Abstract
We have shown previously that N-(4-hydroxyphenyl)retinamide (4HPR, fenretinide), a retinoic acid derivative, induces neuronal differentiation in cultured human retinal pigment epithelial (RPE) cells (Chen et al., 2003, J Neurochem 84:972–981). We asked the question whether the mitogen-activated protein kinase (MAPK) pathway is involved in the regulation of the 4HPR-induced neuronal differentiation of RPE (ARPE-19) cells. When we treated ARPE-19 cells with 4HPR, c-Raf and MEK1/2 kinase were activated resulting in activation of the downstream effector ERK1/2 and of SAPK/JNK. By blocking the upstream kinase MEK1/2 with specific inhibitor U0126 we abrogated the 4HPR-induced phosphorylation of ERK1/2 and SAPK/JNK, indicating that the neuronal differentiation occurs through a positive cross-talk between the ERK and the SAPK/JNK pathways. Both U0126 and the suppression of ERK1/2 expression with small interfering RNA effectively blocked the 4HPR-induced neuronal differentiation of RPE cells and the expression of calretinin. The activated ERK1/2 then induced a sequential activation of p90RSK, and increase in phosphorylation of transcription factors c-Fos and c-Jun leading to transcriptional activation of AP-1. Taken together, our results clearly demonstrate that c-Raf/MEK1/2 signaling cascade involving ERK1/2 plays a central role in mediating the 4HPR-induced neuronal differentiation and calretinin expression in the human ARPE-19 retinal pigment epithelial cell line.
Keywords: RPE, calretinin, ERK, SAPK, JNK, c-Fos, c-Jun, AP-1
Introduction
Retinal degeneration results in irreversible visual loss because, like other neuronal cells, terminally differentiated retinal cells will not reenter the cell cycle. On the other hand, retinal pigment epithelium (RPE) from several species, including human, can reenter the cell cycle, and under certain conditions can be induced to dedifferentiate or transdifferentiate into cell types other than RPE (Dutt et al. 1993, Zhao et al. 1997, Yan et al. 2001). The RPE, a homogenous monolayer of cells, is located between the choroid and the neural retina in the eye (Bok 1993). It has been shown that RPE cells can be transdifferentiated into neuronal retinal cells by basic fibroblast growth factor (bFGF) in vitro (Pittack et al. 1991). In addition, it has been suggested that RPE cells could serve as a source of retinal stem cells (Fischer & Reh 2001).
The molecular mechanisms involved in the differentiation of RPE cells into neuronal cells still remain unclear. Among the key signaling pathways, the mitogen-activated protein kinases (MAPKs) comprising a family of serine/threonine kinases named the extracellular signal-regulated kinase 1 and 2 (ERK1/2), the stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK), and the p38 kinase (p38), are known to play a crucial role in the regulation of cell growth and differentiation (Pearson et al. 2001). Once activated by a phosphorylation cascade, these protein kinases function in specialized pathways by transducing signals from cell surface receptors to the nucleus. Furthermore, studies have shown that MAP kinases regulate neuronal differentiation by activating transcription factors such as AP-1, a regulatory protein involved in cell growth and differentiation (Karin 1995, Whitmarsh & Davis 1996).
ARPE-19, a human RPE cell line (Dunn et al. 1996), retains many structural and functional characteristics of RPE cells in vivo. These cells exhibit epithelial cell morphology and express several genes specific for the RPE, such as RPE65 (mRNA only), and cellular retinaldehyde-binding protein (CRALBP; mRNA and protein), both involved in the regeneration of visual pigment. ARPE-19 cells show noticeable pigmentation, polarized distribution of cell surface markers, and produce variety of growth factors and cytokines (Dunn et al. 1996, Holtkamp et al. 1998). These cells perform some of the known functions of human RPE, including assimilation of photoreceptor outer segments by phagocytosis (Finnemann et al. 1997). We have shown that retinoic acid induced the expression of novel retinal pigment epithelial cell gene (NORPEG/RAI14) in ARPE-19 cells (Kutty et al. 2001). In addition, we have shown that retinoic acid and transforming growth factor-β (TGF-β) induce the expression of stearoyl coenzyme A desaturase (SCD), a microsomal enzyme known to be involved in the regulation of cell growth and differentiation (Samuel et al. 2001, Samuel et al. 2002).
Retinoic acid (RA), a natural derivative of vitamin A, and its synthetic analogs have profound effects on cell growth, differentiation, and apoptosis, and are required for many cellular functions (Chambon 1994). Of the synthetic analogues, N-(4-hydroxyphenyl)retinamide (4HPR, fenretinide), has emerged as one of the most promising alternatives to the natural retinoids. In particular, it has been used in a number of clinical trials as a chemopreventive agent due to its ability to induce apoptosis in number of cancer cell lines (Malone et al. 2003). Recently, we observed that a high concentration of 4HPR induces apoptosis in cultured RPE (ARPE-19) cells, through generation of reactive oxygen species (Samuel et al. 2006). On the other hand, there is also compelling evidence from our earlier work that relatively low concentrations of 4HPR induce neuronal type differentiation of ARPE-19 cells associated with an increased expression of neurofilament proteins, NF160 and NF200, as well as calretinin (calbindin 2), a protein generally expressed in retinal and other neuronal cells (Chen et al. 2003). At present, the molecular mechanism underlying this neuronal differentiation is still unknown. Since MAPK signaling cascades play a crucial role in regulating mammalian cell growth and differentiation, the objective of the present study was to investigate the specific contribution of MAPK signaling pathways in the 4HPR-induced neuronal differentiation of ARPE-19 cells. Here we present evidence that the 4HPR-induced neuronal differentiation of ARPE-19 cells is associated with the activation of both ERK1/2 and SAPK/JNK. U0126, a specific inhibitor of MEK, blocks both neuronal differentiation and the increase in the expression of the neuronal marker calretinin. Our results further indicate that the signaling through both the ERK1/2 and SAPK/JNK pathways converge in the transactivation of AP-1. Thus, we conclude that the 4HPR-induced neuronal differentiation of ARPE-19 cells is mediated through the MAPK pathway.
Materials and methods
Materials
4HPR (N-(4-hydroxyphenyl)retinamide), fenretinide) was obtained from Biomol (Plymouth Meeting, PA). Rabbit antibodies and phospho-specific antibodies of c-Raf, MEK1/2, ERK1/2, SAPK/JNK, c-Jun, PathScan® Phospho-p44/42 MAPK sandwich ELISA kit, and human specific SignalSilence® p44/p42 MAPK siRNA kit were from Cell Signaling Technologies (Beverly, MA). Monoclonal anti-calretinin, anti-β-actin and anti-phospho-c-Fos were from US Biologicals (Swampscott, MA). The enhanced chemiluminescence (ECL) detection system and peroxidase-conjugated anti-rabbit and anti-mouse antibody were from GE Healthcare Life Sciences (Piscataway, NJ). PD98059 and U0126 were purchased from Sigma (St. Louis, MO). TransAM™ AP-1 quantitative ELISA and Nuclear Extract Kits were from Active Motif (Carlsbad, CA).
Cells and culture conditions
The ARPE-19 human retinal pigment epithelial cell line, obtained from ATCC (Manassas, VA), were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing nutrient mixture F12, 50/50 mix (Cellgro, Herndon, VA) supplemented with 5% fetal bovine serum, 2 mM L-glutamine, 1 mM sodium pyruvate, 0.1 mM non-essential amino acids and penicillin (100 U/ml) and streptomycin (100 μg/ml), as described previously (Samuel et al. 2001). Cells were seeded onto tissue culture plates at a density of 2 × 105 cells/ml in complete medium and allowed to grow overnight. The culture medium was replaced next day with fresh serum-free medium containing penicillin (100 U/ml) and streptomycin (100 μg/ml) before adding 4HPR (1 μM) in the presence or absence of 1 μM of U0126, and the cells were allowed to grow for additional indicated time intervals. U0126 was added 1 h prior to the addition of 4HPR. Treatments were performed under subdued light and other conditions as reported previously (Samuel et al. 2001). All compounds were dissolved at a concentration of 10 mM in DMSO before adding to the cell culture medium. The controls received the same amount of DMSO. The cells were maintained at 37°C in a humidified environment of 5% CO2 in air.
Analysis of neurite outgrowth
Cells were examined using an inverted microscope (model IX 70; Olympus, Tokyo, Japan) every day using criteria as described previously (Chen et al. 2003). The cells were judged to be differentiated when the length of their processes was longer than the diameter of the soma or at least two neurites extending from the soma. Cells bearing bidirectional or multidirectional neurite-like processes were counted in minimum 10 randomly selected fields. Each randomly selected area was about 1 mm2 and contained 15–25 cells at the start of the treatment. The percentage of differentiation was calculated from the number of cells that showed neurite outgrowth divided by the total number of cells in each field. Three dishes were used in each experiment, which was repeated three times.
Western immunoblot analysis
Equal amounts of total protein (50 μg) from each sample were subjected to SDS-polyacrylamide gel electrophoresis using 4–12% NUPAGE Bis-Tris gels and then transferred to a nitrocellulose membrane (Invitrogen, Carlsbad, CA). After blocking in 5% non-fat milk in Tris-buffered saline (TBS) containing 0.05% Tween 20 for 1 h, the membranes were incubated overnight at 4°C with non-phospho or phospho-specific antibodies (1:1000) of c-Raf (Ser338), MEK1/2 (Ser217/221), p44/42 MAP kinase (Thr202/Tyr204), SAPK/JNK (Thr183/Tyr185), p90RSK (Ser380), c-Jun (Ser73) or c-Fos (Thr232). Membranes were also incubated at room temperatures for 1 h with monoclonal anti-calretinin or anti-β-actin antibody at 1:1000 dilutions (US Biological). Peroxidase-conjugated goat anti-rabbit or anti-mouse IgG antibody (1:5000) was used as secondary antibody. Immunocomplexes were visualized by a chemiluminescence method using the ECL Plus Western blotting Detection Kit (Amersham Biosciences).
Phospho-p44/42 MAPK ELISA
The quantity of Phospho-p44/42 (ERK1/2) protein was measured using PathScan® Phospho-p44/42 MAPK (T202/Y204) sandwich ELISA kit (Cell Signaling, Danvers, MA). Control and treated the cells were lysed using Cell Lysis Buffer (Cell Signaling) containing protease and phosphatase inhibitors (500 μl/well). After a brief sonication on ice, the cell lysates were centrifuged at 21,000 × g for 10 min at 4°C. Supernatants (100 μl) were analyzed by ELISA using phospho-ERK1/2 antibody to detect the captured phospho-ERK1/2 protein, by measuring the absorbance at 450 nm (Victor2 Multilabel Counter).
RNA interference
RNA interference (RNAi) of the ERK1/2 (p44/p42) transcript was performed in ARPE-19 cells plated in 6-well plates at 50–60% confluence using equimolar concentrations of SignalSilence™ p44 MAPK and SignalSilence® Pool p42 MAPK siRNA from Cell Signaling Technology (Danvers, MA). Small interfering RNA (siRNA) duplexes were transiently transfected with TransIT-TKO® transfection reagent (Mirus). Mock siRNA was targeted to an unrelated gene. After an overnight exposure, the RNAi complex was removed, the cells were washed one time in growth medium, and the medium was replaced with serum free medium before adding 1 μM of 4HPR. Effects on neuronal differentiation, ERK1/2 phosphorylation and calretinin mRNA expression were assessed 72 h after transfection.
Quantitaive Real-Time RT-PCR
For quantitative real-time RT-PCR, 2 μg of total RNA extracted from ARPE-19 cells with RNeasy Protect Mini Kit (Qiagen) was reverse transcribed using High Capacity cDNA Archive Kit (Applied Biosystems). After reverse transcription, 5 μl of cDNA preparations were used as templates for quantitative real-time PCR performed on an Applied Biosystems 7500 Real-Time PCR System using TaqMan Universal PCR Master Mix and other reagents from Applied Biosystems. Each PCR reaction was set up in 50 μl using validated TaqMan probe and primers of calretinin (assay identification number Hs00418693_m1). Human GAPDH gene (catalog number 4326317E) was used as endogenous control. The gene specific probe was labeled with reporter dye FAM, and the endogenous control GAPDH was labeled with a different reporter dye VIC at the 5′ end. Gene amplification data were analyzed with an Applied Biosystems 7500 System Sequence Detection Software version 1.2.3. The results were expressed as n-fold change in gene expression relative to endogenous control calculated using the ΔΔCT method.
Transcription factor assay
AP-1 binding activity in nuclear extracts was measured with a TransAM™ AP-1 family transcription factor ELISA kit (Active Motif). Nuclear extracts (10 μg proteins) from control and treated samples were added to a 96-well plate immobilized with TPA-responsive element (TRE, 5′-TGAGTCA-3′) consensus binding site, and incubated for 1 h at room temperature. For competition experiments, wild-type (WT) or mutated oligonucelotide (MO) were added to the well prior to the addition of the nuclear extract. After washing, phospho-specific c-Jun or c-Fos antibodies were added to the wells, and incubated further for 1 h at room temperature. Peroxidase conjugated secondary antibody at 1:1000 dilutions was used to detect the antibody binding. AP-1 activation was quantified by measuring the absorbance within 5 minutes at 450 nm with a reference wavelength of 655 nm using a Victor2 Multilabel Counter (Perkin Elmer).
Statistical analysis
All values have been expressed as mean ± SD, n = 4. For statistical significance, paired Student’s t-test in Excel was used. P < 0.05 denotes statistically significant differences. The results shown are representative of 3 or more independent experiments.
Results
4HPR-Induces differentiation of RPE cells into a neuronal phenotype
The morphology of ARPE-19 cells treated with 1 μM of 4HPR in serum free medium for various time points was examined by phase-contrast microscopy. Neurite outgrowth was employed as a marker for neuronal differentiation (Chen et al. 2003). 4HPR treatment resulted in visible changes in cell morphology such as shrinkage of the cell body and appearance of processes longer than the cell body (Fig. 1A). This morphological change was time dependent and more than 80% of the cells were differentiated and produced long processes that are characteristic of neurites. The concentration at which 4HPR (1 μM) induced the neuronal differentiation in ARPE-19 cells is similar to our earlier report observed in the presence of serum (Chen et al. 2003). However, a single addition of 1 μM 4HPR was more than enough to induce the neuronal differentiation of cells grown in serum-free condition as compared to daily addition of 4HPR for cells cultured in the presence of serum. In addition, the cells treated under serum-free condition tend to differentiate within 3 days of treatment compared to 5–7 days for the cells treated in the presence of the serum.
Fig. 1. Differentiation of human RPE cells into a neuronal phenotype by 4HPR.
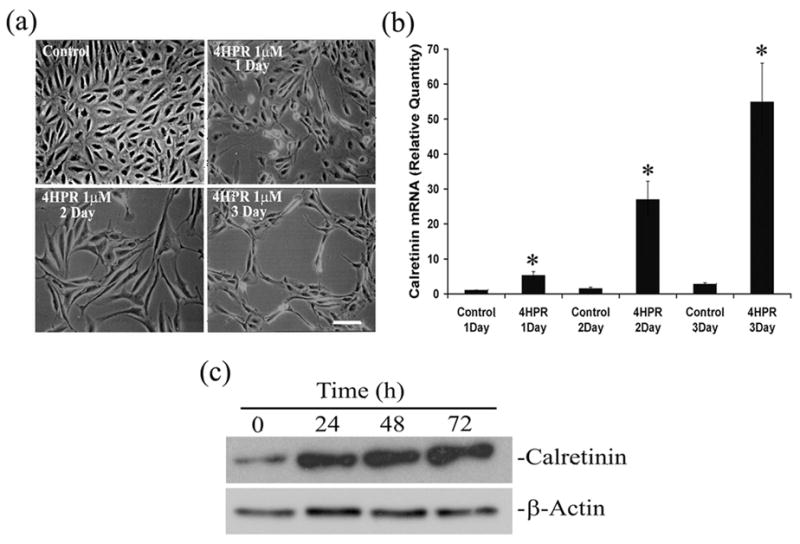
Panel A, Phase-contrast microscopic analysis of ARPE-19 cells treated with indicated concentrations of 4HPR for 1, 2 and 3 days. Scale bar = 100 μm. Panel B, 4HPR-induced calretinin mRNA expression is time-dependent. Cultured cells were treated with 1 μM 4HPR for indicated time points, and the total RNA preparations were analyzed by real-time quantitative PCR as described under Materials and Methods. Panel C, the time-dependent increase in calretinin (protein) expression by 4HPR. Cytoplasmic proteins were extracted from the cultured ARPE-19 cells treated with 1 μM 4HPR for indicated time points, protein levels were determined by Western blot analysis using calretinin antibody. β-Actin expression shows that the amount of protein used in different samples were similar. The values are mean ± SD, n = 4. *P < 0.001 compared with respective control.
To correlate the observed morphological changes with neuronal differentiation, we analyzed the expression of calretinin, a Ca2+-binding protein normally expressed in retinal ganglion cells and other retinal neurons (Pochet et al. 1989, Nag & Wadhwa 1999), by RT-PCR. Treatment of ARPE-19 cells with 4HPR induced the expression of calretinin as expected (Fig. 1B). The calretinin expression was markedly increased in a time-dependent manner, supporting the light microscopy observations. Significant increase in calretinin mRNA expression was observed even after 24 h of treatment and more than 25 and 50-fold increase over the control were observed when cells were treated with 1 μM of 4HPR for 48 and 72 h, respectively.
To see whether the increase in calretinin mRNA expression induced by 4HPR is translated into a corresponding increase in calretinin protein expression, we performed Western blot analysis. As seen in Fig. 1C, a ~ 29 kDa immunoreactive band specific for calretinin was detected. 4HPR treatment increased the calretinin protein expression in a time-dependent manner. No increase in the immunoreactivity for β-actin, used as endogenous control, was detected in control or treated cells.
Involvement of MAPK pathway in 4HPR-induced neuronal differentiation
It is well established that MAPK signaling cascades play a crucial role in regulating mammalian cell growth and differentiation (Pearson et al. 2001). To elucidate how 4HPR mediates the normal differentiation of ARPE-19 cells, the cells were treated in the presence or absence of various inhibitors specific for three major groups of MAP kinases JNK, p38 MAPK and ERK1/2 (p44/p42 MAPK). Morphological changes and the expression of calretinin were analyzed by phase-contrast microscopy and by RT-PCR, respectively. JNK and p38 MAPK are unlikely to be involved as SP600125, an inhibitor of JNK, and SB202190, an inhibitor of p38 MAPK, were ineffective in preventing the neurite outgrowth induced by 4HPR (data not shown). U0126, which specifically inhibits both the inactive and active forms of MEK1/2 (Favata et al. 1998), was used to assess the role of ERK1/2. As shown in Fig. 2A, cells pretreated with 1 μM of U0126 effectively blocked the differentiation induced by 4HPR. Further, the cells treated for 72 h with U0126 alone retained their normal epithelial morphology and appeared viable.
Fig. 2. 4HPR-induced neuronal differentiation of human RPE cells is blocked by U0126.
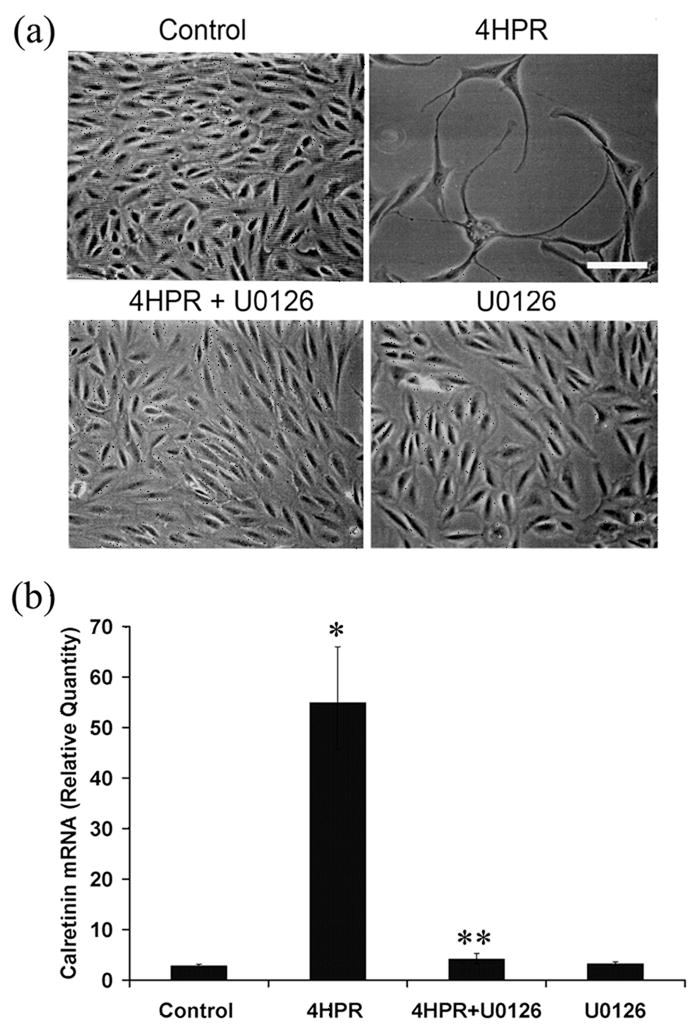
ARPE-19 cells in culture were pretreated with 1 μM of U0126, a selective inhibitor of MEK1/2, for 1 h followed by incubation with 4HPR for additional 72 h. Panel A, Phase-contrast microscopic analysis of the inhibition of 4HPR-induced neuronal differentiation of ARPE-19 cells by U0126. Scale bar = 100 μm. Panel B, the inhibition of 4HPR-induced calretinin mRNA expression by U0126. Total RNA extracted from treated cells was analyzed by real-time quantitative PCR as described under Materials and Methods. The values are mean ± SD, n = 4. *P < 0.001 compared with control; **P < 0.001 compared with 4HPR treatment.
To address whether the MAPK pathway is involved in 4HPR-induced calretinin expression, we examined the effect of ERK1/2 inhibitor U0126 on calretinin expression. After pre-treating the cells for 1 h with 1 μM U0126, the calretinin expression level was analyzed in 4HPR-treated and untreated cells by RT-PCR (Fig. 2B). As expected, more than a 50-fold increase in calretinin expression was observed with 1 μM of 4HPR after 72 h. U0126 pretreatment caused a marked decrease in calretinin expression induced by 4HPR.
ERK1/2 mediates 4HPR-induced neuronal differentiation
It is known that ERK1/2 are dual specificity kinases that function in a mitogen activated protein kinase cascade controlling cell growth and differentiation (Pearson et al. 2001). To establish whether the 4HPR-induced neuronal differentiation involves the phosphorylation of ERK1/2, cell extracts from ARPE-19 cells, treated with 1 μM of 4HPR for different period of time, were analyzed by Western blotting (Fig. 3A) for p-ERK1/2 (phospho-ERK1/2, upper panel) and total ERK1/2 (ERK1/2, lower panel). An increase in ERK1/2 phosphorylation was detectable as early as 6 h after the 4HPR treatment, and the maximum level reached at 48 h. This increase in phosphorylation of ERK1/2 was transient and decreased substantially at 72 h; however, it remains elevated over its control in the fully differentiated cells. On the other hand, we did not observe any noticeable change in the total ERK1/2 level with 4HPR treatment. These results indicate that 4HPR induces a time-dependent phosphorylation of ERK1/2.
Fig. 3. 4HPR-induced phosphorylation of ERK1/2 is blocked by U0126.
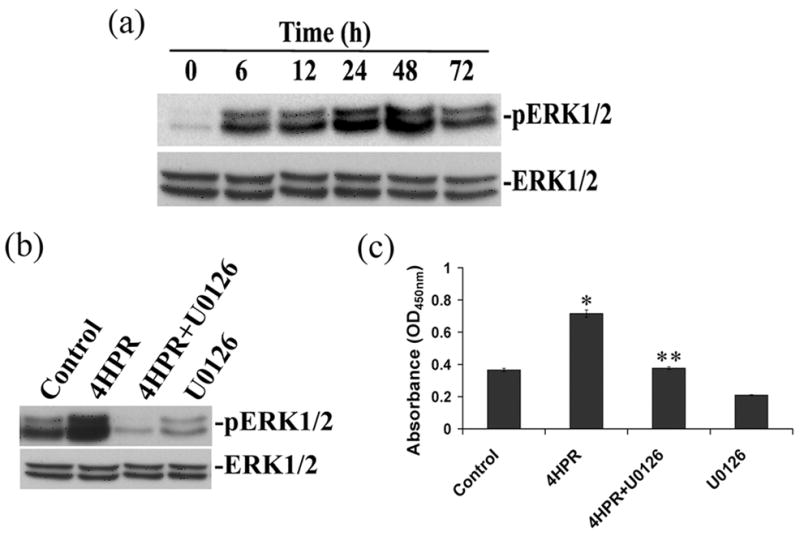
Panel A, 4HPR-induced phosphorylation of ERK1/2 is time-dependent. ARPE-19 cells in culture were treated with 1 μM of 4HPR for indicated time points, and the cell extracts were analyzed for phosphorylation of ERK1/2 by Western blotting described under Materials and Methods. Panel B, the inhibition of 4HPR-induced phosphorylation of ERK1/2 by U0126. Cells were pretreated with 1 μM of U0126 for 1 h followed by 4HPR for additional 48 h, then analyzed by Western blotting. Panel C, ELISA analysis of the inhibition of 4HPR-induced phosphorylation of ERK1/2 by U0126. The cultured ARPE-19 cells were treated with 1 μM of 4HPR for 48 h in the presence or absence of U0126, and the phosphorylation of p44/42 MAPK was measured by PathScan®Phospho-p44/42 MAPK sandwich ELISA kit as described under Materials and Methods. The values are mean ± SD, n = 4. *P < 0.01 compared to control; **P < 0.01 compared with 4HPR treatment.
To determine whether the activation or phosphorylation of ERK1/2 is associated with 4HPR-induced neuronal differentiation, cells were treated with 4HPR in the presence or absence of U0126 added 1 h prior to stimulation. After 48 h, phosphorylation of ERK1/2 was analyzed in cell lysates by Western blotting. As shown in Fig. 3B, an increase in ERK1/2 phosphorylation was observed with 4HPR treatment. Under similar conditions, the increase in ERK1/2 phosphorylation was completely inhibited in U0126 pretreated cells
To further demonstrate the differentiation associated phosphorylation of ERK1/2, we measured phospho-ERK1/2 protein formation in 4HPR treated cells in the presence or absence of U0126 by ELISA. As expected, a significant induction in phospho-ERK1/2 protein formation was observed with 4HPR treatment (Fig. 3C). By contrast, inhibition of ERK1/2 activation with its selective inhibitor U0126 had completely blocked the phospho-ERK1/2 protein formation.
ERK1/2 siRNAs specifically inhibit 4HPR-induced neuronal differentiation
Numerous studies performed on primary cell cultures have suggested that ERK activation is a prerequisite for neurite outgrowth and cell transformation (Pang et al. 1995, Robinson et al. 1998). To rule out the possibility that importance of ERK1/2 signaling is not due exclusively to a pharmacologic inhibitor, and assess further the hypothesis that p-ERK1/2 plays an important role in 4HPR-induced neuronal differentiation, we used small interfering RNAs to specifically knockdown ERK1/2 expression in ARPE-19 cells. After exposure of each transfected cell line with 1 μM 4HPR for 72 h, the morphology of the cell was examined using a phase-contrast microscope. As shown in Fig. 4A, the 4HPR-induced neuronal differentiation of ARPE-19 cells was completely blocked in ERK1/2 silenced cells. The suppression was specific, since the cells transfected with mock siRNA or with transfection reagent alone did not block the neuronal differentiation induced by 4HPR.
Fig. 4. 4HPR-induced neuronal differentiation is blocked by ERK1/2 siRNA.
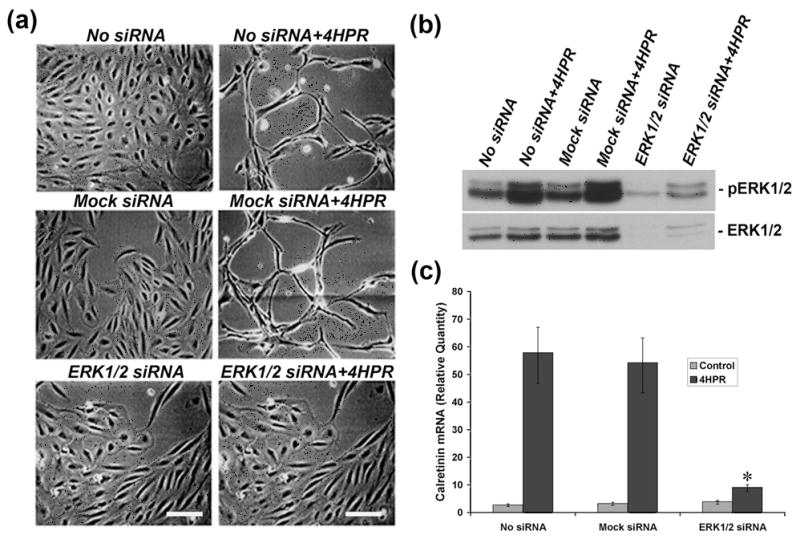
Panel A, ERK1/2 siRNA blocked the neuronal differentiation induced by 4HPR. Cultured ARPE-19 cells were transfected with ERK1/2 siRNA, and after 24 h of post-transfection the cells were treated with 1 μM 4HPR for 72 h, and then analyzed by phase contrast microscopy as described under Materials and Methods. Scale bar = 100 μm. Panel B, ERK1/2 siRNA blocked the ERK1/2 phosphorylation induced by 4HPR. After 24 h of post-transfection with ERK1/2 or mock siRNA, the cells were treated with 1 μM of 4HPR for 72 h. Cell lysates were analyzed for phosphorylation of ERK1/2 by Western blotting. Panel C, ERK1/2 siRNA attenuated the expression of calretinin induced by 4HPR. Cells transfected with ERK1/2 or mock siRNA were treated with 1 μM 4HPR for 72 h after 24 h of post-transfection. Total RNA was extracted and analyzed by real time quantitative PCR as described under Materials and Methods. The values are mean ± SD, n = 4. *P < 0.001 compared with 4HPR treatment.
To examine whether silencing ERK1/2 cause a decrease in ERK1/2 phosphorylation induced by 4HPR, we performed Western blot analysis on the cell lysates made from the transfected cell line treated with 1 μM of 4HPR for 72 h. As shown in Fig. 4B, in ERK1/2 siRNA transfected cells, the amount of p-ERK1/2 was greatly decreased. However, the level of p-ERK1/2 was not significantly altered in cells transfected with mock siRNA or in the cell transfected with reagent alone. In addition, the basal level expression of ERK1/2 was significantly inhibited in the cells transfected with ERK1/2 siRNA duplexes.
Lastly, we examined the effect of 4HPR on calretinin expression in ERK1/2 silenced cells. The cells transfected with siRNA duplexes specific for p44 (ERK1) and p42 (ERK2) at equimolar concentration or mock siRNA were treated with 4HPR for 72 h, and calretinin expression was analyzed by RT-PCR. As shown in Fig. 4C, 4HPR-induced calretinin expression was significantly blocked (~70%) in ERK1/2 silenced cells. The calretinin expression induced by 4HPR was not blocked in cells transfected with mock siRNA or transfection reagent alone under standard culture conditions.
4HPR induces ERK activation through a Raf-dependent pathway
Since neuronal differentiation induced by 4HPR is associated with activation of ERK1/2, we next analyzed whether the activation of ERKs induced by 4HPR is mediated through the common Raf/MEK cascade or via an alternative route. To address these questions, ARPE-19 cells in culture were treated with 4HPR (1 μM) in the presence or absence of U0126 (1 μM) for 48 h. Using phospho-specific antibodies, the activation of c-Raf and MEK1/2 was determined by Western blot analysis. As shown in Fig. 5A, we observed an increase in phospho-c-Raf (upper panel) compared to total c-Raf (lower panel) with 4HPR treatment. Under similar conditions, 4HPR treatment increased the phosphorylation of MEK1/2 in RPE cells significantly. However, the finding that 4HPR-induced activation of c-Raf and MEK1/2 was not inhibited by U0126 pretreatment further supports the evidence that U0126 inhibits the catalytic activity of MEKs but not its phosphorylation (Favata et al. 1998). In addition, it also supports the notion that the activation of c-Raf precedes MEK1/2 activation due to the correlation between the activation of MEK1/2 and the corresponding increase in the level of p-ERKs. Furthermore, the increase in activation of c-Raf and MEK1/2 (Fig. 5A) observed after 48 h of treatment with 4HPR was similar to ERK1/2 phosphorylation, which reached its maximum level at 48 h (Fig. 3B). Taken together, these results suggest that 4HPR-induced activation of ERKs is mediated by c-Raf and MEK1/2, the upstream components involved in MAPK signal transduction pathway.
Fig. 5. MEK1/2 pathway mediates 4HPR-induced neuronal differentiation of ARPE-19 cells.
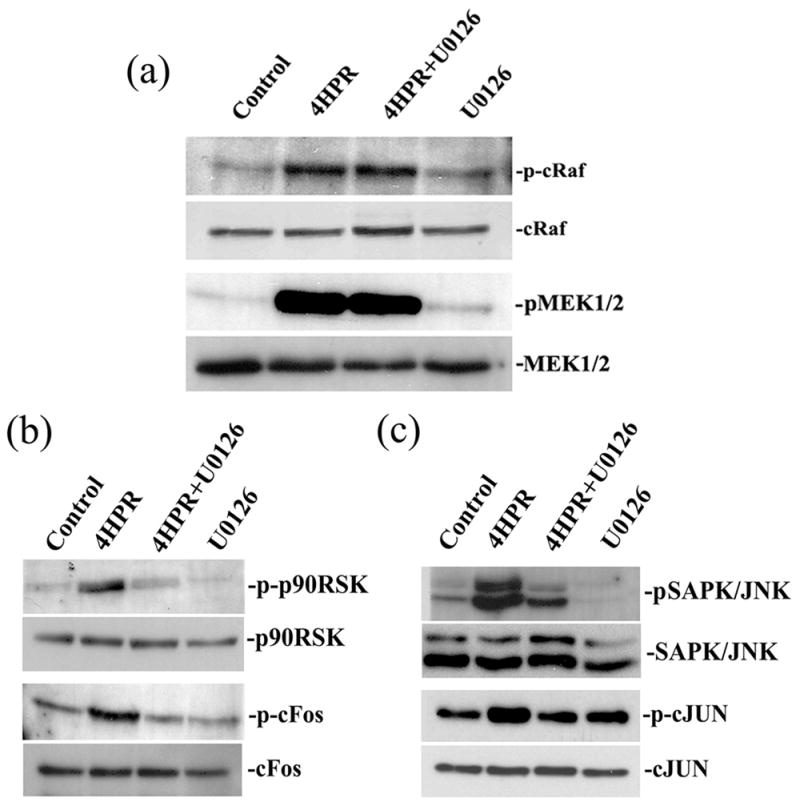
Cultured ARPE-19 cells were pretreated with 1 μM U0126 for 1 h followed by incubation with 1μM 4HPR for additional 72 h. Cell lysates were prepared, and then analyzed by Western blotting using non-phospho or phospho-specific antibodies of MAPK/ERK pathway as described under Materials and Methods. Panel A, 4HPR-induced phosphorylation of c-Raf and MEK1/2 were not blocked by U0126. Panel B, the inhibition of 4HPR-induced phosphorylation of p90RSK and c-Fos by U0126. Panel C, the inhibition of 4HPR-induced phosphorylation of SAPK/JNK and c-Jun by U0126.
To further determine whether the induction of differentiation induced by 4HPR is associated with the classical MAPK phosphorylation cascade of Raf/MEK/ERK, we studied the effect of 4HPR on the phosphorylation of the down-stream components of this pathway. To ensure identical activation range between each condition within the experiment, the same batch of cells were treated with 1 μM 4HPR in the presence or absence of U0126 (1 μM) for 48 h, and the phosphorylation of 90-kDa ribosomal S6 kinase (p90RSK) and c-Fos were analyzed by Western blotting (Fig. 5B). Interestingly, the phosphorylation of p90RSK, a potential downstream target of ERK1/2, was increased with 4HPR treatment in differentiating cells. In addition, treatment of the cells with 4HPR also enhanced the phosphorylation of c-Fos, a downstream target of p90RSK. This increase in phosphorylation of both p90RSK and c-Fos was dependent on the activation of ERK1/2 because U0126, a selective inhibitor of MEK1/2, completely blocked p90RSK and c-Fos phosphorylation. This shows that ERK1/2, p90RSK and c-Fos activation closely parallel each other, and that the inhibition of ERKs activation by U0126 decreases the levels of both p-p90RSK and p-c-Fos. Together, these experiments provide compelling evidence that activated p90RSK and c-Fos are downstream mediators of ERK1/2 in the neuronal differentiation induced by 4HPR.
The signaling connection between SAPK/JNK, another family member of MAPK pathway, and ERK, and the existence of multiple interactions between them have been reported (Cowan & Storey 2003). Therefore, we explored whether 4HPR induced the phosphorylation of SAPK/JNK and c-Jun in ARPE-19 cells using Western blot analysis (Fig. 5C). The 4HPR treatment also induced the phosphorylation of SAPK/JNK and c-Jun. However, in the presence of U0126 the phosphorylation of SAPK/JNK and c-Jun induced by 4HPR was significantly decreased. Thus, our results indicate the possible involvement of JNK signal transduction pathways in the neuronal differentiation of ARPE-19 cells induced by 4HPR. Additionally, the inhibition of SAPK/JNK phosphorylation by U0126, suggests that Raf/MEK1/2 are upstream components involved in this signaling cascade.
ERK regulates 4HPR-induced neuronal differentiation of ARPE-19 cells through AP-1-activation
As the signal transduction pathway regulated by both SAPK/JNK and ERK1/2 terminates with the activation of the transcription factor activator protein-1 (AP-1), an ELISA-based transcription factor activation assay was performed to determine which of the AP-1 members were activated. Nuclear extracts prepared from ARPE-19 cells treated with 4HPR in the presence or absence of U0126, which block ERKs activation, was used. Binding of AP-1 components present in the nuclear extracts to its immobilized AP-1 consensus oligonucleotide on the 96-well plate was detected using specific phosphorylated antibodies. As shown in Fig. 6A, 4HPR treatment significantly increased AP-1 binding to its specific responsive element sequence when c-Jun was used as a primary antibody. The binding of AP-1 is specific since an excess of AP-1 binding element (4HPR-WT) blocked AP-1/DNA complex formation whereas the addition of mutated oligonucleotide (4HPR-MO) had no competitive effect. No significant AP-1 activation by 4HPR was observed in nuclear extracts of U0126 pretreated cells. These results suggest that c-Jun/AP-1 complex is formed when the cells are stimulated with 4HPR.
Fig. 6. MEK1/2 inhibitor U0126 attenuates 4HPR-induced AP-1 transactivation.
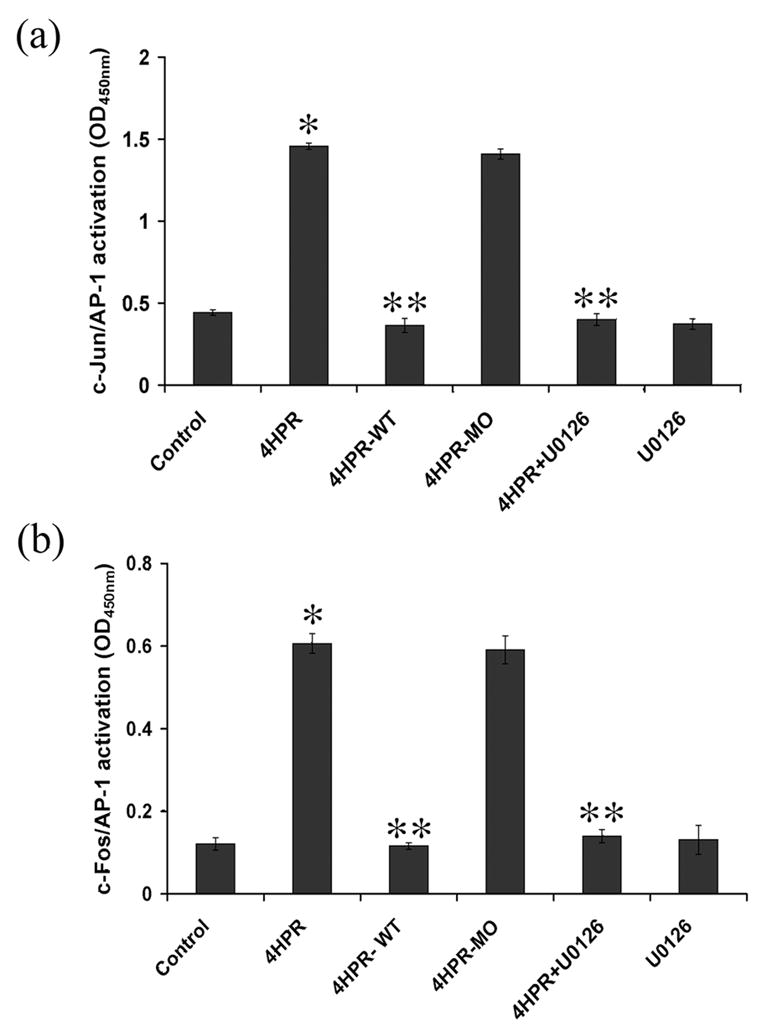
ARPE-19 cells in culture were pretreated with 1 μM of U0126 for 1 h followed by incubation with 1 μM 4HPR for additional 72 h. Nuclear extracts prepared were used for analyzing the AP-1 transcription factor activation by quantitative ELISA using antibodies specific for the activated form of c-Fos or phosphorylated c-Jun as described under Materials and Methods. The specificity of AP-1 activation was verified using wild type oligonucleotide (4HPR-WT) or mutated oligonucleotide (4HPR-MO). Panel A, c-Jun mediates 4HPR-induced transactivation of AP-1. Panel B, c-Fos mediates 4HPR-induced transactivation of AP-1. The values are mean ± SD, n = 4. *P < 0.01 compared to control; **P <0.001 compared with 4HPR treatment.
As illustrated in Fig. 6B, AP-1 activation was also significantly increased with 4HPR treatment when c-Fos was used as primary antibody. Furthermore, the binding of AP-1 is specific since excess of AP-1 blocked the AP-1/DNA complex formation. As expected, the addition of mutated oligonucleotide prior to nuclear extract did not block the AP-1/DNA complex formation. Nuclear extracts from U0126 treated cells also had no significant AP-1 activation by 4HPR further suggesting that the phosphorylation of AP-1 is mediated through ERK1/2 of MAPK kinases. Thus, 4HPR treatment activates c-Fos/AP-1 complex formation in ARPE-19 cells. Together, these findings suggest a role for AP-1 signaling in mediating the 4HPR-induced neuronal differentiation of ARPE-19 cells.
Discussion
RPE cells from many vertebrate species can be induced to differentiate both in vivo and in vitro into neuronal cells using different biochemical and other stimuli (Pittack et al. 1991, Zhao et al. 1997, Fischer & Reh 2001, Yan et al. 2001), but little is known about the neuronal type differentiation of mammalian RPE cells. In the present study, we have provided evidence that 4HPR-induced neuronal differentiation of ARPE-19 cells, a human RPE cell line, is predominantly mediated through the MAP kinase pathway (Fig. 7). However, it should be noted that this line may not be representative of the effects of 4HPR on an intact RPE monolayer. The neuronal differentiation induced by 4HPR in ARPE-19 cells is evident not only by the appearance of the neuron-like morphology but also by the observed increase in the expression of calretinin, a retinal neuronal marker (Nag & Wadhwa 1999). Treatment of the ARPE-19 cells with 4HPR resulted in the activation of c-Raf and MEK1/2, the typical all-purpose signaling kinases. This activation was accompanied by an increase in the phosphorylation of their downstream mediators SAPK/JNK and ERK1/2. Importantly, the activation of both SAPK/JNK and ERK1/2 were blocked by U0126, a potent inhibitor of the dual-specific protein kinase MEK 1 and 2. The activated ERK1/2 then induced the phosphorylation of key regulatory protein p90RSK, and of transcription factors c-Fos and c-Jun. The phosphorylation of c-Fos and c-Jun, the main AP-1 proteins present in mammalian cells, resulted in increased AP-1 transcriptional activity. This in turn could regulate the expression of many genes such as the neuronal marker calretinin, and thereby elicit the neuronal differentiation of ARPE-19 cells.
Fig. 7. Schematic representation of the postulated MAP kinase signaling pathways involved in 4HPR-induced neuronal differentiation of ARPE-19 cells.
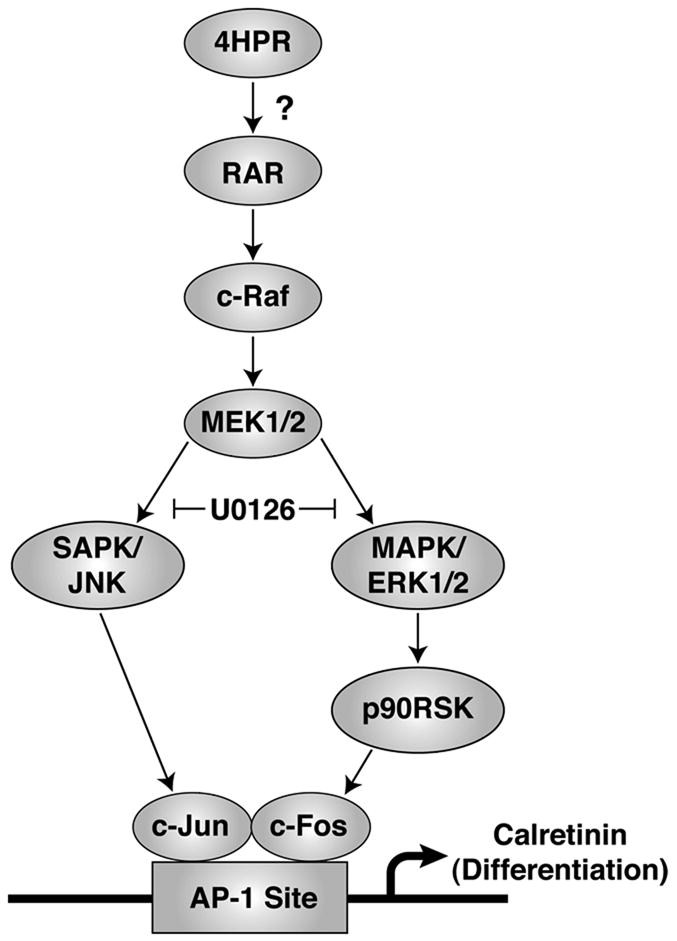
4HPR mediates the neuronal differentiation of ARPE-19 cells by activating both c-Raf and MEK1/2, while the activation of its downstream targets such as SAPK/JNK and MAPK/ERK1/2 was regulated by MEK1/2. The activation of ERK1/2 appears to activate its downstream targets such as p90RSK and SAPK/JNK, and resulted in the phosphorylation of c-Fos and c-Jun, respectively. The activated form of c-Fos and c-Jun mediates the transactivation of AP-1, one of the effectors of differentiation, perhaps through an AP-1 response element present in the promoter of the neuronal marker calretinin. The question mark (?) indicates that the involvement of retinoid receptors in this process is not yet known.
N-(4-hydroxyphenyl)retinamide is known to exert its chemotherapeutic effects in cancer cells through induction of apoptosis (Malone et al. 2003). Administration of 4HPR for long term in cancer patients causes a dose-dependent decline of plasma retinol levels and retinol binding protein, which can lead to night blindness and dermatologic disorders (Camerini et al. 2001). However, a recent study raises the potential of 4HPR in long-term treatment of lipofuscin-based retinal diseases by blocking the formation of A2E in the RPE of ABCA4 knockout mice (Radu et al. 2005). In the present study, we have observed that relatively low concentration of 4HPR (1 μM) induces morphological alteration of cultured ARPE-19 cells. These morphological alterations, elongation of cells equal to or longer than the axis of the cell body, and cells bearing bidirectional or multidirectional neurite-like processes, are a typical phenomenon associated with cells undergoing neuronal type differentiation (Munch et al. 2003). Another important finding in the present study is that 4HPR tends to induce the neuronal type differentiation very early when the cells are cultured in medium lacking fetal bovine serum (FBS) compared to cells cultured in the presence of the serum (data not shown). The reason may be that FBS has a growth promoting effect rather than a differentiation effect (Ikegami et al. 2002). In addition, the concentration at which 4HPR (1 μM) induced the neuronal differentiation in ARPE-19 cells is similar to our earlier report observed in the presence of serum (Chen et al. 2003). This concentration was found to be optimal and does not result in appreciable apoptosis or necrosis. However, 4HPR at high concentrations (5 and 10 μM) induces membrane blebbing and the change into round morphology, which is a typical phenomenon associated with cells undergoing apoptosis or necrosis in ARPE-19 cells (Samuel et al. 2006). Apart from the observation that RPE cells differentiate into neuronal-like cells much earlier under our culture conditions, there was no other noticeable difference between the cells grown in the presence or absence of serum. The 4HPR-induced neuronal differentiation and the associated increase in calretinin expression observed in the presence of serum were also blocked by the MEK1/2 specific inhibitor, U0126 (data not shown).
Neuronal differentiation induced by retinoic acid (RA) has been shown in a variety of embryonic neuronal cell types, and RA receptors may be involved in this process (Guan et al. 2001). Further, RA promotes activation of MAPK pathways, and activated MAPKs differentially regulate the induction of neurite outgrowth and expression of neuronal markers (Singh et al. 2003). Like other retinoids, 4HPR exerts its chemotherapeutic effect through the nuclear retinoid receptor pathway (Sabichi et al. 1998). We have also shown recently that 4HPR-induced apoptosis in human RPE cells were mediated through a signal transduction pathway involving retinoid receptors (Samuel et al. 2006). Its ability to induce apoptosis in RA-resistant cells raises the possibility that 4HPR mediates its effect through a retinoid receptor-independent mechanism (Dmitrovsky 1997). Thus, 4HPR can mediate its effects through both receptor-dependent and receptor-independent mechanisms. Such a situation is seen in F9 carcinoma cells treated with 4HPR. At 10 μM, 4HPR induces apoptosis, while at 1 μM it induces differentiation of these cells into primitive endodermal phenotype (Clifford et al. 1999). The hypothesis that 4HPR mediates the neuronal differentiation of ARPE-19 cells through activation of nuclear retinoid receptors is an attractive one, but remains to be investigated (Fig. 7).
The signal transduction pathway of MAP kinases has been shown to play an important role in the neuronal differentiation and expression of neuronal markers in PC12, cultured embryonic stem and neural precursor cells (Morooka & Nishida 1998, Wang et al. 2007, Li et al. 2006). We observed an increase in ERK1/2 phosphorylation in response to 4HPR treatment that was completely blocked by U0126, suggesting that the phosphorylation of ERK1/2 occurs via MEKs. In addition, U0126 also blocked the increase in calretinin expression associated with 4HPR-induced neuronal differentiation, which then seems to be specifically mediated by the activation of the ERK MAPK signaling pathway. It is possible that the increase in calretinin expression evokes alteration in Ca2+ concentrations, and calcium ions could act as a second messenger in the signal transduction pathway. Fluctuation in intracellular Ca2+ concentrations has been proposed to be a critical event in both apoptosis and necrosis. 4HPR-induced apoptosis in glioma cells has been shown to be mediated by an increase in free cytosolic as well as mitochondrial Ca2+ (Tiwari et al. 2006). Ca2+ signaling may act through its downstream effectors such as MAPKs to regulate neuronal growth and differentiation (Zheng & Poo 2007). Furthermore, when we silenced ERK1/2 by siRNA, 4HPR neither induced the neuronal differentiation of ARPE-19 cells nor increased the expression of calretinin.
Activation of SAPK/JNK has been shown to play a crucial role in neurite outgrowth (Waetzig & Herdegen 2003), and in the proliferation and differentiation of multipotent neural precursor cells (Wang et al. 2007, Yu et al. 2003). In the present study, we have also observed that 4HPR induces the phosphorylation of SAPK/JNK in ARPE-19 cells in addition to the phosphorylation of ERK1/2. Furthermore, the observed inhibition of SAPK/JNK phosphorylation by MEK1/2 specific inhibitor U0126 indicates that 4HPR-induced neuronal differentiation of ARPE-19 cells occurs through a crosstalk between the ERK1/2 and SAPK/JNK pathways. An active cross-talk between SAPK/JNK and ERK1/2 pathways has been shown to mediate the neuronal differentiation of PC12 cells induced by NGF (Leppa et al. 1998), and is necessary for increased expression of neuronal cytoskeleton proteins during NGF-induced neuronal differentiation of PC12 cells (Zentrich et al. 2002). The SAPK/JNK signaling pathways are activated by dual phosphorylation at its regulatory Tyr and Thr motif by MEKKs 1–4 (Hirai et al. 1996). Although, the three MAPK modules, ERK1/2, SAPK/JNK and p38 are in parallel, there is considerable crosstalk at the upstream level specific to their activation (Cowan & Storey 2003). It is possible that the phosphorylation of SAPK/JNK could be mediated via MEK1/2, since U0126 blocked the phoshorylation of SAPK/JNK, and its downstream target c-Jun.
Activated Raf, either through the downstream MEK and ERK or independently of MEK and ERK, plays a critical role in mediating the signals from cell surface receptors to transcription factors (McCubrey et al. 2006). In the present study, we observed an increase in c-Raf phosphorylation at Ser259 following 4HPR treatment, suggesting that the activation of the downstream components occurs via the c-Raf pathway. The activation of c-Raf precedes that of MEK1/2 as U0126 was unable to inhibit the c-Raf phosphorylation associated with the 4HPR-induced neuronal differentiation of ARPE-19 cells. We have also observed an increase in MEK1/2 phosphorylation associated with 4HPR-induced neuronal differentiation. This correlates with the report that activated Raf phosphorylates the dual-specificity kinase MEK, and produces a fully active MEK (McCubrey et al. 2006). Also we have observed that the 4HPR-induced phosphorylation of MEK1/2 was not blocked by U0126 as previously observed (Favata et al. 1998). Thus, the c-Raf/MEK1/2 plays a central role in mediating the 4HPR-induced neuronal differentiation of ARPE-19 cells.
p90RSK, a downstream component of the typical Raf/MEK1/2 signaling cascade, functions as an important intermediate in mediating the ERK1/2 signals (Moor et al. 2001, Luo et al. 2007). In this study, we observed an increase in the phosphorylation of p90RSK with 4HPR treatment, and complete inhibition of this by U0126. Thus, ERK1/2 lies upstream of p90RSK since the phosphorylation of p90RSK depends on ERK1/2 activation. The activation of p90RSK has been shown to regulate gene expression via phosphorylation of transcription factors such as c-Fos (Moor et al. 2001), and members of the ERK pathway translocated into the nucleus phosphorylate c-Fos (Monje et al. 2003). Similarly, SAPK/JNK translocated to the nucleus phosphorylates c-Jun (Leppa & Bohmann 1999), and c-Jun can also be phosphorylated by ERK1/2 (Pearson et al. 2001). Thus, the observed increase in phosphorylation of both c-Fos and c-Jun with 4HPR treatment correlates well with these observations. Further, the phosphorylation of both c-Jun and c-Fos was inhibited by U0126, suggesting a role for complex formation between AP-1 components c-Jun/c-Fos in mediating the neuronal differentiation (Karin 1995, Whitmarsh & Davis 1996, Shaulian & Karin 2002). In the present study, an increase in AP-1 transcriptional activation was observed during the 4HPR-induced neuronal differentiation of ARPE-19 cells. Thus, our findings point to a critical function of the AP-1 transcription factors in this process.
In summary, we show that neuronal differentiation and calretinin expression induced by 4HPR in human RPE cells is blocked by the MEK1/2 inhibitor U0126. This was accompanied by a marked decrease in phosphorylation of ERK1/2 and SAPK/JNK, the downstream mediators of the MAPK pathway. This resulted in the suppression of phosphorylation of the transcriptional mediators c-Fos/c-Jun, and led to the inhibition of AP-1 transcriptional activity. Further, the suppression of ERK1/2 expression with small interfering RNA effectively blocked the 4HPR-induced neuronal differentiation of RPE cells, and the expression of neuronal marker, calretinin. Thus, both neuronal differentiation and the associated increase in calretinin expression induced by 4HPR are mediated through a signal transduction pathway involving MAPKs. c-Raf/MEK1/2, in particular, play a central role in this process.
Acknowledgments
We are grateful to Todd Duncan (Biochemistry section, Laboratory of Retinal Cell and Molecular Biology, NEI) for help and valuable suggestions. This research was supported by the Intramural Research Program of the NIH, NEI.
Abbreviations used are
- RPE
retinal pigment epithelium
- 4HPR
N -(4-Hydroxyphenyl)-retinamide
- MAPK
mitogen-activated protein kinase
- ERK1/2
extracellular signal-regulated kinase 1 and 2
- SAPK/JNK
stress-activated protein kinase/c-Jun N-terminal kinase
- p38 MAPK
38-kDa mitogen-activated protein kinase
- MEK1/2
mitogen activated protein kinase/extracellular signal-regulated kinase 1 and 2 kinase
- p-
phosphorylated
- p90RSK
90-kDa ribosomal S6 kinase
- AP-1
activator protein-1
- siRNA
small interfering RNA
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
References
- Bok D. The retinal pigment epithelium: a versatile partner in vision. J Cell Sci. 1993;17:189–195. doi: 10.1242/jcs.1993.supplement_17.27. [DOI] [PubMed] [Google Scholar]
- Camerini T, Mariani L, De Palo G, Marubini E, Di Mauro MG, Decensi A, Costa A, Veronesi U. Safety of the synthetic retinoid fenretinide: long-term results from a controlled clinical trial for the prevention of contralateral breast cancer. J Clin Oncol. 2001;19:1664–1670. doi: 10.1200/JCO.2001.19.6.1664. [DOI] [PubMed] [Google Scholar]
- Chambon P. The retinoid signaling pathway: molecular and genetic analyses. Semin Cell Biol. 1994;5:115–125. doi: 10.1006/scel.1994.1015. [DOI] [PubMed] [Google Scholar]
- Chen S, Samuel W, Fariss RN, Duncan T, Kutty RK, Wiggert B. Differentiation of human retinal pigment epithelial cells into neuronal phenotype by N-(4-hydroxyphenyl)retinamide. J Neurochem. 2003;84:972–981. doi: 10.1046/j.1471-4159.2003.01608.x. [DOI] [PubMed] [Google Scholar]
- Clifford JL, Menter DG, Wang M, Lotan R, Lippman SM. Retinoid receptor-dependent and -independent effects of N-(4-hydroxyphenyl)retinamide in F9 embryonal carcinoma cells. Cancer Res. 1999;59:14–18. [PubMed] [Google Scholar]
- Cowan KJ, Storey KB. Mitogen-activated protein kinases: new signaling pathways functioning in cellular responses to environmental stress. J Exp Biol. 2003;206:1107–1115. doi: 10.1242/jeb.00220. [DOI] [PubMed] [Google Scholar]
- Dmitrovsky E. N-(4-hydroxyphenyl)retinamide activation of a distinct pathway signaling apoptosis. J Natl Cancer Inst. 1997;89:1179–1181. doi: 10.1093/jnci/89.16.1179. [DOI] [PubMed] [Google Scholar]
- Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- Dutt K, Scott M, Sternberg PP, Linser PJ, Srinivasan A. Transdifferentiation of adult human pigment epithelium into retinal cells by transfection with an activated H-ras proto-oncogene. DNA Cell Biol. 1993;12:667–673. doi: 10.1089/dna.1993.12.667. [DOI] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, et al. Identification of a novel inhibitor of mitogen- activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alpha(v)beta5 integrin for binding but not for internalization. Proc Natl Acad Sci. 1997;94:12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Transdifferentiation of pigmented epithelial cells: a source of retinal stem cells? Dev Neurosci. 2001;23:268–276. doi: 10.1159/000048710. [DOI] [PubMed] [Google Scholar]
- Guan K, Chang H, Rolletschek A, Wobus AM. Embryonic stem cell-derived neurogenesis. Retinoic acid induction and lineage selection of neuronal cells. Cell Tissue Res. 2001;305:171–176. doi: 10.1007/s004410100416. [DOI] [PubMed] [Google Scholar]
- Hirai S, Izawa M, Osada S, Spyrou G, Ohno S. Activation of the JNK pathway by distantly related protein kinases, MEKK and MUK. Oncogene. 1996;12:641–650. [PubMed] [Google Scholar]
- Holtkamp GM, Van Rossem M, de Vos AF, Willekens B, Peek R, Kijlstra A. Polarized secretion of IL-6 and IL-8 by human retinal pigment epithelial cells. Clin Exp Immunol. 1998;112:34–43. doi: 10.1046/j.1365-2249.1998.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami Y, Mitsuda S, Araki M. Neural cell differentiation from retinal pigment epithelial cells of the newt: an organ culture model for the urodele retinal regeneration. J Neurobiol. 2002;50:209–220. doi: 10.1002/neu.10031. [DOI] [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Kutty RK, Kutty G, Samuel W, Duncan T, Bridges CC, El-Sherbeeny A, Nagineni CN, Smith SB, Wiggert B. Molecular characterization and developmental expression of NORPEG, a novel gene induced by retinoic acid. J Biol Chem. 2001;276:2831–2840. doi: 10.1074/jbc.M007421200. [DOI] [PubMed] [Google Scholar]
- Leppa S, Bohmann D. Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene. 1999;18:6158–6162. doi: 10.1038/sj.onc.1203173. [DOI] [PubMed] [Google Scholar]
- Leppa S, Saffrich R, Ansorge W, Bohmann D. Differential regulation of c-Jun by ERK and JNK during PC12 cell differentiation. Embo J. 1998;17:4404–4413. doi: 10.1093/emboj/17.15.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Theus MH, Wei L. Role of ERK 1/2 signaling in neuronal differentiation of cultured embryonic stem cells. Dev Growth Differ. 2006;48:513–523. doi: 10.1111/j.1440-169X.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- Luo J, Kintner DB, Shull GE, Sun D. ERK1/2-p90RSK-mediated phosphorylation of Na+/H+ exchanger isoform 1. A role in ischemic neuronal death. J Biol Chem. 2007;282:28274–28284. doi: 10.1074/jbc.M702373200. [DOI] [PubMed] [Google Scholar]
- Malone W, Perloff M, Crowell J, Sigman C, Higley H. Fenretinide: a prototype cancer prevention drug. Expert opinion on investigational drugs. 2003;12:1829–1842. doi: 10.1517/13543784.12.11.1829. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Abrams SL, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249–279. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Monje P, Marinissen MJ, Gutkind JS. Phosphorylation of the carboxyl-terminal transactivation domain of c-Fos by extracellular signal-regulated kinase mediates the transcriptional activation of AP-1 and cellular transformation induced by platelet-derived growth factor. Mol Cell Biol. 2003;23:7030–7043. doi: 10.1128/MCB.23.19.7030-7043.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor AN, Gan XT, Karmazyn M, Fliegel L. Activation of Na+/H+ exchanger-directed protein kinases in the ischemic and ischemic-reperfused rat myocardium. J Biol Chem. 2001;276:16113–16122. doi: 10.1074/jbc.M100519200. [DOI] [PubMed] [Google Scholar]
- Morooka T, Nishida E. Requirement of p38 mitogen-activated protein kinase for neuronal differentiation in PC12 cells. J Biol Chem. 1998;273:24285–24288. doi: 10.1074/jbc.273.38.24285. [DOI] [PubMed] [Google Scholar]
- Munch G, Gasic-Milenkovic J, Dukic-Stefanovic S, Kuhla B, Heinrich K, Riederer P, Huttunen HJ, Founds H, Sajithlal G. Microglial activation induces cell death, inhibits neurite outgrowth and causes neurite retraction of differentiated neuroblastoma cells. Experimental brain research. 2003;150:1–8. doi: 10.1007/s00221-003-1389-5. [DOI] [PubMed] [Google Scholar]
- Nag TC, Wadhwa S. Developmental expression of calretinin immunoreactivity in the human retina and a comparison with two other EF-hand calcium binding proteins. Neuroscience. 1999;91:41–50. doi: 10.1016/s0306-4522(98)00654-x. [DOI] [PubMed] [Google Scholar]
- Pang L, Sawada T, Decker SJ, Saltiel AR. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem. 1995;270:13585–13588. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Pittack C, Jones M, Reh TA. Basic fibroblast growth factor induces retinal pigment epithelium to generate neural retina in vitro. Development. 1991;113:577–588. doi: 10.1242/dev.113.2.577. [DOI] [PubMed] [Google Scholar]
- Pochet R, Blachier F, Malaisse W, Parmentier M, Pasteels B, Pohl V, Resibois A, Rogers J, Roman A. Calbindin-D28 in mammalian brain, retina, and endocrine pancreas: immunohistochemical comparison with calretinin. Adv Exp Med Biol. 1989;255:435–443. doi: 10.1007/978-1-4684-5679-0_46. [DOI] [PubMed] [Google Scholar]
- Radu RA, Han Y, Bui TV, Nusinowitz S, Bok D, Lichter J, Widder K, Travis GH, Mata NL. Reductions in serum vitamin A arrest accumulation of toxic retinal fluorophores: a potential therapy for treatment of lipofuscin-based retinal diseases. Invest Ophthalmol Vis Sci. 2005;46:4393–4401. doi: 10.1167/iovs.05-0820. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Stippec SA, Goldsmith E, White MA, Cobb MH. A constitutively active and nuclear form of the MAP kinase ERK2 is sufficient for neurite outgrowth and cell transformation. Curr Biol. 1998;8:1141–1150. doi: 10.1016/s0960-9822(07)00485-x. [DOI] [PubMed] [Google Scholar]
- Sabichi AL, Hendricks DT, Bober MA, Birrer MJ. Retinoic acid receptor beta expression and growth inhibition of gynecologic cancer cells by the synthetic retinoid N-(4-hydroxyphenyl) retinamide. J Natl Cancer Inst. 1998;90:597–605. doi: 10.1093/jnci/90.8.597. [DOI] [PubMed] [Google Scholar]
- Samuel W, Kutty RK, Nagineni S, Gordon JS, Prouty SM, Chandraratna RA, Wiggert B. Regulation of stearoyl coenzyme A desaturase expression in human retinal pigment epithelial cells by retinoic acid. J Biol Chem. 2001;276:28744–28750. doi: 10.1074/jbc.M103587200. [DOI] [PubMed] [Google Scholar]
- Samuel W, Kutty RK, Nagineni S, Vijayasarathy C, Chandraratna RA, Wiggert B. N-(4- hydroxyphenyl)retinamide induces apoptosis in human retinal pigment epithelial cells: retinoic acid receptors regulate apoptosis, reactive oxygen species generation, and the expression of heme oxygenase-1 and Gadd153. J Cell Physiol. 2006;209:854–865. doi: 10.1002/jcp.20774. [DOI] [PubMed] [Google Scholar]
- Samuel W, Nagineni CN, Kutty RK, Parks WT, Gordon JS, Prouty SM, Hooks JJ, Wiggert B. Transforming growth factor-beta regulates stearoyl coenzyme A desaturase expression through a Smad signaling pathway. J Biol Chem. 2002;277:59–66. doi: 10.1074/jbc.M108730200. [DOI] [PubMed] [Google Scholar]
- Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- Singh US, Pan J, Kao YL, Joshi S, Young KL, Baker KM. Tissue transglutaminase mediates activation of RhoA and MAP kinase pathways during retinoic acid-induced neuronal differentiation of SH-SY5Y cells. J Biol Chem. 2003;278:391–399. doi: 10.1074/jbc.M206361200. [DOI] [PubMed] [Google Scholar]
- Tiwari M, Kumar A, Sinha RA, et al. Mechanism of 4-HPR-induced apoptosis in glioma cells: evidences suggesting role of mitochondrial-mediated pathway and endoplasmic reticulum stress. Carcinogenesis. 2006;27:2047–2058. doi: 10.1093/carcin/bgl051. [DOI] [PubMed] [Google Scholar]
- Waetzig V, Herdegen T. The concerted signaling of ERK1/2 and JNKs is essential for PC12 cell neuritogenesis and converges at the level of target proteins. Mol Cell Neurosci. 2003;24:238–249. doi: 10.1016/s1044-7431(03)00126-x. [DOI] [PubMed] [Google Scholar]
- Wang X, Fu S, Wang Y, Yu P, Hu J, Gu W, Xu XM, Lu P. Interleukin-1beta mediates proliferation and differentiation of multipotent neural precursor cells through the activation of SAPK/JNK pathway. Mol Cell Neurosci. 2007;36:343–354. doi: 10.1016/j.mcn.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- Yan RT, Ma WX, Wang SZ. neurogenin2 elicits the genesis of retinal neurons from cultures of nonneural cells. Proc Natl Acad Sci. 2001;98:15014–15019. doi: 10.1073/pnas.261455698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YM, Han PL, Lee JK. JNK pathway is required for retinoic acid-induced neurite outgrowth of human neuroblastoma, SH-SY5Y. Neuroreport. 2003;14:941–945. doi: 10.1097/01.wnr.0000074341.81633.b8. [DOI] [PubMed] [Google Scholar]
- Zentrich E, Han SY, Pessoa-Brandao L, Butterfield L, Heasley LE. Collaboration of JNKs and ERKs in nerve growth factor regulation of the neurofilament light chain promoter in PC12 cells. J Biol Chem. 2002;277:4110–4118. doi: 10.1074/jbc.M107824200. [DOI] [PubMed] [Google Scholar]
- Zhao S, Rizzolo LJ, Barnstable CJ. Differentiation and transdifferentiation of the retinal pigment epithelium. Int Rev Cytol. 1997;171:225–266. doi: 10.1016/s0074-7696(08)62589-9. [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Poo MM. Calcium signaling in neuronal motility. Annual review of cell and developmental biology. 2007;23:375–404. doi: 10.1146/annurev.cellbio.23.090506.123221. [DOI] [PubMed] [Google Scholar]


