Abstract
Penile erection is a complex physiologic event resulting from the interactions of the nervous system on a highly specialized vascular organ. Activation of central nervous system melanocortinergic (MC) receptors with either endogenous or synthetic melanotropic ligands may initiate and/or facilitate spontaneous penile erection.
While the CNS contains principally the MC3 and MC4 receptor subtypes, there is conflicting data as to which receptor mediates erection. Although the MC4R is emerging as the principle effector of MC induced erection, the role of the MC3R is poorly understood. Manipulation of each receptor subtype with newly synthesized receptor specific agonists and antagonists, as well as knockout mice, has elucidated their individual contributions. Novel data from our laboratories suggests that antagonism of forebrain MC3R may enhance melanocortin-induced erections. Furthermore, melanocortin agents may interact with better-studied systems such as oxytocinergic pathways at the hypothalamic, brainstem or spinal level.
Current therapies for erectile dysfunction target end organ vascular tissue. Manipulation of MC receptors may provide an alternative, centrally mediated therapeutic approach for erectile and other sexual dysfunctions. The non-specific “superpotent” MC agonist, PT-141, which is the carboxylate derivative of MT-II, has reached phase II human trials. Through their centrally mediated activity, melanocortin agonists have potential to treat erectile dysfunction as well as possible applications to the unmet medical needs of decreased sexual motivation and loss of libido.
INTRODUCTION
The melanocortinergic (MC) system mediates a wide and complex array of physiological effects including skin pigmentation, salt regulation, food intake regulation, pain nerve regeneration, sexual behavior and penile erection [1-5]. These vastly different effects occur through selective activation of five known receptor subtypes by unique peptides derived from alternate posttranslational modification of proopiomelanocortin (POMC) gene products including ACTH, α-MSH, β-MSH and γ-MSH. The recognition that differential manipulation of specific receptor subtypes could lead to specifically desired physiological outcomes has led to the development of a variety of synthetic compounds, many of which are being actively studied for potential therapeutic effects.
The proerectile effects of MC compounds have been recognized since the mid-20th century when studies by Ferrari and colleagues showed increased sexual excitement after intracerebral delivery of α-MSH and ACTH in a variety of mammalian species[6]. Interest in human applications of melanocortinergic agents toward penile erection did not occur until fortuitous events of the 1980s. During initial testing of a novel synthetic agent intended for artificial tanning, melanotan-II, a self described “human pincushion/ guinea pig” inadvertently self-administered a dose twice the expected concentration. To his surprise, he experienced an 8 hour-long erection, along with some nausea and vomiting [7]. Gastrointestinal effects aside, the potential therapeutic effect of this agent for erectile dysfunction was immediately recognized.
In the ensuing years, much effort has been spent toward understanding the relationship between the melanocortinergic system and penile erection. This paper focuses on general and MC specific neuroerectile pathways, receptor subtypes and a detailed discussion of actively studied melanocortin agonists and antagonists. Although several important investigative groups have established MC related neural pathways and implicated specific MC receptors, there continues to be areas of active debate as well as a significant potential for drug development as treatment for sexual dysfunctions.
Normal Erectile Physiology
Penile erection is the final endpoint of a complex coordination between the central nervous system, peripheral nervous system, endocrine system, voluntary and involuntary pelvic musculature and the highly specialized vascular tissue of the penis [8-10]. Supraspinal centers in the brain integrate sensory input and hormonal cues as part of the initiation of sexual desire, arousal and libido. These centrally initiated pro-erectile signals are relayed to sympathetic and parasympathetic centers in the thoracolumbar and sacral spinal cord in order to regulate vascular tone in the penile tissues. Alternately, direct genital afferents to the lumbosacral spinal cord can initiate a reflexogenic erection independent of supraspinal input. Inhibition of sympathetic vasoconstriction coordinated with vasodilatory parasympathetic activation greatly increases blood flow through the paired cavernosal arteries. As the cavernous spaces within the corpora cavernosa expand, they compress the venous outflow pathways leading to marked increase in intracavernosal pressure with subsequent tissue expansion. Voluntary contraction of the bulbocavernosus muscle further increases intracavernous pressures to produce a rigid erection while periurethral and bulbospongiosus muscular contractions assist with seminal ejaculation.
The most important end-organ neurotransmitter modulating erection is now recognized as nitric oxide (NO) [11-13]. Release of NO from the terminals of non adrenergic non cholinergic parasympathetic nerve fibers results in activation of cavernosal smooth muscle cell guanalyl cyclase (GC). This leads to increased production of the cyclic nucleotide guanosine monophosphate (cGMP), which in turn leads to cellular relaxation through direct calcium regulating mechanisms. Smooth muscle contraction and penile detumescence is in turn regulated by phosphodiesterase type 5 enzyme degradation of cGMP, as well as sympathetic activation at the moment of ejaculation.
Erectile Neural Circuits and Melanocortins
Neural control of erection results from a complex interaction between the forebrain, midbrain, spinal cord and peripheral nervous system. Although MC agonists are known to induce penile erection, whether or not endogenous melanocortins are necessary for normal physiologic penile erection remains unknown. A broader knowledge of neural erectile pathways, including the non-melanocortinergic pathways may lead to a greater understanding of areas where the melanocortinergic system may exert influence. Giuliano and Rampin provide an excellent review of the known pathways and neuropharmacology involved in penile erection [14].
Forebrain and Hindbrain
Erections occur in many different physiological and behavioral contexts and in response to a variety of stimuli, some of which clearly are relevant to reproduction while others seemingly have little to do with reproduction. Examples of the former may include erections during copulation and genital stimulated erections while examples of the latter include erections observed in-utero, nocturnal erections and “non-contact erections” [8, 15]. The supraspinal CNS is primarily responsible for sexual motivation as related to penile erection. Multiple areas of the forebrain and hindbrain may be responsible for erections in each of these contexts. In particular, based on their projections to the lumbosacral spinal cord, the following forebrain regions have been identified as potential initiators or facilitators of penile erection: the medial preoptic area (MPOA), the parvocellular portion of the paraventricular nucleus (PVN), the nucleus paragigantocellularis (NPGI) and the medial nucleus of the amygdala (MeA)[9].
The MPOA of the hypothalamus is well recognized for its role in male sexual behavior, likely through the integration and redistribution of information to other hypothalamic and brainstem nuclei. Electrical stimulation of this area as well as the PVN elicits complex sexual responses and erection in male monkeys and rats [14]. Neurons of the PVN are activated by dopamine and send oxytocinergic and vasopressinergic projections to the lumbosacral spinal cord. Lesions of this area decrease non-contact erections while having little effect on copulatory erections [16, 17]. Brainstem NPGI neurons send serotonergic signals to the lumbosacral spinal cord. Lesions of this area remove inhibition of both reflex erections and copulatory erections [18, 19]. PVN projections to the NPGI may be responsible for physiological release of this tonic inhibition of erection. Lesions of the MeA facilitate reflexive erections, depress non-contact erections, and have no effect on copulatory erections [20].
Complex circuits involving many neurotransmitters, including oxytocin and dopamine, have been described with potential effects related to erection, most of which are beyond the scope of this article.
Within this complex neural network, the melanocortinergic system has multiple potential sites for regulation. In male rats exposed to sexual situations, α-MSH is secreted in the MPOA [21]. The arcuate nucleus (Arc) of the hypothalamus is a primary source of POMC secreting neurons in the CNS with projections to the lateral hypothalamus, dorsal medial nucleus and the PVN [22]. POMC expression has been documented in regions of the PVN known to send oxytocinergic (OT) projections to the spinal cord, implicating possible regulatory interactions between the OT and MC systems. Male rats show increased expression of the immediate-early gene, Fos, in magnocellular oxytocin neurons in the PVN when exposed to either intromission or direct α-MSH intracerebral exposure. Central administration of an MC4R antagonist attenuated the increased Fos expression in these PVN neurons and inhibited copulatory behaviors [23].
Indirect evidence that hindbrain melanocortin signaling may contribute to supraspinal erection may be provided by the localization of POMC neurons in the nucleus tractus solitareus (NTS) [24, 25]. An independent caudal brainstem melanocortin receptor trigger for sympathetically stimulated metabolic responses has been reported [26].
Spinal Cord
The spinal cord contains neurons which project to the penis and are linked with penile erection. These include thoracolumbar sympathetic, sacral parasympathetic and sacral pudendal pathways. Sensory afferents from the penis project primarily to the lumbosacral spinal cord while some corpus cavernosal afferents have been traced to the thoracolumbar spinal cord[14].
The spinal cord coordinates ascending and descending inputs affecting penile erection utilizing a variety of neurotransmitters. A well-documented pro-erectile pathway involves the aforementioned OT neurons projections from the paraventricular nucleus to the sacral parasympathetic nuclei expressing the OT receptors. These sacral nuclei directly innervate the corpus cavernosum. [27, 28].
More recently, proerectile functions of spinal melanocortin receptors have been proposed. Spinal MC4R mRNA has been demonstrated in multiple studies [29, 30]. Intrathecal injection of the melanocortin agonist, MT-II, to the lumbar spinal cord dose-dependently increased spontaneous erections in male rats [31]. This effect was abolished by intrathecal co-administration of the melanocortin antagonist, SHU-9119. When SHU-9119 was given intracereroventricularly (ICV), it did not block MT-II spinally induced erections. These results suggest that MC agonists act on independent spinal loci for initiation of erection. Notably, these results are in contrast with a study of intrathecal administration of α-MSH, which failed to affect intracavernosal pressure in anesthetized rats [32]. However, the lack of effect of α-MSH may be attributable to its relatively lower affinity for the MC4R and/or its rapid metabolism.
While MT-II clearly induces erections at the supraspinal level, Giuliano and colleagues have shown both inductive and facilitative effects at the spinal level. In acutely spinally transected rats (T8 level) as well those with bilateral transaction of pelvic or dorsal penile nerves, systemic MT-II facilitated erections induced by cavernous nerve stimulation as measured by increased inter-cavernous pressures. However, the facilitator effect of MT-II was abolished by removal of the lumbar paravertebral sympathetic chain [33]. These results suggest that the facilitatory effects of MT-II act principally to modulate the sympathetic efferents to the pelvis, with little effect on the parasympathetics. The mechanism by which sympathetic modulation promotes increased cavernosal pressures is unclear, highlighting the need for further studies of spinal melanocortin action.
Peripheral
Many studies demonstrate pro-erectile effects of melanocortinergic agents after systemic delivery. However, MC agonists have yet to demonstrate modulation of erection through direct action on the cavernosum. One study used in-situ hybridization to localize MC4R mRNA to stretch activated mechanoreceptors and sensory afferent nerves of the penis [30]. However, in anesthetized male rats, MC agonists injected intracavernosally neither increased intracavernous pressure nor augmented neurostimulated erectile responses [31]. Direct application of an MC4R agonist failed to produce relaxation of cavernosal strips in organ bath experiments [34] or alter calcium currents of isolated cavernosal smooth muscle cells in vitro [30].
MC Receptor Agonists
A variety of research modalities have been used to elucidate the action of MC compounds on penile erection (see Table 1). MC compound affinity and activity properties are determined by cell culture and membrane receptor assays. In general, MC agonists bind strongly to subsets of the five G-protein coupled MC receptors and cause increased intracellular production of cAMP while MC antagonists bind strongly but do not stimulate cAMP production. Notably MCRs 1, 3, 4 and 5 have high constitutive (ligand-independent) activity enabling antagonists to decrease basal levels of cAMP production.
Table 1.
Models for study of MC compounds on Penile Erection
| Model | Comment |
|---|---|
| In vitro | |
| Cell culture |
|
| Organ bath | • Measure corpus cavernosal (end organ) relaxation in response to direct drug application |
| In vivo | |
| Animal studies | |
| Spontaneous erections | • Site specific drug delivery: intracerebral, intrathecal, intracavernosal or systemically followed by observation period. May or may not mimic behaviorally stimulated erection. Observer dependent results |
| Mating behaviors | • Site-specific delivery as above. Place trained male in contact with trained receptive female in artificial estrous or separated by barrier (non-contact erections). Measure specific aspects of erection: mounts, intromissions, ejaculations, etc |
| Reflexogenic erections | • Site-specific delivery in a restrained or anesthetized animal. Monitor specific penile responses (cups, flares, flips) or intracorporal pressure respectively |
| Nerve stimulation studies | • Site-specific drug delivery with nerve stimulation (usually cavernous nerve) in an anesthetized animal. Evaluation for facilitation or inhibition of erection by drug |
| Human studies | |
| Laboratory based | • Drug delivery IV, SQ or intranasal. Monitor penile tumescence w/ Rigi-scan with or without visual sexual stimulation. (VSS) |
| At home trials | • Phase II and III trials to determine safety, pharmacokinetics and efficacy using validated self report questionnaires and event diaries |
Endogenous MC Receptor Agonists
ACTH, α-MSH, β-MSH and γ-MSH are the known endogenous agonists of the MC system. Each hormone is a product of posttranslational modification of the POMC gene transcript and contains the sequence of His-Phe-Arg-Trp, considered to be the “core” of agonist activity [35, 36]. Only ACTH and α-MSH have shown the ability to generate sexual stimulation and penile erection in various animal species including rats, rabbits, cats, dogs and monkeys [14]. These pro-erectile effects appear to be androgen-dependent as castration abolishes the aforementioned response [37]. Notably, many of the synthetic MC agonists contain the “core” sequence present in ACTH and α-MSH, particularly the agents MT-II and PT-141.
MT-II (Melanotan-II)
Pharmacology
MT-II is a synthetic cyclic heptapeptide that was initially designed as an artificial tanning agent. Its structure is based on an earlier linear peptide, Melanotan-I, however cyclization was introduced to prevent degradation and allow both N and C terminal truncation of the peptide [38]. The pro-erectile activity of MT-II was reported as a significant unexpected reaction during a phase-I human trial of human tanning [39]. MT-II contains a seven amino acid sequence with homology to receptor binding portions of α-MSH and ACTH. The compound is thought to cross the blood brain barrier and has high affinity for the MC1R, MC3R and MC4R. MT-II has a similar affinity for MC4R compared with MC3R and may be considered “superpotent” because of its relatively high affinity for MC4R compared with the endogenous peptides α-MSH and ACTH (10-100 fold difference).
In-vitro and animal studies
The pro-erectile activity of MT-II appears to be both forebrain and spinally mediated, with little, if any, peripheral effect. Dose dependent increases in spontaneous erections in awake Long-Evans rats were noted with administration of MT-II intracerebrally, intrathecally and intravenously [31]. Increases in yawning and grooming behaviors paralleled erectile activity with intracerebral administration but not spinal administration. As discussed previously, when the non-selective MCR antagonist SHU-9119 was given spinally, it blocked spinal MT-II induced erections, however intrathecal SHU-9119 failed to block intracerebral MT-II induced erections. This indicates potentially independent sites of melanocortin action along the CNS axis with intracerebral sites activating multiple downstream pathways including those independent of melanocortinergic activation.
In parallel with the above observations, Vemulapalli et al found that isolated corpus cavernosal strips from rabbits had no relaxation in response to electrical field stimulation in the presence or absence of MT-II. [34] As well, MT-II associated elevations in intracavernosal pressure were abolished when anesthetized rabbits underwent bilateral pudendal nerve ablation. Inhibition of NO-synthase enzymes with L-NAME also blocked the erectile effect of systemic MT-II. These pieces of data indicate that MT-II exerts direct actions through CNS brain and spinal activation, which is then mediated peripherally through established NO vasodilatory pathways.
Human studies
A double blind placebo-controlled crossover study by Wessells et al. demonstrated the safety and pro-erectile activity of subcutaneous MT-II in humans [40]. In the absence of erotic stimulation, 10 men with psychogenic (non-organic) erectile dysfunction received subcutaneous doses ranging from 0.025 to 0.157 mg/kg, while erections were monitored by RigiScan over a 6-hour period. Eight of the 10 men developed clinically apparent erections with greater than 80% rigidity of an average duration of 38 minutes compared with 3 minutes for placebo controls. The time to onset ranged from 15 to 270 minutes. Side effects were dose dependent included nausea, stretching, yawning and decreased appetite. At the preferred dose of 0.025 mg/kg side effects were mild.
The above study documented erectogenic effects of MT-II in men with presumed normal underlying physiology. A subsequent study of MT-II was carried out on men with organic ED [41]. In a similar double blind, placebo-controlled crossover study, 10 men received 2 subcutaneous doses of 0.025 mg/kg MT-II and 2 doses of vehicle. MT-II initiated subjectively reported erections following 63% of the drug injection verses 5% of the placebo injections. The mean rigidity score of the responders was 6.9 on a scale of 0 to 10. Mean duration of tip rigidity greater than 80% was 45 minutes with Melanotan II compared to two minutes for placebo. There was increased subjective reporting of sexual desire after MT-II administration compared with placebo, although the question used to assess desire was not designed specifically to measure desire in men not engaging in sexual intercourse.
PT 141 (Bremelanotide®)
Pharmacology
PT-141 (Bremelanotide®) is currently the most studied melanocortinergic compound with regard to therapeutic potential for treatment of erectile dysfunction. PT-141 is a synthetic heptapeptide. It is a deaminated derivative and likely metabolite of MT-II. This compound has strong binding to MC receptors 1, 3 and 4, with a higher affinity for MC4R over MC3R. Application of PT-141 to HEK-293 cells expressing MC4R increases cAMP production, indicating that this compound, like MT-II, acts as an agonist [42].
Animal studies
Studies with adult male Sprague-Dawley rats indicate pro-erectile responses through multiple modes of delivery [42]. Intranasal injection of 50μg/kg PT-141 produced a significant increase in spontaneous erections compared with saline controls in rats observed over a 30-minute period. As well, 100% of the drug treated rats had at least one erection. In this study the pro-erectile effect of PT-141 was attributed to hypothalamic stimulation of MC3R and/or MC4R. Two hours after PT-141 (50μg/kg IN) administration, immunostaining for FOS, a measure of neural activation, showed increased expression in the paraventricular nucleus compared with rats administered saline.
Human studies
Preliminary trials in humans have established both safety and efficacy of PT-141. A phase 1 randomized double-blind placebo controlled trial involved 24 healthy male subjects without erectile dysfunction [42, 43]. Intranasal doses of 4 to 20mg were delivered to patients in the absence of visual sexual stimulation (VSS). Safety and tolerability were monitored revealing no significant hemodynamic changes or side effects, including priapism. Serum concentration of drug was dose dependent and peaked at 30 minutes in the maximum dose group. Serum half-life was measured at 120 minutes. Rigi-Scan monitoring of erectile response revealed a significantly increased duration of rigid erections of 140 minutes compared to 22 minutes in the placebo group. Time to onset of erection ranged from 34 to 63 minutes. Erections were considered rigid if they were more than 60% of base rigidity.
Based on the above results, phase II studies were initiated in patients with mild to moderate ED who showed positive erectile response to PDE-5 inhibitors [44]. RigiScan monitoring in the presence of VSS detected a 3-fold increase in erectile activity with PT-141 (20mg intranasal) administration. The duration of base rigidity was significantly increased utilizing both a 60% and 80% cut-off versus placebo [43]. Timing of erections corresponded well to visual stimulation indicating a potential facilitator mechanism of drug action.
In a first Phase IIB at home study, PT-141 induced dose dependent improvements in erectile function as assessed by the International Index of Erectile Function (IIEF) [45]. Of the patients who completed at least 3 at home attempts (n = 203), the mean IIEF erectile function domain score increased in a dose dependent fashion (p < 0.05 for 10, 15, and 20 mg). Normal erectile function (EF>26) was achieved by 10, 30, 36, 53, and 50% of patients in the placebo, 5, 10, 15, and 20 mg groups respectively. Improved erections as defined by a global assessment question were reported by 17, 49, 67, 66, and 66% of patients in the placebo, 5, 10, 15, and 20 mg groups respectively. There were no episodes of syncope or hypotension. The only serious adverse event reported in this study occurred in one patient who reported a prolonged erection that was painless and required no treatment. Gastrointestinal side-effects were the primary reasons for discontinuation in the higher two higher dose groups.
In summary, PT-141 is a potent initiator of erection with minimal side effects, a rapid onset of action and a sufficiently long duration of action. Notably, the recent Phase II studies confirm that the erectile responses are augmented by sexual stimulation. With its central mechanism of action, PT-141 may act independent or synergistically with PDE-5 inhibitors and provide a useful alternative therapy for erectile dysfunction, both from organic and psychogenic origin. A randomized prospective placebocontrolled study compared treatement of ED with sildenafil alone verses sildenafil with 7.5mg intranasal PT-141 [46]. Co-administration of the two agents resulted in significantly prolonged time of increased base rigidity (>60%) compared with sildenafil alone during a 2.5 hour monitoring session. The combination of drugs was well tolerated with no significantly increased side-effects over either sildenafil or PT-141 alone. The ability of the peptide to safely and effectively induce high quality erections allowed Palatin Technologies to initiate a second Phase IIB study in 2006 and propose Phase III studies in 2007 [47].
THIQ
THIQ is a synthetic small molecule hMC4R agonist with moderate bioavailability (14%), rapid absorption (Tmax = 1hr) and a short T1/2 (0.5hrs) [48]. THIQ has a >600 selectivity for the MC4R compared with MC3R [49, 50]. Studies by Martin and Van der Ploeg evaluated the effects of THIQ delivered both systemically and intracerebrally in rodents. Systemic administration (1mg/kg) potentiated electrically stimulated erections as well as decreasing mounting and intromission latency mating behaviors in wild type mice [30]. In an ex copula model using male rats, systemic THIQ dose dependently increased total numbers of erections [48]. This effect was blocked by central administration of the non-specific antagonist, AgRP, as well as the MC4R specific antagonist, MBP-10. ICV delivery of 20μg of THIQ increased reflexive penile erections. There are no studies of THIQ in humans to date. Interestingly, and in contradistinction to MT-II, THIQ has not been reported to initiate spontaneous erections in rodents.
MC Receptor Antagonists
Endogenous and synthetic antagonists have been used to explore melanocortin signaling. When MCR antagonists bind to the MC receptors they either decrease constitutive levels of cAMP production or prevent agonist induced increases in cAMP production. In studies of penile erection, MCR antagonists have been primarily utilized to identify the mechanisms and location of action of MCR agonists as well as parcel out specific receptor subtype activity. These compounds have not been utilized as therapeutic agents.
Endogenous melanocortin receptor inhibitors include agouti or agouti-related peptide (AgRP). AgRP is a 132 amino acid peptide which competitively antagonizes both MC3R and MC4R [51]. While AgRP has primarily been studied for its role in energy homeostasis, this peptide is principally expressed in the arcuate nucleus of the hypothalamus, a potential site for regulation of melanocortin mediated erection [14]. As mentioned, intracerebral delivery of AgRP (5.5μg) was shown to block erections in rats induced by the MC4R agonist, THIQ [48]. There have been no studies in humans with regard to erection.
SHU-9119 is a classical inhibitor of both the MC3R and the MC4R. This synthetic cyclic lactam α-MSH analogue is closely related in structure to MT-II [52]. SHU-9119 actually has agonist properties at MC1R and MC5R, but for the purposes of discussing erection, this compound is considered primarily an antagonist because of the lack of these receptors in the CNS. In rabbits this highly potent compound readily blocked MT-II induced erections when administered systemically [34]. In rats, SHU-9119 blocked erections and grooming/yawning behaviors stimulated by MT-II both at supraspinal and spinal locations [31]. This compound has not been studied in humans.
Two other synthetic MC receptor antagonists that have been utilized in studies of erectogenisis include MPB-10 and HS014. Both of these compounds preferentially block the MC4 receptor. Their use in animal studies has primarily been related to determination of receptor specification as described in the following section. These compounds have not been studied in humans.
MC3R vs. MC4R
Of the 5 melanocortin receptor subtypes, only the MC3R and MC4R have been identified in CNS regions associated with activation of penile erection [1], particularly the PVN of the hypothalamus. Many of the more commonly studied compounds, such as MT-II and αMSH, activate both MC3 and MC4 receptor subtypes to some degree. This lack of receptor specificity has limited our understanding of each receptor’s contribution toward erectile behaviors and has prompted studies utilizing receptor specific agonists and antagonists as well as receptor knock out mouse models. Contrary evidence has pointed to each receptor as the principle subtype mediating erection. Although the weight of evidence leans towards MC4R activation being responsible for activation of erection, the debate remains unresolved.
Evidence of MC3Rs participation in sexual stimulation and erection comes from a series of studies in the late 1990s utilizing an MC4R specific antagonist, HS014 [53]. Vergoni et al. administered ACTH and α-MSH into the lateral ventricle of adult male Sprague-Dawley rats and showed predictable responses with grooming, stretching, yawning and erections [2]. Co-administration of these compounds with HS014 completely blocked grooming, stretching and yawning behaviors, but only partially reduced erections. Argiolas et al. studied this effect further with ACTH, α-MSH and HS014 microinjections into regions surrounding the 3rd ventricle of adult rats [54]. The effect was a dose dependent elicitation of yawns, grooms and erections when only ACTH and α-MSH were administered. Co-administration of these compounds with HS014 significantly blocked yawns and grooms but erections were unaffected. This evidence suggested that the MC4R was not involved in the sexual response to ACTH and α-MSH. As the only other MC receptor in the region, the MC3R was attributed partial credit for the erectile response. However, HS014 does have MC3R antagonist activity and the relatively small difference in affinity for MC4 vs. MC3 receptors makes interpretation difficult. If MC3R were the primary mediator of erection, one would have expected some diminution of erections with this compound. Another possible consideration in the interpretation of these studies is that a different level of MC4R occupancy may stimulate yawning/ grooming behaviors and erection. Thus a higher dose of antagonist may be required to block penile erection. Finally, the proerectile effects of MSH are not as potent as synthetic analogs such as MT-II, raising the possibility that an inadequate stimulatory dose of the agonist prevented a measurable effect of the antagonist (floor effect).
Evidence in support of MC4R as the principle receptor mediating erections is provided by multiple converging lines of evidence using MC4R knock out mice as well as rat studies with MC4R specific agonists and antagonists. Van der Ploeg et al stimulated the cavernous nerves of wild type and MC4R knockout mice in the presence or absence of systemic THIQ (10mg/kg IV), an agonist with >1000-fold selectivity for MC4R compared with MC3R [30]. Wild-type mice had significant augmentation of erectile activity while knockout mice did not show any changes. Copulatory behavior in these mice was evaluated as well. MC4R wild type mice received THIQ (2.5mg/kg IP) or vehicle and were paired with estrous females. THIQ treatment decreased mounting and intromission latencies in WT mice. Interestingly, MC4R knock out mice had impaired copulatory behavior compared with littermate wild type controls, showing baseline dysfunctional mating characteristics with loss of the MC4R. The systemic delivery of THIQ in these studies led the authors to question whether the effect could be related to direct activation of cavernosal tissue. Although they demonstrated MC4R mRNA in rat cavernosum, application of THIQ to cavernosal strips did not result in tissue relaxation.
Martin et al. utilized a slightly different approach to investigate MC3/4R question, by administering selective and non-selective antagonists to MC4R in combination with the MC4R agonist THIQ [48]. MBP10 is a synthetic MCR antagonist with at least a 125-fold selectivity for MC4R over MC3R [55] while AgRP is an endogenous antagonist with comparable inhibition of both MC3R and MC4R. In this study, an ex copula model of monitoring penile reflexes was measured after drug delivery. Consistent with the work of Van der Ploeg et al., systemic THIQ increased intracavernosal pressures and dose-dependently increased reflex erectile activity in restrained rats. Central administration of THIQ into the 3rd ventricle of rats increased reflexive penile erections. This centrally mediated effect was blocked by pretreatment with both AgRP as well as MPB10. Unfortunately, an MC3R selective antagonist was not employed in this study. The conclusion of this study was that MC4R activation was sufficient for penile erectile activity, but did not exclude a possible role for MC3R.
Although the pro-erectile effects of MC4R activation appear well established, the contribution of MC3R towards erection is incompletely understood. An alternative hypothesis to the above studies is that stimulation of the MC3R may actually be inhibitory toward erectile activity. In support of this hypothesis are neuroanatomical pathways involving AgRP (endogenous melanocortin antagonist) and POMC neurons, which travel in parallel throughout much of the central nervous system. MC3R mRNA has been co-localized to both AgRP and POMC neurons in a rostrocaudal gradient in the arcuate nucleus [56]. In contrast, MC4R mRNA was not found in AgRP or POMC expressing cells. This implies a potential negative feedback loop whereby “expression of MC3R by POMC neurons provides a potential circuit for amplification of AgRP-mediated signals, because AgRP-induced inhibition of POMC neurons via the MC3R would reinforce the postsynaptic effects of AGRP. Furthermore, the expression of the MC3R by AGRP neurons provides a potential circuit for negative autoregulation of POMC-mediated signals, because POMC-induced activation of AGRP neurons via the MC3R would terminate the postsynaptic effects of POMC”.
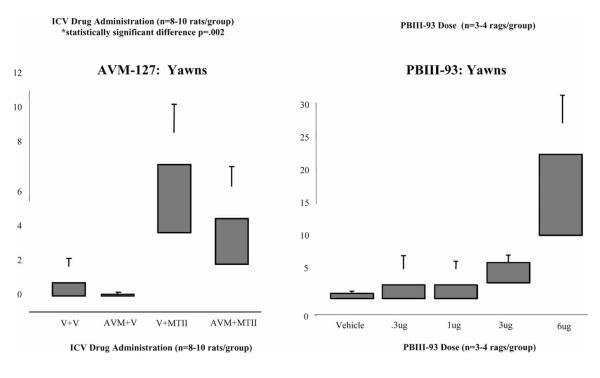
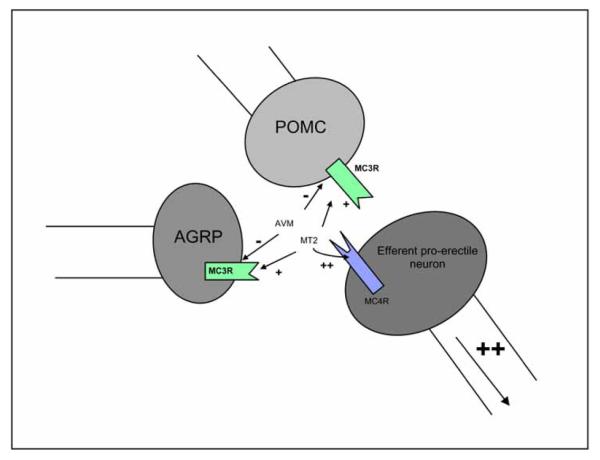
Preliminary studies in our laboratory using MC3R specific agonists and antagonists may have unmasked a proposed autoinhibitory pathway of the MC3 receptor. AVM-127 is a cyclic γ-MSH analog with selective MC3R/ MC5R Antagonist activities in vitro; it is a synthetic compound with antagonist activity and 100 fold selectivity for MC3R compared to MC4 [57]. A study of intracerebroventricular administration of AVM-127 (750ng) prior to MT-II (1μg) in adult male rats was performed with the initial hypothesis that antagonism of the MC3 receptor would either reduce or have no effect on MT-II stimulated erections. This hypothesis was based on previous studies indicating that MC3R activation was “pro-erectile”. Paradoxically, rats administered AVM-127 in combination with MT-II had significantly more erections over a 90 minute observation period when compared with rats given MT-II alone (see Fig. 1). Rats given AVM-127 alone had no significant erectile activity compared with controls. AVM-127 had no effect on MT-II induced yawns or genital grooming behaviors. These results were highly surprising and unexpected. An inverse approach to this study involved specific stimulation of central MC3 receptors with a novel specific MC3R agonist, the γ-MSH analog PBIII-93 [58]. When administered ICV to male rats, this compound failed to produce erections despite dose-dependently stimulating yawns. These results taken together suggest that MC3 activation does not stimulate erections but rather inhibits erections. As well, MC3 receptor antagonism (inhibition of inhibition) may not be sufficient to induce erections, but may facilitate erections initiated by MC4R activation. A proposed model for this can be seen in Fig. (2). An alternative interpretation of the data is simply that by occupying the MC3R, the antagonist allows greater amounts of MT-II to bind to MC4R.
Fig. (1).
ICV testing of novel MC3R antagonist (AVM-127, 750ng) in combination with the non-specific MC3/4R agonist MT-II (1μg). AVM in combination with MT-II produced more erections than MT-II alone. A novel MC3R specific agonist, PBIII-93, was dose-response tested. This compound failed to produce significant erections at any of the doses from .3μg to 6μg. V= vehicle (normal saline).
Fig. (2).
Possible hypothalamic neuronal configuration and interaction of MC3 and MC4 receptors. AVM-127 inhibits both the pro-erectile α-MSH delivery from the POMC neuron as well as the hypothesized anti-erectile AgRP delivery from a parallel neuron. In the absence of basal levels of the inhibitory influence of AgRP, the downstream pro-erectile neuron is free to be stimulated strongly by MT-II. Although MT-II also stimulates the MC3R on the AgRP neuron, it does so with relatively weak affinity.
The data and experience with these compounds, AVM-127 and PBIII-93, are limited in breadth and further validation of these studies is pending. However, these novel results may indicate future directions for study of melanocortin receptor interactions in the CNS. Testing with novel MC3R agonists and antagonists is needed. Another approach to this question may involve use of conditional knockout mice for MC3R and MC4R.
Erectile Dysfunction (ED) and Current Therapies
Erectile dysfunction (ED) is defined as the inability to produce or maintain a penile erection with rigidity sufficient for intercourse. Risk factors include advanced age, diabetes mellitus, hypertension, obesity, dyslipidemia, pharmacologic side effects and cardiovascular disease. The prevalence increases with age and may affect up to one third of men over the age of 50, representing a significant source of morbidity in an aging population.
Currently medical therapies for ED are limited to direct manipulation of cavernosal smooth muscle relaxation. Selective pharmacological inhibition of phosphodiesterase-5 enzyme in penile smooth muscle cells prevents breakdown of cGMP leading to higher intracellular levels of this molecule resulting in increased smooth muscle relaxation and erection. Available PDE-5 inhibitors include sildenafil (Viagra®), vardenafil (Levitra®) and tadalafil (Cialis®). Other pharmacologic options for ED include intracavernosal (Caverject) or intraurethral (MUSE) alprostadil delivery.
The aforementioned therapies each have drawbacks leading to the search for alternative treatment methods. The PDE-5 inhibitors have been the most successful pharmacotherapy class, however up to 50% of diabetic men with ED remain refractory to such agents. Importantly, while each of the above therapies addresses the mechanical issue of rigidity necessary for penetration and intercourse, none of the above therapies is known to affect sexual desire/libido, an important component to the overall treatment of ED. Thus, there is an unmet medical need to study alternative pathways and agents, such as the melanocortinergic compounds, which may fill in the gaps left by current forms of treatment.
SUMMARY
Complex interactions between the supraspinal, spinal and peripheral nervous system lead to the highly specific and regulated vasculogenic event of penile erection. Of the many neurotransmitters involved, melanocortins appear to play a significant role in regulation of erection, particularly at the supraspinal and spinal levels. MC agents may regulate physiologic erection, and could also have as yet unexplored effects on sexual motivation and libido. Much knowledge has been gained of MC receptor sites and MC receptor subtypes involved in erection, particularly through the utilization of novel compounds which activate and/or inhibit specific MC receptors. However, further detailed studies are necessary, particularly if new therapeutic agents are to be developed. The two superpotent synthetic MC agonists, MT-II and PT-141, have been tested in human subjects, with PT-141 showing promise in early clinical trials for treatment of ED.
Table 2.
Sample of Melanocortin Receptor Binding Agents Studied with Erection
| Structure | Preferential MCR Binding (3 vs. 4) |
MC3R affinity Ki (nM)* |
MC4R affinity Ki (nM)* |
|
|---|---|---|---|---|
| Agonists | ||||
| ACTH | 39 amino acid peptide SYSMEHFRWGKPVGKKRRPVKVYP DAGEDQSAEAFPLEF |
3,4 | ||
| α-MSH | Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2 | 3,4 | 30 | 5 |
| MT-II | Ac-Nle-c (Asp-His-D-Phe-Arg-Trp-Lys)-NH2 | 3,4 | 1.3 | 1.1 |
| PT-141 | Ac-Nle-c (Asp-His-D-Phe-Arg-Trp-Lys)-OH | 3,4 | N/A | N/A |
| PB-III-93 | H-Tyr-Val-Met-Gly-His-Phe-Arg-D-Trp-Asp-Arg-Phe-Gly-OH | 3 | 6.7 | 600 |
| THIQ | N-[(3R)-1,2,3,4-tetrahydroisoquinolinium-3-ylcarbonyl]-(1R)-1-(4-chlorobenzyl)- 2-[4-cyclohexyl-4-(1H-1,2,4-triazol-1-ylmethyl)piperidin-1-yl]-2-oxoethylamine |
4 | 761 | 1.2 |
| Antagonists | ||||
| AgRP | 132 amino acid peptide | 3,4 | 4.5 | 3.5 |
| SHU-9119 | Ac-Nle-c[Asp-His-D-Nal(2′)-Arg-Trp-Lys]-NH2 | 3,4 | 0.23 | 0.06 |
| MPB 10 | c(6β→10ε)[succinyl6-D-(2′)Nal7-Arg8-Trp9-Lys10]-NH2 | 4 | 150 | 0.5 |
| AVM-127 | c[Nle-Val-D-Nal(2′)-Arg-Trp-Glu]-NH2 | 3 | 1.7 | 180 |
| HS-014 | Ac-c[Cys-Glu-His-D-Nal(2′)-Arg-Trp-Gly-Cys]-Pro-Pro-Lys-Asp-NH2 | 4 | 54.4 | 3.16 |
Acknowledgments
Dedicated to Professor Mac E. Hadley
REFERENCES
- [1].Wikberg JE, et al. New aspects on the melanocortins and their receptors. Pharmacol. Res. 2000;42(5):393–420. doi: 10.1006/phrs.2000.0725. [DOI] [PubMed] [Google Scholar]
- [2].Vergoni AV, et al. Differential influence of a selective melanocortin MC4 receptor antagonist (HS014) on melanocortin-induced behavioral effects in rats. Eur. J. Pharmacol. 1998;362:95–101. doi: 10.1016/s0014-2999(98)00753-5. [DOI] [PubMed] [Google Scholar]
- [3].Fan W, et al. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- [4].van der Kraan M, et al. Expression of melanocortin-5 receptor in secretory epithelia supports a functional role in exocrine and endocrine glands. Endocrinology. 1998;139:2348–2355. doi: 10.1210/endo.139.5.6008. [DOI] [PubMed] [Google Scholar]
- [5].Schioth HB, Watanobe H. Melanocortins and reproduction. Brain Res. Brain Res. Rev. 2002;38(3):340–50. doi: 10.1016/s0165-0173(01)00159-x. [DOI] [PubMed] [Google Scholar]
- [6].Ferrari W, Gessa GL, Vargiu L. Behavioral effects induced by intracisternally injected ACTH and MSH. Ann. N. Y. Acad. Sci. 1963;104:330–45. doi: 10.1111/j.1749-6632.1963.tb17677.x. [DOI] [PubMed] [Google Scholar]
- [7].Hadley ME. Discovery that a melanocortin regulates sexual functions in male and female humans. Peptides. 2005;26(10):1687–9. doi: 10.1016/j.peptides.2005.01.023. [DOI] [PubMed] [Google Scholar]
- [8].Meisel R, Sachs B. Chapter 35: The Physiology of Male Sexual Behavior. In: E K, JD N, editors. The Physiology of Reproduction. 2nd Ed Raven Press Ltd.; New York: 1994. pp. 3–105. [Google Scholar]
- [9].Giuliano F, Rampin O. Neural control of erection. Physiol. Behav. 2004;83(2):189–201. doi: 10.1016/j.physbeh.2004.08.014. [DOI] [PubMed] [Google Scholar]
- [10].Hart BL. Testosterone regulation of sexual reflexes in spinal male rats. Science. 1967;155(767):1283–4. doi: 10.1126/science.155.3767.1283. [DOI] [PubMed] [Google Scholar]
- [11].Burnett AL, et al. Nitric oxide: a physiologic mediator of penile erection. Science. 1992;257(5068):401–3. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- [12].Kim N, et al. A nitric oxide-like factor mediates nonadrenergic-noncholinergic neurogenic relaxation of penile corpus cavernosum smooth muscle. J. Clin. Invest. 1991;88(1):112–8. doi: 10.1172/JCI115266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ignarro LJ, et al. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem. Biophys. Res. Commun. 1990;170(2):843–50. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- [14].Giuliano F. Control of penile erection by the melanocortinergic system: experimental evidences and therapeutic perspectives. J. Androl. 2004;25(5):683–91. doi: 10.1002/j.1939-4640.2004.tb02842.x. [DOI] [PubMed] [Google Scholar]
- [15].Koyanagi T, Horimoto N, Nakano H. REM sleep determined using in utero penile tumescence in the human fetus at term. Biol. Neonate. 1991;60(Suppl 1):30–5. doi: 10.1159/000251014. [DOI] [PubMed] [Google Scholar]
- [16].Monaghan EP, Arjomand J, Breedlove SM. Brain lesions affect penile reflexes. Horm. Behav. 1993;27(1):122–31. doi: 10.1006/hbeh.1993.1009. [DOI] [PubMed] [Google Scholar]
- [17].Liu YC, Salamone JD, Sachs BD. Impaired sexual response after lesions of the paraventricular nucleus of the hypothalamus in male rats. Behav. Neurosci. 1997;111(6):1361–7. doi: 10.1037//0735-7044.111.6.1361. [DOI] [PubMed] [Google Scholar]
- [18].Marson L, List MS, McKenna KE. Lesions of the nucleus paragigantocellularis alter ex copula penile reflexes. Brain Res. 1992;592(12):187–92. doi: 10.1016/0006-8993(92)91675-5. [DOI] [PubMed] [Google Scholar]
- [19].Liu YC, Sachs BD. Erectile function in male rats after lesions in the lateral paragigantocellular nucleus. Neurosci. Lett. 1999;262(3):203–6. doi: 10.1016/s0304-3940(99)00070-1. [DOI] [PubMed] [Google Scholar]
- [20].Kondo Y, Sachs BD, Sakuma Y. Importance of the medial amygdala in rat penile erection evoked by remote stimuli from estrous females. Behav. Brain Res. 1998;91(12):215–22. [PubMed] [Google Scholar]
- [21].Hughes AM, Everitt BJ, Herbert J. The effects of simultaneous or separate infusions of some pro-opiomelanocortin-derived peptides (beta-endorphin, melanocyte stimulating hormone, and corticotrophin-like intermediate polypeptide) and their acetylated derivatives upon sexual and ingestive behaviour of male rats. Neuroscience. 1988;27(2):689–698. doi: 10.1016/0306-4522(88)90298-9. [DOI] [PubMed] [Google Scholar]
- [22].Wessells H, Blevins JE, Vanderah TW. Melanocortinergic control of penile erection. Peptides. 2005;26(10):1972–7. doi: 10.1016/j.peptides.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Caquineau C, et al. Effects of alpha-melanocyte-stimulating hormone on magnocellular oxytocin neurones and their activation at intromission in male rats. J. Neuroendocrinol. 2006;18(9):685–91. doi: 10.1111/j.1365-2826.2006.01465.x. [DOI] [PubMed] [Google Scholar]
- [24].Jacobowitz DM, O’Donohue TL. alpha-Melanocyte stimulating hormone: immunohistochemical identification and mapping in neurons of rat brain. Proc. Natl. Acad. Sci. USA. 1978;75(12):6300–4. doi: 10.1073/pnas.75.12.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Joseph SA, Pilcher WH, Bennett-Clarke C. Immunocytochemical localization of ACTH perikarya in nucleus tractus solitarius: evidence for a second opiocortin neuronal system. Neurosci. Lett. 1983;38(3):221–5. doi: 10.1016/0304-3940(83)90372-5. [DOI] [PubMed] [Google Scholar]
- [26].Williams DL, et al. Brainstem melanocortin 3/4 receptor stimulation increases uncoupling protein gene expression in brown fat. Endocrinology. 2003;144(11):4692–7. doi: 10.1210/en.2003-0440. [DOI] [PubMed] [Google Scholar]
- [27].Giuliano F, et al. Spinal proerectile effect of oxytocin in anesthetized rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280(6):R1870–7. doi: 10.1152/ajpregu.2001.280.6.R1870. [DOI] [PubMed] [Google Scholar]
- [28].Veronneau-Longueville F, et al. Oxytocinergic innervation of autonomic nuclei controlling penile erection in the rat. Neuroscience. 1999;93(4):1437–47. doi: 10.1016/s0306-4522(99)00262-6. [DOI] [PubMed] [Google Scholar]
- [29].van der Kraan M, et al. Expression of melanocortin receptors and pro-opiomelanocortin in the rat spinal cord in relation to neurotrophic effects of melanocortins. Brain Res. Mol. Brain Res. 1999;63(2):276–86. doi: 10.1016/s0169-328x(98)00291-5. [DOI] [PubMed] [Google Scholar]
- [30].Van der Ploeg LH, et al. A role for the melanocortin 4 receptor in erectile function. Proc. Natl. Acad. Sci. USA. 2002;99(17):11381–11386. doi: 10.1073/pnas.172378699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wessells H, et al. Ac-Nle-[Asp-His-DPhe-Arg-Trp-Lys]-NH2 induces penile erection via brain and spinal melanocortin receptors. Neuroscience. 2003;118(3):755–62. doi: 10.1016/s0306-4522(02)00866-7. [DOI] [PubMed] [Google Scholar]
- [32].Mizusawa H, Hedlund P, Andersson KE. alpha-Melanocyte stimulating hormone and oxytocin induced penile erections, and intracavernous pressure increases in the rat. J. Urol. 2002;167(2 Pt 1):757–60. doi: 10.1016/S0022-5347(01)69140-7. [DOI] [PubMed] [Google Scholar]
- [33].Giuliano F, et al. Melanotan-II: Investigation of the inducer and facilitator effects on penile erection in anaesthetized rat. Neuroscience. 2006;138(1):293–301. doi: 10.1016/j.neuroscience.2005.11.008. [DOI] [PubMed] [Google Scholar]
- [34].Vemulapalli RK,S, Salisbury B, Parker E, Davis H. Activation of central melanocortin receptors by MT-II increases cavernosal pressure in rabbits by the neuronal release of NO. Br. J. Pharmacol. 2001;134:1705–1710. doi: 10.1038/sj.bjp.0704437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Grieco P, et al. Structure-activity studies of the melanocortin peptides: discovery of potent and selective affinity antagonists for the hMC3 and hMC4 receptors. J. Med. Chem. 2002;45(24):5287–94. doi: 10.1021/jm0202526. [DOI] [PubMed] [Google Scholar]
- [36].Haskell-Luevano C, et al. Structure activity studies of the melanocortin antagonist SHU9119 modified at the 6,7,8, and 9 positions. Peptides. 2000;21:49–57. doi: 10.1016/s0196-9781(99)00167-9. [DOI] [PubMed] [Google Scholar]
- [37].Bertolini A, et al. Induction of sexual excitement with intraventricular ACTH; permissive role of testosterone in the male rabbit. Life Sci. 1968;7:1203–1206. doi: 10.1016/0024-3205(68)90231-2. [DOI] [PubMed] [Google Scholar]
- [38].Al-Obeidi F, et al. Potent and prolonged acting cyclic lactam analogues of ∝— melanotropin: design based on molecular dynamics. J. Med. Chem. 1989;32:2555–2561. doi: 10.1021/jm00132a010. [DOI] [PubMed] [Google Scholar]
- [39].Dorr RT, et al. Evaluation of Melanotan-II, a superpotent cyclic melanotropic peptide in a pilot phase-I clinical study. Life Sci. 1996;58(20):1777–1784. doi: 10.1016/0024-3205(96)00160-9. [DOI] [PubMed] [Google Scholar]
- [40].Wessells H, et al. Synthetic melanotropic peptide initiates erections in men with psychogenic erectile dysfunction: double-blind, placebo controlled crossover study. J. Urol. 1998;160(2):389–93. [PubMed] [Google Scholar]
- [41].Wessells H, et al. Effect of an alpha-melanocyte stimulating hormone analog on penile erection and sexual desire in men with organic erectile dysfunction. Urology. 2000;56(4):641–6. doi: 10.1016/s0090-4295(00)00680-4. [DOI] [PubMed] [Google Scholar]
- [42].Molinoff PB, et al. PT-141: a melanocortin agonist for the treatment of sexual dysfunction. Ann. N. Y. Acad. Sci. 2003;994:96–102. doi: 10.1111/j.1749-6632.2003.tb03167.x. [DOI] [PubMed] [Google Scholar]
- [43].Rosen RC, et al. Evaluation of the safety, pharmacokinetics and pharmacodynamic effects of subcutaneously administered PT-141, a melanocortin receptor agonist, in healthy male subjects and in patients with an inadequate response to Viagra. Int. J. Impot. Res. 2004;16(2):135–42. doi: 10.1038/sj.ijir.3901200. [DOI] [PubMed] [Google Scholar]
- [44].Diamond LE, et al. Double-blind, placebo-controlled evaluation of the safety, pharmacokinetic properties and pharmacodynamic effects of intranasal PT-141, a melanocortin receptor agonist, in healthy males and patients with mild-to-moderate erectile dysfunction. Int. J. Impot. Res. 2004;16(1):51–9. doi: 10.1038/sj.ijir.3901139. [DOI] [PubMed] [Google Scholar]
- [45].Wessells H, Padma-Nathan H, Raifer J, Feldman R, Rosen R, Molinoff P, Diamond L, Earle D, Powers B. At-home efficacy of an intranasally administered melanocortin receptor agonist, PT-141, in men with erectile dysfunction (ED). American Urology Association Annual Meeting; San Francisco, California. 2004. [Google Scholar]
- [46].Diamond LE, et al. Co-administration of low doses of intranasal PT-141, a melanocortin receptor agonist, and sildenafil to men with erectile dysfunction results in an enhanced erectile response. Urology. 2005;65(4):755–9. doi: 10.1016/j.urology.2004.10.060. [DOI] [PubMed] [Google Scholar]
- [47].Palatin Technologies, company communications.
- [48].Martin WJ, et al. Activation of melanocortin MC(4) receptors increases erectile activity in rats ex copula. Eur. J. Pharmacol. 2002;454(1):71–9. doi: 10.1016/s0014-2999(02)02479-2. [DOI] [PubMed] [Google Scholar]
- [49].Martin WJ, MacIntyre DE. Melanocortin receptors and erectile function. Eur. Urol. 2004;45(6):706–13. doi: 10.1016/j.eururo.2003.03.001. [DOI] [PubMed] [Google Scholar]
- [50].Sebhat IK, et al. Design and pharmacology of N-[(3R)-1,2,3,4-tetrahydroisoquinolinium-3-ylcarbonyl]-(1R)-1-(4-chlorobenzyl)-2-[4-cyclo-hexyl-4-(1H-1,2,4-triazol- 1-ylmethyl)piperidin-1-yl]-2-oxoethylamine (1), a potent, selective, melanocortin subtype-4 receptor agonist. J. Med. Chem. 2002;45(21):4589–93. doi: 10.1021/jm025539h. [DOI] [PubMed] [Google Scholar]
- [51].Arora S, Anubhuti Role of neuropeptides in appetite regulation and obesity - A review. Neuropeptides. 2006 doi: 10.1016/j.npep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- [52].Schioth HB, et al. Selectivity of cyclic [D-Nal7] and [D-Phe7] substituted MSH analogues for the melanocortin receptor subtypes. Peptides. 1997;18(7):1009–13. doi: 10.1016/s0196-9781(97)00079-x. [DOI] [PubMed] [Google Scholar]
- [53].Schioth HB, Muceniece R, Wikberg JE. Characterization of the binding of MSH-B, HB-228, GHRP-6 and 153N-6 to the human melanocortin receptor subtypes. Neuropeptides. 1997;31(6):565–71. doi: 10.1016/s0143-4179(97)90002-0. [DOI] [PubMed] [Google Scholar]
- [54].Argiolas A, et al. ACTH- and alpha-MSH- induced grooming, stretching, yawning and penile erection in male rats: Site of action in the brain and role of melanocortin receptors. Brain Res. Bull. 2000;51(5):425–431. doi: 10.1016/s0361-9230(99)00270-1. [DOI] [PubMed] [Google Scholar]
- [55].Bednarek MA, et al. Selective, high affinity peptide antagonists of alpha-melanotropin action at human melanocortin receptor 4: their synthesis and biological evaluation in vitro. J. Med. Chem. 2001;44(22):3665–72. doi: 10.1021/jm010165y. [DOI] [PubMed] [Google Scholar]
- [56].Bagnol D, et al. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J. Neurosci. 1999;19(18):RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mayorov AV, et al. Development of cyclic gamma-MSH analogues with selective hMC3R agonist and hMC3R/hMC5R antagonist activities. J. Med. Chem. 2006;49(6):1946–52. doi: 10.1021/jm0510326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Grieco P, et al. D-Amino acid scan of gamma-melanocyte-stimulating hormone: importance of Trp(8) on human MC3 receptor selectivity. J. Med. Chem. 2000;43(26):4998–5002. doi: 10.1021/jm000211e. [DOI] [PubMed] [Google Scholar]
- [59].Ye Z, et al. Discovery and activity of (1R,4S,6R)-N-[(1R)-2-[4-cyclohexyl-4-[[(1,1-dimethylethyl)amino]carbonyl]- 1-piperidinyl]-1-[(4-fluorophenyl)methyl]-2-oxoethyl]-2-methyl-2-azabicycl o[2.2.2]octane-6-carboxamide (3, RY764), a potent and selective melanocortin subtype-4 receptor agonist. Bioorg. Med. Chem. Lett. 2005;15(15):3501–5. doi: 10.1016/j.bmcl.2005.05.109. [DOI] [PubMed] [Google Scholar]
- [60].Tota MR, et al. Molecular interaction of Agouti protein and Agouti-related protein with human melanocortin receptors. Biochemistry. 1999;38(3):897–904. doi: 10.1021/bi9815602. [DOI] [PubMed] [Google Scholar]