Abstract
Objective
To monitor hematological indices in otherwise normal children with central precocious puberty (CPP) who underwent frequent venous sampling as part of a longitudinal clinical research study.
Study design
34 female subjects underwent frequent venous sampling (q10-20 min for 8-16 hrs) every 6 months for ≥ 3 years during and after their treatment with a GnRH analogue. Hgb, MCV and ferritin were measured prior to and following each phlebotomy session.
Results
At baseline, average Hgb was 12.5 ± 0.7 g/l. At conclusion of the first sampling session, Hgb fell 1.2 ± 0.1 g/l, remaining within the normal range for age. Upon 3-month follow-up, there was complete recovery of Hgb (12.6 ± 0.2 g/l). Longitudinal evaluation every 6-months for up to 3-years showed no significant differences in Hgb, MCV or ferritin levels from baseline. No clinically significant adverse effects attributable to phlebotomy were reported.
Discussion
When appropriate safety guidelines were followed, both acute and long-term frequent venous sampling in a pediatric population was safe. Guidelines include monitoring of hematological indices, phlebotomy volume <10cc/kg/24 hours, and iron replacement.
Keywords: Pediatric Research Risk, Precocious Puberty, Frequent Venous Sampling
Frequent venous sampling is an important assessment tool in neuroendocrinology. Specifically, it is indispensable when investigating the secretory patters of pulsatile hormones such as lutenizing hormone, growth hormone, insulin and prolactin (1). Consequently, understanding the safety of this method is critical for many clinical researchers. Despite this need, the short and long-term hematologic impact of this technique in a pediatric population has not been previously described. We assessed hemoglobin (Hgb), mean corpuscular volume (MCV), and ferritin levels in children with CPP undergoing repeated frequent venous samplings in collaboration with our IRB. We evaluated 34 female subjects over a period of 36-48 months of episodic sampling to assess both the short and long-term impact of this technique.
Methods
34 female subjects with central precocious puberty (CPP) were evaluated for the impact of phlebotomy. Due to the possible effects of suppression of serum testosterone levels on hemoglobin concentrations, male subjects were excluded from this study as were subjects on other chronic medications besides GnRHa. Complete initial clinical and hematologic characteristics of our cohorts were obtained as well as follow-up data for inclusion in serial analysis. Informed consent was obtained from all patients in the study and these serial evaluations were reported to our IRB as they evolved.
In 17 of these subjects' admissions, pre-admission hemoglobin (PreHgb), post-admission hemoglobin (PostHgb) and Hgb at 3-month follow-up were measured. At enrollment, this cohort ranged in age from 1.6 to 9.2 years (6.6 ± 1.8 years) and in body weight from 13.7 to 53.5 kg (32 ± 9.7 kg). All subjects were Caucasian.
34 female subjects ranging in age from 1.6 to 9.2 years (6.3 ± 2.2 years) and in weight from 13.7 to 45.1 kg (29.2 ± 8.5 kg) were followed for at least 36 months. This cohort included 32 white, 1 African American and 1 Asian female. 8 subjects were followed after ceasing GnRHa therapy, from 30-48 months post enrollment.
Between 1981 and 1991, subjects with CPP were evaluated at one of two academic centers (Massachusetts General Hospital or Children's Hospital Boston) as part of an IRB-approved, NIH-funded clinical research study to evaluate the impact of gonadotropin-releasing hormone analogue (GnRHa) induced pituitary-gonadal suppression (2). Hematologic indices were monitored in subjects who were evaluated in the GCRCs at 3 to 6 month intervals: (pretherapy), 3, 6, 12, 18, 24, 30, 36, 42, and 48 months. Permission was obtained from parents prior to the enrollment of their child and assent was obtained from children 7-years of age and older. To characterize gonadotropin secretory profiles in detail, sampling for LH and FSH was obtained every 10 minutes when secretion was active (prior to and following discontinuation of GnRHa) and every 20 minutes when secretion was suppressed (during GnRHa therapy) for 16 hours spanning night and day periods (2200 to 1400 hours). An indwelling venous catheter was placed at the beginning of each GCRC admission to obtain serial samples. The frequency and duration of phlebotomy was adjusted to be within a 9-10cc/kg limit, derived by scaling down the 500cc blood bank donation routinely drawn in adults weighing as little as 47 kg. Actual phlebotomy losses averaged approximately 9 cc/kg at initial admission and 6 cc/kg for subsequent visits during treatment. Following the withdrawal of each individual sample, a comparable volume of normal saline was infused. All children were treated with iron supplementation (elemental iron 1-6 mg/kg/day) for 6 weeks following each hospitalization.
Blood samples for all laboratory determinations were obtained after discarding 3 cc of heparinized saline-diluted residua from an indwelling venous catheter. Hgb and MCV determinations were carried out using a whole blood counter at both hospitals. Ferritin was determined by RIA, chemiluminescence and/or ELISA at MGH and CHB. Using the laboratory-specific reference ranges, iron deficiency was considered to be present when the serum ferritin was either <13 ug/L (MGH) or <10 ug/L (CHB).
Group data are described by the mean ± SEM in the text unless otherwise stated. Changes in hematologic indices during short-term studies were assessed by paired t-test. To determine if a hematologic variable changes significantly over multiple, longitudinal observations, we performed ANOVA with post-hoc analysis when p<0.05. Normative data reported by Dallman and Siimes (3) was used to calculate an age and sex-appropriate Hemoglobin Standard Deviation Score (HgbSDS) and MCV Standard Deviation Score (MCVSDS) for each Hgb and MCV value from the population mean, divided by the standard deviation of the normative population.
Results
During inpatient evaluation that included phlebotomy losses averaging 9.6 ± 0.4 cc/kg over a 24-hour period, the acute fall in Hgb in 17 subjects was 1.2 ± 0.1 g/L with a maximal fall in Hgb of 2.3 g/L in an individual subject (PreHgb v. PostHgb, p<0.001, Figure 1). Subjects were restudied 3 months after initial evaluation. Hgb levels were 12.6 ± 0.2 g/l, not significantly different from baseline values (p=0.72). One subject that had a normal Hgb value at baseline had an Hgb value that was greater than 2 standard deviations (SD) below the mean at 3-month follow-up (baseline 12.2 g/l; 3-month follow-up 12.0 g/l). Baseline MCV values (81.9 ± 1.3 fl) were also similar at 3-month follow-up (83.8 ± 0.6 fl, p=0.27) while ferritin (baseline 28.50 ± 2.6 ug/l) showed a moderate, but not statistically significant increase at 3-months (39.92 ± 5.1 ug/l, p=0.06).
Figure 1.
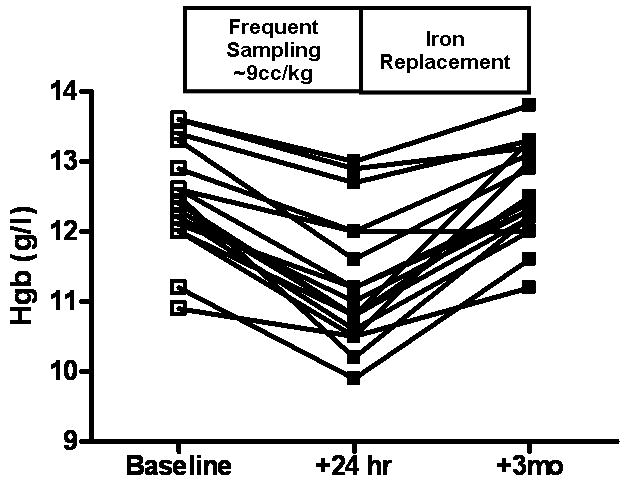
Acute decline and subsequent full recovery of hemoglobin (Hgb) concentration in subjects with CPP who underwent frequent venous blood sampling as part of their research study participation.
Hgb and MCV values of 34 subjects evaluated every 3-6 months over 48 months were not significantly different at any time point (p>0.05, Figure 2) during this period. Mean PreHgb was 12.6 ± 0.2 g/l. Over 48 months of follow-up, Hgb levels averaged 12.5 ± 0.05 g/l with a minimal value of 10.2 g/l in a single subject. Six subjects had Hgb levels that were more than 2SD below the mean during 48-months. Four of these subjects' Hgb levels recovered by their next follow-up (6-months later). Two subjects had two or more Hgb measurements in a row that were more than 2SD below the mean. All subjects had Hgb levels that recovered to within the normal range by the end of the study. Mean PreMCV was 84.2 ± 0.6 fl in 34 subjects, and also showed no significant difference at any time point evaluated over 48 months (84.2 ± 0.2 fl over 48 months follow-up). In addition, ferritin levels increased mildly during the study, but values were not significantly different (baseline, 27.7 ± 2.3 ug/l; 48-months 37.8 ± 1.5, p=0.08).
Figure 2.
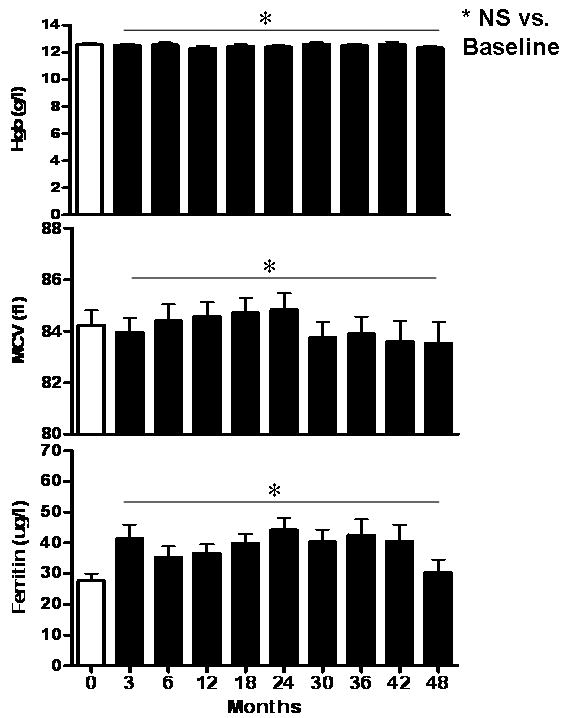
Serial hematological indices in children with CPP who underwent frequent blood sampling every 3-6 months. * NS, no significant difference over 48-months.
Because both Hgb and MCV values normally increase during childhood, we evaluated these indices in an age-adjusted manner by calculating an age and sex appropriate Hemoglobin Standard Deviation Score (HgbSDS) and MCV Standard Deviation Score (MCVSDS) for each determination. Baseline HgbSDS was -0.63 ± 0.81 and baseline MCVSDS was 1.08 ± 0.15. At 36-month follow-up, HgbSDS was -0.89 ± 0.17 and MCVSDS was 0.60 ± 0.20. Although both groups showed a moderate decline over 36 months, this did not reach statistical significance (p>0.10 for both comparisons).
Discussion
Frequent venous sampling is an important tool for clinical research in neuroendocrinology yet its safety in children has not been previously reported. Here we demonstrate that, when adequate precautions are taken, this tool is safe in a pediatric population.
Pediatric subjects are considered a vulnerable group under federal regulations and therefore require special protection when enrolled in research studies (4). Subpart D of the Code of Federal Regulations specifies four categories defining risk assessment in pediatric subjects (5). These include: 1) Research not involving greater than minimal risk (46.404), 2) Research involving greater than minimal risk but presenting the prospect of direct benefit to the individual subjects (46.405), 3) Research involving greater than minimal risk and no prospect of direct benefit to individual subjects, but likely to yield generalizable knowledge about the subject's disorder or condition (46.406) and 4) Research not otherwise approvable which presents an opportunity to understand, prevent, or alleviate a serious problem affecting the health or welfare of children (46.407). Although all risk determinations allow for research to proceed, each successive category requires an increasing level of scrutiny by an institution's IRB. Therefore, understanding the level of risk posed by a specific research technique is paramount in the design and risk-stratification of any IRB-approved study.
Withdrawal of a single blood sample is generally considered minimal risk (46.404) by IRBs (5), but no universal guidelines exist for frequent phlebotomy undertaken as part of clinical pediatric research. With frequent sampling, initial pain at the site of venous catheter placement, emotional stress, and infection risk are all similar to that of a single blood sample. Therefore, in this study, our primary concern was to ensure that participation in longitudinal studies involving serial phlebotomy did not result in a clinically significant decline in iron stores.
In addition, beginning in early childhood, both Hgb and MCV increase with age (6). Because of these developmental changes, we sought to adjust our subjects' Hgb and MCV values for age using normative data derived from the literature (3). Two findings became apparent from these evaluations. First, although well within the normal range, our subjects with CPP entered the protocol with mean values of Hgb and MCV that were significantly different from the mean of chronologic age-matched controls (HgbSDS -0.63 ± 0.81 and MCVSDS 1.08 ± 0.15). Secondly, both Hgb and MCV standard deviation scores declined moderately during our study. In considering the applicability of Dallman's normative data to our study population, it is doubtful that differences in subject population or laboratory techniques contributed to our findings. However, CPP and/or its suppression may have had some impact on the hematologic status of our subjects. Given that anemia most commonly occurs during periods of rapid growth, the baseline negative HgbSDS in our subjects with CPP is not entirely unexpected (7). There is no evidence to implicate phlebotomy-induced iron deficiency as the cause of the negative trends in HgbSDS and MCVSDS observed during participation in this study given that only 2 subjects with low Hgb levels and none with low MCV levels had associated decrease in ferritin. Even though drug effect is difficult to rule out with certainty, no direct effects of GnRH agonists on hematologic variables have been reported. Alternatively, GnRHa-induced suppression of pituitary-gonadal function might inhibit factors that are important in orchestrating the normal, age-related increases in Hgb and MCV values. Several factors related to the activity of the hypothalamic-pituitary-gonadal axis are known to stimulate or inhibit erythropoiesis (8-11). Therefore, although there are limited data available on the effect of CPP and its treatment on hematological indices in children, the calculated SD scores from this cohort reiterate the results of the present analysis: that when specific guidelines for phlebotomy are followed, and appropriate iron replacement is provided, such studies can be undertaken without significant adverse hematologic effects.
Acknowledgments
This work was supported in part through NIH grants HD-18169, RR-01066 and RR-08847. Broder-Fingert and Boepple declare no conflicts of interest. Crowley is a consultant for Abbott Diagnostics, Quest Diagnostics, and Co-founder and Chair of the Scientific Advisory Board of Combinent[H1] Biomedical Systems, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sam S, Frohman LA. Normal physiology of hypothalamic pituitary regulation. Endorinol Metab Clin North Am. 2008 Mar;37:1–22. doi: 10.1016/j.ecl.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Boepple PA, Mansfield MJ, Wierman ME, Rudlin CR, Bode HH, Crigler JF, Jr, Crawford JD, Crowley WF., Jr Use of a potent, long acting agonist of gonadotropin-releasing hormone in the treatment of precocious puberty. Endocr Rev. 1986 Feb;7:24–33. doi: 10.1210/edrv-7-1-24. [DOI] [PubMed] [Google Scholar]
- 3.Dallman PR, Siimes MA. Percentile curves for hemoglobin and red cell volume in infancy and childhood. J Pediatr. 1979 Jan;94:26–31. doi: 10.1016/s0022-3476(79)80344-3. [DOI] [PubMed] [Google Scholar]
- 4.Steinbrook R. Testing Medications in Children. N Engl J Med. 2002 Oct;347:1462–70. doi: 10.1056/NEJMhpr021646. [DOI] [PubMed] [Google Scholar]
- 5.Department of Health and Human Services, Food and Drug Administration. Additional safeguards for children in clinical investigations of FDA-regulated products. Fed Regist. 2001;66:20589–600. [PubMed] [Google Scholar]
- 6.Fairbanks VF, Beutler E. Iron Deficiency. In: Willams WJ, Beutler E, Erslev AJ, Lichtman MD, editors. Hematology. New York: McGraw-Hill; 1990. pp. 482–505. [Google Scholar]
- 7.Lukens JN. Iron metabolism and iron deficiency anemia. In: Miller DR, Baehner RL, McMillan CW, editors. Blood diseases of infancy and childhood. St. Louis: CV Mosby co; 1984. pp. 170–98. [Google Scholar]
- 8.Yamamoto M, Hayashi Y, Yamada K. Sex hormones and bone marrow functions. Japanese Journal of Clinical Medicine. 1974 Nov;32:3346–9. [PubMed] [Google Scholar]
- 9.Yu J, Maderazo L, Shao LE, Frigon NL, Vaughan J, Vale W, Yu A. Specific roles of activin/inhibin in human erythropoiesis in vitro. Ann N Y Acad Sci. 1991;628:199–211. doi: 10.1111/j.1749-6632.1991.tb17247.x. [DOI] [PubMed] [Google Scholar]
- 10.Dukes PP, Goldwasser E. Inhibition of erythropoiesis by estrogens. Endocrinology. 1961 Jul;69:21–9. doi: 10.1210/endo-69-1-21. [DOI] [PubMed] [Google Scholar]
- 11.Kurtz A, Zapf J, Eckardt K, Clemons G, Froesch ER, Bauer C. Insulin-like growth factor I stimulates erythropoiesis in hypophysectomized rats. Proc Natl Acad Sci USA. 1988 Oct;85:7825–9. doi: 10.1073/pnas.85.20.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]


