Abstract
Purpose
To evaluate longer-term response in subjects with interstitial cystitis (IC) who initially responded to intravesical bacillus Calmette-Guerin (BCG) or placebo in a randomized clinical trial.
Materials and Methods
Subjects with IC, who responded positively to treatment with either BCG or placebo after 34 weeks of follow-up in a double-blind clinical trial, were followed for an additional 34 weeks in an observational study to assess the durability of response. Outcomes at 68 weeks included a patient-reported global response assessment, a 24-hour voiding diary, pain, urgency, and validated IC symptom indices.
Results
Thirty-eight of the responders to BCG or placebo in the clinical trial continued extended follow-up in the observational study. Twelve (75%) responders who received placebo and 19 (86%) responders who received BCG considered themselves to remain moderately or markedly improved at Week 68. Improved symptom outcomes were also generally maintained for the duration of follow-up in both groups.
Conclusions
The majority of subjects who respond to therapy with intravesical BCG or placebo maintain improved symptoms for up to 68 weeks after the initiation of therapy. However, initial response rates are low and placebo responders demonstrate essentially the same durability of response as BCG responders. These results argue against the routine use of BCG in this patient group.
Keywords: bacillus Calmette Guerin, BCG, interstitial cystitis, pain, quality of life
Introduction
Interstitial cystitis (IC) is a chronic condition characterized by bladder pain and bladder storage symptoms, in association with characteristic cystoscopic findings.1 The etiology of this condition is unknown and treatment remains suboptimal. The National Institutes of Health established the Interstitial Cystitis Clinical Trials Group (ICCTG) to conduct randomized controlled clinical trials among patients with IC. The ICCTG recently reported the results of a randomized, double-blind placebo controlled clinical trial of intravesical bacillus Calmette-Guerin (BCG).2 After 34 weeks follow-up there was no statistically significant difference between BCG and placebo in the rate of responders, based on a Global Response Assessment (GRA). A report of extended follow-up for a previous clinical trial of BCG showed the beneficial effect to be durable after an average of 27 months.3 We report the results of an observational study of a subset of the ICCTG clinical trial participants who responded to either BCG or placebo, in order to determine whether the effects persisted. These subjects remained masked to their original treatment assignment throughout the long-term follow-up.
Methods
Details of the design of the trial and primary and secondary outcomes have been published previously.2 Briefly, women and men who had at least moderate symptoms of IC were randomized to either intravesical BCG or intravesical saline (placebo) and received up to six instillations of treatment, over a six to ten week period. The 34-week period following randomization comprised the primary randomized portion of the study. The follow-up period of 34 weeks was chosen to correspond to the previous studies of BCG3, and to make follow-up comparable for all subjects since length of treatment could vary somewhat. At Week 34, participants rated their symptoms as compared to baseline on a Global Response Assessment (GRA), a seven-point scale ranging from markedly worse to markedly improved. Subjects were classified as responders if they reported “moderate” or “marked” improvement on the GRA, supplemented with information on medication use.2 Subjects who withdrew from the study without completing the GRA were classified as non-responders. Responders, who remained blinded to initial treatment allocation, were invited to participate in follow-up for an additional 34 weeks. Non-responders were offered treatment with open-label BCG; the results of that study are reported elsewhere. Informed consent was obtained for all participants and the protocol was approved by the Institutional Review Board at each site.
The following secondary outcome measures were obtained at baseline, the end of the clinical trial (34 weeks post-randomization), the end of the observational phase of the study (68 weeks post-randomization), and selected intermediate intervals: pain and urgency scores, urinary frequencies recorded on 24-hour voiding diaries, the University of Wisconsin IC Symptom Inventory4, and the O’Leary-Sant IC Symptom (ICSI) and Problem (ICPI) Indices5. The GRA was also obtained at Week 68. The GRA asked respondents to evaluate their symptoms as compared to when they “started this study”; we presumed that the participants interpreted this to mean the time of randomization. Adverse events were not assessed during the observational phase.
Since the subjects were a subset of the original randomized cohort, the presentation of results is primarily descriptive. Standard 95% confidence intervals (CIs) were produced for the mean changes from Week 34 to Week 68 for those subjects with data at both time points; confidence intervals that include zero are consistent with no statistically significant change. Fisher’s exact test (two-sided) was used to compare proportions between groups.
Results
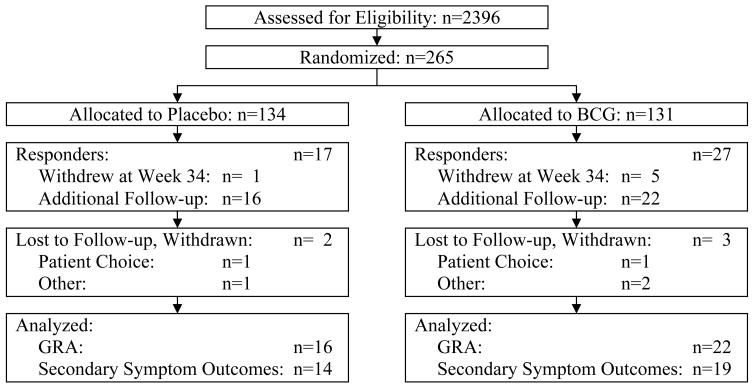
The disposition of the subjects included in this report is shown in Figure 1. It should be noted that the classification of subjects as responders eligible for inclusion in this report was based on the determination of response status at the clinical site. The primary endpoint for the randomized clinical trial was based on later central review of the responder status and yielded a slightly different number.2 Of the 44 responders in the clinical trial, by clinical site assessment, 38 (86%) participated in the observational study. The median age at time of randomization for these 38 responders was 52 years for those randomized to placebo and 46 years for those assigned to BCG; approximately 80% were female in both arms. Eleven (69%) of the placebo responders were white or Caucasian as compared to 21 (95%) of the BCG responders but this difference was of borderline statistical significance (p=0.065) and the numbers are very small. Five subjects withdrew during long-term follow-up, yielding 33 subjects (87%) assessed at 68 weeks.
Figure 1.
Subject accounting through all phases of the study. The clinical site determination of response status at the end of the randomized clinical trial (Week 34) was used to evaluate whether a subject was a responder eligible for long-term follow-up or a non-responder eligible for open-label BCG. The primary endpoint for the randomized clinical trial was based on central review of the responder status.
Twelve out of 16 responders (75%) from the placebo arm and 19 out of 22 responders from the BCG arm (86%) considered themselves to remain moderately or markedly improved by the GRA at Week 68. This includes six subjects from the placebo arm and 13 subjects from the BCG arm who reported that they remained markedly improved. Note that the five subjects who withdrew are included in the denominator for these response rates. There was no statistically significant difference between the two arms (p=0.43), although the sample sizes are small.
Summaries of individual symptoms and the three symptom indices, at the beginning (Week 34) and the end (Week 68) of the observational study, are shown in Table 1. For reference, the baseline values (at randomization) for these subjects are also shown. The mean values for all of these efficacy outcomes were lower at Week 34 than at baseline among these subjects, reflecting their status as treatment responders in the randomized trial. Mean changes during follow-up from Week 34 to Week 68 were much smaller than in the randomized trial. Although it appeared that symptom improvements were better maintained in those subjects who responded to BCG as compared to responders to placebo, the confidence intervals indicate that all of the changes were consistent with a mean change of zero. It is important to note that for all of the secondary efficacy endpoints, the sample sizes are small and the standard deviations are large, such that the confidence intervals are wide and the impact of the withdrawals may be large.
Table 1.
Symptom Outcomes over Time
| Mean ± s.d. | Change from Week 34 to Week 68 | |||||
|---|---|---|---|---|---|---|
| Outcome | Arm | Baseline | Week 34 | Week 68 | Mean ± s.d. | 95% CI |
| # of Subjects | Placebo | 16 | 16 | 14 | 14 | |
| BCG | 22 | 22 | 19 | 19 | ||
| Pain | Placebo | 6.2 ± 1.5 | 2.1 ± 1.4 | 3.1 ± 2.2 | 0.8 ± 2.7 | (−0.6, 2.2) |
| BCG | 6.5 ± 1.1 | 2.0 ± 1.5 | 2.3 ± 1.5 | 0.2 ± 1.2 | (−0.3, 0.7) | |
| Urgency | Placebo | 6.8 ± 1.3 | 2.9 ± 1.7 | 3.1 ± 2.0 | 0.1 ± 2.1 | (−1.0, 3.2) |
| BCG | 6.8 ± 1.2 | 2.5 ± 1.4 | 2.3 ± 1.6 | −0.2 ± 1.2 | (−0.7, 1.7) | |
| 24-hour Frequency | Placebo | 15.1 ± 5.7 | 10.7 ± 3.2 | 12.4 ± 5.2 | 1.5 ± 4.6 | (−0.9, 3.9) |
| BCG | 16.9 ± 5.9 | 11.1 ± 5.5 | 10.4 ± 3.3 | 0.0 ± 3.1* | (−1.5, 1.5) | |
| Wisconsin IC Inventory | Placebo | 31.0 ± 7.9 | 12.1 ± 7.7 | 13.8 ± 11.4 | 1.2 ± 7.4† | (−2.8, 6.2) |
| BCG | 29.8 ± 7.7‡ | 9.1 ± 6.9‡ | 8.1 ± 6.6 | −1.3 ± 5.3 | (−3.7, 1.1) | |
| ICSI | Placebo | 13.1 ± 3.5 | 7.0 ± 3.1 | 8.1 ± 4.4 | 1.1 ± 3.9 | (−0.9, 3.1) |
| BCG | 12.4 ± 3.4 | 6.4 ± 3.3 | 6.4 ± 2.3 | −0.1 ± 2.4* | (−1.2, 1.0) | |
| ICPI | Placebo | 12.6 ± 2.5 | 5.5 ± 3.4 | 6.3 ± 3.6 | 0.6 ± 2.8 | (−0.9, 2.1) |
| BCG | 11.5 ± 3.0 | 4.0 ± 3.0 | 4.1 ± 3.1 | −0.2 ± 3.2* | (−1.7, 1.3) | |
n=17;
n=13;
n=21
Discussion
Although we observed no statistically significant beneficial effect of treatment with BCG as compared to placebo in the randomized clinical trial (with low response rates in both groups), we continued to observe the subset of subjects who responded to either treatment in order to evaluate the duration of effects. Previously, Peters, et al., followed nine subjects who had previously responded to BCG in a randomized clinical trial.3 With a mean follow-up of 27 months, eight (89%) continued to demonstrate improvement as compared to pre-treatment symptoms. (Longer-term follow-up of the responders to placebo in that trial was not reported.) In the ICCTG trial reported here, most of the subjects initially responsive to BCG who participated in the current observational study also maintained their responder status and individual improved symptom measures up to 68 weeks. Thus, although the response rate for BCG was low (21%) in the randomized clinical trial, the positive effect appeared to be durable for most of the study subjects for the period of time in which they were observed.
Interestingly, a high proportion of subjects who responded to the administration of intravesical saline also maintained their response for a period of 68 weeks. Although spontaneous remission of symptoms is frequently mentioned in IC, this result is provocative in that it is distinctly unusual to see long-lasting, dramatic symptom improvement in such refractory patients in the absence of specific therapy. It suggests that the placebo response in IC may also be long-term, especially for invasive modes of administration of masked treatment. Although BCG is considered to have a pharmacological action, we expected that the route of administration with catheterization would likely have a higher placebo effect than an oral medication, and accordingly designed the original randomized clinical trial expecting a 30% placebo rate. However, the initial placebo response rate was only 12%, similar to the 13% placebo response rate observed in a previous ICCTG trial of oral therapies.6
Few other studies have shown benefits of placebo or sham therapies up to six months after treatment. A recent study of transdermal posterior tibial nerve laser therapy in IC, which showed no difference between laser and sham treatments, demonstrated significant improvement in voiding measures, the O’Leary-Sant Symptom Indices, and quality of life at twelve weeks after initiation of treatment.7 In a trial of microwave thermoablation for benign prostatic hyperplasia, a reduction in the mean AUA symptom score was observed by six weeks and was maintained up to six months, with a larger improvement in the microwave treatment group as compared to sham therapy.8 Of the 35 subjects on sham therapy in that trial, five (14%) had mild symptoms at six months, representing maintained improvement from baseline. A Canadian study of 25 months of follow-up after placebo therapy for benign prostatic hyperplasia showed a similar long-term placebo effect.9
Both the magnitude and duration of placebo effects may be affected by treatment blinding. At the end of the randomized portion of the trial at 34 weeks, subjects were asked to guess their treatment assignments; they were not informed of their actual treatments until all follow-up through 68 weeks had been completed. All of the responders who provided a guess as to assigned treatment guessed that they had been assigned to BCG, whereas two-thirds of the non-responders guessed that they had received placebo. However, there was no evidence that unblinding occurred and it is likely that many of the responders guessed that they received BCG simply because they improved.
It could be argued that the beneficial effect observed in both the BCG and placebo groups is due to hydrodistention from administration of these agents intravesically. However, only 50cc of placebo or BCG solution was instilled and the mean dwell times for retention were 1.6 and 1.7 hours, respectively. It is generally recognized that hydrodistention not performed under general anesthesia provides no lasting benefits in terms of symptom amelioration. Rather than bladder distention, another possible explanation for the extended effect for both BCG and placebo is through the process of learning to postpone urination through holding the instillation. This may provide an effective behavioral therapy that some patients are then able to utilize long-term to control their disease. It is also possible that the sustained improvement in both groups was partially due to participation in a clinical trial, working with a caring responsive research coordinator, and concentration on bladder symptoms possibly leading to behavior changes not part of the clinical trial protocol. However, to our knowledge there are no published data from a randomized clinical trial to evaluate the long-term effects of behavioral modifications in refractory patients with IC.
The major limitation of this study is that it is essentially an observational cohort of a subgroup of subjects, and thus there may be a selection bias. Although 86% of the responders from the clinical trial participated in this long-term follow-up, these subjects no longer represent the original randomization and any comparisons of the placebo and BCG arms should be interpreted accordingly. Furthermore, due to the low response rate for both therapies in the original trial, the sample size for long-term follow-up of the responders is also small, such that there is limited statistical power for most comparisons.
Conclusions
The majority of subjects who responded to treatment with either intravesical BCG or placebo maintained their improvement for up to 68 weeks after the initiation of treatment. However, the overall response rates were low and BCG was similar to placebo, even with extended follow-up. These longer-term results provide more evidence that that BCG should not be routinely used in IC. The duration of the placebo effect emphasizes the importance of including a placebo group in IC trials, at least until reliably effective therapy is developed.
- BCG
bacillus Calmette-Guerin
- CI
confidence interval
- GRA
Global Response Assessment
- IC
interstitial cystitis
- ICCTG
Interstitial Cystitis Clinical Trials Group
- ICPI
O’Leary-Sant Interstitial Cystitis Problem Index
- ICSI
O’Leary-Sant Interstitial Cystitis Symptom Index
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
Appendix: Interstitial Cystitis Clinical Trials Group Membership
University of Pennsylvania
Alan J. Wein, MD (PI)
George W. Drach, MD
Philip Hanno, MD
Eric Rovner, MD
Marilou Foy, RN
Gloria McNamara, RN
Lilliam Ribeiro, BA
New England Medical Center
Grannum R. Sant, MD (PI)
Erol Onel, MD
T.C. Theoharides, PhD, MD
Patricia Radgowski
Carolyn Shea-O’Malley, RN
University of Rochester
Edward M. Messing, MD (PI)
Robert Mayer, MD
Kay Rust, RN, MSN, FNP
Elizabeth Smith, BS
University of Maryland
John Warren, MD (PI)
Toby Chai, MD
Susan Keay, MD
Linda Horne
Theresa Jackson
University of Oklahoma
Daniel J. Culkin, MD (PI)
James F. Donovan, Jr., MD
Lynda Kelsey, RN, MS
Karen Mataranglo, RN
William Beaumont Hospital
Ananias C. Diokno, MD (PI)
Kenneth Peters, MD
Eleanor Anton, RN
Loni Lampkins
Henry Ford Hospital
David Burks, MD (co-PI)
Rifaat Dagher, MD
Michelle Peabody, RN
Jill Sullivan, RN, BSN
Queen’s University
J. Curtis Nickel, MD (PI)
Alvaro Morales, MD
Joe Downey, BSc, MSc
Laurel Emerson, RN
Sylvia Robb, RN
Stanford University Medical Center
Christopher K. Payne, MD (PI)
Kathryn Azevedo, PhD
Gilbert Rigaud, MD
Rajesh Shinghal, MD
Debra Clay, RN, BSN
University of Pennsylvania Data Coordinating Center
J. Richard Landis, PhD (PI)
Kathleen J. Propert, ScD (co-PI)
Deborah R. Erickson, MD (Pennsylvania State University)
John T. Farrar, MD
John E. Tomaszewski, MD
Rosemary A. Madigan, RN, MPH
Ted Barrell, BA
Denise Cifelli, BS
James Dattilo, BS
Stephen B. Durborow, BS
Lori Fanelli, RT, BA
Christine Hardy, MS
Raymond W. Hilderbrand, MS, MBA
Chris Helker, RN, MSPH
Gina Norwood, BS
Gargi Parikh, MS
Yanlin Wang, MS
Yawei Zhang, MD, MS
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
John W. Kusek, PhD
Christopher Mullins, PhD
Leroy M. Nyberg, Jr., PhD, MD
Interstitial Cystitis Association (ICA)
Vicki Ratner, MD
University of Virginia
William A. Steers, MD (Chairman of ICCTG Steering Committee from 1998–2001)
Yale University
Harris E. Foster, MD (Chairman of ICCTG Steering Committee from 2001–2004)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourology and Urodynamics. 2002;21:167. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 2.Mayer R, Propert KJ, Peters KM, Payne CK, Zhang Y, Burks D, et al. A randomized controlled trial of intravesical bacillus Calmette Guerin for treatment refractory interstitial cystitis. J Urol. 2005;173:1186. doi: 10.1097/01.ju.0000152337.82806.e8. [DOI] [PubMed] [Google Scholar]
- 3.Peters KM, Diokno AC, Steinert BW, Gonzalez JA. The efficacy of intravesical bacillus Calmette-Guerin in the treatment of interstitial cystitis: long-term follow-up. J Urol. 1998;159:1483. doi: 10.1097/00005392-199805000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Goin JE, Olaleye D, Peters KM, Steinert B, Habicht K, Wynant G. Psychometric analysis of the University of Wisconsin Interstitial Cystitis Scale: implications for use in randomized clinical trials. J Urol. 1998;159:1085. [PubMed] [Google Scholar]
- 5.O’Leary M, Sant GR, Fowler FJ, Whitmore KE, Spolarich-Kroll J. The interstitial cystitis symptom index and problem index. Urology. 1997;49(Suppl 5A):58. doi: 10.1016/s0090-4295(99)80333-1. [DOI] [PubMed] [Google Scholar]
- 6.Sant GR, Propert KJ, Hanno PM, Burks D, Culkin D, Diokno AC, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170:810. doi: 10.1097/01.ju.0000083020.06212.3d. [DOI] [PubMed] [Google Scholar]
- 7.O’Reilly BA, Dwyer PL, Hawthorne G, Cleaver S, Thomas E, Rosamilia A, et al. Transdermal posterior tibial nerve laser therapy is not effective in women with interstitial cystitis. J Urol. 2004;172:1880. doi: 10.1097/01.ju.0000142846.47245.16. [DOI] [PubMed] [Google Scholar]
- 8.Larson TR, Blute ML, Bruskewitz RC, Mayer RD, Ugarte RR, Utz WJ. A high-efficiency microwave thermoablation system for the treatment of benign prostatic hyperplasia: results of a randomized, sham-controlled, prospective, double-blind, multicenter clinical trial. Urology. 1998;51:731. doi: 10.1016/s0090-4295(97)00710-3. [DOI] [PubMed] [Google Scholar]
- 9.Nickel JC. Placebo therapy of benign prostatic hyperplasia: a 25-month study. Canadian PROSPECT Study Group. Br J Urol. 1998;81:383. doi: 10.1046/j.1464-410x.1998.00554.x. [DOI] [PubMed] [Google Scholar]