Abstract
Recent studies in species that fertilize externally have demonstrated that fertilization triggers localized activation of Src-family protein kinases in the egg cortex. However, the requirement for Src-family kinases in activation of the mammalian egg is different from lower species and the objective of this study was to characterize changes in the distribution and activity of Src-family protein tyrosine kinases (PTKs) during zygotic development in the mouse. Immunofluorescence analysis of mouse oocytes and zygotes with an anti-phosphotyrosine antibody revealed that fertilization stimulated accumulation of P-Tyr-containing proteins in the egg cortex and that their abundance was elevated in the region overlying the MII spindle. In addition, the poles of the MII spindle exhibited elevated P-Tyr levels. As polar body extrusion progressed, P-Tyr-containing proteins were especially concentrated in the region of cortex adjacent to the maternal chromatin and the forming polar body. In contrast, P-Tyr labeling of the spindle poles eventually disappeared as meiosis II progressed to anaphase II. In approximately 24% of cases, the fertilizing sperm nucleus was associated with increased P-Tyr labeling in the overlying cortex and oolema.
To determine whether Src-family protein tyrosine kinases could be responsible for the observed changes in the distribution of P-Tyr containing proteins, an antibody to the activated form of Src-family PTKs was used to localize activated Src, Fyn, or Yes. Activated Src-family kinases were found to be strongly associated with the meiotic spindle at all stages of meiosis II, however no concentration of labeling was evident at the egg cortex. The absence of cortical Src-family PTK activity continued until the blastocyst stage when strong cortical activity became evident. At the pronuclear stage, activated Src-family PTKs became concentrated around the pronuclei in close association with the nuclear envelope. This pattern was unique to the earliest stages of development and disappeared by the eight cell stage. Functional studies using chemical inhibitors and a dominant-negative Fyn construct demonstrated that Src-family PTKs play an essential role in completion of meiosis II following fertilization and progression from the pronuclear stage into mitosis.
This data suggests that while Src-family PTKs are not required for fertilization induced calcium oscillations, they do play a critical role in development of the zygote. Furthermore, activation of these kinases in the mouse egg is limited to distinct regions and occurs at specific times after fertilization.
Keywords: Fertilization, mouse, oocyte, phosphotyrosine, Src, Fyn, protein kinase
INTRODUCTION
Src-family protein tyrosine kinases (PTKs) are cytoplasmic enzymes that can be targeted to plasma membrane microdomains where they typically act to transduce signals from external stimuli (Bromann, et al., 2004). Signal transduction cascades involving Src-PTKs such as Fyn, Src, and Yes have been shown to play a major role during egg activation and early development in species that fertilize externally such as marine invertebrates, amphibians, and fish (Sato, et al., 2000; Wu, and Kinsey, 2001; Runft, et al., 2002). In these species, Src-family PTKs are activated rapidly after fertilization and function in triggering the sperm-induced calcium transient that initiates the egg activation process (Giusti, et al., 1999; Giusti, et al., 2000; Kinsey, and Shen, 2000; Sato, et al., 1998; Sato, et al., 2000; Kinsey, et al., 2003). In the zebrafish oocyte, kinase activation was shown to be initiated at the point of sperm-egg fusion and to progress through the egg cortex (Sharma, and Kinsey, 2006). Later stages of egg activation such as pronuclear fusion and mitosis also require PTK activity although the specific kinases involved in these steps have not been identified (Moore, and Kinsey, 1995; Wright and Schatten, 1995). Once development has begun, Fyn and Yes are required for cell movements involved in epiboly (Tsai et al., 2005; Sharma, et al., 2005) while Src and Yes function during cell intercalation and blastopore closure (Denoyelle, et al., 2001). The role of Src-family PTKs in mammalian fertilization is clearly different from that in externally fertilizing species. For example, while mammalian eggs express Fyn, Yes, and in some cases, Src (Talmor, et al., 1998; Talmor-Cohen, et al., 2004a) these kinases are not required for the unique sperm-induced calcium oscillations (Mehlmann, et al., 1998; Kurokawa, et al., 2004; Mehlmann, and Jaffe, 2005) which trigger egg activation in mammals (Carroll, 2001). Instead, these calcium oscillations are initiated directly by a sperm-borne phospholipase that does not require PTK regulation (Cox, et al., 2002). The function of Src-family PTKs in later stages of mammalian fertilization has been addressed primarily through the use of parthenogenetic activation. Studies in mouse and rat demonstrate that agents which suppress Src-family kinase activation also inhibit the MII/anaphase transition induced by parthenogenetic activation in vitro. In addition, microinjection of active Fyn kinase has been shown to stimulate meiosis resumption in mouse and rat (Sette, et al., 2002; Talmor-Cohen, et al., 2004a). A second requirement for Src-family PTK activity at S or S/G2 phase of the first mitotic division has been demonstrated through the use of chemical inhibitors such as genistein (Besterman, and Schultz, 1990; Jacquet, et al., 1995). Further analysis using GST fusion proteins encoding the SH2 domain of Fyn have confirmed the importance of Src-family kinase activity for development past the pronuclear stage (Meng, et al., 2006). Together, these observations indicate that Src-family kinases such as Fyn may play an important role in development of the mammalian zygote, but it is unclear which specific pathways are regulated during zygotic development.
The objective of the present study was to determine whether fertilization of the mouse oocyte triggers the global activation of Src-family PTKs in the egg cortex as occurs in lower species (Sharma and Kinsey, 2006). The approach was to first use antibodies to phosphotyrosine to establish which parts of the egg contain elevated levels of phosphotyrosine, and secondarily to use an antibody to activated Src-family PTKs to establish the distribution and activation pattern of this family of kinases. The results indicate that fertilization does stimulate PTK signaling in localized regions of the mouse egg. The activated Src-family kinases are associated with meiotic and mitotic spindle microtubules and the pronuclear envelope. The activation pattern of these kinases is different from that in lower species such as zebrafish since even though P-Tyr labeling indicated that some tyrosine kinase was active in the egg cortex, the activated Src-family PTKs were restricted primarily to the spindle and the pronuclear envelope. One potential application of these studies could be to evaluate the quality of egg activation and zygote development under different conditions used in assisted reproductive technologies. We therefore we have focused our morphological studies primarily on zygotes produced by in vivo fertilization and not exposed to in vitro culture during recovery and fixation.
MATERIALS AND METHODS
Embryo and oocyte collection
Oocyte-Cumulus-Complexes (OCC) and zygotes were collected from 6–7 week old CF1 female mice (Harlan Sprague-Dawley, Indianapolis IN USA). Females were stimulated with 5 IU eCG (Calbiochem, San Diego CA USA) followed by 5 IU hCG 48h later. To produce in vivo fertilized embryos, female mice were mated with mature B6D2F1 (C57BL/6 × DBA/2) male mice. Embryos were collected every 15–30 minutes between 13.0 and 16.0 h post-hCG to provide a range of developmental stages from non-fertilized OCC to early pronuclei. Embryos were also collected at later stages of development (times post-hCG: 24h = 2 pronuclei, 48h = late 2cell, 72h = compacting 8cells, 120h blastocyst). Embryos and OCC were released from the oviducts directly into fixative with phosphatase inhibitors to avoid the possibility that the ex vivo environment or culture conditions might influence the activity of protein kinases or phosphatases in the egg.
Western Blot analysis
MII oocytes collected as above were incubated in FHM (HEPES buffered KSOM, Specialty Media Phillipsburgh NJ USA) containing 0.3mg/ml hyaluronidase and 40 μM phenylarsine oxide and 100 μM sodium orthovanadate to remove attached cumulus cells. The oocytes were then washed three times in FHM medium, then excess medium was removed with a pulled glass pipet. The oocytes were immediately solubilized in a final volume of 10ul of SDS-gel sample buffer containing 40 μM phenylarsine oxide and 100 μM sodium orthovanadate and stored at −70°C. Samples were resolved on a 10% SDS-PAGE with a 4% stacking gel. The wells in the stacking gel were formed with a comb containing teeth 1mm in width which facilitated analysis of very small sample volumes (typically 1μl). Immunoblotting and detection was performed as previously described (Sharma and Kinsey, 2006).
Fixation and immunohistochemical staining
All eggs and embryos were fixed for 5 min at room temperature in FHM medium with 2% paraformaldehyde followed by 30 min at 35 °C in microtubule stabilization buffer (0.1 M PIPES, pH 6.9, 5 mM MgCl2.6H2O, 2.5 mM EGTA) containing 2% formaldehyde, 0.1% Triton X-100, 1 μM taxol, 10 units/ml aprotinin and 50% deuterium oxide (Messinger and Albertini, 1991). After fixation, eggs and embryos were transferred into wash solution (PBS containing 2% BSA, 2% powdered milk, 2% normal goat serum, 0.1 M glycine, and 0.01% Triton X-100) and held overnight at 4°C. All fixatives and wash solutions were supplemented with 40 μM phenylarsine oxide and 100 μM sodium orthovanadate to inhibit phosphatase activity. Cumulus cells were removed after fixation and immediately before staining by adding 0.3 mg/ml hyaluronidase to the wash solution for less than 1 minute. Embryos were washed twice without hyaluronidase before labeling.
To limit the potential effects of storage time on phosphorylated epitopes, all embryos were labeled within 24h of fixation and imaged within 2 days after labeling. The clone 28 mouse monoclonal antibody (Biosource International, Camarillo CA USA) was used to localize activated forms of Src, Fyn and Yes, while a monoclonal antibody to the autophosphorylation site at Tyr 416 (Nonphospho-Src(Tyr416),Cell Signaling Technology Inc, Danvers, MA) was used to detect inactive Src-family PTKs. An anti-phosphotyrosine antibody (clone 4G10, Upstate, Lake Placid NY, USA) was used to localize tyrosine phosphorylated proteins. Embryos were co-labeled with either rat monoclonal anti-tubulin (YOL 1/34, Abcam Inc, Cambridge MA USA) or phalloidin conjugated with Alexa 568 (to label f-actin) to display cytoskeletal structures. Secondary antibodies were goat anti-mouse Alexa 488 or goat anti-rat Alexa 568 (Molecular Probes, Eugene OR USA). Negative controls were prepared identically to the labeled samples however primary antibodies were pre-mixed with either Phospho-L-Tryosine (clone 4G10) or clone 28 blocking peptide (EPQYQPGENL-COOH) synthesized by (Synpep Corp. Dublin, CA) at 1.0 μM. In experiments where both clones 28 and 4G10 were used, the embryos from each treatment were divided randomly after fixation and all embryos from each replicate were labeled and imaged at the same time. Embryos were labeled with primary antibodies at 35°C for 1h, washed 3x then labeled with secondary antibody for 1h. After secondary labeling, embryos were transferred to a wash solution containing 1 ug/ml Hoechst 33258 with or without Alexa 568-phalloidin and stored in the dark overnight at 4°C. Embryos were mounted the following morning and imaged (mounting medium consisted of 1:1 glycerol: PBS supplemented with 5 mg/ml sodium azide and 1 μg/ml Hoechst 33258). All chemicals, hormones and reagents were purchased from Sigma Chemical Company, St. Louis, MO unless otherwise stated.
Imaging and data analysis
Samples were imaged by serial z-sections (8 μm depth) on an inverted Zeiss LSM500 confocal microscope. The serial z-sections were used to detect 3-dimensional relationships between the egg cortex, meiotic spindle and fertilizing sperm. Fluorescence intensity was quantitated by linescan and by area measurement analysis using Metamorph 6.2 (Universal Imaging Corp. Downington, PA).
Pharmacological treatment of eggs fertilized in vitro
In order to test the effects of Src-family PTK inhibitors on fertilization and initiation of embryonic development, a synchronized pool of zygotes was produced by in vitro fertilization, using methods previously published (Summers, et al., 2005). Briefly, oocyte cumulus complexes (OCC) were collected 14h post-hCG from superovulated CF1 female mice. Sperm were collected from the cauda epidydimis of mature B6D2F1 male mice and capacitated for 90 minutes in modified tyrode’s medium. OCC were released directly into fertilization drops of mKSOMaa medium (KSOMaa, Chemicon, Temecula, CA) supplemented with 4 mg/ml BSA, 5.56 mM glucose, 1 mM glycyl-glutamine (Biggers, et al., 2004). Capacitated sperm were added to OCC (1×106/ml) and allowed 5 hours for fertilization and early pronuclear formation. Several PTK inhibitors were tested including: PP2 (10 & 100 uM; Calbiochem, San Diego, CA), SKI-606 (1 and 10 uM; Calbiochem), PD168393 (0.5 and 5 uM: Calbiochem), GTP-14564 (2 and 20 uM; Calbiochem). Inhibitors were prepared as stock solutions in DMSO and stored at −20C. Culture medium was equilibrated in the CO2 incubator in 15ml tubes for at least 2 hours before culture. Immediately before adding embryos, each inhibitor was thawed and mixed with pre-equilibrated mKSOMaa medium and 50 ul drops were placed into NUNC 4-well plates. Each treatment drop was overlain with mineral oil (sterile filtered Sigma Embryo Tested Mineral Oil, cat. M8410) containing the same concentration of the inhibitor. After 24h culture, all embryos were fixed for immunofluorescence analysis as described above. Statistical analysis of developmental success was performed using SigmaStat software (Jandel Scientific, San Rafael, CA).
RESULTS
Evidence for PTK activity in the mouse oocyte
It is well established that the mammalian oocyte expresses active protein tyrosine kinases with the result that P-Tyr-containing proteins accumulate in the egg (Besterman and Schultz, 1990), (Kurokawa, et al., 2004). In order to gain an insight into the regions of the egg that are actively involved in PTK signaling during fertilization, we used a well established monoclonal antibody to phosphotyrosine (4G10) to localize P-Tyr in mouse oocytes and zygotes. Our procedure entailed the aggressive use of phosphotyrosyl phosphatase inhibitors at all stages of sample preparation to prevent dephosphorylation of tyrosine residues by phosphatases present in the egg and in the reagents used for immunofluorescence. Initial observations recorded at lower magnification Fig. 1, revealed that P-Tyr residues were distributed uniformly in the cytoplasm of the MII oocyte with some concentration in the cortex adjacent to the MII spindle (Fig. 1A, white arrows). Fertilization triggered accumulation of P-Tyr in the egg cortex which was evident by early anaphase (Fig. 1B), remained elevated through telophase (Fig. 1C) and became less intense by the pronuclear stage (Fig. 1D). Visual analysis of over 60 oocytes and zygotes indicated that fertilization triggered increased accumulation of P-Tyr-containing proteins in the egg cortex. These changes in fluorescence intensity were quantitated in 22 oocytes or zygotes which were oriented such that the meiotic spindle or polar bodies could be clearly identified. The ratio of anti-P-Tyr fluorescence intensity in the egg cortex relative to that in the central cytoplasm was determined by quantitation of average pixel intensity (integrated pixel intensity/pixel number). These measurements were made in the cortex (egg surface and cytoplasm 5μm deep) and the central cytoplasm (region deep to the cortex) using Metamorph 6.2. As seen in Table 1, fertilization resulted in a 1.5-fold increase in the concentration of P-Tyr-containing proteins detected in the cortex over that in the central cytoplasm. The magnitude of the changes in P-Tyr content of the egg cortex relative to the central cytoplasm were also evident when fluorescence intensity was quantitated by linescan analysis through the equator of the egg. As seen in Fig. 2, fluorescence intensity was not concentrated in the egg cortex prior to fertilization. However, zygotes collected at anaphase II and telophase II exhibited a marked concentration of P-Tyr proteins in the egg cortex represented in Fig. 2 as the left and right extremes of the X axis.
Figure 1.

Effect of Fertilization on the distribution of P-Tyr-containing egg proteins. MII oocytes were recovered from the oviducts of superovulated mice at 14 hrs post-hCG and fertilizing eggs and zygotes were collected from superovulated mated females at 13.0–16.0 hrs post hCG. The oocytes or zygotes were dissected free of the oviduct in the presence of fixative and processed for immunofluorescence as described in “Materials and Methods”. The samples were stained with the mouse anti-P-Tyr monoclonal 4G10 followed by Alexa-488-goat anti-mouse IgG (green) as well as Hoechst 33258 (white) to detect DNA. In some cases, the spindle was stained with rat monoclonal YOL 1/34 anti-tubulin which was detected by Alexa-568-goat anti-rat (red). Samples were examined on a Zeiss LSM500 confocal microscope as described in “Methods”. (A), MII oocyte; (B), fertilized, early anaphase; (C) fertilized telophase; (D) fertilized, early pronuclear stage (16 hrs post-hCG). Specificity was demonstrated by incubating eggs in the presence of the 4G10 antibody + 1mM P-Tyr (E). Magnification is indicated by the bars which represent 10μm.
Table 1.
Comparison of immunofluorescence intensity in the cortical and central ooplasm.
| Sample | n | Cortical/Central | S.E.M. |
|---|---|---|---|
| Unfertilized | 7 | 1.144 | 0.0284 |
| Fertilized | 12 | 1.671 | 0.0721 |
In order to compare the level of bound anti-P-Tyr antibody in MII oocytes with that of zygotes fixed prior to the pronuclear stage, the fluorescence intensity per unit area of the egg cortex was calculated and expressed as a ratio to the intensity of the central cytoplasm of each egg or zygote. Fluorescence intensity was quantitated as pixel intensity in the green fluorescence channel using the region measurement tool in Metamorph 6.2. The region of each egg cortex was traced by hand and included the plasma membrane and the underlying cytoplasm approximately 5μm deep. The region of the central cytoplasm was the remainder of the egg. The integrated pixel intensity of each region was divided by the number of pixels in each region. The ratio of cortical fluorescence intensity to central cytoplasmic fluorescence intensity was then calculated and the values represent the mean obtained from (n) oocytes or zygotes and is expressed +/− S.E.M. Analysis by t-test revealed that there is a statistically significant difference between the input groups (P = <0.001).
Figure 2. Changes in the relative distribution of P-Tyr in response to fertilization.
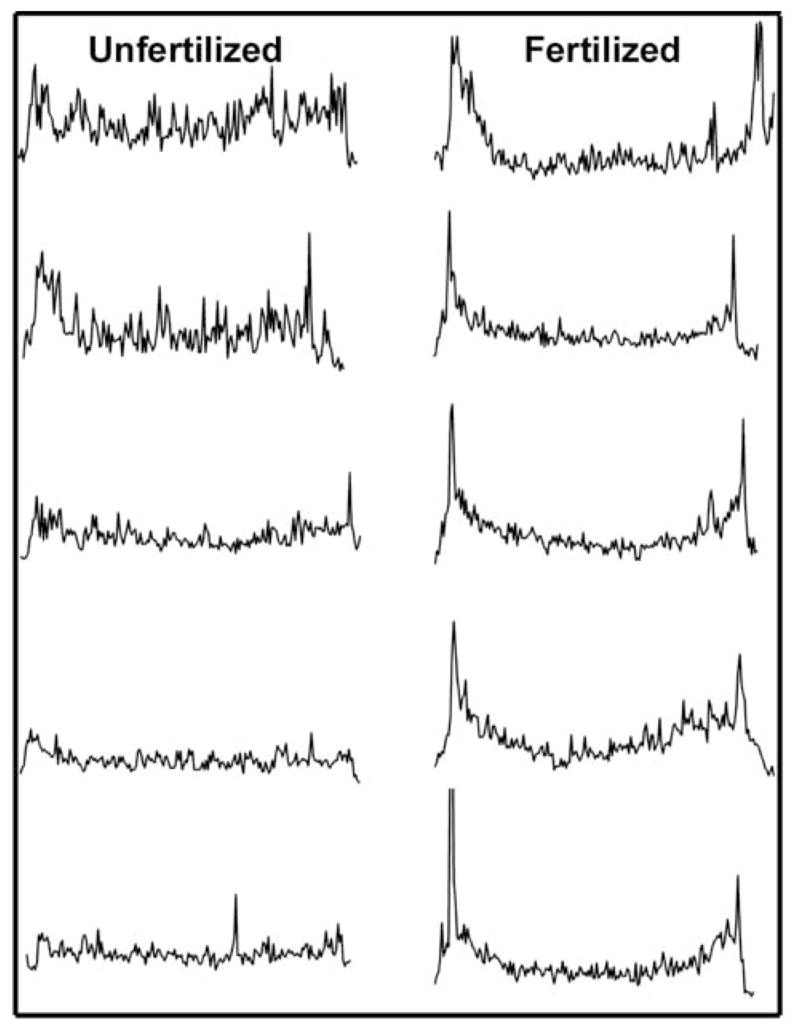
In order to demonstrate the effect of fertilization on the distribution of P-Tyr residues, transverse linescan analysis was performed on images from eggs and zygotes labeled with the anti-P-Tyr antibody as in Figure 1. Measurements lines were drawn originating from a point adjacent to the meiotic spindle and passing through the center of the egg to the opposite side. Pixel intensity along the course of the lines is presented in the vertical axis. Measurements made from representative unfertilized eggs are presented on the left and those made from different stages of zygote development up to the pronuclear stage.
In most cases, the cortical fluorescence intensity was not uniform over the entire egg, but appeared more intense over the half of the zygote containing the meiotic spindle (Fig. 1). In order to demonstrate the asymmetric nature of the cortical P-Tyr specific fluorescence, the fluorescence intensity of the entire egg cortex was quantitated by circumferential linescan analysis and presented as a two dimensional graph in Fig. 3. Linescans were initiated in the cortex of the egg 180° opposite the meiotic spindle and progressed clockwise around the egg. The pixel intensity was averaged over a region of cortex approximately 5μm deep beginning at the egg surface. In this analysis, the cortex adjacent to meiotic spindle appears near the middle of each graph and is indicated by arrows. These measurements were not intended to compare fluorescence intensity from egg to egg but rather to show the changes in relative fluorescence intensity from region to region in a single egg cortex. As predicted from the images presented in Fig. 1, the P-Tyr specific fluorescence in the cortex appeared as a collection of microspikes of varying intensity across the circumference of the egg. In unfertilized oocytes, the amplitude of the microspikes near the meiotic spindle was, for the most part, similar in intensity to those elsewhere in the cortex. However, during anaphase/telophase, cortical fluorescence intensity was 2 to 2.5 fold higher in the hemisphere containing the maternal chromatin and spindle (arrows). Highly localized concentrations of P-Tyr containing proteins were consistently observed at the margins of the site of polar body extrusion possibly reflecting the remnants of the contractile ring. At the pronuclear stage, fluorescence intensity in the cortex showed little evidence of polarity and was highly variable with scattered regions of more intense fluorescence distributed around the entire egg. Further observations made at higher magnification showed more clearly that, in the unfertilized egg, P-Tyr was concentrated in the cortex overlying the MII spindle (Fig. 4A). In addition, P-Tyr was concentrated in regions of cytoplasm adjacent to the poles of the MII spindle (Fig. 4B–D). P-Tyr was found concentrated at the spindle poles in the majority (19 of 24, 79%) of unfertilized eggs and completely disappeared after fertilization by anaphase (0 of 18).
Figure 3.
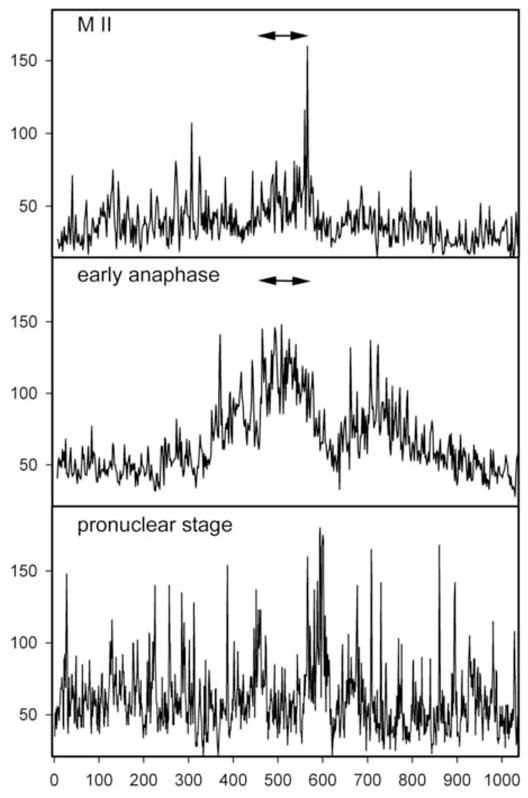
Distribution of P-Tyr-containing proteins in the egg cortex. In order to demonstrate the asymmetric distribution of P-Tyr in the egg cortex, the fluorescence intensity of eggs and zygotes labeled with the anti-P-Tyr antibody as in Figure 1, was quantitated by circumferential linescan analysis using Metamorph 6.2 and is indicated on the vertical axis. A measurement line was traced on images beginning from a position at the egg surface opposite the meiotic spindle and progressed clockwise around the egg cortex to include the entire cortex (horizontal axis). The position of the spindle is therefore near the center of each scan and is indicated by the arrows.
Figure 4.

Localization of P-Tyr proteins in the region of the meiotic spindle. Oocytes were collected from the same replicates as shown in Figure 1. Panel A is a compression of 3 serial confocal images showing the overlying cortex and the full length spindle with a small spot of P-Tyr label visible at the bottom spindle pole (Panel A). Panels B and C are two individual scans of a single MII spindle showing P-Tyr specific labeling at both spindle poles, top (B) and bottom (C). Panel D is an MII spindle from a second egg in which the layers have been compressed to allow visualization of both spindle poles on a single image. Panels A-C were co-labeled with YOL 1/34 anti-tubulin and Alexa-568 (red) to show spindle microtubules, while Panel D co-labeled with Alexa-568-Phalloidin to identify f-actin. Magnification is indicated by the bar which represents 10μm.
Another feature that became obvious at higher magnification was the observation of intense P-Tyr fluorescence in the plasma membrane or cortex immediately overlying some sperm that had recently fused with the egg (Fig. 5). Sperm in which the head appeared to be external to the egg plasma membrane (Fig. 5A) were not associated with increased cortical fluorescence (0 of 7). However, approximately 24% (5 of 21) of the cases in which the sperm head was positioned just beneath the egg plasma membrane exhibited elevated P-Tyr labeling in the overlying egg cortex and plasma membrane (Fig. 5B–E). The fact that this feature was detected in only a subset of eggs that had incorporated sperm indicated that the P-Tyr accumulation may be a transient event. Examination of the sperm themselves indicated that while little or nor P-Tyr was detected in the head region, the entire sperm flagellum labeled with the P-Tyr antibody which was especially pronounced in the region of the midpiece (Fig. 5A).
Figure 5.
P-Tyr-containing proteins in the egg cortex overlying the decondensing sperm head. Samples prepared as in Fig. 1 were stained with anti-P-Tyr followed by Alexa-488-goat anti-mouse IgG (green) and Hoechst 33258 (white) to label DNA. Fertilizing (capacitated) sperm bound to the oolema exhibited P-Tyr proteins in the sperm mid-piece, however specific P-Tyr label was not detected in the head region (Panel A). As fertilization progressed, decondensing sperm heads located deep to the oolema were associated with an increase in P-Tyr-containing proteins within the egg cortex immediately overlying the sperm nucleus (Panel B, C and D). Occasionally, the male pronucleus formed and remained adjacent to the egg cortex for some time and the P-Tyr label continued to be detected in the cortex near the paternal chromatin (Panel E). As the sperm DNA migrated away from the cortex, the P-Tyr label disappeared (not shown). Magnification is indicated by the bar which represents 10μm.
In summary, these results clearly indicate that the MII oocyte responds to fertilization with intense PTK signaling that is localized to the cortex overlying the spindle as well as specific sites associated with the spindle poles, the site of sperm incorporation, and the site of polar body extrusion. These signaling events are transient and are no longer detected at the mid-late pronuclear stage.
Evidence for Activation of Src-family PTKs in the Egg
In order to determine whether the above increase in P-Tyr-containing proteins could result from localized activation of Src-family PTKs, we used a phosphorylation site-specific antibody to detect activated Src-family members in the egg by immunofluorescence. The clone 28 antibody recognizes the dephosphorylated, C-terminal tyrosine (QYQPG) and flanking sequence common to several Src-family PTKs (Kawakatsu, et al., 1996). This antibody can detect activated Src, Fyn, and Yes, and possibly other Src-family members (Wu, et al., 2000) and we have used it recently to detect activated Src-family PTKs in the zebrafish egg (Sharma, and Kinsey, 2006). The specificity of this antibody in the mouse egg system was demonstrated by Western blot analysis of MII oocytes (Fig. 6) which showed that the clone 28 antibody bound a single band of 59–60KDa and that binding was blocked by excess peptide epitope (EPQYQPEGNL).
Figure 6.
Detection of Src-family PTKs by Western blot of mouse oocytes. Samples of unfertilized, cumulus-free oocytes were loaded on multiple lanes of a 10% SDS-PAGE gel (7.5eggs/lane) and electrophoresed, then blotted to a nylon membrane and blocked with TTBS+5% dried milk containing phosphatase inhibitors as described in “Materials and Methods”. Lanes were incubated with a control mouse monoclonal IgG (lane A), clone 28 IgG + 1mM blocking peptide (lane B), or clone 28 IgG (lane C), at a concentration of 1μg/ml overnight. The blots were then washed, incubated with goat anti-mouse IgG-peroxidase, and bound antibody was localized by chemiluminescence. The position of molecular weight standards is indicated (in KDa) at left, and the position of the Src-family PTK(s) detected with the clone 28 antibody is indicated by the arrow at right.
Immunofluorescence analysis of mouse oocytes before and at different times after fertilization demonstrated that activated Src-family PTKs were distributed uniformly throughout the cytoplasm of MII oocytes (Fig 7A) and early zygotes (Fig 7D–G). In distinct contrast to the P-Tyr labeling pattern, clone 28 binding exhibited no significant concentration in the egg cortex. However, the meiotic spindle was heavily labeled by the clone 28 antibody in all eggs examined (n=59) as seen at higher magnification in Fig. 7B. The localization of Src-family PTKs to the spindle resembled the results of a recent report (Zheng et al., 2007) in which a monoclonal antibody (Nonphospho-Src(Tyr416) against the autophosphorylation site of Src-family PTKs labeled the spindle in mouse oocytes. We have repeated their results (Fig. 7C) and it is clear that the clone 28 antibody and the Nonphospho-Src(Tyr416)antibody label the spindle with similar morphology. Since the Nonphospho-Src(Tyr416) antibody binds to inactive Src-family kinases, the result presented in Fig 7B,C demonstrate that the spindle is associated with a population of Src-family PTKs that includes both inactive and active kinases. As development of the zygote progressed, the distribution of clone-28 labeling did not change during anaphase II (Fig. 7D), telophase (Fig. 7E), and the early pronuclear stage (Fig. 7F). The specificity of clone 28 binding to the spindle was demonstrated by incubating the antibody with a blocking peptide (Fig 7G) duplicating the epitope against which the antibody was designed.
Figure 7.
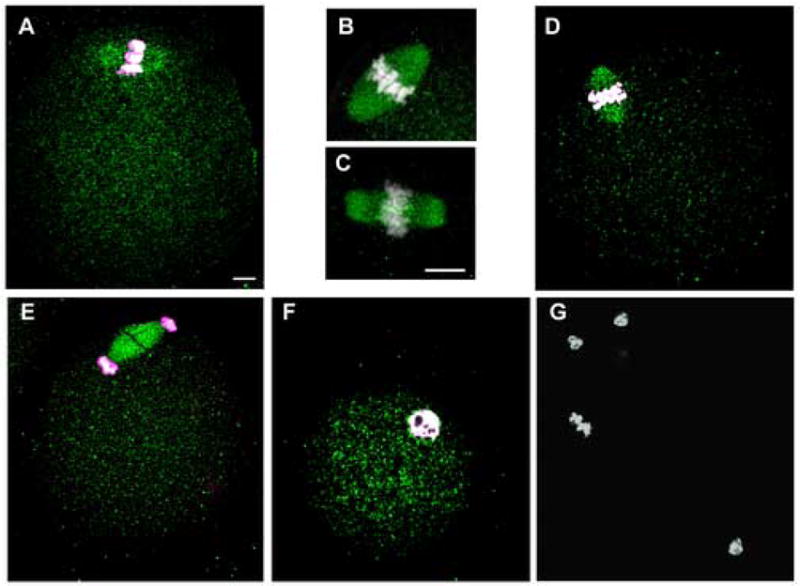
Active Src-family PTKs associate with spindle microtubules. Oocytes and zygotes fixed at different stages of zygote development were labeled with a monoclonal antibody against activated Src-family PTKs (clone 28), or with the Nonphospho-Src(Tyr416)antibody specific for inactive Src-family PTKs (Panel C only). Bound antibody was detected with Alexa-488-goat anti-mouse IgG (green) and Hoechst 33258 (white) was used to demonstrate chromatin. Active SFKs were detected as a low level of uniform fluorescence in the cytoplasm of oocytes as well as zygotes up to the early pronuclear stage. In contrast, high levels of both activated SFKs (panel A,B) and inactive SFKs (panel C) were associated with the spindle microtubules of the unfertilized MII oocytes indicating that the spindle is associated with a population of SFKs, some of which are active. Active Src-family PTKs detected with the clone 28 antibody remained associated with the spindle from early anaphase (Panel D) and telophase (Panel E). When the male and female pronuclei formed, the active SFKs became uniformly distributed in the cytoplasm again (Panel F). Specificity was demonstrated by incubating the sample with the clone 28 antibody in the presence of the synthetic peptide (EPQYQPGENL) at 1mM (G). Magnification is indicated by the bar which represents 10μm.
Once meiosis was complete and zygotes reached the late pronuclear stage, the clone-28 antibody was found to label the pronuclear envelope (Fig. 8A & 8D). In cleavage stage mouse embryos, activated Src-family PTKs continued to be associated with the nuclear envelope of the 2-cell embryo (Fig. 8B & 8E) although the labeling was less intense than at the pronuclear stage. Embryos that were fixed during the process of mitotic division displayed activated Src-family PTKs associated with the mitotic spindle and midbody (Fig. 8C,G).
Figure 8.

Activated SFKs associate with the nuclear envelope at the late pronuclear stage. Later pronuclear stage zygotes (24h post-hCG), 2-cell (48h) and 3- to 4-cell (60h) embryos were fixed and labeled with the clone-28 antibody as described for Figure 5. Activated SFKs were localized at or near the pronuclear envelope (Panel A, D). This peri-nuclear localization was still detectable at the 2-cell stage (Panel B, E). During mitosis (Panel C,F), activated SFKs were associated with the spindle (open arrow) and mid-body microtubules (closed arrow). Magnification is indicated by the bar which represents 10μm.
At compaction, activated Src-family PTKs were no longer associated with the nuclear envelope (Fig 9A,D) and the cortex remained devoid of Src-family PTK activity. Blastocyst stage embryos also exhibited activated Src-family PTKs associated with mitotic spindles and midbodies (Fig. 9B,E arrows). In addition, the blastocyst was the first developmental stage in which active Src-family PTKs were concentrated in the cortical cytoplasm of individual cells. This was most prominent in the inner cell mass cells but also visible in the trophoblast cells (Fig 9E,F). Mitotic cells exhibited increased Src-family PTK activity in all regions of the cytoplasm (Fig 9E,F arrows).
Figure 9.
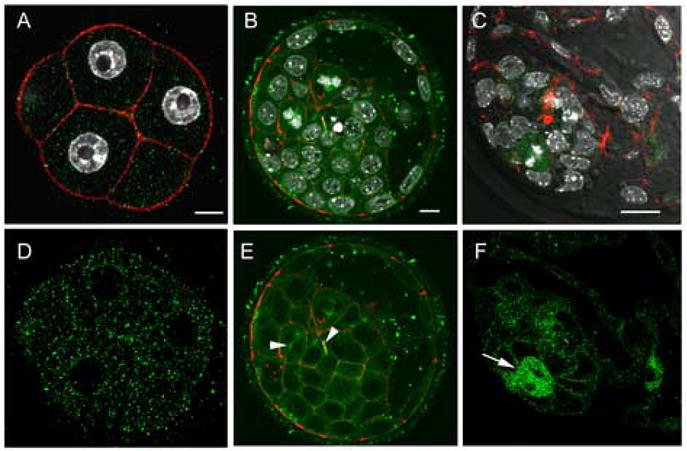
Distribution of activated SFKs in the morula and blastocyst. Embryos were fixed at the compaction (~48h post-hCG) and the expanded blastocyst (120h post-hCG) stages, then labeled with the clone-28 antibody (green) as well as Alexa-568-Phalloidin (red) to visualize f-actin. At the 8-cell compacted stage, activated SFKs were no longer associated with the nuclear envelope and were evenly distributed throughout the cytoplasm. In blastocyst stage embryos (Panel B,C,E,F), activated Src-family PTKs were concentrated at the cortex of most inner cell mass and trophoblast cells as well as at mitotic spindles and midbodies (arrow heads). At higher magnification, (Panels C, F) activated Src-family PTKs were concentrated in cells actively undergoing mitosis (arrows). Magnification is indicated by the white bar which represents 10μm.
In summary, even though the zygote cortex displayed fertilization-dependent accumulation of P-Tyr-containing proteins, activated Src-family PTKs were not detected in the zygote cortex. Instead, activated Src-family PTKs were localized to the meiotic and mitotic spindles as well as the nuclear envelope at the late pronuclear stage and early 2-cell stages. Once the embryo reached the blastocyst stage, cortical localization of activated Src-family PTKs was obvious in all cells of the blastocyst. Blastocysts also exhibited intense activation of Src-family PTKs in cells undergoing mitosis.
Functional Requirement for Src-family PTKs in Zygote Development
In order to determine whether the catalytic activity of Src-family PTKs plays an important role in development of the mammalian zygote, we tested the effect of several PTK inhibitors as well as a dominant-negative mutant Fyn construct on zygote development. These experiments were performed on eggs fertilized in vitro to ensure a synchronized population. MII oocytes were fertilized by incubation with capacitated sperm for a period of five hours, then transferred to culture drops containing different concentrations of PTK inhibitor and overlain with oil equilibrated with the same concentration of inhibitor. Zygotes were cultured for an additional 24 hours then scored for developmental progress. As seen in Table 2, 80% of the control zygotes treated with DMSO as a solvent control had reached the two cell stage. Zygotes treated with GTP14564 (Murata, et al., 2003) an inhibitor of class III receptor tyrosine kinases (IC50 0.3μM); or PD168393, an inhibitor of the EGFr kinase (IC50 0.7nM) (Fry, et al., 1998) successfully developed to the two cell stage within 24 hours. The Src-family PTK inhibitor PP2 had a small, but significant effect on zygote development at concentrations between 10 and 100μM, a range at which it could have non-specific effects on other protein kinases (Tatton, et al., 2003) (Bain, et al., 2003). However, the recently developed Src-family kinase inhibitor SKI-606 was much more effective at concentrations known to exhibit specificity in cell culture systems. Zygotes treated with SKI-606 exhibited a reduced rate of cleavage at 1 μM and were almost completely inhibited at 10μM. Cell division was reduced to 44% at 2.5 μM, a concentration similar to the IC50 reported to inhibit proliferation of cultured somatic cells (Boschelli, et al., 2004; Golas, et al., 2005). Examination of the zygotes that failed to cleave as a result of treatment with SKI-606 revealed that most were arrested prior to completion of second polar body emission. Typically, the MII spindle had rotated until it was perpendicular to the egg surface and all zygotes exhibited misplaced chromosomes and aberrant spindle microtubules. In addition, the treatment seemed to cause disruption of the microtubule dynamics in the egg resulting in some monopolar structures and astral arrays of microtubules under the egg cortex (Fig 10). Zygotes remained arrested in this configuration for as long as 24 hours. When SKI-606 was added after meiosis was complete and two pronuclei had formed, development was still arrested prior to the first mitotic division (Table 2) demonstrating that zygotes require Src-family PTK activity at a second point during the first cell cycle.
Table 2.
Effect of Different PTK Inhibitors on Zygote Development.
| Treatment | Stage Treated | n | % 2-cell | S.E.M. |
|---|---|---|---|---|
| DMSO | MII/anaphase | 80 | 82.1 | 0.045 |
| PD168393 (5μM) | “ | 49 | 80.1 | 0.071 |
| GTP14564 (20μM) | “ | 55 | 78.9 | 0.071 |
| PP2 (10μM) | “ | 34 | 72.5 | 0.185 |
| (100μM) | “ | 37 | 33.7* | 0.086 |
| SKI-606 (1μM) | “ | 72 | 72.3 | 0.154 |
| (2.5μM) | “ | 21 | 43.5* | 0.165 |
| (10μM) | “ | 89 | 14.9* | 0.086 |
| (10μM) | pronuclear | 106 | 13.3* | 0.033 |
| c-Fyn RNA (1.5μg/ul) | “ | 77 | 83.2 | 0.650 |
| dn-Fyn RNA (1.5μg/ml) | “ | 69 | 37.9* | 4.550 |
Mature, MII oocytes were collected from superovulated females and fertilized by incubation with capacitated sperm for 4.5 hours as described in “Materials and Methods”. The eggs were then transferred to small droplets of mKSOMAA containing the indicated inhibitor and overlain with oil equilibrated with the same inhibitor. This transfer was done either immediately after fertilization (MII/anaphase) or at the early pronuclear stage (pronuclear). For RNA injection, mRNA was transcribed in vitro and prepared as previously described (Sharma, et al., 2005), then pronuclear stage zygotes were injected with approximately 1–2pg of RNA and cultured in normal mKSOMAA. The status of the zygotes was assessed at 24 hours by examination by Hoffmann modulation or confocal fluorescence microscopy to establish whether sperm incorporation did occur and whether cell division had occurred. Values represent the mean from at least two experiments (*) indicates that the value is significantly different from the DMSO control group as determined by t test (P<0.05).
Figure 10.
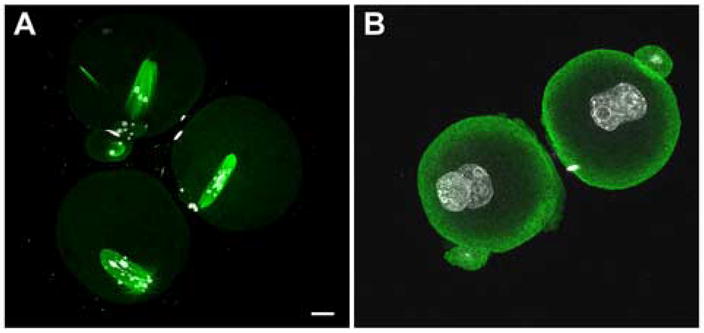
Inhibition of Src family PTK activity disrupted normal microtubule dynamics during meiosis II. Mature, MII oocytes were fertilized in vitro for five hours, then transferred to medium containing SKI-606 (10μm) (A) or DMSO (B). Zygotes were examined at 24 hours post-IVF when most controls were at the two cell stage. All embryos which failed to cleave to the two cell stage were fixed and stained with anti-tubulin (green) and Hoechst 33258 (white). Panel A demonstrates typical SKI-606 treated zygotes, and panel B demonstrates the morphology of DMSO treated controls that failed to cleave within 24hours. Magnification is indicated by the bar which represents 10 μm.
In an effort to confirm the role of Src-family PTKs in zygotic development without the use of chemical inhibitors, we tested the effect of a dominant-negative form of the Fyn kinase produced by mutation of Lysine 399 which is critical to catalytic activity (Sharma, et al., 2005). As seen in Table 2, injection of pronuclear stage zygotes with mRNA encoding the dominant-negative Fyn blocked development to the two cell stage with the majority of zygotes arrested with intact pronuclei (not shown). Those zygotes injected with mRNA encoding native Fyn as a control reached the two cell stage normally. Together, these results indicate that the observed association of activated Src-family PTKs with the MII spindle and pronuclear envelope are likely to represent functional signaling events important for zygote development.
DISCUSSION
Numerous studies involving chemical inhibitors, dominant-negative fusion proteins, and exogenous, recombinant kinases have demonstrated that PTKs including Src family PTKs, play an important role in activation of eggs from non-mammalian species. These species typically exhibit a rapid activation of Src-family PTKs which may play a role in sperm-egg fusion (Sakakibara, et al., 2005), and are required for the rapid, high amplitude calcium transient that triggers egg activation (Runft, et al., 2002). In addition, PTK activation has been observed later in zygotic development and is required for steps involved in pronuclear migration and fusion (Moore, and Kinsey, 1995; Wright, and Schatten, 1995) as well as developmental competence (Livingston, et al., 1998; Sharma, et al., 2005). The extent to which these pathways are required in mammalian fertilization is now the subject of much investigation. Recent studies have clearly shown that Src-family PTK activity is not required for the repetitive calcium oscillations that trigger activation of the mouse oocyte (Kurokawa, et al., 2004; Mehlmann, and Jaffe, 2005) highlighting differences between fertilization in mammals and lower species that fertilized externally. However, the experiments to date do indicate that PTKs are important for later aspects of zygotic development. Analysis of mammalian egg activation through the use of chemical inhibitors and SH2 domain-containing fusion proteins has demonstrated that MII resumption induced parthenogenetically required Src-family PTK activity (Sette, et al., 2002; Talmor-Cohen, et al., 2004a). However, MII resumption induced by sperm injection has proven more difficult to inhibit with these reagents (Meng, et al., 2006). Evidence for PTK functions later in zygote development was first obtained with the chemical inhibitor genistein (Besterman, and Schultz, 1990; Jacquet, et al., 1995) which blocked development prior to the exit from S-phase of the first zygotic cell cycle. Similarly, microinjection of a fusion protein encoding the SH2 domain of Fyn kinase caused developmental arrest at the late pronuclear stage (Meng, et al., 2006). Together these studies suggest that, while Src family PTKs may not be critical for calcium signaling at fertilization in mammals, they do play significant roles in later events critical to development of the mammalian zygote.
The signaling mechanisms present in the mammalian oocyte prior to fertilization include receptor protein tyrosine kinases such as EGF receptor (Hill, et al., 1999) and c-Kit (Motro, and Bernstein, 1993), as well as the Src-family PTKs Yes, Fyn, and, in some cases, Src (Talmor-Cohen, et al., 2004a; Mehlmann, and Jaffe, 2005). While direct measurement of PTK activity in mammalian eggs has proven difficult (Talmor, et al., 1998), the presence of active PTK signaling (phosphorylation greater than dephosphorylation) can also be inferred by the accumulation of the P-Tyr reaction product which can be detected either by chemical means (Besterman, and Schultz, 1990) or with anti-P-Tyr antibodies (Kurokawa, et al., 2004). These studies have indicated that since the amount of P-Tyr in the egg increased after fertilization, the balance between PTK and PTPase shifts after fertilization such the P-Tyr is allowed to accumulate. In the present study, we have used immunofluorescence localization of P-Tyr-containing proteins as a method to detect increased PTK signaling within different subcellular compartments of the zygote. This study was made possible by careful attention to controlling post-fixation dephosphorylation of egg proteins through the use of the covalent PTPase inhibitor phenylarsine oxide at all stages of fixation and processing. The results demonstrated that increased PTK signaling occurs in several different subcellular compartments following fertilization of the mouse egg.
Immunofluorescence analysis of P-Tyr accumulation during fertilization and zygote development revealed that fertilization is followed by highly localized changes (both increases and decreases) in PTK signaling in the egg. The unfertilized MII oocyte was relatively quiescent with a low level of P-Tyr distributed fairly evenly throughout the oocyte with only a slight concentration of P-Tyr in the cortex adjacent to the MII spindle and at the spindle poles. Fertilization resulted in a significant accumulation of P-Tyr in the zygote cortex indicating that increased PTK signaling was occurring in this compartment. The distribution of cortical P-Tyr exhibited a distinct polarity in most zygotes with the highest concentration of P-Tyr in the hemisphere associated with the meiotic spindle. During anaphase, the region directly overlying the spindle (corresponding generally with the cortical granule free domain (Deng et al., 2003)) was usually most intensely labeled by the anti-P-Tyr antibody. This suggests that PTK signaling may play an important role in either actin-mediated events that modify this region of cortex, or in other signaling pathways such as MAPK (Deng, et al., 2005) or PAR-3 (Duncan, et al., 2005) which characterize this important region. Within the zygote cortex, further concentrations of P-Tyr were detected at the shoulders of the emerging polar body associated with the region occupied by the contractile ring. The contractile ring in sea urchin embryos has been associated with ganglioside-1 and cholesterol-rich microdomains that are characterized by intense PTK signaling (Ng, et al., 2005), and it is likely that a similar mechanism is employed during polar body extrusion.
While fertilization caused an increase in PTK signaling in the egg cortex, the highly localized accumulation of P-Tyr at the spindle poles decreased as anaphase progressed and disappeared at telophase. The fact that PTK signaling at the spindle poles declined as meiosis progressed indicates that this pathway is likely linked to the cell cycle events that control spindle function. Enzymes such as MAPK (Hatch, and Capco, 2002) and polo like kinase (Wianny, et al., 1998; Tong, et al., 2002; Fan, et al., 2003) which are substrates for PTKs and have been localized to spindle poles are likely targets for PTK signaling in this region
Another highly localized PTK signaling event was detected in the cortex overlying the site of sperm incorporation. Since P-Tyr concentration was found in only 24% of the sperm incorporation sites observed, we infer that it could represent a transient signaling event. This event was not detected in the early stages of sperm incorporation such as sperm-egg attachment or formation of the fertilization cone. Instead it was apparent only after the sperm head was fully incorporated and generally when nuclear decondensation had begun. It is not clear whether the P-Tyr containing proteins observed overlying the sperm head were egg proteins or were contributed by the sperm. It is also unclear whether they were phosphorylated by a PTK derived from the egg or the sperm. The timing of this localized signaling event indicates that it does not represent the phosphorylation of uroplakin III, which occurs rapidly in the Xenopus oocyte and is thought to function in sperm-egg fusion (Sakakibara, et al., 2005). In any case, this interesting finding demonstrates that the site of sperm incorporation/decondensation is associated with highly localized PTK signaling
The above results raise the question of which PTKs in the egg are responsible for the localized changes in protein tyrosine phosphorylation. The recent development of phosphorylation site-specific antibodies that recognize the activated form of different PTKs has provided a method to identify which PTKs are activated in different subcellular compartments. As an initial step, we have used the clone 28 antibody (Kawakatsu, et al., 1996) which is specific for the activated form of Src-family PTKs and has been used to demonstrate highly localized changes in Src-family PTK activity in tissues ranging from kidney, peripheral nerve, endometrium, and various cancers (Takikita-Suzuki, et al., 2003; Zhao, et al., 2003; Ito, et al., 2002). One limitation is that, since the sequence of amino acids flanking the C-terminal tyrosine is highly conserved among Src-family members, this antibody cannot differentiate among the different family members. However, only a limited number of Src-family PTKs have been detected in eggs of different species (O’Neill, et al., 2004; Mehlmann, et al., 2001; Talmor-Cohen, et al., 2004a). Therefore, the kinases detected by the clone 28 antibody in the egg probably include Fyn, Yes, and possibly Src, although other Src family PTKs may contribute to our results. The antibody would not be able to detect the closely related Abl, the receptor type PTKs such as Kit or EGF receptor, or other PTKs such as FAK or JAK.
The most surprising observation was that activated Src-family PTKs were not highly localized to the zygote cortex which exhibited such a dramatic increase in P-Tyr after fertilization. We have previously shown that Fyn kinase is concentrated at the cortex of the mouse egg (Meng, et al., 2006) but the present results indicate that in the mouse egg, Fyn must remain inactive in this compartment until later in development. This observation contrasts with our results in the zebrafish egg which exhibited cortical activation of Src-family PTKs at fertilization (Sharma, and Kinsey, 2006). Instead, the results in mouse demonstrated a very striking association of activated Src-family PTKs with active spindle structures (both meiotic and mitotic) as well as midbodies. This finding confirms earlier results obtained with this same antibody in somatic cells (Wu, et al., 2000; Yamada, et al., 2000; Yamamoto, et al., 2002). Localization of active Src-family PTKs to the spindle also correlated well with the demonstration that Fyn kinase was tightly bound to the meiotic spindle in rat oocytes (Talmor-Cohen, et al., 2004b). A similar approach was used by Zheng et al., 2007 to detect Src-family PTKs in the mouse oocytes. Their results demonstrated that an antibody to non-activated Src-family PTKs (Nonphospho-Src(Tyr416)) also bound to the MII spindle with a morphology similar to that reported here for the clone 28 antibody. The fact that both active and inactive Src-family PTKs were associated with the spindle at all stages of spindle function, suggests that this signaling mechanism may play a role in maintenance of the spindle structure or function. For example, tyrosine phosphorylation of CDC2 has been linked to its ability to drive assembly of the mitotic spindle in yeast (Gould, and Feoktistova, 1996).
In addition to the spindle, the second most obvious site of Src-family PTK activation was at the pronuclear envelope of late pronuclear stage zygotes. We have previously demonstrated that Fyn kinase associated with the pronuclear envelope (Meng, et al., 2006), so it is likely that Fyn represents some of the activated Src-family PTKs detected by the clone 28 antibody near the pronuclear membranes. The association of active PTKs with the nuclear envelope remained high at the 2-cell stage but was much reduced by the four-cell stage and was not evident in the nuclei of blastocysts, or in somatic cells such as cumulus cells. The demonstration of activated Src-family kinases located at the surface of the nuclear envelope during pronuclear through the two cell stage correlates well with functional data demonstrating that PTK activity and Src-family PTK activity specifically is required at this stage. For example, chemical PTK inhibitors have been shown to cause zygotic arrest at this stage (Besterman, and Schultz, 1990; Jacquet, et al., 1995) and GST fusion proteins encoding the SH2 domain of Fyn caused mouse zygotes to arrest at the late pronuclear or two cell stage. While Src-family PTK activation has been shown to play a role in cell cycle events in somatic cells (Courtneidge, 2002), the fact that intense, nuclear envelope-associated PTK activation was primarily observed at the 1–2 cell stage indicates that this must represent a zygote-specific function, possibly involving alterations in nuclear structure associated with zygotic gene activation.
The use of pharmacological inhibitors specific for Src-family PTKs has included studies of the PP2 which blocks MII resumption in response to parthenogenetic stimuli but only at high concentrations (Sette, et al., 2002; Talmor-Cohen, et al., 2004a). SU6656, which appears to become sequestered in vacuoles in the egg, has little effect on fertilization (Mehlmann and Jaffe, A. 2005). In the present study, we have tested the recently developed quinolinecarbonitrile derivative SKI-606 which combines high specificity for Src family PTKs with superior aqueous solubility and stability (Boschelli, et al., 2004). The compound inhibits Src in vitro with an IC 50 of 1.2nM, while it exhibited an in vitro IC50 of 2.6μM for the receptor PTK ErbB-2, and 19 μM for the Ser/THr kinase Cdk4 (Boschelli, et al., 2001). Like PP2 (Clark and Peterson, 2003), SKI-606 also inhibits the closely related Abl kinase with an IC50 of 1.0nM The compound readily penetrates the plasma membrane, blocking Src kinase activity in cultured cells with an IC50 of 250nM and Src-dependent cell proliferation with an IC50 of 1.5–2.5 μM. The stability of this compound in aqueous media leads to its ability to inhibit tumor growth in vivo with an IC50 of 250nM (Golas, et al., 2005) and the compound is now undergoing clinical trials for treatment of human cancer (ClinicalTrials.gov). We found this compound to block meiosis II completion and cause abnormal spindle structures and a high frequency of misplaced chromosomes. When added after meiosis was complete, it blocked development to the two cell stage. In order to confirm this result by another means and begin the process of determining which Src-family PTKs are involved in different stages of zygotic development, we used a dominant-negative form of Fyn kinase introduced by RNA injection. The results of this experiment indicated that Fyn is likely to be a key component of those Src-family PTKs required for zygotic development. The use of kinase-inactivating mutations to produce dominant-negative forms of Src-family PTKs has been successfully employed in developmental studies before (Denoyelle et al., 2001). The kinase inactivating point mutation used in FynK299M has the advantage that the U, SH3 and SH2 protein interaction domains remain intact and can compete with the native Fyn for protein interactions occurring both upstream and downstream. To the extent that the specificity of these interactions is common to other Src-family members, the dominant-negative construct can be expected to compete with other Src-family members and thereby block compensation by these kinases. This provides an advantage over single gene knockout or RNAi knockdown studies but makes it more difficult to identify the role of each specific kinase. While Src family PTKs are well known to share overlapping specificity, it is unlikely that non-Src-family PTKs would be affected by this dominant-negative construct since they would not share the combination of SH2, SH3 and U domain specificities.
In summary, the present study has demonstrated that fertilization in mammals results in highly localized PTK signaling events associated with specific regions of the egg cortex, meiotic spindle, and pronuclear envelope. Their localized nature and differential timing indicates that they are likely under different control mechanisms in the zygote. The Src-family PTKs, which are highly concentrated in the egg cortex, were not activated significantly in this compartment and it is likely that other PTKs are responsible for the intense tyrosine phosphorylation of proteins in the cortex of the mouse egg. Instead, the Src-family PTKs appeared to play roles in spindle structure or function as well as nuclear events unique to the zygote and early cleavage stages.
Acknowledgments
We are indebted to Dr. Li Meng and Dr. J. Luo who made initial observations that led to the present study. Supported by NICHD 14846 to W.H.K. and NICHD 42076 to D. F. A. Additional support for D.F.A. from the The Hall Family Foundation and The ESHE Fund.
Abbreviations
- MII
meiosis II
- GST
glutathione S transferase
- SH2
Src homology 2
- eCG
equine chorionic gonadotropin
- hCG
human chorionic gonadotropin
- KSOM
potassium simplex optimized medium
- PIPES
piperazine-N,N′-bis[2-ethanesulfonic acid]
- EGTA
ethylene glycol-bis(β-aminoethyl ether) N,N,N,N-tetraacetic acid
- PBS
phosphate buffered saline
- BSA
bovine serum albumin
- HEPES
N-(2hydroxyethyl)piperazine-N′-[2-ethanesulfonic acid])
- DMSO
dimethylsuphoxide
- TTBS
NaCl 0.1M, Tris, 0.01M, 0.1% Tween-20, pH 7.5
- EGFr
epidermal growth factor receptor
- PTPase
protein tyrosine phosphatase
- MAPK
mitogen activated protein kinase
- PTK
protein tyrosine kinase
- FAK
focal adhesion kinase
- JAK
janus kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besterman B, Schultz RM. Regulation of mouse preimplantation development: inhibitory effect of genistein, an inhibitor of tyrosine protein phosphorylation, on cleavage of one-cell embryos. J Exp Zool. 1990;256:44–53. doi: 10.1002/jez.1402560107. [DOI] [PubMed] [Google Scholar]
- Biggers JD, McGinnis LK, Lawitts JA. Enhanced effect of glycyl-L-glutamine on mouse preimplantation embryos in vitro. Reprod Biomed Online. 2004;9:59–69. doi: 10.1016/s1472-6483(10)62111-6. [DOI] [PubMed] [Google Scholar]
- Boschelli DH, Wang YD, Johnson S, Wu B, Ye F, Barrios Sosa AC, Golas JM, Boschelli F. 7-Alkoxy-4-phenylamino-3-quinolinecar-bonitriles as dual inhibitors of Src and Abl kinases. J Med Chem. 2004;47:1599–1601. doi: 10.1021/jm0499458. [DOI] [PubMed] [Google Scholar]
- Boschelli DH, Ye F, Wang YD, Dutia M, Johnson SL, Wu B, Miller K, Powell DW, Yaczko D, Young M, Tischler M, Arndt K, Discafani C, Etienne C, Gibbons J, Grod J, Lucas J, Weber JM, Boschelli F. Optimization of 4-phenylamino-3-quinolinecarbonitriles as potent inhibitors of Src kinase activity. J Med Chem. 2001;44:3965–3977. doi: 10.1021/jm0102250. [DOI] [PubMed] [Google Scholar]
- Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 2004;23:7957–7968. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- Carroll J. The initiation and regulation of Ca2+ signalling at fertilization in mammals. Semin Cell Dev Biol. 2001;12:37–43. doi: 10.1006/scdb.2000.0215. [DOI] [PubMed] [Google Scholar]
- Clark DD, Peterson BR. Analysis of protein tyrosine kinase inhibitors in recombinant yeast lacking the ERG6 gene. Chembiochem. 2003 Jan 3;4:101–107. doi: 10.1002/cbic.200390001. [DOI] [PubMed] [Google Scholar]
- Courtneidge SA. Role of Src in signal transduction pathways. Biochem Soc Trans. 2002;30:11–17. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Cox LJ, Larman MG, Saunders CM, Hashimoto K, Swann K, Lai FA. Sperm phospholipase Czeta from humans and cynomoigus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction. 2002;124:611–623. doi: 10.1530/rep.0.1240611. [DOI] [PubMed] [Google Scholar]
- Deng M, Williams CJ, Schultz RM. Role of MAP kinase and myosin light chain kinase in chromosome-induced development of mouse egg polarity. Dev Biol. 2005 Feb 15;278:358–366. doi: 10.1016/j.ydbio.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Denoyelle M, Valles A, Lentz D, Thiery JP, Boyer B. Mesoderm-independent regulation of gastrulation movements by src tyrosine kinase. Differentiation. 2001;69:38–48. doi: 10.1046/j.1432-0436.2001.690104.x. [DOI] [PubMed] [Google Scholar]
- Duncan FE, Moss SB, Schultz RM, Williams CJ. PAR-3 defines a central subdomain of the cortical actin cap in mouse eggs. Dev Biol. 2005 Apr 1;280:38–47. doi: 10.1016/j.ydbio.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Fan HY, Tong C, Teng C, Lian L, Li SW, Yang ZM, Chen DY, Schatten H, Sun QY. Characterization of polo-like kinase-1 in rat oocytes and early embryos implies its functional roles in the regulation of meiotic maturation, fertilization, and cleavage. Mol Reprod Dev. 2003;65:318–329. doi: 10.1002/mrd.10283. [DOI] [PubMed] [Google Scholar]
- Fry DW, Bridges AJ, Denny WA, Doherty A, Greis KD, Hicks JL, Hook KE, Keller PR, Leopold WR, Loo JA, McNamara DJ, Nelson JM, Sherwood V, Smaill JB, Trumpp-Kallmeyer S, Dobrusin EM. Specific, irreversible inactivation of the epidermal growth factor receptor and erbB2, by a new class of tyrosine kinase inhibitor. Proc Natl Acad Sci U S A. 1998;95:12022–12027. doi: 10.1073/pnas.95.20.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusti AF, Carroll DJ, Abassi YA, Terasaki M, Foltz KR, Jaffe LA. Requirement of a Src family kinase for initiating calcium release at fertilization in starfish eggs. J Biol Chem. 1999;274:29318–29322. doi: 10.1074/jbc.274.41.29318. [DOI] [PubMed] [Google Scholar]
- Giusti AF, Xu WQ, Hinkle B, Terasaki M, Jaffe LA. Evidence that fertilization activates starfish eggs by sequential activation of a Src-like kinase and phospholipase Cgamma. J Biol Chem. 2000;275:16788–16794. doi: 10.1074/jbc.M001091200. [DOI] [PubMed] [Google Scholar]
- Golas JM, Lucas J, Etienne C, Golas J, Discafani C, Sridharan L, Boghaert E, Arndt K, Ye F, Boschelli DH, Li F, Titsch C, Huselton C, Chaudhary I, Boschelli F. SKI-606, a Src/Abl inhibitor with in vivo activity in colon tumor xenograft models. Cancer Res. 2005 Jun 15;65:5358–5364. doi: 10.1158/0008-5472.CAN-04-2484. [DOI] [PubMed] [Google Scholar]
- Gould KL, Feoktistova A. Characterization of novel mutations at the Schizosaccharomyces pombe cdc2 regulatory phosphorylation site, tyrosine 15. Mol Biol Cell. 1996;7:1573–1586. doi: 10.1091/mbc.7.10.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch KR, Capco DG. Colocalization of CaM KII and MAPK on architectural elements of the mouse egg: Potentiation of MAP kinase by CaM KII. Mol Reprod Dev. 2002;58:69–77. doi: 10.1002/1098-2795(200101)58:1<69::AID-MRD10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Hill JL, Hammar K, Smith PJ, Gross DJ. Stage-dependent effects of epidermal growth factor on Ca2+ efflux in mouse oocytes. Mol Reprod Dev. 1999;53:244–253. doi: 10.1002/(SICI)1098-2795(199906)53:2<244::AID-MRD13>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Ito Y, Kawakatsu H, Takeda T, Tani N, Kawaguchi N, Noguchi S, Sakai T, Matsuura N. Activation of c-Src is inversely correlated with biological aggressiveness of breast carcinoma. Breast Cancer Res Treat. 2002;76:261–267. doi: 10.1023/a:1020860221099. [DOI] [PubMed] [Google Scholar]
- Jacquet P, Saint-Georges L, Barrio S, Baugnet-Mahieu L. Morphological effects of caffeine, okadaic acid and genistein in one-cell mouse embryos blocked in G2 by X-irradiation. Int J Radiat Biol. 1995;67:347–358. doi: 10.1080/09553009514550401. [DOI] [PubMed] [Google Scholar]
- Kawakatsu H, Sakai T, Takagaki Y, Shinoda Y, Saito M, Owada H, Yano J. A new monoclonal antibodu which selectively recognizes the active form of Src Tryosine kinase. J-Biol-Chem. 1996;271:5680–5685. doi: 10.1074/jbc.271.10.5680. [DOI] [PubMed] [Google Scholar]
- Kinsey WH, Shen SS. Role of the Fyn kinase in calcium release during fertilization of the sea urchin egg. Devel Biol. 2000;225:253–264. doi: 10.1006/dbio.2000.9830. [DOI] [PubMed] [Google Scholar]
- Kinsey WH, Wu W, Macgregor E. Activation of Src-family PTK activity at fertilization: role of the SH2 domain. Dev Biol. 2003;264:255–262. doi: 10.1016/j.ydbio.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Kurokawa M, Sato K, Smyth J, Wu H, Fukami K, Takenawa T, Fissore RA. Evidence that activation of Src family kinase is not required for fertilization-associated [Ca2+]i oscillations in mouse eggs. Reproduction. 2004;127:441–454. doi: 10.1530/rep.1.00128. [DOI] [PubMed] [Google Scholar]
- Livingston BT, VanWinkle CE, Kinsey WH. Protein tyrosine kinase activity following fertilization is required to complete gastrulation, but not for initial differentiation of endoderm and mesoderm in the sea urchin embryo. Dev Biol. 1998;193:90–99. doi: 10.1006/dbio.1997.8743. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Carpenter G, Rhee SG, Jaffe LA. SH2 domain-mediated activation of phospholipase Cgamma is not required to initiate Ca2+ release at fertilization of mouse eggs. Dev-Biol. 1998;203:221–232. doi: 10.1006/dbio.1998.9051. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Chattopadhyay A, Carpenter G, Jaffe LA. Evidence that phospholipase c from the sperm is not responsible for initiating Ca2+ release at fertilization in mouse eggs. Dev Biol. 2001;236:492–501. doi: 10.1006/dbio.2001.0329. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Jaffe LA. SH2 domain-mediated activation of an SRC family kinase is not required to initiate Ca2+ release at fertilization in mouse eggs. Reproduction. 2005;129:557–564. doi: 10.1530/rep.1.00638. [DOI] [PubMed] [Google Scholar]
- Meng L, Luo JP, Li C, Kinsey WH. Role of SH2 domain-mediated PTK signaling in mouse zygotic development. Reproduction. 2006;132:413–421. doi: 10.1530/rep.1.01151. [DOI] [PubMed] [Google Scholar]
- Messinger S, Albertini D. Centrosome and microtubule dynamics during meiotic progression in the mouse oocyte. J Cell Sci. 1991;100:289–298. doi: 10.1242/jcs.100.2.289. [DOI] [PubMed] [Google Scholar]
- Moore KL, Kinsey WH. Effects of protein tyrosine kinase inhibitors on egg activation and fertilization-dependent protein tyrosine kinase activity. Dev-Biol. 1995;168:1–10. doi: 10.1006/dbio.1995.1056. [DOI] [PubMed] [Google Scholar]
- Motro B, Bernstein A. Dynamic changes in ovarian c-kit and Steel expression during the estrous reproductive cycle. Dev Dyn. 1993;197:69–79. doi: 10.1002/aja.1001970107. [DOI] [PubMed] [Google Scholar]
- Murata K, Kumagai H, Kawashima T, Tamitsu K, Irie M, Nakajima H, Suzu S, Shibuya M, Kamihira S, Nosaka T, Asano S, Kitamura T. Selective cytotoxic mechanism of GTP-14564, a novel tyrosine kinase inhibitor in leukemia cells expressing a constitutively active Fms-like tyrosine kinase 3 (FLT3) J Biol Chem. 2003;278:32892–32898. doi: 10.1074/jbc.M210405200. [DOI] [PubMed] [Google Scholar]
- Ng MM, Chang F, Burgess DR. Movement of membrane domains and requirement of membrane signaling molecules for cytokinesis. Dev Cell. 2005;9:781–790. doi: 10.1016/j.devcel.2005.11.002. [DOI] [PubMed] [Google Scholar]
- O’Neill FJ, Gillett J, Foltz KR. Distinct roles for multiple Src family kinases at fertilization. J Cell Sci. 2004;117:6227–6238. doi: 10.1242/jcs.01547. [DOI] [PubMed] [Google Scholar]
- Runft LL, Jaffe LA, Mehlmann LM. Egg activation at fertilization: Where it all begins. Dev Biol. 2002;245:237–254. doi: 10.1006/dbio.2002.0600. [DOI] [PubMed] [Google Scholar]
- Sakakibara K, Sato K, Yoshino K, Oshiro N, Hirahara S, Mahbub Hasan AK, Iwasaki T, Ueda Y, Iwao Y, Yonezawa K, Fukami Y. Molecular identification and characterization of Xenopus egg uroplakin III, an egg raft-associated transmembrane protein that is tyrosine-phosphorylated upon fertilization. J Biol Chem. 2005;280:15029–15037. doi: 10.1074/jbc.M410538200. [DOI] [PubMed] [Google Scholar]
- Sato K, Iwasaki T, Tamaki I, Aoto M, Tokmakov AA, Fukami Y. Involvement of Protein Tyrosine phosphorylation in sperm-induced xenopus egg activation. Febs-Lett. 1998;424:113–118. doi: 10.1016/s0014-5793(98)00123-9. [DOI] [PubMed] [Google Scholar]
- Sato K, Tokmakov AA, Fukami Y. Fertilization signalling and protein-tyrosine kinases. Comp Biochem Physiol[B] 2000;126:129–148. doi: 10.1016/s0305-0491(00)00192-9. [DOI] [PubMed] [Google Scholar]
- Sette C, Paronetto MP, Barchi M, Bevilacqua A, Geremia R, Rossi P. Tr-kit-induced resumption of the cell cycle in mouse eggs requires activation of a Src-like kinase. EMBO J. 2002;21:5386–5395. doi: 10.1093/emboj/cdf553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Holets L, Zhang X, Kinsey WH. Role of Fyn kinase in signaling associated with epiboly during zebrafish development. Dev Biol. 2005;285:462–476. doi: 10.1016/j.ydbio.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Sharma D, Kinsey WH. Fertilization triggers localized activation of Src-family protein kinases in the zebrafish egg. Dev Biol. 2006;295:604–614. doi: 10.1016/j.ydbio.2006.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers MC, McGinnis LK, Lawitts JA, Biggers JD. Mouse embryo development following IVF in media containing either L-glutamine or glycyl-L-glutamine. Hum Reprod. 2005;20:1364–1371. doi: 10.1093/humrep/deh756. [DOI] [PubMed] [Google Scholar]
- Takikita-Suzuki M, Haneda M, Sasahara M, Owada MK, Nakagawa T, Isono M, Takikita S, Koya D, Ogasawara K, Kikkawa R. Activation of Src kinase in platelet-derived growth factor-B-dependent tubular regeneration after acute ischemic renal injury. Am J Pathol. 2003;163:277–286. doi: 10.1016/S0002-9440(10)63651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmor A, Kinsey WH, Shalgi R. Expression and immunolocalization of p59c-fyn tyrosine kinase in rat eggs. Dev-Biol. 1998;194:38–46. doi: 10.1006/dbio.1997.8816. [DOI] [PubMed] [Google Scholar]
- Talmor-Cohen A, Tomashov-Matar R, Eliyahu E, Shapiro R, Shalgi R. Are Src family kinases involved in cell cycle resumption in rat eggs? Reproduction. 2004a;127:455–463. doi: 10.1530/rep.1.00104. [DOI] [PubMed] [Google Scholar]
- Talmor-Cohen A, Tomashov-Matar R, Tsai WB, Kinsey WH, Shalgi R. Fyn kinase-tubulin interaction during meiosis of rat eggs. Reproduction. 2004b;128:387–393. doi: 10.1530/rep.1.00266. [DOI] [PubMed] [Google Scholar]
- Tatton L, Morley GM, Chopra R, Khwaja A. The Src-selective kinase inhibitor PP1 also inhibits Kit and Bcr-Abl tyrosine kinases. J Biol Chem. 2003;278:4847–4853. doi: 10.1074/jbc.M209321200. [DOI] [PubMed] [Google Scholar]
- Tong C, Fan HY, Lian L, Li SW, Chen DY, Schatten H, Sun QY. Polo-like kinase-1 is a pivotal regulator of microtubule assembly during mouse oocyte meiotic maturation, fertilization, and early embryonic mitosis. Biol Reprod. 2002;67:546–554. doi: 10.1095/biolreprod67.2.546. [DOI] [PubMed] [Google Scholar]
- Tsai WB, Zhang X, Sharma D, Wu W, Kinsey WH. Role of Yes kinase during early zebrafish development. Dev-Biol. 2005;277:129–141. doi: 10.1016/j.ydbio.2004.08.052. [DOI] [PubMed] [Google Scholar]
- Wianny F, Tavares A, Evans MJ, Glover DM, Zernicka-Goetz M. Mouse polo-like kinase 1 associates with the acentriolar spindle poles, meiotic chromosomes and spindle midzone during oocyte maturation. Chromosoma. 1998;107:430–439. doi: 10.1007/s004120050327. [DOI] [PubMed] [Google Scholar]
- Wright SJ, Schatten G. Protein tyrosine phosphorylation during sea urchin fertilization: microtubule dynamics require tyrosine kinase activity. Cell Motil Cytoskeleton. 1995;30:1122–1135. doi: 10.1002/cm.970300204. [DOI] [PubMed] [Google Scholar]
- Wu W, Kinsey WH. Fertilization triggers activation of Fyn kinase in the zebrafish egg. Int J Dev Biol. 2001;44:837–841. [PubMed] [Google Scholar]
- Wu Y, Ozaki Y, Inoue K, Satoh K, Ohmori T, Yatomi Y, Owada K. Differential activation and redistribution of c-Src and Fyn in platelets, assessed by MoAb specific for C-terminal tyrosine-dephosphorylated c-Src and Fyn. Biochim Biophys Acta Mol Cell Res. 2000 Jun 2;1497:27–36. doi: 10.1016/s0167-4889(00)00043-4. [DOI] [PubMed] [Google Scholar]
- Yamada T, Aoyama Y, Owada MK, Kawakatsu H, Kitajima Y. Scraped-wounding causes activation and association of C-Src tyrosine kinase with microtubules in cultured keratinocytes. Cell Struct Funct. 2000;25:351–359. doi: 10.1247/csf.25.351. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Maruyama T, Sakai N, Sakurai R, Shimizu A, Hamatani T, Masuda H, Uchida H, Sabe H, Yoshimura Y. Expression and subcellular distribution of the active form of c-Src tyrosine kinase in differentiating human endometrial stromal cells. Mol Hum Reprod. 2002;8:1117–1124. doi: 10.1093/molehr/8.12.1117. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Takagawa K, Oya T, Sata M. Active Src expression is induced after rat peripheral nerve injury. Glia. 2003;42:184–193. doi: 10.1002/glia.10223. [DOI] [PubMed] [Google Scholar]
- Zheng KG, Meng X, Yang Y, Yu Y, Liu D, Li Y. Requirements of Src family kinase during meiotic maturation in mouse oocytes. Mol Reprod Devel. 2007;74:125–130. doi: 10.1002/mrd.20613. [DOI] [PubMed] [Google Scholar]




