368–376 (Beckman Coulter, Fullerton CA). Samples were analyzed by flow-cytometry and data was processed using Cellquest® software (Becton-Dickinson, Franklin Lakes, NJ). Gates were set on lymphocytes that were CD4, CD14 and CD19 (PC5) negative. Immunophenotyping of lymphocyte subsets (CD3, CD4, CD8, CD20, CD22, and CD14) was performed using FITC or PE labeled monoclonal antibodies in accordance to manufacturer’s instructions (Becton-Dickinson, Franklin Lakes, NJ). Samples were analyzed by flow-cytometry using the same software as described above. Inter assay CV was consistently <10% for all tetramers.
Results
Clinical outcomes
The presented clinical trial enrolled a total of 40 patients. Four patients declined participation prior to receiving treatment. One patient was found to be ineligible due to inadequate FEV1 levels. A 35 year old male who entered the study at the 500 ug dose level died of rapid progression of disease within 13 days of registration. This patient’s data were considered non-informative in terms of the study endpoint and thus, not included in the analysis. Another patient was recruited to the 500 ug dose level to ensure 5 evaluable patients were treated at this dose level. Patient and tumor characteristics at registration are presented in Table 1 by dose level.
Table 1.
Patient characteristics at study entry
| Patient characteristics | Dose of GM-CSF per cohort | ||||||
|---|---|---|---|---|---|---|---|
| 500 ug (n=5) |
750 ug (n=5) |
1000 ug (n=5) |
1250 ug (n=5) |
1500 ug (n=3) |
1750 ug (n=5) |
2000 ug (n=6) |
|
| Male | 80% | 80% | 80% | 60% | 100% | 60% | 60% |
|
Median age (range) |
48 (37–78) |
55 (23–74) |
56 (48–77) |
60 (52–67) |
80 (61–84) |
72 (57–84) |
56 (38–79) |
| ECOG PS | |||||||
| 0 | 80% | 100% | 40% | 100% | 67% | 40% | 83% |
| 1 | 20% | 0% | 60% | 0% | 33% | 60% | 17% |
| Prior Radiation Therapy | 40% | 20% | 40% | 20% | 0% | 40% | 33% |
| Prior systemic therapy | 60% | 80% | 60% | 60% | 33% | 60% | 50% |
| Metastatic sites other than lung (M1c) | 80% | 40% | 20% | 100% | 33% | 60% | 50% |
Dose escalation was not terminated due to excessive toxicity and continued through all planned doses without reaching the above defined IE dose. Three patients developed severe toxicities (≥ grade 3) considered “probably”, “possibly”, or “definitely” related to treatment. Grade 4 dyspnea with grade 3 cough led to the discontinuation of study treatment in one patient after the their first administration of treatment at the 2000ug dose level and in a second patient after 1 cycle of treatment at the 1750ug dose level. Both patients immediately/completely responded to discontinuation of GM-CSF treatment and therapy with nebulized albuterol. Another patient who completed the first cycle of treatment at the 1000ug dose level developed grade 3 fatigue and grade 2 dyspnea but did not continue treatment due to disease progression. Grade 2 toxicities reported as probably”, “possibly”, or “definitely” related to treatment included: cough (one at the 750ug dose), anemia (one at 750ug), rash (one at 750ug), constipation (one at 1500ug), fatigue (one at 1750ug), dizziness (one at 2000ug) and nausea (one at 2000ug). None of the patients exhibited sufficient changes in lung volumes/flow rates to necessitate discontinuation of therapy based on remote spirometry (data not shown).
With regard to clinical benefit, one patient enrolled at the 2000ug dose level continues to receive study treatment ≥19.5 months post-registration maintaining a partial tumor response. Five patients demonstrated stable disease for more than 6 months (1 patient each of the following dose levels: 500ug, 750ug, 1500ug, 1750ug and 2000ug) prior to disease progression. Progression free survival and overall survival data, per dose level are presented in Table 2.
Table 2.
Clinical outcomes
| Dose level | Median number of cycles (range) | Immune response | Individual patient PFS in months | Individual patient OS in months |
|---|---|---|---|---|
|
500 ug (n=5) |
4 (2–6) |
unknown | 1.4, 4.8, 4.9 | 7.8, 19.3, 20.6 |
| no | 4.5 | 14.9 | ||
| yes | 8.3 | 48.9 | ||
|
750 ug (n=5) |
4 (1–9) |
no | 1.1, 1.9, 3.7,15.8 | 3.4, 4.0, 7.2, 51.2+ |
| yes | 4.6 | 24.7 | ||
|
1000 ug (n=5) |
1 (1–2) |
unknown | 0.9 | 2.1 |
| no | 1.0, 1.0, 1.1, 1.8 | 6.3, 7.5, 9.9, 18.9 | ||
|
1250 ug (n=5) |
1 (1–3) |
unknown | 0.9, 0.9, 1.0 | 1.4, 3.1, 5.3 |
| no | 2.7, 2.8 | 7.6, 17.4 | ||
|
1500 ug (n=3) |
4 (3–7) |
no | 2.8 | 7.2 |
| yes | 4.2, 10.3 | 6.2, 24.0 | ||
|
1750 ug (n=5) |
2 (1–6) |
unknown | 1.0 | 18.6 |
| no | 1.1, 1.9, 2.1, 8.2 | 2.7, 3.2, 10.4, 27.2+ | ||
|
2000 ug (n=6) |
4 (1–12+) |
unknown | 0.7 | 7.2 |
| no | 1.1, 4.8, 5.6, 8.3, | 7.5, 14.1+,16.8+, 17.9+ | ||
| yes | 19.5+ | 22.2+ |
bold faced numbers indicate patients who had a 2–4 fold increase in at least one tetramer positive CTL
Immune response
Effects of aerosolized GM-CSF on the frequency of melanoma specific CTL in peripheral blood
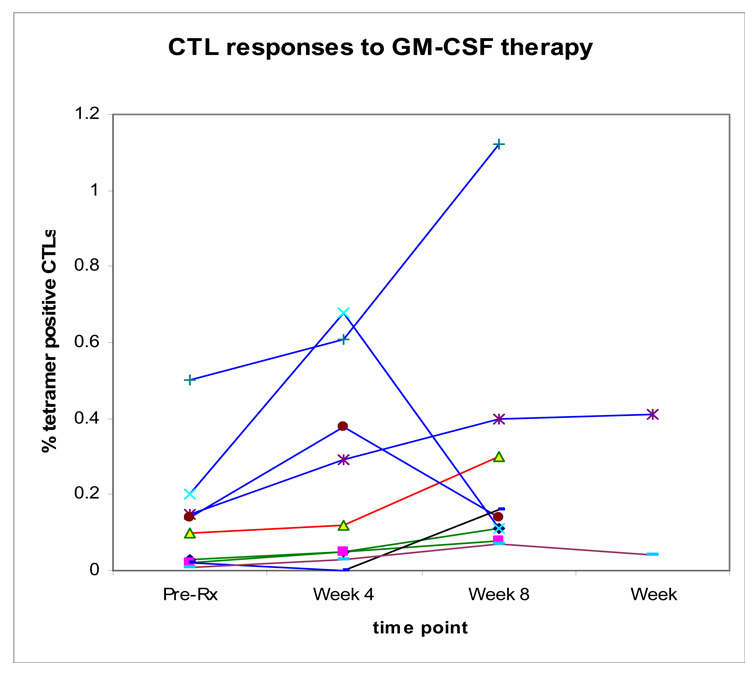
The primary immunologic objective of this study was to find a dose that would produce an immune response in a majority of patients without excessive toxicity (an IE dose). Post-treatment tetramer data were not available for 9 patients due to progression during the first 4 weeks of treatment (6 patients), adverse reaction on day 1 (1 patient), or failure to submit post-treatment blood specimens (2 patients). Of the 13 patients with tetramer data among the 20 patients enrolled on the 1250ug dose or lower, none of 4 patients that were tetramer positive to at least one melanoma specific peptide prior to treatment developed a immune response and 2 of 9 patients tetramer negative to the melanoma specific peptides prior to treatment developed an immune response to gp100. Of the 12 patients with tetramer data among the 14 patients enrolled at the 1500ug dose and higher, all 12 patients were tetramer positive to at least one melanoma specific peptide prior to treatment but the 3 patients who developed an immune response did so against peptides they were tetramer negative prior to treatment (gp100: 2 patients; tyrosinase: 1 patient). In addition, 1 of 4 patients at the 1750ug and 4 of 5 at the 2000ug dose had 2–4 fold increases in at least one melanoma specific tetramer positive CTL (MART-1: 1 patient; gp100: 4 patients; tyrosinase: 2 patients) (Table 3, Figure 1).
Table 3.
Changes in tetramer frequencies to melanoma differentiation antigens
| Dose level | Antigen reactivity | Non-detectable pre- and post-treatment | Non-detectable pre-treatment/detectable post treatment | Detectable pre-treatment/ non-detectable post treatment | Detectable pre-and post treatment |
|---|---|---|---|---|---|
|
500 (n=2) |
MART-1 | 1 | 0 | 1 [0.13↓] |
0 |
| gp100 | 1 |
1 [↑ 0.16] |
0 | 0 | |
| tyrosinase | 2 | 0 | 0 | 0 | |
|
1000 (n=5) |
gp100 | 3 |
1 [↑ 0.07] |
1 [0.06↓ ] |
0 |
| MART-1 & tyrosinase | 5 | 0 | 0 | 0 | |
|
1250 (n=2) |
gp100 | 0 | 0 | 2 [0.06↓ ;0.05↓] |
0 |
| MART-1 & tyrosinase | 2 | 0 | 0 | 0 | |
|
1500 (n=3) |
MART-1 | 0 | 0 | 0 | 3 [0.11↑0.12 ; 0.14↑0.17 ; 0.13↑0.23] |
| gp100 | 0 |
2 [↑ 0.08 ;↑ 0.11] |
0 | 1 [0.08↑0.13] |
|
| tyrosinase | 0 | 0 | 0 | 3 [0.09↑0.16 ; 0.26↓0.17; 0.20↑0.29] |
|
|
1750 (n=4) |
MART-1 | 0 | 0 | 3 [ 0.08↓ ; 0.07↓ ; 0.05↓] |
1 [0.18↓0.10] |
| gp100 | 0 | 0 | 1 [ 0.06↓] |
3 [0.08↑0.11 ; 0.08↓0.07; 0.09↓0.06] |
|
| tyrosinase | 1 | 0 | 0 | 3 [0.16↓0.06; 0.07↑0.11; 0.10↑0.30] |
|
|
2000 (n=5) |
MART-1 | 0 | 0 | 0 | 5 [0.06↑0.18; 0.12↑0.14; 0.86↓0.61; 0.16↓0.12; 0.28↓0.22] |
| gp100 | 0 | 0 | 0 | 5 [0.15↑0.40; 4.70↑20.1; 0.14↑0.38; 0.11↑0.12; 0.20↑0.68] |
|
| tyrosinase | 1 |
1 [↑ 0.18] |
3 [0.06↑0.10; 0.50↑1.12; 0.44↓0.16] |
Figure 1.
Changes in CTL frequency of to melanoma differentiation antigen peptides in 9 individual patients that demonstrated at least doubling of CTL frequency relative to baseline measurements: 1 patient treated at 500ug (black), 1 patient treated at 750ug ( ), 2 patients treated at 1500 (
), 2 patients treated at 1500 ( ), 1 patient treated at 1750ug (
), 1 patient treated at 1750ug ( ), and 4 patients treated at 2000ug (
), and 4 patients treated at 2000ug ( ).
).
There were immune cell subset immunophenotyping data for 22 of the 34 eligible patients. Two-fold or more increases from pre-treatment levels were most often seen in CD3/CD69 expressing activated T cells (6 of 22). Dendritic cell analysis revealed an increase in the frequency (at least doubling) of the following cell subsets: CD40/DR (6 of 22); CD11c/CD80 (5 of 22); CD11c/CD83 (7 of 22); and CD11c/CD86 (6 of 22) (Table 4).
Table 4.
Changes in select immune cell subsets demonstrating at least 2-fold change (pre vs post treatment measurements)
| Dose level | Percent positive cells | ||||
|---|---|---|---|---|---|
| CD3/69 | CD11c/CD80 | CD11c/CD83 | CD11c/CD86 | CD40/DR | |
|
500 (n=2) |
0.21 ↑ 0.44 | 0.01 ↑ 0.02 | 0.03 ↑ 0.12 | 0.24 ↑ 0.83 | |
|
750 (n=2) |
0.03 ↑ 0.16 | ||||
|
1000 (n=4) |
0.24 ↑ 0.83 0.25 ↑ 0.74 |
0.14 ↑ 0.93 | |||
|
1250 (n=2) |
0.03 ↑ 0.07 | 0.01 ↑ 0.03 | |||
|
1500 (n=3) |
0.01 ↑ 0.04 | 0.02 ↑ 0.05 | 0.20 ↑ 0.58 | 0.09 ↑ 0.27 0.13 ↑ 0.96 |
|
|
1750 (n=4) |
0.04 ↑ 0.10 | 0.01 ↑ 0.02 | 0.02 ↑ 0.06 0.07 ↑ 0.20 |
||
|
2000 (n=5) |
0.04 ↑ 0.14 0.12 ↑ 0.54 |
0.01 ↑ 0.03 | 0.16 ↑ 0.64 0.27 ↑ 0.75 0.33 ↑ 0.85 |
0.13 ↑ 0.39 0.20 ↑ 0.70 |
|
Clinical Benefit
In this phase I clinical trial of an otherwise un-selected population of patients with metastatic melanoma involving the lung, clinical outcomes data was only descriptive. In terms of clinical benefit, a 57 year old male with metastases limited to the lung enrolled at the 2000ug dose level developed tetramer positive CTL to tyrosinase but no significant changes in immune cell subsets, continues to receive study treatment ≥19.5 months post-registration maintaining a partial tumor response. Five other males aged 37 to 72 years had stable disease for more than 6 months (1 patient at 500ug; 750ug; 1500ug; 1750ug and 2000ug doses) prior to disease progression. The patient enrolled at the 500ug had multiple sites of metastases including the lung and developed tetramer positive CTL to gp100 and ≥2-fold increases in CD3/CD69 and CD11c/CD83 cell counts. The patient enrolled at the 750ug dose had lung metastases and little change in any immunologic parameters. The patient enrolled at the 1500ug dose had multiple sites of metastases including the lung and developed tetramer positive CTL to gp100 and little change in immune cell subsets. The patient enrolled at the 1750ug dose had metastases to the lung and little change in tetramer positive CTL but a 3-fold increase in CD11c/CD83 counts. The patient enrolled at the 2000ug dose had metastases to the lung and had a >2-fold increase in tetramer positive CTL to gp100 and tyrosinase and in CD11c/CD86 counts.
All patients have been followed to death or a minimum of 14 months. The reasons patients discontinued study treatment included tumor progression (31 patients) and grade 4 dyspnea (2 patients). At last contact, 1 patient was alive without disease, progression, 5 were alive with disease progression, and 28 were deceased due to disease progression. PFS and OS are presented by dose level in Table 2.
With regard to changes in immune (CTL-tetramer) response and associated clinical benefit of therapy, progression-free survival ranged from 4 to >19.5 months (median: 8.4 months) among the 5 patients who developed an immune response; 1–15.8 months (median: 2 months) in the 20 patients who did not develop an immune response; and 1–4.6 months (median: 1 month) among the 9 patients who did not have immune data (Table 2)
Discussion
The presented data demonstrate the safety of aerosolized GM-CSF therapy up to a dose of 2000ug twice/day and lend support to its hypothesized mechanism of action as an inducer of systemic anti-tumor immunity when administered to the tumor-immune system interface in the lung. The small numbers of patients, limited biospecimen availability for more extensive laboratory study and modest change in measured parameters (tetramers) are only suggestive and not definitive confirmation of effective anti-tumor immunomodulation (auto-immunization). The suggestion of clinical and immunological benefits in this phase I study in patients demonstrating at least doubling of the frequencies of melanoma specific CTL lend further support to efforts in the study of aerosol GM-CSF therapy as a means of cancer immunotherapy.
Our first experience with aerosolized GM-CSF suggested that the agent was safe at dose levels of up to 240ug delivered twice per day on days 1–7 & 15–21 of a 28 day treatment cycle8. All patients tolerated therapy very well with no significant toxicity. Interestingly, two of the treated patients with metastatic melanoma (stage M1c) demonstrated a partial remission and stabilization of disease progression for over 9 months. Encouraged by these results we embarked on two phase II clinical trials further evaluating the safety of the 240ug dose of aerosol GM-CSF for patients with either metastatic melanoma or metastatic renal cell carcinoma involving the lungs (submitted for publication). In both studies, aerosolized GM-CSF was well tolerated. However, clinical benefit was only anecdotal. In the few HLA-A2+ melanoma patients from whom we had available peripheral blood lymphocytes, we were able to identify a trend towards an increase in the frequency of melanoma specific CTL suggesting anti-tumor auto-immunization. These data were consistent with the observations in our “compassionate use” protocol for patients with a number of different metastatic cancers to the lungs undergoing aerosol GM-CSF therapy6. Additionally, data demonstrating alveolar macrophage activation using aerosolized GM-CSF for the treatment of pulmonary alveolar proteinosis further supported the hypothesis of aerosol GM-CSF driven anti-tumor auto-immunization and gave justification for further dose escalation of aerosolized GM-CSF. 9 Our current study further supports the notion of modulation of immune cell function (anti-tumor autoimmunization) by aerosolized GM-CSF delivered to metastatic melanoma in the lungs. Although the exact mechanisms remain unclear, the emergence of peripheral blood tumor specific CTL as a result of aerosol GM-CSF therapy, suggests at least an improvement in tumor antigen presentation by tumor-associated antigen presenting cells (e.g. alveolar macrophages). The modest systemic emergence of tumor specific CTL was associated with a trend towards improved clinical outcomes as has been suggested previously10.
Albeit limited in scope, our current study demonstrates the safety of aerosol GM-CSF delivery at doses up to 200 ug/treatment and further illustrates the potential of induction of anti-tumor auto-immunization with the emergence of peripheral blood tumor specific CTL. To fully understand the clinical and immunological benefits of this approach to therapy, dose escalation to a maximally tolerated dose (MTD) is indicated. We were unable to extend the dose escalation schema of the current study due to the prohibitive cost of further increases in GM-CSF dose resulting from the poor efficiency of the utilized aerosol delivery apparatus (Pari LC Plus). We are currently exploring alternate, more efficient means of aerosol drug delivery.
The current study demonstrates the plausibility of anti-tumor auto-immunization using aerosolized GM-CSF therapy of patients with metastatic melanoma to the lungs. The potential clinical benefits/applications of fully defining an effective/reproducible aerosol GM-CSF auto-immunization treatment protocol are not insignificant. Such efforts may complement ongoing T-cell immunomodulatory strategies in cancer therapy (e.g. anti-CTLA4). Utilization of aerosol GM-CSF as “adjuvant” treatment following tumor-ablative procedures (e.g. local radiation or cryoablation) may lead to greater anti-tumor auto-immunization efficacy (i.e. disruption of the immunosuppressive tumor microenvironment with local/regional release of antigens). Likewise, the ability to modulate pulmonary antigen presenting cells with aerosolized GM-CSF raises the possibility of aerosol GM-CSF as an immune adjuvant to aerosolized vaccines. Simultaneous aerosol delivery of GM-CSF with vaccine antigens may result in the development of both systemic and mucosal immunity, not achievable by other modes of immunization 11–15. This may be particularly relevant following the success of nasal mucosal immunization for influenza 16–23.
In summary, the presented data from our phase I clinical trial illustrates the safety of aerosol GM-CSF therapy and suggests a possible clinical and immunologic benefit of aerosolized GM-CSF therapy in patients with metastatic melanoma to the lungs. The data also suggest that further dose escalation (or prolonged treatment schedule) to maximally tolerated doses of GM-CSF may be warranted in an effort to identify a more clinically and immunologically effective dose that could be considered for further therapeutic efficacy, phase II testing.
Acknowledgments
Additional participating institutions include: Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA 52403 (Martin Wiesenfeld, M.D.); Sioux Community Cancer Consortium, Sioux Falls, SD 57105 (Loren K. Tschetter, M.D.); Mayo Clinic Scottsdale, Scottsdale, AZ 85259–5404 (Tom R. Fitch, M.D.); Missouri Valley Cancer Consortium, Omaha, NE 68106 (Gamini S. Soori, M.D.)
Footnotes
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-37417, CA-35195, CA-35101, CA-35415, CA-52352, CA-35103, CA-63849, and CA-60276 from the National Cancer Institute, Department of Health and Human Services as well as partial support from Berlex Labs Inc.
References
- 1.Dranoff G. GM-CSF-based cancer vaccines. Immunol Rev. 2002;188:147–154. doi: 10.1034/j.1600-065x.2002.18813.x. [DOI] [PubMed] [Google Scholar]
- 2.Dranoff G. GM-CSF-secreting melanoma vaccines. Oncogene. 2003;22:3188–3192. doi: 10.1038/sj.onc.1206459. [DOI] [PubMed] [Google Scholar]
- 3.Ridolfi L, Ridolfi R, Ascari-Raccagni A, et al. Intralesional granulocyte-monocyte colony-stimulating factor followed by subcutaneous interleukin-2 in metastatic melanoma: a pilot study in elderly patients. J Eur Acad Dermatol Venereol. 2001;15:218–223. doi: 10.1046/j.1468-3083.2001.00254.x. [DOI] [PubMed] [Google Scholar]
- 4.Ridolfi L, Ridolfi R. Preliminary experiences of intralesional immunotherapy in cutaneous metastatic melanoma. Hepatogastroenterology. 2002;49:335–339. [PubMed] [Google Scholar]
- 5.Hoeller C, Jansen B, Heere-Ress E, et al. Perilesional injection of r-GM-CSF in patients with cutaneous melanoma metastases. J Invest Dermatol. 2001;117:371–374. doi: 10.1046/j.0022-202x.2001.01427.x. [DOI] [PubMed] [Google Scholar]
- 6.Rao RD, Anderson PM, Arndt CA, et al. Aerosolized granulocyte macrophage colony-stimulating factor (GM-CSF) therapy in metastatic cancer. Am J Clin Oncol. 2003;26:493–498. doi: 10.1097/01.coc.0000037664.04141.D0. [DOI] [PubMed] [Google Scholar]
- 7.Rao RD, Markovic SN, Anderson PM. Aerosol therapy for malignancy involving the lungs. Curr Cancer Drug Targets. 2003;3:239–250. doi: 10.2174/1568009033481895. [DOI] [PubMed] [Google Scholar]
- 8.Anderson PM, Markovic SN, Sloan JA, et al. Aerosol granulocyte macrophage-colony stimulating factor: a low toxicity, lung-specific biological therapy in patients with lung metastases. Clin Cancer Res. 1999;5:2316–2323. [PubMed] [Google Scholar]
- 9.Tazawa R, Hamano E, Arai T, et al. Granulocyte-macrophage colony-stimulating factor and lung immunity in pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2005;171:1142–1149. doi: 10.1164/rccm.200406-716OC. [DOI] [PubMed] [Google Scholar]
- 10.Markovic SN, Suman VJ, Ingle JN, et al. Peptide vaccination of patients with metastatic melanoma: improved clinical outcome in patients demonstrating effective immunization. Am J Clin Oncol. 2006;29:352–360. doi: 10.1097/01.coc.0000217877.78473.a4. [DOI] [PubMed] [Google Scholar]
- 11.Sabin AB. Immunization against measles by aerosol. Rev Infect Dis. 1983;5:514–523. doi: 10.1093/clinids/5.3.514. [DOI] [PubMed] [Google Scholar]
- 12.Sabin AB, Albrecht P, Takeda AK, et al. High effectiveness of aerosolized chick embryo fibroblast measles vaccine in seven-month-old and older infants. J Infect Dis. 1985;152:1231–1237. doi: 10.1093/infdis/152.6.1231. [DOI] [PubMed] [Google Scholar]
- 13.Sabin AB, Fernandez de Castro J, Flores Arechiga A, et al. Clinical trials of inhaled aerosol of human diploid and chick embryo measles vaccine. Lancet. 1982;2:604. doi: 10.1016/s0140-6736(82)90673-0. [DOI] [PubMed] [Google Scholar]
- 14.Sabin AB, Flores Arechiga A, Fernandez de Castro J, et al. Successful immunization of infants with and without maternal antibody by aerosolized measles vaccine. II. Vaccine comparisons and evidence for multiple antibody response. Jama. 1984;251:2363–2371. [PubMed] [Google Scholar]
- 15.Sabin AB, Flores Arechiga A, Fernandez de Castro J, et al. Successful immunization of children with and without maternal antibody by aerosolized measles vaccine. I. Different results with undiluted human diploid cell and chick embryo fibroblast vaccines. Jama. 1983;249:2651–2662. [PubMed] [Google Scholar]
- 16.Stephenson I, Zambon MC, Rudin A, et al. Phase I evaluation of intranasal trivalent inactivated influenza vaccine with nontoxigenic Escherichia coli enterotoxin and novel biovector as mucosal adjuvants, using adult volunteers. J Virol. 2006;80:4962–4970. doi: 10.1128/JVI.80.10.4962-4970.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh Y, Ohta K, Kuno-Sakai H, et al. Local and systemic influenza haemagglutinin-specific antibody responses following aerosol and subcutaneous administration of inactivated split influenza vaccine. Vaccine. 1992;10:506–511. doi: 10.1016/0264-410x(92)90348-n. [DOI] [PubMed] [Google Scholar]
- 18.O'Hagan DT. Recent advances in vaccine adjuvants for systemic and mucosal administration. J Pharm Pharmacol. 1998;50:1–10. doi: 10.1111/j.2042-7158.1998.tb03298.x. [DOI] [PubMed] [Google Scholar]
- 19.Muszkat M, Yehuda AB, Schein MH, et al. Local and systemic immune response in community-dwelling elderly after intranasal or intramuscular immunization with inactivated influenza vaccine. J Med Virol. 2000;61:100–106. [PubMed] [Google Scholar]
- 20.McCarthy MW, Kockler DR. Trivalent intranasal influenza vaccine, live. Ann Pharmacother. 2004;38:2086–2093. doi: 10.1345/aph.1E191. [DOI] [PubMed] [Google Scholar]
- 21.Kuno-Sakai H, Kimura M, Ohta K, et al. Developments in mucosal influenza virus vaccines. Vaccine. 1994;12:1303–1310. doi: 10.1016/s0264-410x(94)80056-6. [DOI] [PubMed] [Google Scholar]
- 22.Denis F, Alain S, Ploy MC. [New routes of administration: epidermal, transcutaneous mucosal ways of vaccination] Med Sci (Paris) 2007;23:379–385. doi: 10.1051/medsci/2007234379. [DOI] [PubMed] [Google Scholar]
- 23.Boyce TG, Hsu HH, Sannella EC, et al. Safety and immunogenicity of adjuvanted and unadjuvanted subunit influenza vaccines administered intranasally to healthy adults. Vaccine. 2000;19:217–226. doi: 10.1016/s0264-410x(00)00171-7. [DOI] [PubMed] [Google Scholar]