Abstract
The endocytosis of AMPA receptors (AMPARs) underlies several forms of synaptic plasticity including NMDA receptor (NMDAR)-dependent long-term depression (LTD) but the molecular mechanisms responsible for this trafficking remain unknown. Here we demonstrate that PSD-95, a major postsynaptic density protein, plays a key role in NMDAR-triggered endocytosis of synaptic AMPARs because of its binding to AKAP150, a scaffold for specific protein kinases and phosphatases. Knockdown of PSD-95 with shRNA blocks NMDAR-triggered, but not constitutive nor mGluR-triggered endocytosis of AMPARs. Deletion of PSD-95’s SH3 and GK domains as well as a point mutation (L460P), both of which inhibit binding of PSD-95 to AKAP150, also block NMDAR-triggered AMPAR endocytosis. Furthermore, expression of a mutant AKAP150 that does not bind calcineurin inhibits this NMDAR-triggered trafficking event. These results suggest that PSD-95’s interaction with AKAP150 is critical for NMDAR-triggered AMPAR endocytosis and LTD, possibly because these scaffolds position calcineurin in the appropriate subsynaptic domain.
Activity-dependent changes in the strength of excitatory synapses are believed to be key cellular mechanisms that contribute to the plasticity of neuronal networks underlying many forms of experience-dependent plasticity including learning and memory1. Long-term potentiation (LTP) and long-term depression (LTD) triggered by activation of NMDA (N-methyl-D-asparatate) receptors (NMDARs) are extensively studied models for such synaptic modifications and compelling evidence suggests that they are due, at least in part, to activity-dependent regulated trafficking of AMPA (α -amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptors (AMPARs) to and away from synapses1–4. The detailed molecular mechanisms underlying such AMPAR trafficking, however, are incompletely understood.
AMPARs are heteromeric complexes composed of combinations of four subunits termed GluR1-GluR4 (also known as GluRA-D)5, 6. They are thought to be clustered in the postsynaptic density (PSD) of synapses via the binding of closely associated accessory proteins, termed TARPS, to members of the membrane-associated guanylate kinase (MAGUK) family of PDZ domain-containing scaffold proteins2–4, 7, 8. The most extensively studied MAGUK is PSD-95/SAP-90, changes in the levels of which influence synaptic AMPAR content. Specifically, overexpression of PSD-95 in cultured hippocampal neurons enhances surface expression of AMPARs9 and in hippocampal slice cultures causes a large increase in AMPAR-mediated excitatory postsynaptic currents (EPSCs)10–13. Conversely, shRNA-mediated knockdown of PSD-95 decreases AMPAR EPSCs11, 14–16. This strong correlation between synaptic PSD-95 levels and synaptic strength suggests that changes in PSD-95 level may be one important component of the mechanisms underlying NMDAR-dependent LTP and LTD. Consistent with this idea, overexpression of PSD-95 “occluded” LTP10, 17 and enhanced LTD17. Furthermore, biochemical modifications of PSD-95 leading to its loss from synapses, specifically ubiquitination18 and depalmitoylation19, have been reported to be critically involved in the agonist-induced endocytosis of AMPARs in cultured neurons, an extensively studied model for synaptically-induced LTD in slices20–22.
Recently, we examined the role of PSD-95 in LTD using expression of different mutant forms of PSD-95 combined with shRNA-mediated knockdown of PSD-95 and were able to molecularly dissociate the roles of PSD-95 in regulating basal synaptic strength and LTD23. Surprisingly, the mutant constructs that were used to demonstrate a role for ubiquitination and depalmitoylation in the endocytosis of AMPARs either were not targeted to synapses or had no effect on LTD. Instead, evidence was presented that the C-terminal Src homology 3 (SH3) and guanylate kinase-like (GK) domains of PSD-95 were required for LTD and in particular their binding to A-kinase-anchoring protein 79/150 (AKAP79/150). AKAP79/150 is a protein that in a range of cell types has been proposed to function as a scaffold for protein kinase A (PKA), protein kinase C (PKC) and the Ca2+/calmodulin-dependent protein phosphatase calcineurin (also known as PP2B) and thus position these enzymes adjacent to key protein substrates24–26. It is a particularly attractive candidate for playing a key role in LTD as PKA and calcineurin have been implicated in the regulation of AMPAR trafficking during this form of synaptic plasticity1,20,27,28.
A limitation of our previous work on the role of PSD-95 in LTD23 is that because electrophysiological assays were used, direct measurements of the effects of molecular manipulations of PSD-95 on NMDAR-triggered AMPAR endocytosis were not made. This is particularly important because the effects of two of the PSD-95 mutant constructs on LTD were not consistent with their effects on agonist-induced endocytosis of AMPARs18, 19. Here we have studied the role of PSD-95 and its interaction with AKAP150 in NMDAR-triggered AMPAR endocytosis using a molecular replacement strategy that allows simultaneous shRNA-mediated acute knockdown of endogenous PSD-95 and expression of mutant forms of recombinant PSD-95 in cultured hippocampal neurons. There are two significant advantages to this approach. First, developmental compensatory adaptations that may occur during synaptogenesis and synapse maturation due to the loss of PSD-95 are minimized. Second, the function of heterologous constructs can be studied without the necessity of a dominant effect as required by a standard overexpression approach.
We find that acute knockdown of PSD-95 dramatically reduces NMDAR-triggered endocytosis of synaptic AMPARs while not affecting constitutive AMPAR endocytosis nor the endocytosis of AMPARs triggered by metabotropic glutamate receptor (mGluR) activation. Disruption of the interaction between PSD-95 and AKAP150 strongly inhibits the NMDAR-dependent endocytosis of AMPARs as does expression of AKAP150 lacking its calcineurin binding domain. Furthermore, loss of PSD-95 from synapses is neither necessary nor sufficient for the endocytosis of synaptic AMPARs caused by NMDAR activation. These results are consistent with a model in which the binding of AKAP150 to PSD-95 is required to position calcineurin at the appropriate subsynaptic location so that it can be activated by Ca2+ influx through NMDARs and contribute to the endocytosis of AMPARs that underlies one major form of LTD.
RESULTS
NMDAR activation triggers endocytosis of synaptic AMPARs
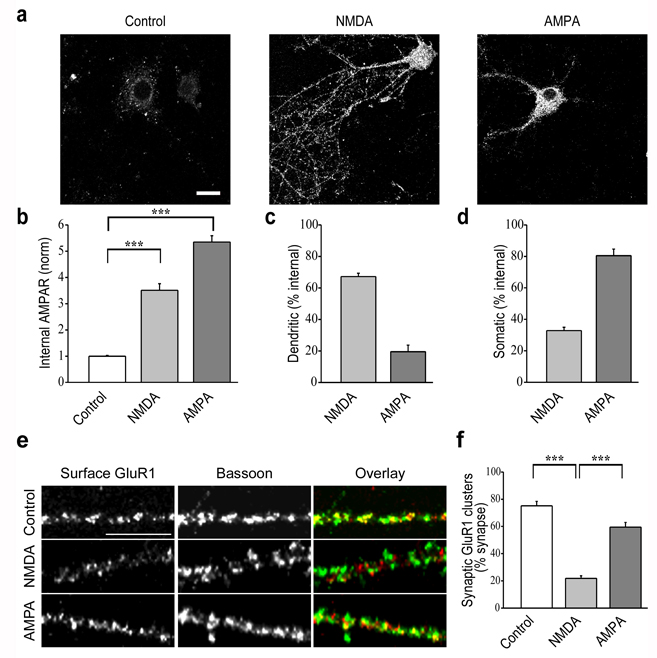
Rapid endocytosis of surface AMPARs can be triggered in cultured hippocampal neurons by application of glutamate receptor agonists including glutamate itself, NMDA, AMPA and group I mGluR agonists18–22, 29, 30. However, the spatial pattern and intracellular trafficking of the endocytosed AMPARs may differ depending on the specific agonist used20, 29, 31. Because the goal of this study was to elucidate the role of PSD-95 and its interaction with AKAP79/150 in the NMDAR-triggered endocytosis of synaptic AMPARs, it was important to first establish that under our experimental conditions NMDAR activation did in fact lead to the loss of synaptic AMPARs. Consistent with previous results using a method that allows visualization of internalized AMPARs20, brief application of NMDA (100 µM for 3 min) in the presence of both AMPAR and mGluR antagonists (20 µM DNQX and 100 µM LY341495, respectively) caused significant internalization of AMPARs throughout the cell (Fig. 1a, b; control: 1.0 ± 0.03, n = 46; NMDA: 3.5 ± 0.25, n = 25; internalized AMPAR immunoreactivity normalized to control condition) including distal and proximal portions of dendrites as well as in the soma. Treatment of cultures with AMPA (100 µM for 5 min) in the presence of the NMDA receptor antagonist, D-APV (50 µM) as well as the mGluR antagonist LY341495 (100 µM), also induced internalization of AMPARs (Fig. 1a, b; AMPA: 5.4 ± 0.24, n = 33). However, the spatial pattern of AMPAR endocytosis was markedly different than that induced by NMDA in that internalized AMPARs were observed primarily in the soma and proximal dendrites but not distal dendrites. To quantify the differences in the localization of internalized AMPARs, we measured total AMPAR immunofluorescence within a 10 µm circumference around the soma (somatic endocytosis) and compared this to the total AMPAR immunofluorescence in the more distal dendrites (outside this 10 µm circumference; dendritic endocytosis) (see Methods). Fifteen minutes after application of NMDA, 67 ± 2% of the internalized AMPAR staining was dendritic whereas 80 ± 4% of the internalized AMPARs were somatic following application of AMPA (Fig. 1c, d).
Figure 1.
NMDAR and AMPAR-triggered endocytosis of AMPARs show distinct patterns of internalization. (a) Cells show internalized AMPAR immunoreactivity following NMDA treatment (100 µM for 3 min) in both distal dendrites and soma, whereas AMPA treatment (100 µM for 5 min) caused endocytosis of AMPARs primarily in proximal dendrites and the soma. Scale bar, 20 µm. (b) Quantitation of total endocytosis of AMPARs in response to different treatments. (c, d) Quantititation of dendritic (>10 µm from soma) versus somatic (soma and <10 µm from soma) AMPAR endocytosis. (e) Examples of dendritic staining for surface GluR1 (red) and Bassoon (green) after NMDA and AMPA treatment. Scale bar, 10 µm. (f) Quantitation of percent synapses (defined by Bassoon staining) that express detectable surface GluR1 puncta in control cells and cells that received NMDA or AMPA treatment. *** indicates p < 0.001 in all panels.
The majority of surface AMPAR puncta in the dendrites of cultured neurons are synaptic as defined by co-localization with pre or postsynaptic markers. Thus it seemed likely that the internalized AMPARs in the dendrites were predominantly of synaptic origin. To test this prediction, we quantified the proportion of synapses containing detectable levels of surface AMPARs by staining for surface GluR1 clusters and counterstaining for Bassoon, a core component of the active zone commonly used to identify presynaptic terminals32. Application of NMDA substantially reduced the proportion of Bassoon puncta that co-localized with surface GluR1 puncta while AMPA application had a modest effect on this measure (Fig. 1e, f; control: 75.2 ± 3.2 %, n = 20; NMDA: 21.8 ± 1.9 %, n = 17; AMPA: 59.5 ± 3.4 %, n = 21; number of puncta per condition = 767–1002). These results confirm that brief application of NMDA caused the internalization of synaptic AMPARs and are consistent with previous work showing that NMDA application in this manner causes a decrease in miniature EPSCs20. Thus in all further experiments we used this manipulation to induce endocytosis of synaptic AMPARs.
PSD-95 knockdown inhibits endocytosis of synaptic AMPARs
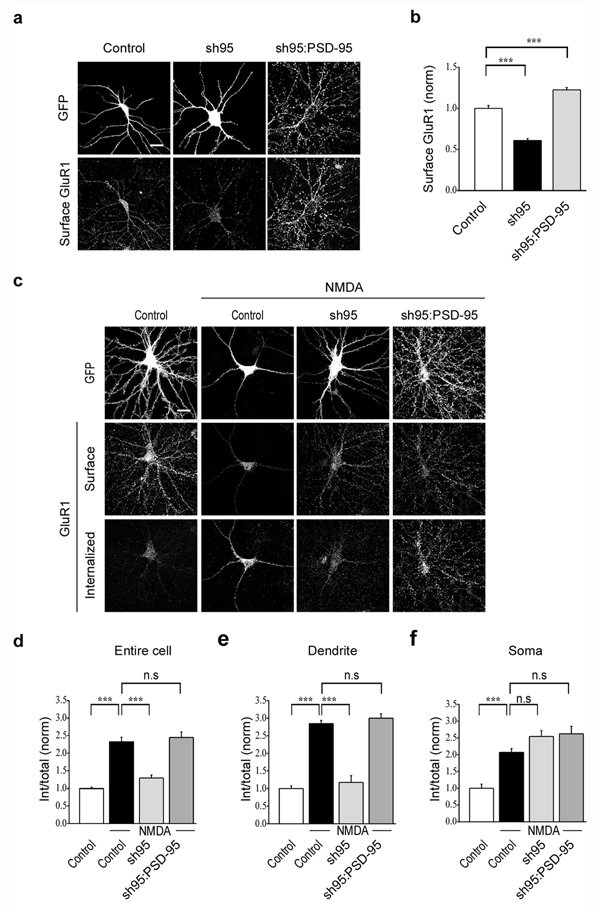
To examine the role of PSD-95 in NMDAR-triggered endocytosis of synaptic AMPARs we initially tested the consequences of acutely knocking down endogenous PSD-95 using a shRNA to PSD-95 (sh95) that was expressed in the cultured neurons using lentiviruses. This sh95 construct was highly effective as evidenced by the almost complete absence of PSD-95 immunofluorescence measured 5–6 days after infection (Supplementary Fig. 1a, b, control: 1.0 ± 0.04, n = 35; sh95: 0.1 ± 0.02, n = 29). Consistent with its effects on AMPAR EPSCs in slice cultures11 the shRNA-mediated knockdown of PSD-95 reduced the surface expression of endogenous AMPARs by ~40% while simultaneously expressing GFP-tagged wildtype PSD-95 enhanced surface AMPAR expression to slightly above control levels (Fig. 2a, b; control: 1.0 ± 0.04, n = 31; sh95: 0.6 ± 0.02, n = 23; sh95:PSD-95GFP: 1.2 ± 0.03, n = 29).
Figure 2.
Acute knockdown of PSD-95 decreases the surface expression and NMDAR-triggered endocytosis of AMPARs. (a) Representative cells showing that surface GluR1 staining is reduced by sh95 compared to control cells expressing GFP and this reduction is rescued by expression of wildtype PSD-95 (fused to GFP). (b) Quantitation of surface GluR1 levels following knockdown and replacement of endogenous PSD-95. (c) Representative examples of surface and internalized AMPARs following NMDA treatment in cells expressing GFP, sh95 or sh95 and wildtype PSD-95. (d–f) Quantitation of the amount of NMDAR-triggered AMPAR endocytosis normalized to original surface levels throughout the cell (d), in the dendrites (e) and in the soma (f). Scale bar, 20 µm. *** indicates p < 0.001 and n.s indicates p > 0.05.
These results confirm the efficacy of the sh95 and the molecular replacement strategy in cultured hippocampal neurons. However, because sh95 dramatically reduced the surface expression of AMPARs, to examine the specific role of PSD-95 in NMDAR-triggered AMPAR endocytosis it was necessary to use an assay that permitted measurement of the proportion of surface AMPARs that were internalized following NMDA application. Using a procedure that allowed staining of both internalized and remaining surface AMPARs with different secondary antibodies22 (see Methods; Supplementary Fig. 2) revealed that knockdown of PSD-95 with sh95 caused an almost complete inhibition of NMDA-triggered internalization of AMPARs (Fig. 2c, d; control + NMDA: 2.3 ± 0.13, n = 36; sh95 + NMDA: 1.3 ± 0.09, n = 21). This reduction in AMPAR endocytosis due to the knockdown of PSD-95 was rescued by expression of recombinant PSD-95 (Fig. 2c, d; sh95:PSD-95GFP + NMDA: 2.4 ± 0.16, n = 23) indicating that it was not due to some non-specific effects of the sh95. Importantly, the knockdown of PSD-95 inhibited the NMDA-triggered endocytosis of AMPARs only in the distal dendrites (10 µm away from the soma) (Fig. 2e; control + NMDA: 2.8 ± 0.09; sh95 + NMDA: 1.2 ± 0.19; sh95:PSD-95GFP + NMDA: 3.0 ± 0.12) but not in the soma (Fig. 2f; control + NMDA: 2.1 ± 0.11; sh95 + NMDA: 2.5 ± 0.17; sh95:PSD-95GFP + NMDA: 2.6 ± 0.22). These results suggest that PSD-95 plays a specific role in the NMDAR-triggered endocytosis of synaptic AMPARs but not somatic, presumably extrasynaptic AMPARs. In this and all subsequent experiments, to control for any possible consequences of lentivirus infections, the effects of sh95 and various PSD-95 and AKAP150 constructs on the surface expression and endocytosis of AMPARs are compared with cells infected with lentivirus containing the control FUGW vector that expresses GFP (labeled “Control” in all figures).
Several previous studies have reported that neither knockdown of PSD-95 with shRNA nor overexpression of PSD-95 affect the synaptic localization of NMDARs nor NMDAR-mediated synaptic currents9,11,12,15,23. Nevertheless, to test whether knockdown of PSD-95 might have affected NMDAR synaptic localization and thereby influenced NMDA-triggered endocytosis of AMPARs in these experiments, we quantified the percentage of Bassoon puncta that colocalized with NMDAR puncta (identified using an antibody against the requisite NMDAR subunit NR1). Acute sh95-mediated knockdown of PSD-95 had no detectable effect on the synaptic localization of NMDARs (Supplementary Fig. 3a, b; control: 74.5 ± 4.7 %, n = 15; sh95: 74 ± 1.2 %, n = 17; number of puncta per condition = 476–622). This lack of effect of sh95 on NMDAR synaptic localization is consistent with previous studies and suggests a specific role for PSD-95 in AMPA receptor surface expression and trafficking.
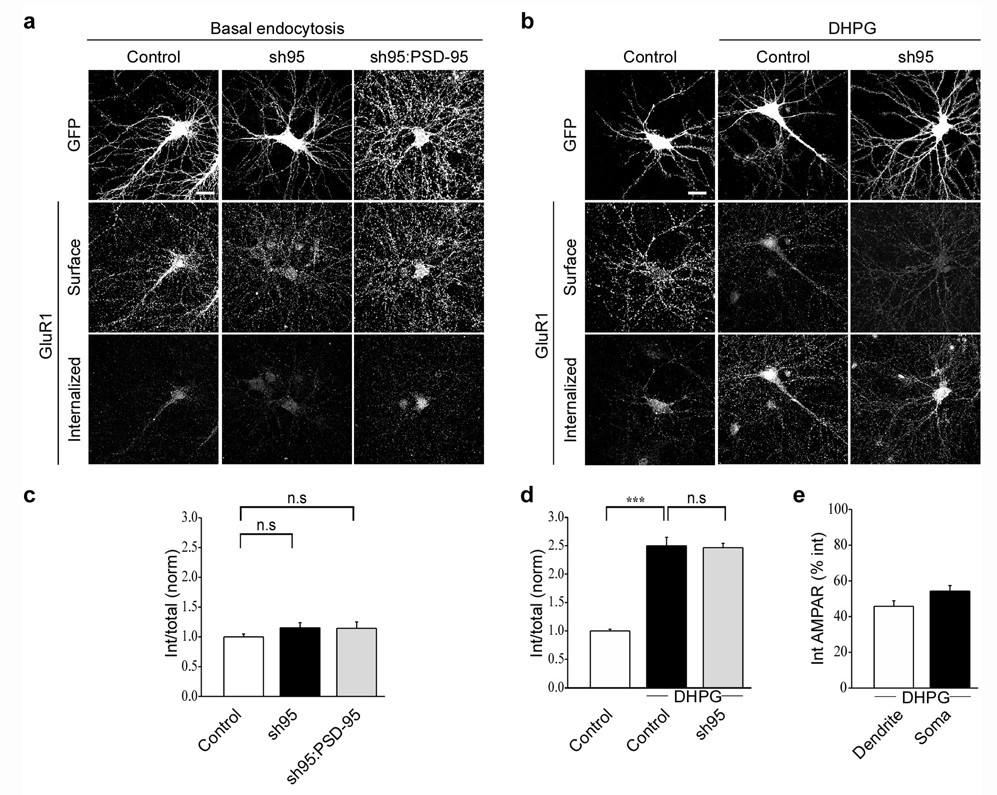
AMPAR endocytosis occurs constitutively and is greatly enhanced not only by activation of NMDARs and AMPARs but also by activation of group I metabotropic glutamate receptors (mGluRs) in a manner that presumably mimics mGluR-triggered LTD30. We therefore examined whether knockdown of PSD-95 influences these forms of AMPAR endocytosis. In contrast to its clear effects on NMDAR-triggered AMPAR endocytosis, expression of sh95 had no detectable effect on constitutive endocytosis of AMPARs nor did expressing recombinant PSD-95 with sh95 (Fig. 3a, c; sh95: 1.2 ± 0.09, n = 28; sh95:PSD-95GFP: 1.1 ± 0.11, n = 21). Similarly sh95-mediated knockdown of PSD-95 had no effect on the increase in AMPAR endocytosis elicited by application of the group I mGluR agonist DHPG (3,5-Dihydroxyphenylglycine) (50 µM) (Fig. 3b, d; control + DHPG: 2.5 ± 0.15, n = 33; sh95 + DHPG: 2.5 ± 0.08, n = 28). Like NMDA application, DHPG application caused endocytosis of AMPARs in both the soma and dendrites (15 min after DHPG application, 45.8 ± 3.1% of internalized AMPAR staining was dendritic, Fig. 3e). Thus PSD-95 appears to be critical specifically for the endocytosis of synaptic AMPARs triggered by activation of NMDARs but not other forms of AMPAR endocytosis.
Figure 3.
Constitutive endocytosis and mGluR-triggered endocytosis of AMPARs are not affected by knockdown of PSD-95. Representative images (a) and quantitation (c) of constitutive endocytosis of AMPARs in cells expressing GFP, sh95 or sh95 and wildtype PSD-95. Representative images (b) and quantitation (d) of AMPAR endocytosis triggered by DHPG application (50 µM for 5 min) in cells expressing GFP or sh95. (e) Bar graph showing that similar to NMDA application, DHPG application causes AMPAR endocytosis in both dendrites and soma. Scale bar, 20 µm. *** indicates p < 0.001 and n.s indicates p > 0.05.
PSD-95 mutations affect NMDAR-triggered AMPAR endocytosis
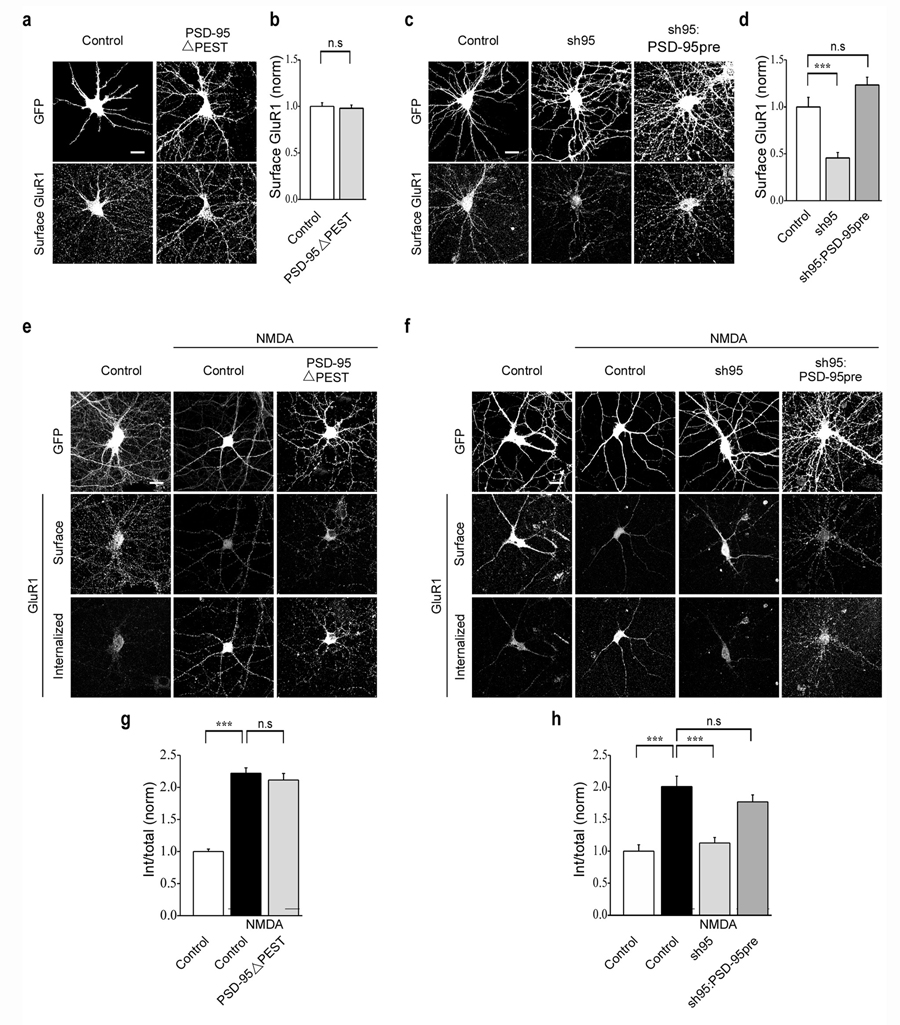
N-terminal domain modifications of PSD-95, specifically its polyubiquitination and depalmitoylation, have been suggested to be important for agonist-induced endocytosis of AMPARs in dissociated cultured neurons and by implication, LTD18, 19. These conclusions were supported by the observations that expression of a mutant form of PSD-95 lacking its PEST motif, which prevented its polyubiquitination (PSD-95ΔPEST), strongly inhibited agonist-induced AMPAR endocytosis18 as did a form of PSD-95 which was prenylated and therefore was resistant to the membrane dissociation caused by depalmitoylation19. However, in hippocampal slice cultures these same mutant forms of PSD-95 were either not targeted to synapses (PSD-95ΔPEST) or did not inhibit LTD (PSD-95 prenyl)23. In an attempt to reconcile these discrepant results, we examined the effects of these PSD-95 constructs on NMDAR-triggered AMPAR endocytosis.
Consistent with our observations in hippocampal slice culture, PSD-95ΔPEST (fused to GFP) did not display the highly punctate dendritic expression typical of wildtype PSD-95 and also did not affect the surface expression of AMPARs (Fig. 4a, b; PSD-95ΔPESTGFP: 0.98 ± 0.03, n = 43). Furthermore, it had no detectable effect on NMDAR-triggered endocytosis of AMPARs (Fig. 4e, g; control + NMDA: 2.2 ± 0.09, n = 47; PSD-95ΔPESTGFP + NMDA: 2.1 ± 0.10, n = 35). We next examined the effect of replacing endogenous PSD-95 with a form of PSD-95 containing a prenylation motif fused to GFP19. Again in agreement with results from experiments using hippocampal slice culture23, this construct rescued the decrease in surface expression of AMPARs due to the knockdown of PSD-95 (Fig. 4c, d; sh95: 0.5 ± 0.06, n = 41; sh95:PSD-95preGFP: 1.2 ± 0.08, n = 22). However, it also rescued the NMDAR-triggered endocytosis of AMPARs (Fig. 4f, h; control + NMDA: 2.0 ± 0.16, n = 18; sh95 + NMDA: 1.1 ± 0.09, n = 22; sh95:PSD-95preGFP + NMDA: 1.8 ± 0.11, n = 27) suggesting that detachment of PSD-95 from the plasma membrane is not required for this form of AMPAR endocytosis.
Figure 4.
PEST motif deletion and prenylation of PSD-95 does not affect NMDAR-triggered endocytosis of AMPARs. Representative images (a) and quantitation (b) of surface AMPAR (GluR1) staining in cells expressing GFP or PSD-95ΔPEST. Representative images (e) and quantitation (g) of NMDAR-triggered endocytosis of AMPARs in cells expressing GFP or PSD-95ΔPEST. Representative images (c) and quantitation (d) of surface AMPAR (GluR1) staining in cells expressing GFP, sh95 or sh95 and PSD-95pre. Note that the prenylated PSD-95 (PSD-95pre) rescues the decrease in AMPAR surface expression caused by sh95. Representative images (f) and quantitation (h) of NMDAR-triggered endocytosis of surface AMPARs in cells expressing GFP, sh95 or sh95 and PSD-95pre. Scale bar, 20 µm. *** indicates p < 0.001 and n.s indicates p > 0.05.
Role of AKAP in NMDAR-triggered AMPAR endocytosis
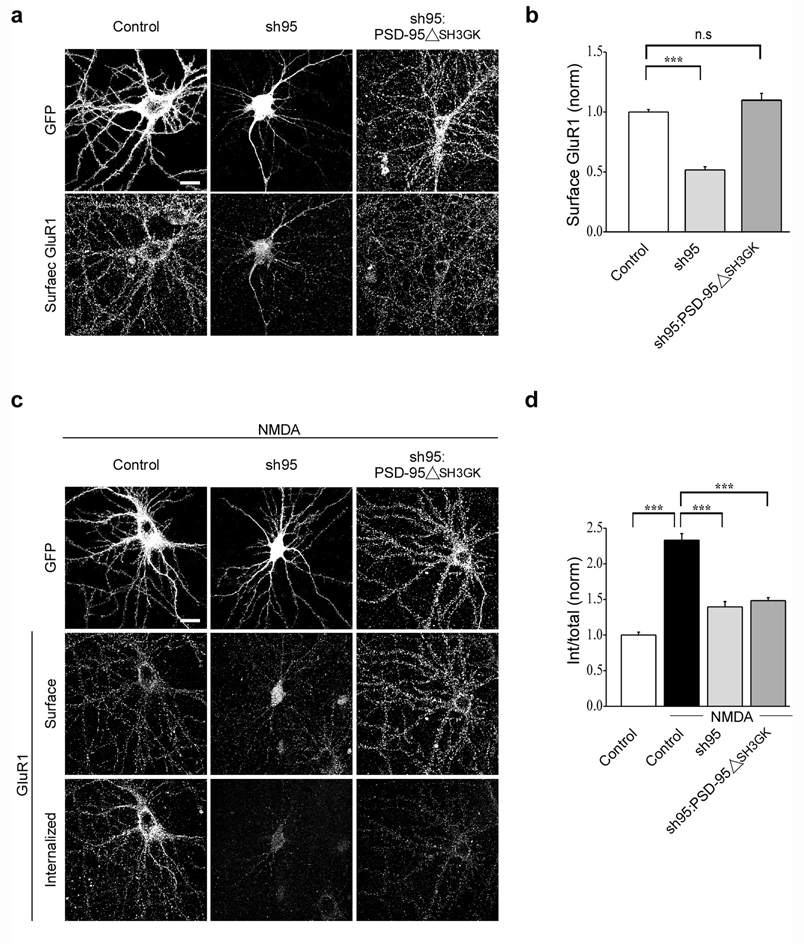
PSD-95 is a modular protein containing three consecutive PDZ domains, an SH3 domain, and an enzymatically inactive guanylate kinase (GK) domain, each of which is thought to interact with specific subsets of cytoplasmic proteins7. One of these, AKAP79/150 binds to the SH3 and/or GK domains and was of particular interest because it binds calcineurin, a protein phosphatase that is required for both NMDAR-dependent LTD and NMDAR-triggered endocytosis of AMPARs20, 27. In addition, AKAP79/150 binds to PKA, an interaction that influences AMPAR trafficking28, 33. To examine whether binding of AKAP150 to PSD-95 is important specifically for NMDAR-triggered endocytosis of AMPARs, we first replaced endogenous PSD-95 with a form of PSD-95 lacking its SH3 and GK domains (PSD-95ΔSH3GK). Despite deletion of its SH3 and GK domains PSD-95ΔSH3GK still rescued the surface expression of AMPARs caused by knockdown of endogenous PSD-95 (Fig. 5a, b; sh95: 0.5 ± 0.03, n = 31; sh95:PSD-95ΔSH3GKGFP: 1.1 ± 0.06, n = 21). However, unlike wildtype PSD-95, it did not rescue the decrease in NMDAR-triggered AMPAR endocytosis (Fig. 5c, d; control + NMDA: 2.3 ± 0.09, n = 24; sh95 + NMDA: 1.4 ± 0.07, n = 29; sh95:PSD-95ΔSH3GKGFP + NMDA: 1.5 ± 0.04, n = 20).
Figure 5.
SH3 and GK domains of PSD-95 are required for NMDAR-triggered endocytosis of AMPARs. Representative images (a) and quantitation (b) of surface AMPARs in cells expressing GFP, sh95 or sh95 and PSD-95ΔSH3GK. Note that PSD-95ΔSH3GK rescues the surface expression of AMPARs. Representative images (c) and quantitation (d) of NMDAR-triggered endocytosis of surface AMPARs in cells expressing GFP, sh95 or sh95 and PSD-95ΔSH3GK. Scale bar, 20 µm. *** indicates p < 0.001 and n.s indicates p > 0.05.
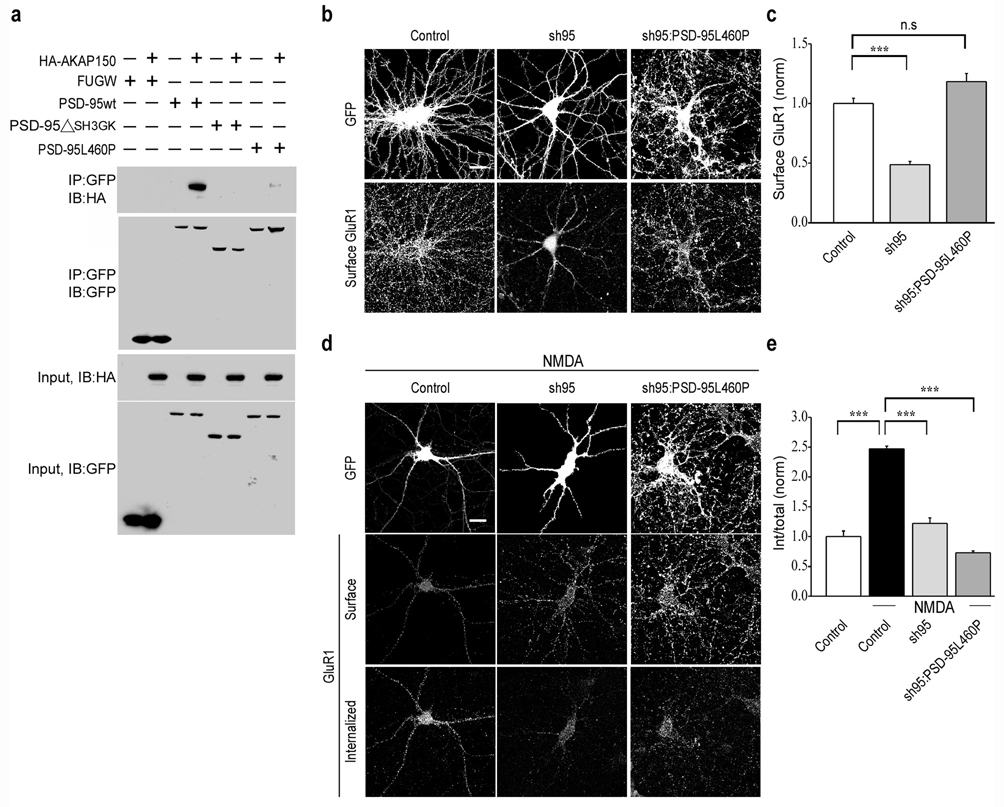
The SH3/GK domains of PSD-95 interact with several proteins in addition to AKAP79/1507. To test the effects of a form of PSD-95 that more specifically impaired its binding to AKAP79/150 we made a point mutation in the SH3 domain (L460P), which has been reported to impair the binding of AKAP79/150 to the isolated SH3 domain33, 34. When placed in full length PSD-95, co-immunoprecipitation experiments in HEK293 cells using HA tagged mouse AKAP150 revealed that this point mutation (PSD-95L460P) did in fact greatly impair the binding of PSD-95 to AKAP150 when compared to wildtype PSD-95 (Fig. 6a). When we replaced endogenous PSD-95 with PSD-95L460P, it rescued the surface expression of AMPARs caused by the acute knockdown of PSD-95 (Fig. 6b, c; sh95: 0.5 ± 0.03, n = 19; sh95:PSD-95L460PGFP: 1.2 ± 0.07, n = 28). However, PSD-95L460P did not rescue the reduction in NMDAR-triggered endocytosis of AMPARs caused by expression of sh95 (Fig. 6d, e; control + NMDA: 2.5 ± 0.05, n = 24; sh95 + NMDA: 1.2 ± 0.09, n = 22; sh95:PSD-95L460PGFP + NMDA: 0.7 ± 0.03, n = 28).
Figure 6.
L460P mutation in PSD-95 disrupts binding to AKAP150 and blocks NMDAR-triggered endocytosis of AMPARs. (a) Co-immunoprecipitation assays demonstrate that both deletion of SH3, GK domains and the L460P point mutation in full length PSD-95 disrupts binding of AKAP150 to PSD-95. Representative images (b) and quantitation (c) of surface AMPARs in cells expressing GFP, sh95 or sh95 and PSD-95L460P. Note that PSD-95L460P rescues the decrease in surface expression of AMPARs caused by sh95. Representative images (d) and quantitation (e) of NMDAR-triggered endocytosis of surface AMPARs in cells expressing GFP, sh95 or sh95 and PSD-95L460P. Scale bar, 20 µm. *** indicates p < 0.001 and n.s indicates p > 0.05.
PP2B binding to AKAP is required for AMPAR endocytosis
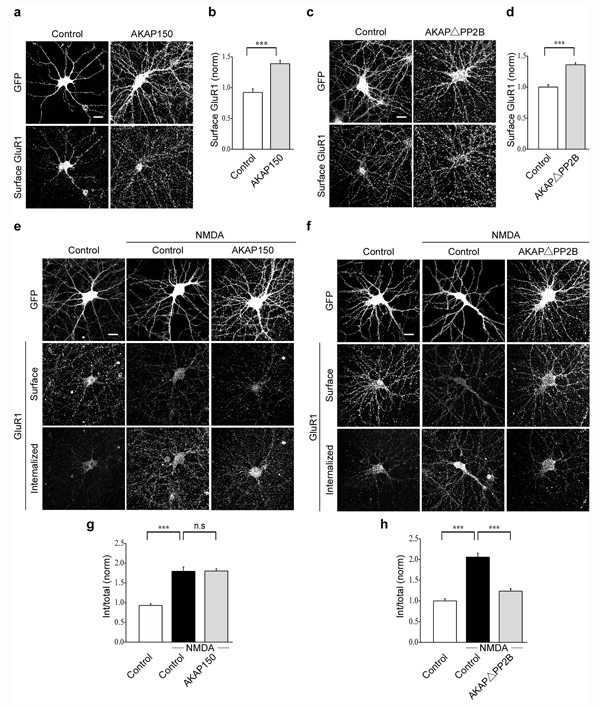
The results thus far suggest that AKAP150 interaction with PSD-95 is necessary for the endocytosis of AMPARs triggered by NMDAR activation. In addition to interacting with calcineurin (PP2B), AKAP150 binds to protein kinase C and protein kinase (PKA)24–26, with the latter interaction being implicated in the delivery of AMPARs to synapses28. To determine whether AKAP150 binding specifically to calcineurin is required for NMDAR-triggered AMPAR endocytosis, we examined the effects of expressing a form of mouse AKAP150 in which the sequence reported to be essential for binding calcineurin (aa 613–655) was deleted35, 36. Before testing this AKAP150 deletion construct (AKAPΔPP2B), as a control we first overexpressed full length, wild-type AKAP150. This modestly increased the surface expression of AMPARs (Fig. 7a, b; AKAP150GFP: 1.4 ± 0.06, n = 45) but had no effect on NMDAR-triggered endocytosis of AMPARs (Fig. 7e, g; control + NMDA: 1.8 ± 0.11, n = 36; AKAP150GFP + NMDA: 1.8 ± 0.06, n = 33). In contrast, while still increasing surface AMPAR expression like wildtype AKAP150 (Fig. 7c, d; AKAPΔPP2B: 1.4 ± 0.03, n = 28), expression of AKAPΔPP2B almost completely blocked the AMPAR endocytosis triggered by NMDAR activation (Fig. 7f, h; control + NMDA: 2.1 ± 0.10, n = 29; AKAPΔPP2B + NMDA: 1.2 ± 0.06, n = 21).
Figure 7.
Binding of calcineurin (PP2B) to AKAP150 is necessary for the NMDAR-triggered endocytosis of AMPARs. Overexpression of AKAP150 increases the surface expression of AMPARs as shown in the representative images (a) and quantitation (b) of surface AMPARs in cells expressing GFP or AKAP150. Representative images (e) and quantitation (g) of NMDAR-triggered endocytosis of surface AMPARs in cells expressing GFP or AKAP150. Representative images (c) and quantitation (d) of surface AMPARs in cells expressing GFP or AKAPΔPP2B. Representative images (f) and quantitation (h) of NMDAR-triggered endocytosis of surface AMPARs in cells expressing GFP or AKAPΔPP2B. scale bar, 20 µm. *** indicates p < 0.001 and n.s indicates p > 0.05.
Loss of synaptic PSD-95 and AKAP during NMDAR activation
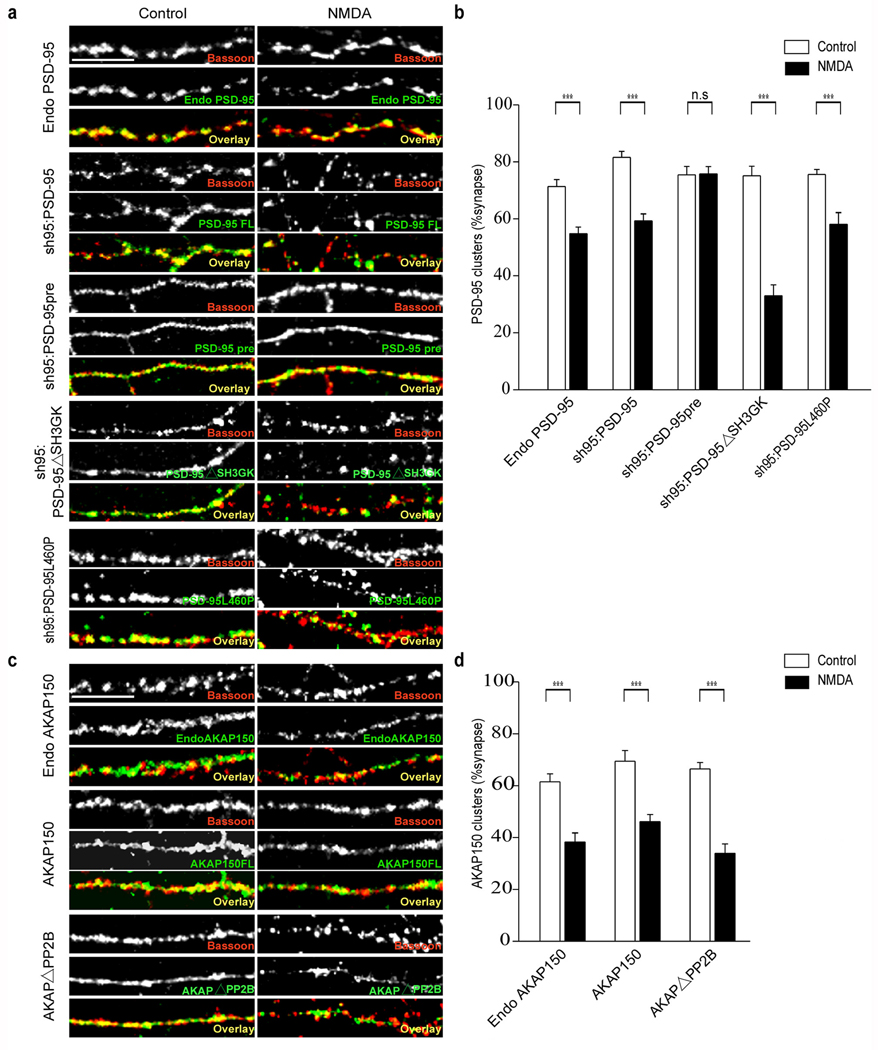
Application of NMDA has been reported to cause trafficking of both PSD-95 and AKAP79/150 away from synapses18, 37. To determine whether the mutant forms of PSD-95 and AKAP150 that impaired NMDA-induced AMPAR endocytosis also impaired the trafficking of these proteins away from synapses, we examined the synaptic localization of these constructs following NMDA application by counterstaining with an antibody to the presynaptic marker protein, Bassoon. Application of NMDA caused a moderate decrease in the proportion of synapses that contain detectable levels of endogenous PSD-95 as well as a similar decrease in the synaptic localization of full length recombinant PSD-95 when expressed with sh95 (Fig. 8a, b; endogenous PSD-95: control, 71.3 ± 2.4 %, n = 18; NMDA, 54.8 ± 2.3 %, n = 21; sh95:PSD-95: control, 81.6 ± 2.1 %, n = 23; NMDA, 59.3 ± 2.5 %, n = 21). Similar to the behavior of wild type PSD-95, NMDA application caused a loss from synapses of sh95:PSD-95ΔSH3GK and sh95:PSD-95L460P, the PSD-95 constructs that impaired or blocked NMDAR-triggered endocytosis of AMPARs (Fig. 8a, b; PSD-95ΔSH3GK: control, 75.1 ± 3.4 %, n = 19; NMDA, 33 ± 3.9 %, n = 14; PSD-95L460P: control, 75.6 ± 1.8 %, n = 20; NMDA, 58 ± 4.2 %, n = 16). In marked contrast, the synaptic localization of the prenylated PSD-95 (sh95:PSD-95pre) was unaffected by NMDA application (Fig. 8a, b; PSD-95pre: control, 75.4 ± 3 %, n = 22; NMDA, 75.7 ± 2.6 %, n = 21). Together these results demonstrate that the loss of PSD-95 from synapses caused by NMDAR activation can be completely dissociated from the NMDAR-triggered endocytosis of synaptic AMPARs. The behavior of PSD-95ΔSH3GK and PSD-95L460P demonstrate that the NMDA-induced loss of PSD-95 from synapses is not sufficient for NMDAR-triggered AMPAR endocytosis while the behavior of PSD-95pre demonstrates that PSD-95 movement away from synapses is not necessary for synaptic AMPAR endocytosis.
Figure 8.
NMDA application causes loss of PSD-95 and AKAP150 from synapses. Application of NMDA causes a decrease in the synaptic localization of endogenous PSD-95, recombinant wildtype PSD-95, PSD-95ΔSH3GK and PSD-95L460P but not prenylated PSD-95 as shown in the representative images (a) and quantitation (b) of PSD-95 puncta that co-localized with presynaptic Bassoon puncta. NMDA application also causes a loss of endogenous AKAP150, recombinant wildtype AKAP150 and AKAPΔPP2B from synapses as shown in the sample images (c) and quantitation (d). Scale bar, 10 µm. *** indicates p < 0.001 and n.s indicates p > 0.05.
The behavior of AKAP150 was similar to PSD-95 in that NMDA application lead to loss of both endogenous as well as full length AKAP150 from synapses (Fig. 8c, d; endogenous AKAP150: control, 61.5 ± 3 %, n = 17; NMDA, 38.3 ± 3.6 %, n = 18; AKAP150: control, 69.4 ± 4.1 %, n = 18; NMDA, 46.1 ± 2.8 %, n = 16). However, the AKAP mutant that greatly impaired NMDAR-triggered AMPAR endocytosis (AKAPΔPP2B) behaved just like wild type AKAP150 indicating that, like PSD-95, movement of AKAP150 away from synapses is not sufficient for eliciting endocytosis of synaptic AMPARs (Fig. 8c, d; AKAPΔPP2B: control, 66.4 ± 2.5 %, n = 19; NMDA, 33.9 ± 3.7 %, n = 23).
Discussion
The detailed molecular mechanisms underlying the trafficking of AMPARs into and out of synapses are of great interest because of their importance in prominent forms of synaptic and experience-dependent plasticity1–4. One key molecular player is PSD-95, a member of the MAGUK family of synaptic scaffolding proteins2–4,7,8. Molecular manipulations have demonstrated that changes in the levels of PSD-95 correlate with synaptic AMPAR content9–17 and it has been suggested that biochemical modifications of PSD-95 leading to its loss from synapses contributes to agonist-induced endocytosis in cultured neurons18,19. However, using electrophysiological assays in hippocampal slice cultures, we found that the mutant forms of PSD-95 that were reported to block AMPAR endocytosis did not influence synaptic strength and LTD in the expected manner. Instead, based on the effects of a series of mutant PSD-95 constructs, we proposed that a key role for PSD-95 is as a scaffold for one or more of the signaling proteins that are required for the triggering of NMDAR-dependent LTD23.
To address whether this hypothesis applies to the endocytosis of AMPARs thought to mediate LTD1–4 it was necessary to directly measure the effects of mutant forms of PSD-95 on agonist-induced endocytosis of endogenous AMPARs, a process that is routinely assayed in cultured neurons. Primarily using a strategy in which mutant forms of PSD-95 replace endogenous PSD-95 that has been knocked down by shRNA we have presented evidence that PSD-95 is important for the NMDAR-triggered endocytosis of synaptic AMPARs at least in part because of its binding to AKAP150. This is an attractive hypothesis because AKAP79/150 binds to calcineurin24–26, a phosphatase that is required for both NMDAR-dependent LTD and NMDAR-triggered AMPAR endocytosis20,27. We propose that the binding of AKAP150 to PSD-95 positions calcineurin at the mouth of the NMDAR channel so that it can be readily activated by the modest rises in Ca2+ thought to be responsible for triggering NMDAR-dependent LTD38.
The NMDAR-triggered AMPAR endocytosis was not affected by expression of two mutant forms of PSD-95, PSD-95ΔPEST and PSD-95 prenyl, which have previously been reported to inhibit agonist-induced AMPAR endocytosis18,19. One explanation for this discrepancy is that the treatment protocols used in the previous experiments primarily caused endocytosis of extrasynaptic AMPARs whereas the protocol we used was shown to elicit loss of synaptic AMPARs in the plasma membrane. It is also possible that the expression levels of the constructs or culture conditions account for the differences. Confidence in our results is greatly increased by the fact that our observations of the effects of these two constructs in dissociated hippocampal neuron cultures are exactly what would be predicted from the results obtained in hippocampal slice cultures23. There is one discrepancy, however, with our previous results in that in the dissociated cultures PSD-95ΔSH3GK caused a clear rescue of the surface expression of AMPARs caused by the PSD-95 knockdown whereas in the hippocampal slice cultures it did not rescue basal AMPAR-mediated synaptic transmission23. This discrepancy may be due to differences in the trafficking of AMPARs to the surface in the two preparations or because of differences in the levels of expression of other MAGUK’s, such as the developmentally regulated SAP-10215, which may have helped target PSD-95 to the plasma membrane in cultured neurons but not slices.
We have presented several lines of evidence that specifically implicate the interactions of PSD-95 with AKAP150 as being critical for NMDAR-triggered endocytosis of synaptic AMPARs. Wildtype PSD-95 completely rescued the consequences of knocking down endogenous PSD-95 on both surface expression of AMPARs and NMDAR-triggered AMPAR endocytosis. In contrast, both PSD-95ΔSH3GK and PSD-95L460P rescued AMPAR surface expression while having no effect on the impairment in AMPAR endocytosis induced by sh95 expression. Although the deletion of the SH3 and GK domains would be expected to impair PSD-95’s interactions with proteins other than AKAP150 such as GKAP/SAPAP and SPAR, the L460P mutation is thought to be highly specific33,34 and we demonstrate that when placed in full length PSD-95, it strongly impaired PSD-95’s ability to interact with AKAP150. Furthermore, expression of a mutant form of AKAP150 lacking its calcineurin binding site35, 36 almost completely blocked NMDAR-triggered AMPAR endocytosis. The scaffolding of calcineurin by AKAP79/150 also appears to regulate L-type Ca2+ channel activity in hippocampal neurons due to the direct binding of AKAP79/150 to Cav1.239. This AKAP-dependent coupling of calcium entry via L-type Ca2+ channels to calcineurin is consistent with previous observations that activation of these channels can induce a form of LTD, which occludes NMDAR-dependent LTD38, and that the endocytosis of AMPARs triggered by Ca2+ channel activation is blocked by calcineurin inhibitors20. It will be important to investigate whether these findings from dissociated hippocampal cultures hold true when LTD is studied in more intact preparations such as hippocampal slices.
The simplest model to explain our results is that the interaction between PSD-95 and AKAP150 is important for NMDAR-triggered AMPAR endoctyosis and LTD because it statically positions calcineurin in the appropriate subsynaptic domain. Several observations, however, suggest that these protein interactions may be much more dynamic and regulated by activity. Calcineurin activity is reduced when it is bound to AKAP79/15040,41 and PSD-95 and calcineurin may compete for binding to AKAP79/15033. Furthermore, the synaptic localization of AKAP79/150 is influenced by NMDAR-dependent activity in a calcineurin-dependent manner25, 37, 42. Thus, it is possible that in the basal state AKAP79/150 is bound to calcineurin, which is largely inactive. Upon appropriate NMDAR activation, AKAP79/150 binds to PSD-95 causing the release of calcineurin, which is activated and can then play its role in AMPAR endocytosis. Subsequently, AKAP79/150 may leave the spine, a step that could contribute to LTD by removing a pool of PKA required for the maintenance of synaptic AMPARs25, 28, 43.
AMPARs also interact with stargazin and related transmembrane AMPAR regulatory proteins (TARPs), which play a critical role in the synaptic clustering of AMPARs via their interactions with MAGUKs such as PSD-958. Furthermore, phosphorylation of stargazin can be dynamically regulated by NMDAR receptor activity in a manner that influences LTP and LTD44 and AMPARs can dissociate from TARPS upon binding glutamate45. Thus the interactions between PSD-95 and AMPARs via TARPS may be another important site of modulation for regulating AMPAR surface expression and NMDAR-triggered endocytosis of AMPARs.
Because loss of PSD-95 and AKAP79/150 from synapses has been suggested to be important for endocytosis of synaptic AMPARs, we examined whether such trafficking was directly correlated with NMDAR-triggered AMPAR endocytosis. Mutant constructs of PSD-95 and AKAP150 that clearly impaired the endocytosis of synaptic AMPARs were lost from synapses upon NMDA application to the same degree as wildtype PSD-95 and AKAP150 indicating that such movements of PSD-95 and AKAP150 are not sufficient to elicit endocytosis of synaptic AMPARs. Conversely, the synaptic localization of the prenylated form of PSD-95, which permitted normal endocytosis of AMPARs upon NMDAR activation, was not affected by NMDA application. This indicates that loss of PSD-95 from synapses is not necessary for NMDAR-triggered endocytosis of synaptic AMPARs and provides further evidence that key roles for PSD-95 and AKAP150 are to function as scaffolds for signaling proteins.
Our results also suggest that the role of PSD-95 in endocytosis of synaptic AMPARs is highly specific. Knockdown of PSD-95 dramatically reduced NMDAR-triggered AMPAR endocytosis in dendrites independent of its effects on surface expression yet did not influence constitutive AMPAR endocytosis, NMDAR-triggered AMPAR endocytosis in the soma, nor mGluR-triggered AMPAR endocytosis. That PSD-95 specifically plays a critical role in NMDAR-dependent processes at synapses is further supported by the observations that mGluR-triggered LTD and AMPAR endocytosis involve signaling cascades distinct from those underlying NMDAR-triggered LTD30, 46–48 in a manner that does not require PSD-95 but instead requires different scaffolding proteins such as Homer49.
METHODS
Hippocampal cultures and lentivirus infection
Primary hippocampal neuron cultures were prepared from P0 Sprague-Dawley rat pups as described previously with minor changes20. Briefly, hippocampi were obtained from P0 rat pups and the dentate gyri were removed. Tissue was dissociated by papain treatment and cells were plated on poly D-lysine-coated cover slips at a density of approximately 75,000 cells per 12 mm well. Cultures were maintained in minimum essential media (MEM) (Invirogen) with 0.5 mM glutamine and N2-supplement. Glial growth was inhibited by adding FUDR after one week in culture. Neurons were infected with lentiviruses on DIV 9–10 and used in experiments on DIV 15–17. An shRNA to PSD-95 was packaged into lentiviruses, which were produced as previously described11. For molecular replacement studies the eGFP in the lentiviral vector containing the shRNA was replaced by the c-terminally eGFP tagged protein of interest (i.e. PSD-95, PSD-95prenyl, PSD-95ΔSH3GK and PSD-95L460P). The overexpression constructs were cloned into FUGW vector and tagged at the c-terminus with eGFP. Detailed strategies for producing these constructs have been described previously23. Mouse AKAP150 cDNA was cloned from total hippocampal RNA. (See Supplementary Information for further details on these procedures.)
Immunocytochemistry and image analysis
Details of the procedures used to assay surface expression and endocytosis of AMPARs can be found in Supplementary Information. All experiments involved labeling of surface AMPARs in live neurons by incubation with a rabbit polycloncal antibody (Calbiochem) directed against the N-terminus of the GluR1 subunit and then inducing endocytosis with various pharmacological manipulations. Synaptic localization of AMPAR, NMDAR, PSD-95 and AKAP150 puncta were quantified by their colocalization with presynaptic Bassoon puncta (see Supplementary Information for further details). For image acquisition, coverslips were mounted in Fluormount (Electron Microscopy Sciences) and cells were imaged with a 63X oil-immersion objective mounted on a Zeiss LSM 510 laser scanning confocal microscope. Images for all conditions in a particular experiment were obtained using identical acquisition parameters and were analyzed with Metamorph software using identical parameters (Meta imaging series 5.0 and 6.1, Universal imaging). All analyses were done blind without knowledge of the experimental manipulation that had been performed using raw images. Untreated and treated cells from the same culture preparation were always compared with one another for each experimental manipulation (see Supplementary Information for further details). Synaptic GluR1, NR1, endogenous PSD-95, endogenous AKAP150, recombinant PSD-95 and recombinant AKAP150 puncta were defined by visual co-localization with presynaptic Bassoon puncta along 50 µm portions of dendrite (see Supplementary Information for further details).
Statistical analysis
The n value given for each experiment refers to the number of cells analyzed. Data are presented as mean ± SEM. Group results were compared by using Student’s t test. P > 0.05 was considered not significant (n.s).
Immunoprecipitation and immunoblotting
HEK293 cells were transfected at 70–80% confluency with expression constructs. Cells were harvested and lysed 24 hr after transfection in RIPA buffer (1% Triton X-100, 0.1% SDS, 0.5% deoxycholic acid, 50 mM NaPO4, 150 mM NaCl and protease inhibitor cocktail [Roche], [pH 7.4]) for 1 hr at 4°C. Immunoprecipitation was done by overnight incubation of the lysates with anti GFP antibody at 4°C (Molecular probes, Invitrogen), and subsequent additional incubation for 1 hr at room temperature with protein A Agarose beads (Roche). The immunoprecipitates were washed and solubilized in SDS-PAGE sample buffer. Samples were separated on 4%–12% gradient Bis-Tris gels (Invitrogen), transferred onto nitrocellulose. Immunoblotting was performed using horseradish peroxidase-conjugated secondary antibodies and the ECL Western blotting detection system (Amersham Biosciences). For immunoblotting, anti GFP antibody (Chemicon) and anti HA antibody (Roche) were used.
Supplementary Material
ACKNOWLEDGEMENT
We thank Arundhati Ghosh and Xiaoyong Cai for expert technical assistance and members of the Malenka lab for helpful discussions. This work was supported by N.I.H. grant MH63394.
Footnotes
COMPETING INTEREST STATEMENT
The authors declare no competing financial interests.
References
- 1.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 3.Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat. Rev. Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell Develop. Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 5.Hollmann M, Heinemann S. Cloned glutamate receptors. Annu. Rev. Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- 6.Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science. 1998;280:1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- 7.Kim E, Sheng M. PDZ domain proteins of synapses. Nat. Rev. Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 8.Nicoll RA, Tomita S, Bredt DS. Auxiliary subunits assist AMPA-type glutamate receptors. Science. 2006;311:1253–1256. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- 9.El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- 10.Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J. Neurosci. 2004;24:916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schluter OM, Xu W, Malenka RC. Alternative N-terminal domains of PSD-95 and SAP97 govern activity-dependent regulation of synaptic AMPA receptor function. Neuron. 2006;51:99–111. doi: 10.1016/j.neuron.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Schnell E, et al. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagawa T, et al. Quaternary structure, protein dynamics, and synaptic function of SAP97 controlled by L27 domain interactions. Neuron. 2004;44:453–467. doi: 10.1016/j.neuron.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Ehrlich I, Klein M, Rumpel S, Malinow R. PSD-95 is required for activity-driven synapse stabilization. Proc. Natl. Acad. Sci. U. S. A. 2007;104:4176–4181. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elias GM, et al. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Futai K, et al. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat. Neurosci. 2007;10:186–195. doi: 10.1038/nn1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein V, House DR, Bredt DS, Nicoll RA. Postsynaptic density-95 mimics and occludes hippocampal long-term potentiation and enhances long-term depression. J. Neurosci. 2003;23:5503–5506. doi: 10.1523/JNEUROSCI.23-13-05503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colledge M, et al. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron. 2003;40:595–607. doi: 10.1016/s0896-6273(03)00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Husseini AD, et al. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 2002;108:849–863. doi: 10.1016/s0092-8674(02)00683-9. [DOI] [PubMed] [Google Scholar]
- 20.Beattie EC, et al. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat. Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- 21.Carroll RC, et al. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc. Natl. Acad. Sci. U. S. A. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin JW, et al. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat. Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- 23.Xu W, et al. Molecular Dissociation of the Role of PSD-95 in Regulating Synaptic Strength and LTD. Neuron. 2008;57:248–262. doi: 10.1016/j.neuron.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beene DL, Scott JD. A-kinase anchoring proteins take shape. Curr. Opin. Cell Biol. 2007;19:192–198. doi: 10.1016/j.ceb.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dell'Acqua ML, et al. Regulation of neuronal PKA signaling through AKAP targeting dynamics. Eur. J. Cell Biol. 2006;85:627–633. doi: 10.1016/j.ejcb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Klauck TM, et al. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- 27.Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- 28.Snyder EM, et al. Role for A kinase-anchoring proteins (AKAPS) in glutamate receptor trafficking and long term synaptic depression. J. Biol. Chem. 2005;280:16962–16968. doi: 10.1074/jbc.M409693200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 30.Snyder EM, et al. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat. Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- 31.Lee SH, Simonetta A, Sheng M. Subunit rules governing the sorting of internalized AMPA receptors in hippocampal neurons. Neuron. 2004;43:221–236. doi: 10.1016/j.neuron.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Regalado MP, Terry-Lorenzo RT, Waites CL, Garner CC, Malenka RC. Transsynaptic signaling by postsynaptic synapse-associated protein 97. J. Neurosci. 2006;26:2343–2357. doi: 10.1523/JNEUROSCI.5247-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colledge M, et al. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron. 2000;27:107–119. doi: 10.1016/s0896-6273(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 34.McGee AW, Bredt DS. Identification of an intramolecular interaction between the SH3 and guanylate kinase domains of PSD-95. J. Biol. Chem. 1999;274:17431–17436. doi: 10.1074/jbc.274.25.17431. [DOI] [PubMed] [Google Scholar]
- 35.Hoshi N, Langeberg LK, Scott JD. Distinct enzyme combinations in AKAP signalling complexes permit functional diversity. Nat. Cell Biol. 2005;7:1066–1073. doi: 10.1038/ncb1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dell'Acqua ML, Dodge KL, Tavalin SJ, Scott JD. Mapping the protein phosphatase-2B anchoring site on AKAP79. Binding and inhibition of phosphatase activity are mediated by residues 315–360. J. Biol. Chem. 2002;277:48796–48802. doi: 10.1074/jbc.M207833200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith KE, Gibson ES, Dell'Acqua ML. cAMP-dependent protein kinase postsynaptic localization regulated by NMDA receptor activation through translocation of an A-kinase anchoring protein scaffold protein. J. Neurosci. 2006;26:2391–2402. doi: 10.1523/JNEUROSCI.3092-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cummings JA, Mulkey RM, Nicoll RA, Malenka RC. Ca2+ signaling requirements for long-term depression in the hippocampus. Neuron. 1996;16:825–833. doi: 10.1016/s0896-6273(00)80102-6. [DOI] [PubMed] [Google Scholar]
- 39.Oliveria SF, Dell'Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coghlan VM, et al. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science. 1995;267:108–111. doi: 10.1126/science.7528941. [DOI] [PubMed] [Google Scholar]
- 41.Kashishian A, et al. AKAP79 inhibits calcineurin through a site distinct from the immunophilin-binding region. J. Biol. Chem. 1998;273:27412–27419. doi: 10.1074/jbc.273.42.27412. [DOI] [PubMed] [Google Scholar]
- 42.Gomez LL, Alam S, Smith KE, Horne E, Dell'Acqua ML. Regulation of A-kinase anchoring protein 79/150-cAMP-dependent protein kinase postsynaptic targeting by NMDA receptor activation of calcineurin and remodeling of dendritic actin. J. Neurosci. 2002;22:7027–7044. doi: 10.1523/JNEUROSCI.22-16-07027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tavalin SJ, et al. Regulation of GluR1 by the A-kinase anchoring protein 79 (AKAP79) signaling complex shares properties with long-term depression. J. Neurosci. 2002;22:3044–3051. doi: 10.1523/JNEUROSCI.22-08-03044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylatoin of stargazin-like TARPs. Neuron. 2005;45:269–277. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 45.Tomita S, Fukata M, Nicoll RA, Bredt DS. Dynamic interaction of stargazing-like TARPs with cycling AMPA receptors at synapses. Science. 2004;303:1508–1511. doi: 10.1126/science.1090262. [DOI] [PubMed] [Google Scholar]
- 46.Gallagher SM, Daly CA, Bear MF, Huber KM. Extracellular signal-regulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. J. Neurosci. 2004;24:4859–4864. doi: 10.1523/JNEUROSCI.5407-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schnabel R, Kilpatrick IC, Collingridge GL. Protein phosphatase inhibitors facilitate DHPG-induced LTD in the CA1 region of the hippocampus. Brit. J. Pharmacol. 2001;132:1095–1101. doi: 10.1038/sj.bjp.0703905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davidkova G, Carroll RC. Characterization of the role of microtubule-associated protein 1B in metabotropic glutamate receptor-mediated endocytosis of AMPA receptors in hippocampus. J. Neurosci. 2007;27:13273–13278. doi: 10.1523/JNEUROSCI.3334-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ronesi JA, Huber KM. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J. Neurosci. 2008;28:543–547. doi: 10.1523/JNEUROSCI.5019-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.