Abstract
BK virus (BKV) is a polyomavirus that ubiquitously infects the human population. Following a typically subclinical primary infection, BKV establishes a lifelong persistent infection in the kidney and urinary tract. BKV is known to reactivate and cause severe disease in immunosuppressed patients, particularly renal and bone marrow transplant patients. Infection of BKV in rodent animal models or cells in culture often results in tumor formation or transformation, respectively. When co-expressed with activated oncogenes, BKV large tumor antigen drives the transformation of primary human cells. An etiological role of BKV in human cancer, however, remains controversial. Multiple reports have demonstrated conflicting results in regards to the presence of BKV sequences and/or proteins in various tumor types. This review compiles the most recent findings of BKV detection in a number of human cancers. Due to the lack of conclusive causality data from these studies, there does not appear to be a definitive association between BKV and human cancers.
Keywords: BK virus, T antigen, polyomavirus, prostate cancer
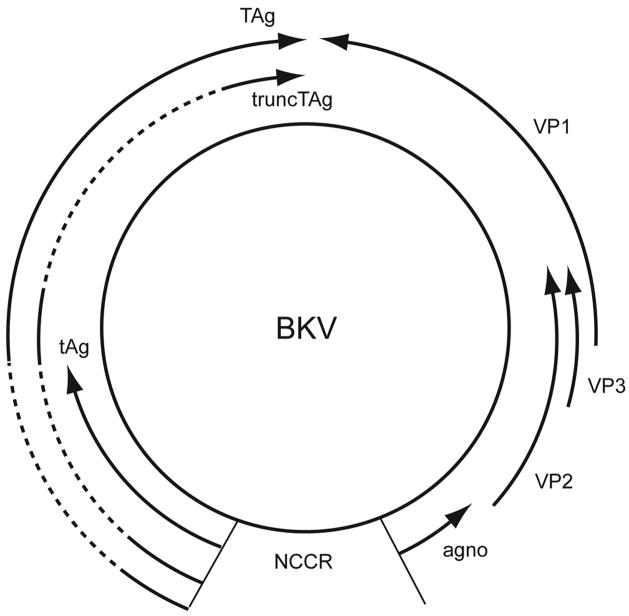
BK virus (BKV) is a human polyomavirus first isolated in 1971 from the urine of an immunocompromised renal transplant patient [1]. It has a non-enveloped, icosahedral capsid containing a small, circular double-stranded DNA genome (~ 5 Kb) [2–4]. The icosahedral capsid is made up of 72 pentamers of the major capsid protein VP1 arranged in a T=7 icosahedral lattice, with each pentamer interacting with a single copy of the minor capsid protein VP2 or VP3 [5–9]. This interaction also links the viral genome to the VP1 outer shell. The BKV genome can be divided into three functional regions, the early region, the late region, and the viral regulatory or non-coding control region (NCCR). The early genes and the late genes are transcribed from opposite strands of the genome (Figure 1). Initiation of transcription occurs from the NCCR, which contains sequences involved in transcriptional regulation of both the early and late genes and also includes the origin of DNA replication [reviewed in 3,10]. Like that of SV40 and JC virus (JCV), the other two well-studied primate polyomaviruses, the BKV genome codes for at least seven viral proteins, three from the early region and four from the late region (Figure 1). The early proteins include the large tumor antigen (TAg), the small tumor antigen (tAg), and the recently discovered truncated tumor antigen (TruncTAg) [11]. The early proteins are translated from alternatively spliced transcripts derived from a common precursor early mRNA (Figure 1). The four late proteins include the capsid proteins VP1, VP2, and VP3, and the agnoprotein [reviewed in 10].
Figure 1. BKV genome.
Schematic view of the double-stranded, circular DNA genome of BKV, showing the early and late regions, and the non-coding control region (NCCR). The solid arrows indicate open reading frames and the dashed lines are early region introns. Transcription of the early and late regions proceeds in the direction of the arrows.
BK virus infection in humans
The life cycle of BKV begins with the interaction of the capsid protein VP1 with cellular ganglioside receptors [12]. The virus then enters the cell through caveolae-mediated endocytosis, passes through a yet-to-be-defined acidic compartment and the caveosome, travels along the microtubule network, and finally reaches the endoplasmic reticulum (ER) [13–15]. Viral uncoating is likely to occur in the ER, which finally leads to the delivery of the genome into the nucleus and subsequent early gene expression. TAg, in particular, performs functions essential for viral replication, including unwinding of the viral origin of replication and recruitment of the host DNA polymerase α/primase complex [16]. At the same time, TAg represses early gene transcription and stimulates late gene expression [reviewed in 10]. After viral DNA replication and structural protein production, new viral particles are assembled and released from the cell.
When a polyomavirus infects a cell, there are two possible outcomes: 1) the host is permissive to viral replication, in which case entry results in viral DNA amplification, production of progeny virions, and cell lysis; 2) the host is nonpermissive to viral replication, leading to an abortive infection or cell transformation, otherwise known as oncogenesis, through the continued expression of the early genes [2–4].
Serological studies indicate that primary BKV infection occurs early in childhood and seroconversion reaches more than 90% by age 10 [17]. The natural transmission route has not been established for BKV. The early age of seroconversion, along with the detection of the virus in tonsillar tissue, has pointed to a respiratory transmission route [18]. Other speculated modes of transmission include urine, semen, blood transfusion, and organ transplantation [19–21]. Primary exposure to BKV is usually asymptomatic and is accompanied by the establishment of a life-long persistent infection, particularly in the kidney and urinary tract [22–25]. Many cells types, including urogenital cells, peripheral blood mononuclear cells, and brain cells, have been suggested to be involved in the dissemination and persistence of BKV [26–32]. Sporadic virus replication resulting in viruria can occur during the persistent state of BKV in a small percentage of healthy individuals and pregnant women [33–36].
Reactivation of BKV infection occurs when the immune response is compromised, mainly due to immunosuppressive treatments, and can result in viruria, viremia and cause severe disease [reviewed in 37]. Reactivation is most commonly seen in bone marrow and kidney transplant patients, and can lead to serious complications such as hemorrhagic cystitis and polyomavirus nephropathy, respectively [38–42]. Moreover, the introduction of new, more potent immunosuppressive regimens in the past decade has led to an increase in the cases of BKV-associated disease [24,43,44]. BKV reactivation has also been observed in other altered immune conditions, such as non-renal solid organ transplant recipients and patients with autoimmune diseases [45,46]. Specific anti-viral treatment is currently unavailable. The usual strategy to treat virus reactivation is to reduce the degree of immunosuppression to allow the immune system to regain control over the infection. Antivirals such as cidofovir and fluoroquinolone are now being tested in cases of BKV-associated disease [47].
Oncogenicity of BK virus
BKV has been implicated as a tumor virus because of its behavior in vitro and in animal models. Expression of the BKV early region oncogenically transforms rodent cells in culture and immortalizes human cells alone or in the presence of other oncogenes including ras, myc and adenovirus E1A [48–62]. Inoculation of BKV into young or newborn hamsters, mice, and rats leads to the development of several different types of tumors, including ependymoma, neuroblastoma, glioma, nephroblastoma, tumors of pancreatic islets, fibrosarcoma, liposarcoma, and osteosarcoma [reviewed in 4,63]. These studies are further discussed elsewhere in this issue.
The early region of BKV encodes two known oncoproteins: TAg and tAg [reviewed in 3,64]. TAg, a nuclear phosphoprotein of 695 amino acids, is a crucial player in both the viral life cycle and transformation of nonpermissive cells. TAg exerts its transforming activities by interacting with and functionally inactivating cellular tumor suppressor proteins including p53, the retinoblastoma susceptibility protein (pRb), and the pRb family members, p107 and p130, leading to uncontrolled host cell proliferation [65,66]. tAg is a cysteine-rich protein of 172 amino acids, the first 80 of which are shared with TAg. tAg plays an important role in transformation by inhibiting protein phosphatase 2A (PP2A), resulting in the stimulation of the MAP kinase pathway and subsequent cellular proliferation [67,68]. A more detailed discussion of the transforming activities of TAg and tAg can be found elsewhere in this issue.
BK virus and human tumors
Reports of the presence or absence of BKV sequences and proteins in human tumors have been accumulating over the past three decades. Technological advances in polymerase chain reaction (PCR) have made it possible to detect DNA and RNA in small biopsy samples with a high degree of sensitivity, and to distinguish BKV sequences from those of JCV and SV40. Extreme care must be taken, however, when using a PCR-based assay to detect viral sequences. Increasing the number of PCR cycles to develop a more sensitive assay can result in a greater susceptibility to false positives due to laboratory contamination. Furthermore, using PCR-based screens of biopsy samples examines a heterogeneous population of cells, in which a small number of normal, BKV-harboring cells in a tumor sample can make the tumor look BKV-positive. Other techniques being used that are less susceptible to contamination include in situ PCR, in situ hybridization (ISH), immunohistochemistry (IHC), or Southern blotting. In addition, in situ methods of analysis help in determining the exact cellular location of the virus in tissue sections, allowing investigators to avoid false positives resulting from nearby normal BKV-infected cells. Despite numerous studies, the role of BKV in human neoplasia is still highly controversial. There are many contradictory reports on the presence of BKV DNA and proteins in human tumor tissues. The following paragraphs and Table 1 summarize the major reports investigating an association between BKV and human tumors.
Table 1.
Detection of BKV in human tumors, tumor cell lines, and normal tissues
| Tissues/Cell lines | BKV DNA-positive samples/samples analyzed (%) | BKV TAg-positive samples/samples analyzed (%) | Method | Reference |
|---|---|---|---|---|
| Tumor tissues | ||||
| Brain | 50/58 (86) | PCR/SBH | [116] | |
| Brain | 74/83 (89) | PCR/SBH | [117] | |
| Brain | 4/5 (80) | SBH | [134] | |
| Brain | 11/24 (46) | SBH | [113] | |
| Brain | 19/74 (26) | SBH | [125] | |
| Brain | 0/80 (0) | PCR | [119] | |
| Brain | 0/274 (0) | PCR | [121] | |
| Brain | 0/80 (0) | IHC | [120] | |
| Brain | 3/225 (1) | PCR/SBH, PCR | [123] | |
| 0/225 (0) | ||||
| Brain | 6/40 (15) | PCR | [118] | |
| Brain | 0/103 (0) | DDrk/SBH | [70] | |
| Medulloblastoma | 0/15 (0) | PCR/SBH | [152] | |
| Neuroblastoma | 18/18 (100) | 16/18 (89) | PCR, ISH, IFA | [114] |
| 17/18 (94)* | ||||
| sPNET | 0/5 (0) | PCR/SBH | [152] | |
| Meningioma | 0/10 (0) | PCR/SBH | [153] | |
| Osteosarcoma | 11/25 (44) | PCR/SBH | [116] | |
| Osteosarcoma | 0/7 (0) | DDrk/SBH | [70] | |
| Bladder | 15/26 (58) | PCR/SBH | [28] | |
| Bladder | 1/1 (100) | SBH | [93] | |
| Bladder | 0/15 (0) | PCR/SBH | [153] | |
| Bladder | 1/1 (100) | 1/1 (100) | PCR/DBH, IHC | [91] |
| Bladder | 4/21 (19) | IHC | [94] | |
| Bladder | 4/76 (5) | 0/4 (0) | PCR, IHC | [96] |
| Bladder | 0/8 (0) | DDrk/SBH | [70] | |
| Bladder | 1/28 (4) | IHC | [92] | |
| Kidney | 10/15 (67) | PCR/SBH | [28] | |
| Kidney | 1/83 (1) | PCR | [102] | |
| Kidney | 0/1 (0) | ISH | [100] | |
| Kidney | 1/1 (100) | 1/1 (100) | PCR, ISH, IHC | [99] |
| Kidney | 0/18 (0) | DDrk/SBH | [70] | |
| Kidney | 0/1 (0) | 1/1 (100) | ISH, IHC | [101] |
| Wilms’ tumor | 0/14 (0) | PCR/SBH | [116] | |
| Renal pelvis | 9/55 (16) | PCR | [102] | |
| Adrenal | 4/107 (4) | 4/4 (100) | PCR | [130] |
| Hepatoblastoma | 0/60 (0) | PCR | [121] | |
| Prostate | 4/7 (57) | PCR/SBH | [28] | |
| Prostate | 3/12 (25) | PCR | [154] | |
| Prostate | 13/16 (81), | 9/21 (43) | PCR, ISH, IHC | [80] |
| 15/21 (71)* | ||||
| Prostate | 7/30 (23) | 0/30 (0) | ISH, IHC | [88] |
| Prostate | 1/200 (0.5) | PCR | [89] | |
| Prostate | 22/26 (85) | 20/26 (77) | PCR, IHC | [87] |
| Ureter | 2/4 (50) | PCR/SBH | [28] | |
| Cervix | 9/14 (64) | PCR/SBH | [29] | |
| Vulvar | 1/7 (14) | PCR/SBH | [29] | |
| Cervix and vulva | 32/42 (76) | PCR/SBH | [135] | |
| Pancreatic islets | 0/4 (0) | DDrk/SBH | [70] | |
| Pancreatic islets | 4/9 (44) | SBH | [125] | |
| Pancreatic islets | 1/1 (100) | SBH | [124] | |
| Colon rectum | 11/18 (61) | ISH | [128] | |
| Kaposi’s sarcoma | 4/20 (20) | SBH | [134] | |
| Kaposi’s sarcoma | 38/38 (100) | PCR/SBH | [135] | |
| Kaposi’s sarcoma | 3/9 (33) | SBH | [133] | |
| Kaposi’s sarcoma | 0/2 (0) | PCR/SBH | [153] | |
| Lymphoma | 0/5 (0) | PCR/SBH | [153] | |
| Lymphoma | 5/83 (6) | 0/5 (0) | PCR, IHC | [129] |
| Extracutaneous melanoma | 0/38 (0) | PCR | [132] | |
| Tumor cell lines | ||||
| Neuroblastoma | 0/10 (0) | PCR | [122] | |
| Medulloblastoma | 0/2 (0) | PCR/SBH | [152] | |
| Glioblastoma | 2/2 (100) | PCR/SBH | [155] | |
| Multiple brain | 21/26 (81) | PCR/SBH | [117] | |
| Multiple brain | 4/19 (21) | SBH | [134] | |
| Multiple | 3/30 (10) | SBH | [134] | |
| Multiple | 28/30 (93) | PCR/SBH | [116] | |
| Multiple | 3/4 (75) | DDrk | [109] | |
| Kaposi’s sarcoma | 6/8 (75) | PCR/SBH | [135] | |
| Kaposi’s sarcoma | 0/14 (0) | PCR/SBH | [153] | |
| Normal tissues | ||||
| Adrenal glands | 0/20 (0) | PCR | [130] | |
| Adrenal glands | 0/5 (0) | 0/5 (0) | PCR, ISH, IHC | [114] |
| 0/5 (0)* | ||||
| PBMC | 25/35 (71) | PCR/SBH | [116] | |
| PBMC | 22/40 (55) | PCR | [30] | |
| PBMC | 53/70 (76) | PCR/SBH | [117] | |
| PBMC | 17/28 (61) | SBH | [32] | |
| Lymphocytes | 12/18 (67) | ISH | [128] | |
| Lymphocytes | 27/30 (90) | PCR/SBH | [117] | |
| Lymphocytes | 0/52 (0) | PCR | [129] | |
| Serum | 0/115 (0) | PCR | [122] | |
| Skin | 25/33 (76) | PCR/SBH | [135] | |
| Brain | 13/13 (100) | PCR/SBH | [117] | |
| Brain | 13/13 (100) | PCR/SBH | [116] | |
| Brain | 0/31 (0) | PCR | [121] | |
| Brain | 0/29 (0) | SBH | [113] | |
| Bone | 2/5 (40) | PCR/SBH | [116] | |
| Colon rectum | 11/18 (61) | ISH | [128] | |
| Bladder | 5/10 (50) | PCR/SBH | [28] | |
| Bladder | 3/9 (33) | IHC | [94] | |
| Bladder | 1/46 (2) | 0/1 (0) | PCR, IHC | [96] |
| Kidney | 0/6 (0) | DDrk/SBH | [70] | |
| Kidney | 5/6 (83) | PCR/SBH | [28] | |
| Prostate | 4/15 (27) | 4/29 (14) | ISH, IHC | [79] |
| Prostate | 11/19 (58) | PCR/SBH | [135] | |
| Prostate | 0/12(0) | 0/12 (0) | PCR, IHC | [87] |
| Ureter | 0/1 (0) | PCR/SBH | [28] | |
| Urethra | 0/1 (0) | PCR/SBH | [28] | |
| Sperm | 18/20 (90) | PCR/SBH | [117] | |
| Sperm | 5/6 (83) | PCR/SBH | [29] | |
| Sperm | 18/19 (95) | PCR/SBH | [135] | |
| Cervix | 13/14 (93) | PCR/SBH | [29] | |
| Vulva | 6/7 (86) | PCR/SBH | [29] | |
| Cervix and vulva | 15/21 (71) | PCR/SBH | [135] | |
Abbreviations used in Table 1: PCR, polymerase chain reaction; SBH, Southern blot hybridization; IHC, immunohistochemistry; IFA, immunofluorescence; ISH, in situ hybridization; DDrk, DNA-DNA reassociation kinetics; sPNET, supratentorial primitive neuroectodermal tumor; DBH, dot blot hybridization; PBMC, peripheral blood mononuclear cells;
, ISH data in cases in which both PCR and ISH were performed.
BKV is known to persist in the kidney and urinary tract [22,23], therefore urinary tract carcinomas would be likely candidates to be associated with BKV. Some earlier studies using immunofluorescent staining for TAg or DNA hybridization analyses on tumor samples or cells derived from tumors did not identify a link between BKV and various urogenital carcinomas [69,70]. More recent reports, however, tend to point to the participation of BKV in the pathogenesis of these tumors, possibly due to improvements in the detection methods. Among all the urinary tract tumors, prostate cancer is one of the leading causes of death from cancers in men worldwide. The relationship between BKV and prostate cancer has been studied by several groups over the years. Monini et al. reported the detection of BKV sequences by PCR and Southern blotting in more than 50% of both normal and tumor tissues obtained from the urinary tract and prostate [28]. In addition, viral DNA load was found to be significantly higher in the neoplastic tissues compared to non-neoplastic tissues, suggesting that there may be a selection for BKV-containing tumor cells [28]. Moreover, BKV DNA in these tumor samples seems to exist in a rearranged episomal and/or integrated form, and the simple restriction pattern of these sequences is suggestive of early acquirement of viral DNA during cancer progression and subsequent clonal expansion [28]. All attempts to rescue virus by transfection of tumor DNA into permissive cells, however, failed, indicating that the viral sequences in these samples may not be capable of productive infection. Interestingly, the sequences of the viral origin of replication obtained from nine positive samples were identical except for two point mutations in one of the samples. They represent a novel strain of BKV designated as URO1, which has NCCR rearrangements that may affect viral DNA replication. Several studies have shown that mutants in this region have altered replication ability and increased transformation potential in cell culture [71–73]. Inhibition of replication in the presence of continued expression of T antigens could prevent host cell death and lead to transformation. It remains to be determined, however, whether the mutations in URO1 have an effect on replication or transformation.
Since that report, five groups have examined prostate tissue for the presence of BKV. Zambrano et al. examined paraffin-embedded and fresh frozen tissue using PCR [74]. While they found the results from fixed tissue to be inconsistent, in frozen tissue they found BKV DNA in three out of twelve patient specimens analyzed. Two of these were tumor tissues and one was normal. JCV and HPV DNA, however, were found at a higher frequency in these samples, leading to the hypothesis that the prostate was a habitat for multiple viral infections.
Prostate cancer develops in several steps, with proliferative inflammatory atrophy (PIA) occurring as the initial stage, which can then progress to prostatic intraepithelial neoplasia (PIN) and/or carcinoma, both of which represent later stages of prostate cancer development [75,76, reviewed in 77,78]. Based on the studies conducted by Das et al., it is speculated that BKV has an etiological role in early stage prostate cancer progression [79,80]. Several lines of evidence support this hypothesis. First, BKV DNA sequences were specifically found in the epithelium of benign ducts and PIA lesions of more than 70% of the neoplastic prostate tissues examined by both PCR and ISH [80]. The frequency of BKV DNA was much lower (< 30%) in normal prostates, ruling out the possibility that the detection of BKV DNA in cancerous tissues was due to its ubiquitous presence in humans [79].
Second, IHC analysis revealed that the percentage of TAg-positive samples was also significantly higher in cancerous prostates than in normal prostates. BKV DNA was found in both the normal ducts and PIA, but not in PIN or tumor cells; TAg was only found in PIA lesions, but not in normal or tumor cells [79,80]. This suggests that BKV may first persistently infect normal epithelium and either the expression of TAg triggers the transition from normal tissues to atrophic lesions or the transition to PIA induces TAg expression. The loss of TAg expression and viral DNA in tumor cells is consistent with a previous report of the loss of TAg expression in prostatic epithelial cell lines derived from TRAMP mice (TRansgenic Adenocarcinoma of Mouse Prostate) [81], and with another report showing that cells cultured from 12 prostate carcinomas were negative for BKV TAg expression by immunofluorescence [69]. It has been postulated that TAg expression might be necessary to initiate transformation, but that continuous TAg expression might not be required for the maintenance of the transformed state in vitro or in vivo [81,82]. A similar loss of viral sequences in tumors has been reported for bovine papillomavirus type 4 [83]. To explain the loss of viral DNA or TAg, it is possible that the ability of TAg to induce chromosomal damage or its pro-apoptotic effects mediated through interactions with the pRb family of proteins, are incompatible with cell growth at advanced stages of carcinogenesis [84–86]. Alternatively, viral sequence loss could be due to dilution of episomal DNA or selection against TAg by the immune system.
Third, the TAg detected in these studies was cytoplasmic, and p53 was found to colocalize with TAg in the cytoplasm. In samples where TAg was not detected, p53 was nuclear when observed. Using laser capture microdissection, it was demonstrated that the p53 gene in laser-captured TAg-positive PIA cells was wild-type, whereas tumor cells expressing nuclear p53 contain a mixture of wild-type and mutant p53 genes [79]. In addition, the cytoplasmic localization of both proteins was not due to mutations in their nuclear localization sequences. Taken together, this indicates that the wild-type p53 may be inactivated by TAg sequestration in the cytoplasm, which can then lead to accumulation of mutations and loss of growth control. Finally, consistent with the cytoplasmic localization of TAg, no VP1 expression was detected in the TAg-positive samples, indicating a lack of viral replication [79].
A recent report by Russo et al. also suggests the role of BKV in the pathogenesis of prostate cancer [87]. BKV DNA was detected in 85% of the prostate cancer specimens but in none of the benign prostatic hyperplasia control group by PCR. Moreover, IHC analysis revealed that both TAg and p53 were present in the cytoplasm in 77% of the cancer samples, whereas in the TAg-negative tumors, p53 was found in the nucleus. These results are consistent with the findings by Das et al. [79,80,87].
Lau et al. used ISH and IHC to examine 30 cancerous prostate tissues [88]. While they did not observe positive TAg expression by IHC in any of their samples, their ISH results detected 4 samples containing BKV DNA in non-neoplastic cells, 2 samples in neoplastic cells and 1 sample in PIN. Using nested PCR, Sfanos et al. analyzed a total of 338 samples from 200 patients for BKV. Surprisingly, only one sample was positive for BKV [89]. It is not clear why there is such a variation in the percentages of BKV DNA- and TAg-positive prostates among different groups.
There are a number of contrasting reports regarding BKV association with the development of bladder cancer [28,90–96]. In studies by Fioriti et al. and Monini et al., BKV sequences were found to be present at a high frequency in bladder carcinoma [28,90]. Renal transplant patients have a higher risk of developing urothelial carcinoma, thereby implicating BKV as a causal transforming agent of metastatic bladder carcinoma in an immunocompromised setting [97,98]. In one case report of a renal and pancreas transplant recipient, TAg expression was detected in almost every single cell in the primary tumor and the metastasis but not in the surrounding stromal cells or in the non-neoplastic urothelium [91]. The presence of BKV was confirmed by PCR followed by dot blot hybridization. p53 showed an identical nuclear staining pattern as the TAg in this sample. In addition, sequence analysis of the p53 gene revealed the absence of mutations, suggesting stabilization and inactivation of this protein by TAg binding. Similarly, in a recent report by Roberts et al., strong nuclear staining for TAg was seen in the urothelial carcinoma of one renal transplant patient, although the percentage of TAg-positive samples among all the urothelial carcinomas was low (1/8 among renal transplant patients and 0/20 among immunocompetent patients) [92]. Herawi et al. detected TAg by immunostaining in both benign and cancerous bladder tissues [94]. Only urothelial carcinoma cells, however, showed strong and diffuse nuclear TAg signals [94]. In another investigation, it was found that immunocompetent patients with detectable BKV-infected “decoy” cells in urine were more likely to develop bladder carcinoma than patients lacking such cells, suggesting BKV may be a risk factor for bladder carcinoma [95]. The caveat of this study, however, is that some urothelial carcinoma cells can be mistaken for decoy cells. In contrast, Rollison et al. concluded that BKV did not play a major role in the pathogenesis of bladder carcinoma, as only 5.5% of the bladder cancer samples were BKV-positive by PCR and none of them showed TAg expression [96].
The involvement of BKV in kidney cancer is also not well established. There are several case studies reporting the presence or absence of BKV DNA sequences or protein expression in renal carcinomas that developed after renal transplants [99–101]. The presence of BKV sequences in larger studies of kidney cancer samples also seem to vary among different groups [28,102].
BKV DNA sequences have also been reported in different tumors that do not correlate with anatomical sites thought to be infected by the virus in humans, including brain tumors, pancreatic tumors, lymphomas, adrenal tumors, colorectal tumors, and Kaposi’s sarcoma [reviewed in 2–4,64,103]. Even the earliest studies investigating the association of BKV and human tumors had conflicting reports of the presence of BKV sequences in similar tissue samples and cell lines. During a five-year span, several groups examined a selection of human tumors, including samples from Wilms’ tumors, lung carcinomas, and brain tumors, as well as a variety of human cell lines, including those derived from lung carcinoma, rhabdomyosarcoma, glioblastoma, and melanoma. The tumors sampled were among those frequently induced by BKV in experimental animals and thus were relevant for examination [104–108]. As these studies pre-dated PCR, the detection methods employed were either nucleic acid reassociation assays or Southern blot analyses. Two of these studies reported BKV sequences in 5 out of 12 human tumors, 3 out of 4 human tumor cell lines, and 43 out of 105 total tissues (tumor and normal) and cell lines [109,110]. In contrast, three other groups using the same or similar tumor tissue types and often the same cell lines reported no BKV DNA sequences present in any of the samples assayed [70,111,112].
Since the closely related polyomavirus JCV naturally infects and causes disease in the brain, researchers also began to look for the presence of BKV in brain tissues and tumors. One study reported that 11 out of 24 brain tumor tissues contained BKV sequences associated with high molecular weight DNA by Southern blot analysis, suggesting integration of the viral genome into chromosomal DNA; normal brain tissue controls were negative [113]. Another group detected BKV sequences in 18 out of 18 neuroblastoma tumors by PCR followed by Southern blot analysis and in 17 out of 18 tumors by ISH; 16 out of 18 tumors also showed cytoplasmic staining for TAg by immunofluorescence [114]. The same group demonstrated that BKV TAg and p53 were cytoplasmic in neuroblastoma cell lines and that reduction of TAg expression in these cells resulted in the restoration of function and nuclear localization of p53 [115]. Other studies using PCR followed by Southern blot analysis detected BKV sequences in 85% of brain tumors and in all normal brain tissues [116], in 57–100% of brain tumors and brain tumor-derived cell lines [117], and in 15% of brain tumor tissues, including astrocytoma, glioblastoma, and meningioma by PCR alone [118], suggesting the possibility of BKV association with brain tumors. Although these reports argue for the involvement of BKV in human tumors, the use of PCR to enhance assay sensitivity, either alone or prior to Southern blot analysis, greatly increases the risk of false positives.
The absence of BKV sequences in human brain tumors and neuroblastoma cell lines has also been extensively reported [119–122]. Rollison et al. attempted to address the inconsistencies of BKV sequence detection in human tumors by conducting an investigation of 225 brain tumor tissues analyzed in two different laboratories. One laboratory reported 3 BKV-positive samples using PCR followed by Southern blot analysis, while the other reported no BKV-positive samples using a real-time PCR assay [123]. This study demonstrates that variability can occur between labs examining the same tumor samples; therefore, it is difficult to claim an association between BKV and brain tumors.
Few studies have made detailed examinations of BKV sequences to attempt to characterize the viruses present in tumor samples. In one report, investigators detected BKV DNA in a pancreatic islet adenoma by Southern blot analysis, and went on to transfect total tumor DNA into human embryonic fibroblasts and rescue infectious BKV (BKV-IR strain). This strain was significantly different from the common laboratory strain (BKV-Dunlop) by restriction endonuclease mapping [124]. Several years later, another study reported that 19 out of 74 brain tumors and 4 out of 9 pancreatic islet tumors contained BKV DNA by Southern blot analysis; transfection of total tumor DNA into human embryonic fibroblasts resulted in the rescue of virus similar to BKV-IR [125]. These findings suggest the possible association of a particular strain of BKV with human tumors. Upon further characterization, it was reported that the BKV-IR strain was less efficient at transformation of hamster cells in culture and induction of tumors in hamsters in vivo, but had a higher propensity for metastasis [126]. BKV-IR contains insertions within its NCCR that, in the context of the genome, resemble a stem-loop structure [126]. It has been suggested that this stem-loop structure may result in excision of the NCCR from the viral genome and integration into cellular DNA, potentially aiding in oncogenic transformation either by activating the expression of cellular oncogenes or by acting as a mutagen by random insertion into cellular genes [126,127].
Other tumors that have been investigated for the presence of BKV sequences include lymphomas, adrenal tumors, melanoma, colorectal tumors, and Kaposi’s sarcoma. Since BKV has been convincingly detected in peripheral blood cells of normal individuals [30,32,128], investigators began to look for an association between BKV and lymphoma. Only 5 out of 83 lymphoma samples were positive for BKV DNA using standard and real-time PCR [129]. Based on the higher percentage of normal BKV-positive lymphocytes routinely observed, this study does not support an association between BKV and lymphoma [30,32,128]. Another study examined adrenal glands and adrenal tumors for the presence of viral sequences, since the adrenal gland is located on top of the kidney, a major site of BKV persistence. This study found 4 out of 107 adrenal tumor samples positive for BKV by both real-time PCR and IHC for TAg [130]. Normal tissue samples were all negative, suggesting that BKV may be associated with adrenal tumors, possibly due to the proximity of the adrenal gland to the kidney and its ability to produce hormones known to stimulate BKV gene expression [131]. There is no clear link between BKV and melanoma, as one study found that 38 non-UV light associated melanomas were all negative for BKV sequences by PCR-based assays [132]. Casini et al. demonstrated the presence of BKV sequences using ISH, in 11 out of 18 colorectal tumors [128]. Out of the 11 BKV-positive samples, 10 samples exhibited positive staining in healthy cells adjacent to cancer cells, suggesting that BKV may contribute to the development of colorectal cancer.
The presence of BKV DNA in Kaposi’s sarcoma (KS), a malignancy associated with immunosuppression, has also been analyzed. A study by Barbanti-Brodano et al. detected BKV sequences by Southern blot analysis in 3 out of 5 KS samples from Uganda but not in 4 KS samples from Italy [133]. Transfection of cellular DNA from KS tissues into human embryonic fibroblasts resulted in the rescue of infectious virus similar to the BKV-IR strain [133,134]. The presence of BKV sequences was also reported in 100% of KS skin lesions, 75% of KS cell lines, and 57–95% of genital tissues and sperm samples, which were examined as potential evidence for a route of transmission [135]. However, given the well-established role of Kaposi’s sarcoma-associated herpesvirus in KS, it is likely that any role for BKV in this disease is secondary.
There are several studies that monitored anti-BKV antibody levels in patient serum samples as a marker for the presence and association of BKV with specific cancers. In these assays, anti-VP1 antibodies are measured by an enzyme-linked immunosorbent assay (ELISA) using yeast- or bacteria-derived virus-like particles as the antigen. Using this assay, there was no association between maternal BKV antibody levels and the development of neuroblastoma in children [122], or between antibody levels and the development of non-Hodgkin lymphoma [136]. Newton et al. observed that anti-BKV antibody levels in patients with prostate cancer were 20% higher than in normal controls, while patients with oral cancer had 17% lower serum antibody levels [137]. In a separate sero-epidemiologic study, the ELISA data did not support a relationship between BKV antibody titers and prostate, kidney, or bladder cancer [138]. A case-controlled study involving 386 male colorectal cancer patients and matching control subjects also did not identify BKV as a risk factor [139]. The shortcomings of using ELISA to detect VP1 antibodies, however, are that polyomaviruses are involved in tumor formation in situations when productive infection is restricted, leading to an over-abundance of TAg but a lack of late protein expression. Furthermore, according to the ‘hit-and-run’ hypothesis (see below), viral genome may be lost from infected cells during different stages of tumor development. Thus, high levels of VP1 expression, and consequently anti-VP1 antibodies, would not be expected during BKV-mediated oncogenesis. Instead, it would be more appropriate to examine anti-TAg antibody levels, as TAg expression would be required at some point during tumor development.
The presence of TAg in a high percentage of cells within a tumor sample would strongly argue for the involvement of BKV in tumor development. The lack of consistent observation of TAg expression, however, could be explained in two ways. First, TAg expression may not be required in every cell due to secretion of paracrine growth signals by TAg-positive cells that act on neighboring and distant cells, stimulating proliferation. This has been shown for SV40-positive mesothelioma cells, where SV40 TAg induces production and release of vascular endothelial growth factor [140–143]. Alternatively, the lack of BKV TAg-positive cells in tumor samples can be explained by the ‘hit-and-run’ mechanism [144]. According to this hypothesis, BKV induces mutations in human cells before the transformed phenotype appears. This could result in irreversible chromosomal damage in host cells, leading to oncogenic transformation. Henceforth, viral sequences may not be required for maintenance of the transformed phenotype and could be eventually lost from the tumor cells.
Conclusions
The clinical studies described above have demonstrated both the presence and absence of BKV sequences in cancerous tissues. As a member of the polyomavirus family, it can be stated that BKV has all the qualifications to be a cofactor in the induction and/or progression of human tumors. A causal role for BKV in human neoplasia, however, still remains to be established. There are several concerns when considering a role for BKV in carcinogenesis: (a) BKV DNA sequences are often found in normal human tissues, thus whole tumor samples must be carefully examined because they contain both normal and tumor cells; (b) the expression of TAg, the viral protein that drives transformation, is infrequently analyzed and reported in cancerous tissues; (c) the inability of BKV to replicate in animal models severely restricts key studies relating to causality; (d) epidemiological studies are impaired by the presence of BKV in nearly the entire population, thus the design of more quantitative studies may help define the role, if any, of BKV in cancer.
Due to these concerns, it is very difficult to apply classical Koch’s postulates to BKV [145]. An alternative set of criteria to define an oncogenic role for viruses in humans has been proposed: (a) epidemiological and clinical evidence that a viral infection puts one at risk for tumor development; (b) presence of the nucleic acid and/or the expression of viral proteins in specific tumor cells; (c) induction of cell transformation or immortalization upon transfection of either the viral genome or its individual sub domains; and (d) demonstration that the phenotypic characteristics of the tumor and the changes caused in cultured cells from the tumor are dependent on the expression of viral proteins [146–151]. Future investigations should be aimed at achieving more consistent detection of BKV DNA and TAg in human tumor samples, developing an animal model that accurately mimics BKV persistent infection and reactivation, and determining whether BKV transitions from a benign persistent pathogen to a virulent infectious agent that causes cancer.
Acknowledgments
We thank members of the Imperiale lab for critical review of the manuscript. This work was supported by AI060584 and CA118970 awarded to M.J.I. from the NIH. J.R.A. was supported by the F.G. Novy Fellowship. M.J. was supported by the American Heart Association Postdoctoral Fellowship 0825806G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gardner SD, Field AM, Coleman DV, Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971;1(7712):1253–7. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- 2.Imperiale MJ. The human polyomaviruses, BKV and JCV: molecular pathogenesis of acute disease and potential role in cancer. Virology. 2000;267(1):1–7. doi: 10.1006/viro.1999.0092. [DOI] [PubMed] [Google Scholar]
- 3.Imperiale MJ. Oncogenic transformation by the human polyomaviruses. Oncogene. 2001;20(54):7917–23. doi: 10.1038/sj.onc.1204916. [DOI] [PubMed] [Google Scholar]
- 4.Tognon M, Corallini A, Martini F, Negrini M, Barbanti-Brodano G. Oncogenic transformation by BK virus and association with human tumors. Oncogene. 2003;22(33):5192–200. doi: 10.1038/sj.onc.1206550. [DOI] [PubMed] [Google Scholar]
- 5.Rayment I, Baker TS, Caspar DL, Murakami WT. Polyoma virus capsid structure at 22.5 A resolution. Nature. 1982;295(5845):110–5. doi: 10.1038/295110a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liddington RC, Yan Y, Moulai J, Sahli R, Benjamin TL, Harrison SC. Structure of simian virus 40 at 3.8-A resolution. Nature. 1991;354(6351):278–84. doi: 10.1038/354278a0. [DOI] [PubMed] [Google Scholar]
- 7.Li TC, Takeda N, Kato K, Nilsson J, Xing L, Haag L, Cheng RH, Miyamura T. Characterization of self-assembled virus-like particles of human polyomavirus BK generated by recombinant baculoviruses. Virology. 2003;311(1):115–24. doi: 10.1016/s0042-6822(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson J, Miyazaki N, Xing L, Wu B, Hammar L, Li TC, Takeda N, Miyamura T, Cheng RH. Structure and assembly of a T=1 virus-like particle in BK polyomavirus. J Virol. 2005;79(9):5337–45. doi: 10.1128/JVI.79.9.5337-5345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stehle T, Gamblin SJ, Yan Y, Harrison SC. The structure of simian virus 40 refined at 3.1 A resolution. Structure. 1996;4(2):165–82. doi: 10.1016/s0969-2126(96)00020-2. [DOI] [PubMed] [Google Scholar]
- 10.Cole CN, Conzen SD. Polyomaviridae: The Viruses and Their Replication. In: Knipe DM, Howley PM, editors. Fields Virology. 2. Vol. 4. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2141–74. [Google Scholar]
- 11.Abend JR, Joseph AE, Das D, Campbell-Cecen DB, Imperiale MJ. A truncated T antigen expressed from an alternatively spliced BK virus early mRNA. J Gen Virol. 2009 doi: 10.1099/vir.0.009159-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Low JA, Magnuson B, Tsai B, Imperiale MJ. Identification of gangliosides GD1b and GT1b as receptors for BK virus. J Virol. 2006;80(3):1361–6. doi: 10.1128/JVI.80.3.1361-1366.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang M, Abend JR, Tsai B, Imperiale MJ. Early events during BK virus entry and disassembly. J Virol. 2009;83(3):1350–8. doi: 10.1128/JVI.02169-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moriyama T, Marquez JP, Wakatsuki T, Sorokin A. Caveolar endocytosis is critical for BK virus infection of human renal proximal tubular epithelial cells. J Virol. 2007;81(16):8552–62. doi: 10.1128/JVI.00924-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moriyama T, Sorokin A. Intracellular trafficking pathway of BK Virus in human renal proximal tubular epithelial cells. Virology. 2008;371(2):336–49. doi: 10.1016/j.virol.2007.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waga S, Bauer G, Stillman B. Reconstitution of complete SV40 DNA replication with purified replication factors. J Biol Chem. 1994;269(14):10923–34. [PubMed] [Google Scholar]
- 17.Knowles WA. Discovery and epidemiology of the human polyomavirus BK virus (BKV) and JC virus (JCV) Adv Exp Med Biol. 2006;577:19–45. doi: 10.1007/0-387-32957-9_2. [DOI] [PubMed] [Google Scholar]
- 18.Goudsmit J, Wertheim-van Dillen P, van Strien A, van der Noordaa J. The role of BK virus in acute respiratory tract disease and the presence of BKV DNA in tonsils. J Med Virol. 1982;10(2):91–9. doi: 10.1002/jmv.1890100203. [DOI] [PubMed] [Google Scholar]
- 19.Bofill-Mas S, Formiga-Cruz M, Clemente-Casares P, Calafell F, Girones R. Potential transmission of human polyomaviruses through the gastrointestinal tract after exposure to virions or viral DNA. J Virol. 2001;75(21):10290–9. doi: 10.1128/JVI.75.21.10290-10299.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bofill-Mas S, Pina S, Girones R. Documenting the epidemiologic patterns of polyomaviruses in human populations by studying their presence in urban sewage. Appl Environ Microbiol. 2000;66(1):238–45. doi: 10.1128/aem.66.1.238-245.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis. 2003;3(10):611–23. doi: 10.1016/s1473-3099(03)00770-9. [DOI] [PubMed] [Google Scholar]
- 22.Chesters PM, Heritage J, McCance DJ. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J Infect Dis. 1983;147(4):676–84. doi: 10.1093/infdis/147.4.676. [DOI] [PubMed] [Google Scholar]
- 23.Heritage J, Chesters PM, McCance DJ. The persistence of papovavirus BK DNA sequences in normal human renal tissue. J Med Virol. 1981;8(2):143–50. doi: 10.1002/jmv.1890080208. [DOI] [PubMed] [Google Scholar]
- 24.Nickeleit V, Singh HK, Mihatsch MJ. Polyomavirus nephropathy: morphology, pathophysiology, and clinical management. Current Opinion in Nephrology and Hypertension. 2003;12:599–605. doi: 10.1097/00041552-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Shinohara T, Matsuda M, Cheng SH, Marshall J, Fujita M, Nagashima K. BK virus infection of the human urinary tract. J Med Virol. 1993;41(4):301–5. doi: 10.1002/jmv.1890410408. [DOI] [PubMed] [Google Scholar]
- 26.Doerries K. Human polyomavirus JC and BK persistent infection. Adv Exp Med Biol. 2006;577:102–16. doi: 10.1007/0-387-32957-9_8. [DOI] [PubMed] [Google Scholar]
- 27.Elsner C, Dorries K. Evidence of human polyomavirus BK and JC infection in normal brain tissue. Virology. 1992;191(1):72–80. doi: 10.1016/0042-6822(92)90167-n. [DOI] [PubMed] [Google Scholar]
- 28.Monini P, Rotola A, Di Luca D, De Lellis L, Chiari E, Corallini A, Cassai E. DNA rearrangements impairing BK virus productive infection in urinary tract tumors. Virology. 1995;214(1):273–9. doi: 10.1006/viro.1995.9928. [DOI] [PubMed] [Google Scholar]
- 29.Martini F, Iaccheri L, Martinelli M, Martinello R, Grandi E, Mollica G, Tognon M. Papilloma and polyoma DNA tumor virus sequences in female genital tumors. Cancer Invest. 2004;22(5):697–705. doi: 10.1081/cnv-200032937. [DOI] [PubMed] [Google Scholar]
- 30.Chatterjee M, Weyandt TB, Frisque RJ. Identification of archetype and rearranged forms of BK virus in leukocytes from healthy individuals. J Med Virol. 2000;60(3):353–62. [PubMed] [Google Scholar]
- 31.Pietropaolo V, Fioriti D, Simeone P, Videtta M, Di Taranto C, Arancio A, Orsi N, Degener AM. Detection and sequence analysis of human polyomaviruses DNA from autoptic samples of HIV-1 positive and negative subjects. Int J Immunopathol Pharmacol. 2003;16(3):269–76. doi: 10.1177/039463200301600313. [DOI] [PubMed] [Google Scholar]
- 32.Dorries K, Vogel E, Gunther S, Czub S. Infection of human polyomaviruses JC and BK in peripheral blood leukocytes from immunocompetent individuals. Virology. 1994;198(1):59–70. doi: 10.1006/viro.1994.1008. [DOI] [PubMed] [Google Scholar]
- 33.Chang D, Wang M, Ou WC, Lee MS, Ho HN, Tsai RT. Genotypes of human polyomaviruses in urine samples of pregnant women in Taiwan. J Med Virol. 1996;48(1):95–101. doi: 10.1002/(SICI)1096-9071(199601)48:1<95::AID-JMV15>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 34.Doerries K. Latent and Persistent Polyomavirus Infection. In: Khalili K, Stoner GL, editors. Human Polyomaviruses: Molecular and Clinical Perspectives. New York: Wiley-Liss; 2001. pp. 197–235. [Google Scholar]
- 35.Arthur RR, Shah KV. The occurrence and significance of papovaviruses BK and JC in the urine. Prog Med Virol. 1989;36:42–61. [PubMed] [Google Scholar]
- 36.Markowitz RB, Eaton BA, Kubik MF, Latorra D, McGregor JA, Dynan WS. BK virus and JC virus shed during pregnancy have predominantly archetypal regulatory regions. J Virol. 1991;65:4515–9. doi: 10.1128/jvi.65.8.4515-4519.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang M, Abend JR, Johnson SF, Imperiale MJ. The role of polyomaviruses in human disease. Virology. 2009 doi: 10.1016/j.virol.2008.09.027. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bressollette-Bodin C, Coste-Burel M, Hourmant M, Sebille V, Andre-Garnier E, Imbert-Marcille BM. A prospective longitudinal study of BK virus infection in 104 renal transplant recipients. Am J Transplant. 2005;5(8):1926–33. doi: 10.1111/j.1600-6143.2005.00934.x. [DOI] [PubMed] [Google Scholar]
- 39.Egli A, Binggeli S, Bodaghi S, Dumoulin A, Funk GA, Khanna N, Leuenberger D, Gosert R, Hirsch HH. Cytomegalovirus and polyomavirus BK posttransplant. Nephrol Dial Transplant. 2007;22(Suppl 8):viii72–viii82. doi: 10.1093/ndt/gfm648. [DOI] [PubMed] [Google Scholar]
- 40.Hirsch HH, Knowles W, Dickenmann M, Passweg J, Klimkait T, Mihatsch MJ, Steiger J. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347(7):488–96. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 41.Fogazzi GB, Cantu M, Saglimbeni L. ‘Decoy cells’ in the urine due to polyomavirus BK infection: easily seen by phase-contrast microscopy. Nephrol Dial Transplant. 2001;16(7):1496–8. doi: 10.1093/ndt/16.7.1496. [DOI] [PubMed] [Google Scholar]
- 42.Bogdanovic G, Priftakis P, Giraud G, Kuzniar M, Ferraldeschi R, Kokhaei P, Mellstedt H, Remberger M, Ljungman P, Winiarski J, et al. Association between a high BK virus load in urine samples of patients with graft-versus-host disease and development of hemorrhagic cystitis after hematopoietic stem cell transplantation. J Clin Microbiol. 2004;42(11):5394–6. doi: 10.1128/JCM.42.11.5394-5396.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Binet I, Nickeleit V, Hirsch HH, Prince O, Dalquen P, Gudat F, Mihatsch MJ, Thiel G. Polyomavirus disease under new immunosuppressive drugs: a cause of renal graft dysfunction and graft loss. Transplantation. 1999;67(6):918–22. doi: 10.1097/00007890-199903270-00022. [DOI] [PubMed] [Google Scholar]
- 44.Mengel M, Marwedel M, Radermacher J, Eden G, Schwarz A, Haller H, Kreipe H. Incidence of polyomavirus-nephropathy in renal allografts: influence of modern immunosuppressive drugs. Nephrol Dial Transplant. 2003;18(6):1190–6. doi: 10.1093/ndt/gfg072. [DOI] [PubMed] [Google Scholar]
- 45.Sundsfjord A, Osei A, Rosenqvist H, Van Ghelue M, Silsand Y, Haga HJ, Rekvig OP, Moens U. BK and JC viruses in patients with systemic lupus erythematosus: prevalent and persistent BK viruria, sequence stability of the viral regulatory regions, and nondetectable viremia. J Infect Dis. 1999;180(1):1–9. doi: 10.1086/314830. [DOI] [PubMed] [Google Scholar]
- 46.Munoz P, Fogeda M, Bouza E, Verde E, Palomo J, Banares R. Prevalence of BK virus replication among recipients of solid organ transplants. Clin Infect Dis. 2005;41(12):1720–5. doi: 10.1086/498118. [DOI] [PubMed] [Google Scholar]
- 47.Rinaldo CH, Hirsch HH. Antivirals for the treatment of polyomavirus BK replication. Expert Rev Anti Infect Ther. 2007;5(1):105–15. doi: 10.1586/14787210.5.1.105. [DOI] [PubMed] [Google Scholar]
- 48.Costa J, Howley PM, Legallais F, Yee C, Young N, Rabson AS. Oncogenicity of a nude mouse cell line transformed by a human papovavirus. J Natl Cancer Inst. 1977;58(4):1147–9. doi: 10.1093/jnci/58.4.1147. [DOI] [PubMed] [Google Scholar]
- 49.Grossi MP, Corallini A, Valieri A, Balboni PG, Poli F, Caputo A, Milanesi G, Barbanti-Brodano G. Transformation of hamster kidney cells by fragments of BK virus DNA. J Virol. 1982;41(1):319–25. doi: 10.1128/jvi.41.1.319-325.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grossi MP, Caputo A, Meneguzzi G, Corallini A, Carra L, Portolani M, Borgatti M, Milanesi G, Barbanti-Brodano G. Transformation of human embryonic fibroblasts by BK virus, BK virus DNA and a subgenomic BK virus DNA fragment. J Gen Virol. 1982;63(2):393–403. doi: 10.1099/0022-1317-63-2-393. [DOI] [PubMed] [Google Scholar]
- 51.Major EO, Di Mayorca G. Malignant transformation of BHK21 clone 13 cells by BK virus--a human papovavirus. Proc Natl Acad Sci U S A. 1973;70(11):3210–2. doi: 10.1073/pnas.70.11.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mason DH, Jr, Takemoto KK. Transformation of rabbit kidney cells by BKV(MM) human papovavirus. Int J Cancer. 1977;19(3):391–5. doi: 10.1002/ijc.2910190317. [DOI] [PubMed] [Google Scholar]
- 53.Pagnani M, Corallini A, Caputo A, Altavilla G, Selvatici R, Catozzi L, Possati L, Barbanti-Brodano G. Co-operation in cell transformation between BK virus and the human c-Harvey-ras oncogene. Int J Cancer. 1988;42(3):405–13. doi: 10.1002/ijc.2910420317. [DOI] [PubMed] [Google Scholar]
- 54.Portolani M, Barbanti-Brodano G, Placa ML. Malignant transformation of hamster kidney cells by BK virus. J Virol. 1975;15(2):420–2. doi: 10.1128/jvi.15.2.420-422.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Portolani M, Borgatti M. Stable transformation of mouse, rabbit and monkey cells and abortive transformation of human cells by BK virus, a human papovavirus. J Gen Virol. 1978;38(2):369–74. doi: 10.1099/0022-1317-38-2-369. [DOI] [PubMed] [Google Scholar]
- 56.Seehafer J, Downer DN, Salmi A, Colter JS. Isolation and characterization of BK virus-transformed rat and mouse cells. J Gen Virol. 1979;42(3):567–78. doi: 10.1099/0022-1317-42-3-567. [DOI] [PubMed] [Google Scholar]
- 57.Seehafer J, Salmi A, Colter JS. Isolation and characterization of BK virus-transformed hamster cells. Virology. 1977;77(1):356–66. doi: 10.1016/0042-6822(77)90432-9. [DOI] [PubMed] [Google Scholar]
- 58.Takemoto KK, Martin MA. Transformation of hamster kidney cells by BK papovavirus DNA. J Virol. 1975;17(1):247–53. doi: 10.1128/jvi.17.1.247-253.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanaka R, Koprowski H, Iwasaki Y. Malignant transformation of hamster brain cells in vitro by human papovavirus Bk. J Natl Cancer Inst. 1976;56(3):671–3. doi: 10.1093/jnci/56.3.671. [DOI] [PubMed] [Google Scholar]
- 60.van der Noordaa J. Infectivity, oncogenicity and transforming ability of BK virus and BK virus DNA. J Gen Virol. 1976;30(3):371–3. doi: 10.1099/0022-1317-30-3-371. [DOI] [PubMed] [Google Scholar]
- 61.Vasavada R, Eager KB, Barbanti-Brodano G, Caputo A, Ricciardi RP. Adenovirus type 12 early region 1A proteins repress class I HLA expression in transformed human cells. Proc Natl Acad Sci U S A. 1986;83(14):5257–61. doi: 10.1073/pnas.83.14.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watanabe S, Kotake S, Nozawa A, Muto T, Uchida S. Tumorigenicity of human BK papovavirus plaque isolates, wild-type and plaque morphology mutant, in hamsters. Int J Cancer. 1982;29(5):583–6. doi: 10.1002/ijc.2910290515. [DOI] [PubMed] [Google Scholar]
- 63.Corallini A, Tognon M, Negrini M, Barbanti-Brodano G. Evidence for BK Virus as a Human Tumor Virus. In: Khalili K, Stoner GL, editors. Human Polyomaviruses: Molecular and Clinical Perspectives. New York: Wiley-Liss, Inc; 2001. pp. 431–60. [Google Scholar]
- 64.Barbanti-Brodano G, Sabbioni S, Martini F, Negrini M, Corallini A, Tognon M. BK virus, JC virus and Simian Virus 40 infection in humans, and association with human tumors. Adv Exp Med Biol. 2006;577:319–41. doi: 10.1007/0-387-32957-9_23. [DOI] [PubMed] [Google Scholar]
- 65.Harris KF, Christensen JB, Imperiale MJ. BK virus large T antigen: interactions with the retinoblastoma family of tumor suppressor proteins and effects on cellular growth control. J Virol. 1996;70(4):2378–86. doi: 10.1128/jvi.70.4.2378-2386.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harris KF, Christensen JB, Radany EH, Imperiale MJ. Novel mechanisms of E2F induction by BK virus large-T antigen: requirement of both the pRb-binding and the J domains. Mol Cell Biol. 1998;18(3):1746–56. doi: 10.1128/mcb.18.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rundell K, Major EO, Lampert M. Association of cellular 56,000- and 32,000-molecular-weight protein with BK virus and polyoma virus t-antigens. J Virol. 1981;37(3):1090–3. doi: 10.1128/jvi.37.3.1090-1093.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sontag E, Fedorov S, Kamibayashi C, Robbins D, Cobb M, Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993;75(5):887–97. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- 69.Shah KV, Daniel RW, Stone KR, Elliott AY. Investigation of human urogenital tract tumors of papovavirus etiology: brief communication. J Natl Cancer Inst. 1978;60(3):579–82. doi: 10.1093/jnci/60.3.579. [DOI] [PubMed] [Google Scholar]
- 70.Grossi MP, Meneguzzi G, Chenciner N, Corallini A, Poli F, Altavilla G, Alberti S, Milanesi G, Barbanti-Brodano G. Lack of association between BK virus and ependymomas, malignant tumors of pancreatic islets, osteosarcomas and other human tumors. Intervirology. 1981;15(1):10–7. doi: 10.1159/000149209. [DOI] [PubMed] [Google Scholar]
- 71.Rubinstein R, Schoonakker BC, Harley EH. Recurring theme of changes in the transcriptional control region of BK virus during adaptation to cell culture. J Virol. 1991;65(3):1600–4. doi: 10.1128/jvi.65.3.1600-1604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watanabe S, Yoshiike K. Change of DNA near the origin of replication enhances the transforming capacity of human papovavirus BK. J Virol. 1982;42(3):978–85. doi: 10.1128/jvi.42.3.978-985.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Watanabe S, Yoshiike K. Decreasing the number of 68-base-pair tandem repeats in the BK virus transcriptional control region reduces plaque size and enhances transforming capacity. J Virol. 1985;55(3):823–5. doi: 10.1128/jvi.55.3.823-825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zambrano A, Kalantari M, Simoneau A, Jensen JL, Villarreal LP. Detection of Human Polyomaviruses and Papillomaviruses in Prostatic Tissue reveals the Prostate as a habitat for Multiple Viral Infections. The Prostate. 2002;53(4):263–76. doi: 10.1002/pros.10157. [DOI] [PubMed] [Google Scholar]
- 75.De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155(6):1985–92. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shah R, Mucci NR, Amin A, Macoska JA, Rubin MA. Postatrophic hyperplasia of the prostate gland: neoplastic precursor or innocent bystander? Am J Pathol. 2001;158(5):1767–73. doi: 10.1016/S0002-9440(10)64132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349(4):366–81. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 78.De Marzo AM, Meeker AK, Zha S, Luo J, Nakayama M, Platz EA, Isaacs WB, Nelson WG. Human prostate cancer precursors and pathobiology. Urology. 2003;62(5):55–62. doi: 10.1016/j.urology.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 79.Das D, Wojno K, Imperiale MJ. BK virus as a cofactor in the etiology of prostate cancer in its early stages. J Virol. 2008;82(6):2705–14. doi: 10.1128/JVI.02461-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Das D, Shah RB, Imperiale MJ. Detection and expression of human BK virus sequences in neoplastic prostate tissues. Oncogene. 2004;23(42):7031–46. doi: 10.1038/sj.onc.1207920. [DOI] [PubMed] [Google Scholar]
- 81.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57(16):3325–30. [PubMed] [Google Scholar]
- 82.Ewald D, Li M, Efrat S, Auer G, Wall RJ, Furth PA, Hennighausen L. Time-sensitive reversal of hyperplasia in transgenic mice expressing SV40 T antigen. Science. 1996;273(5280):1384–6. doi: 10.1126/science.273.5280.1384. [DOI] [PubMed] [Google Scholar]
- 83.Campo MS, Moar MH, Sartirana ML, Kennedy IM, Jarrett WF. The presence of bovine papillomavirus type 4 DNA is not required for the progression to, or the maintenance of, the malignant state in cancers of the alimentary canal in cattle. EMBO J. 1985;4:1819–25. doi: 10.1002/j.1460-2075.1985.tb03856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Theile M, Grabowski G. Mutagenic activity of BKV and JCV in human and other mammalian cells. Arch Virol. 1990;113:221–33. doi: 10.1007/BF01316675. [DOI] [PubMed] [Google Scholar]
- 85.Tognon M, Casalone R, Martini F, Mattei MD, Granata P, Minelli E, Arcuri C, Collini P, Bocchini V. Large T antigen coding sequences of two DNA tumor viruses, BK and SV40, and non random chromosome changes in two glioblastoma cell lines. Cancer Genet Cytogenet. 1996;90:17–23. doi: 10.1016/0165-4608(96)00067-2. [DOI] [PubMed] [Google Scholar]
- 86.Trabanelli C, Corallini A, Gruppioni R, Sensi A, Bonfatti A, Campioni D, Merlin M, Calza N, Possati L, Barbanti-Brodano G. Chromosomal aberrations induced by BK virus T antigen in human fibroblasts. Virology. 1998;243(2):492–6. doi: 10.1006/viro.1998.9080. [DOI] [PubMed] [Google Scholar]
- 87.Russo G, Anzivino E, Fioriti D, Mischitelli M, Bellizzi A, Giordano A, Autran-Gomez A, Di Monaco F, Di Silverio F, Sale P, et al. p53 gene mutational rate, Gleason score, and BK virus infection in prostate adenocarcinoma: Is there a correlation? J Med Virol. 2008;80(12):2100–7. doi: 10.1002/jmv.21312. [DOI] [PubMed] [Google Scholar]
- 88.Lau SK, Lacey SF, Chen YY, Chen WG, Weiss LM. Low frequency of BK virus in prostatic adenocarcinomas. Apmis. 2007;115(6):743–9. doi: 10.1111/j.1600-0463.2007.apm_601.x. [DOI] [PubMed] [Google Scholar]
- 89.Sfanos KS, Sauvageot J, Fedor HL, Dick JD, De Marzo AM, Isaacs WB. A molecular analysis of prokaryotic and viral DNA sequences in prostate tissue from patients with prostate cancer indicates the presence of multiple and diverse microorganisms. Prostate. 2008;68(3):306–20. doi: 10.1002/pros.20680. [DOI] [PubMed] [Google Scholar]
- 90.Fioriti D, Pietropaolo V, Dal Forno S, Laurenti C, Chiarini F, Degener AM. Urothelial bladder carcinoma and viral infections: different association with human polyomaviruses and papillomaviruses. Int J Immunopathol Pharmacol. 2003;16(3):283–8. doi: 10.1177/039463200301600315. [DOI] [PubMed] [Google Scholar]
- 91.Geetha D, Tong BC, Racusen L, Markowitz JS, Westra WH. Bladder carcinoma in a transplant recipient: evidence to implicate the BK human polyomavirus as a causal transforming agent. Transplantation. 2002;73(12):1933–6. doi: 10.1097/00007890-200206270-00015. [DOI] [PubMed] [Google Scholar]
- 92.Roberts IS, Besarani D, Mason P, Turner G, Friend PJ, Newton R. Polyoma virus infection and urothelial carcinoma of the bladder following renal transplantation. Br J Cancer. 2008;99(9):1383–6. doi: 10.1038/sj.bjc.6604711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Monini P, de Lellis L, Rotola A, Di Luca D, Ravaioli T, Bigoni B, Cassai E. Chimeric BK virus DNA episomes in a papillary urothelial bladder carcinoma. Intervirology. 1995;38(5):304–8. doi: 10.1159/000150455. [DOI] [PubMed] [Google Scholar]
- 94.Herawi M, Parwani AV, Chan T, Ali SZ, Epstein JI. Polyoma virus-associated cellular changes in the urine and bladder biopsy samples: a cytohistologic correlation. Am J Surg Pathol. 2006;30(3):345–50. doi: 10.1097/01.pas.0000179117.38787.57. [DOI] [PubMed] [Google Scholar]
- 95.Weinreb DB, Desman GT, Amolat-Apiado MJ, Burstein DE, Godbold JH, Jr, Johnson EM. Polyoma virus infection is a prominent risk factor for bladder carcinoma in immunocompetent individuals. Diagn Cytopathol. 2006;34(3):201–3. doi: 10.1002/dc.20429. [DOI] [PubMed] [Google Scholar]
- 96.Rollison DE, Sexton WJ, Rodriguez AR, Kang LC, Daniel R, Shah KV. Lack of BK virus DNA sequences in most transitional-cell carcinomas of the bladder. Int J Cancer. 2007;120(6):1248–51. doi: 10.1002/ijc.22494. [DOI] [PubMed] [Google Scholar]
- 97.Birkeland SA, Storm HH, Lamm LU, Barlow L, Blohme I, Forsberg B, Eklund B, Fjeldborg O, Friedberg M, Frodin L, et al. Cancer risk after renal transplantation in the Nordic countries, 1964–1986. Int J Cancer. 1995;60(2):183–9. doi: 10.1002/ijc.2910600209. [DOI] [PubMed] [Google Scholar]
- 98.Buzzeo BD, Heisey DM, Messing EM. Bladder cancer in renal transplant recipients. Urology. 1997;50(4):525–8. doi: 10.1016/S0090-4295(97)00305-1. [DOI] [PubMed] [Google Scholar]
- 99.Narayanan M, Szymanski J, Slavcheva E, Rao A, Kelly A, Jones K, Jaffers G. BK virus associated renal cell carcinoma: case presentation with optimized PCR and other diagnostic tests. Am J Transplant. 2007;7(6):1666–71. doi: 10.1111/j.1600-6143.2007.01817.x. [DOI] [PubMed] [Google Scholar]
- 100.Kausman JY, Somers GR, Francis DM, Jones CL. Association of renal adenocarcinoma and BK virus nephropathy post transplantation. Pediatr Nephrol. 2004;19(4):459–62. doi: 10.1007/s00467-003-1407-7. [DOI] [PubMed] [Google Scholar]
- 101.Emerson LL, Carney HM, Layfield LJ, Sherbotie JR. Collecting duct carcinoma arising in association with BK nephropathy post-transplantation in a pediatric patient. A case report with immunohistochemical and in situ hybridization study. Pediatr Transplant. 2008;12(5):600–5. doi: 10.1111/j.1399-3046.2007.00855.x. [DOI] [PubMed] [Google Scholar]
- 102.Knoll A, Stoehr R, Jilg W, Hartmann A. Low frequency of human polyomavirus BKV and JCV DNA in urothelial carcinomas of the renal pelvis and renal cell carcinomas. Oncol Rep. 2003;10(2):487–91. [PubMed] [Google Scholar]
- 103.Lee W, Langhoff E. Polyomavirus in human cancer development. Adv Exp Med Biol. 2006;577:310–8. doi: 10.1007/0-387-32957-9_22. [DOI] [PubMed] [Google Scholar]
- 104.Chenciner N, Meneguzzi G, Corallini A, Grossi MP, Grassi P, Barbanti-Brodano G, Milanesi G. Integrated and free viral DNA in hamster tumors induced by BK virus. Proc Natl Acad Sci U S A. 1980;77(2):975–9. doi: 10.1073/pnas.77.2.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Corallini A, Altavilla G, Carra L, Grossi MP, Federspil G, Caputo A, Negrini M, Barbanti-Brodano G. Oncogenity of BK virus for immunosuppressed hamsters. Arch Virol. 1982;73(3–4):243–53. doi: 10.1007/BF01318078. [DOI] [PubMed] [Google Scholar]
- 106.Corallini A, Altavilla G, Cecchetti MG, Fabris G, Grossi MP, Balboni PG, Lanza G, Barbanti-Brodano G. Ependymomas, malignant tumors of pancreatic islets, and osteosarcomas induced in hamsters by BK virus, a human papovavirus. J Natl Cancer Inst. 1978;61(3):875–83. [PubMed] [Google Scholar]
- 107.Corallini A, Barbanti-Brodano G, Bortoloni W, Nenci I, Cassai E, Tampieri M, Portolani M, Borgatti M. High incidence of ependymomas induced by BK virus, a human papovavirus: brief communication. J Natl Cancer Inst. 1977;59(5):1561–4. doi: 10.1093/jnci/59.5.1561. [DOI] [PubMed] [Google Scholar]
- 108.Uchida S, Watanabe S, Aizawa T, Furuno A, Muto T. Polyoncogenicity and insulinoma-inducing ability of BK Virus, a human Papovavirus, in Syrian golden hamsters. J Natl Cancer Inst. 1979;63(1):119–26. [PubMed] [Google Scholar]
- 109.Fiori M, Di Mayorca G. Occurrence of BK virus DNA in DNA obtained from certain human tumors. Proc Natl Acad Sci U S A. 1976;73(12):4662–6. doi: 10.1073/pnas.73.12.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pater MM, Pater A, Fiori M, Slota J, Di Mayorca G. BK virus DNA sequences in human tumors and normal tissues and cell lines. In: Essex M, Todaro G, Zur Hausen H, editors. Viruses in Naturally Occurring Cancers. Vol. 7. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1980. pp. 329–41. [Google Scholar]
- 111.Israel MA, Martin MA, Takemoto KK, Howley PM, Aaronson SA, Solomon D, Khoury G. Evaluation of normal and neoplastic human tissue for BK virus. Virology. 1978;90(2):187–96. doi: 10.1016/0042-6822(78)90302-1. [DOI] [PubMed] [Google Scholar]
- 112.Wold WS, Mackey JK, Brackmann KH, Takemori N, Rigden P, Green M. Analysis of human tumors and human malignant cell lines for BK virus-specific DNA sequences. Proc Natl Acad Sci U S A. 1978;75(1):454–8. doi: 10.1073/pnas.75.1.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dorries K, Loeber G, Meixensberger J. Association of polyomaviruses JC, SV40, and BK with human brain tumors. Virology. 1987;160(1):268–70. doi: 10.1016/0042-6822(87)90071-7. [DOI] [PubMed] [Google Scholar]
- 114.Flaegstad T, Andresen PA, Johnsen JI, Asomani SK, Jorgensen GE, Vignarajan S, Kjuul A, Kogner P, Traavik T. A possible contributory role of BK virus infection in neuroblastoma development. Cancer Res. 1999;59(5):1160–3. [PubMed] [Google Scholar]
- 115.Jorgensen GE, Johnsen JI, Ponthan F, Kogner P, Flaegstad T, Traavik T. Human polyomavirus BK (BKV) and neuroblastoma: mechanisms of oncogenic action and possible strategy for novel treatment. Med Pediatr Oncol. 2000;35(6):593–6. doi: 10.1002/1096-911x(20001201)35:6<593::aid-mpo22>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 116.De Mattei M, Martini F, Corallini A, Gerosa M, Scotlandi K, Carinci P, Barbanti-Brodano G, Tognon M. High incidence of BK virus large-T-antigen-coding sequences in normal human tissues and tumors of different histotypes. Int J Cancer. 1995;61(6):756–60. doi: 10.1002/ijc.2910610603. [DOI] [PubMed] [Google Scholar]
- 117.Martini F, Iaccheri L, Lazzarin L, Carinci P, Corallini A, Gerosa M, Iuzzolino P, Barbanti-Brodano G, Tognon M. SV40 early region and large T antigen in human brain tumors, peripheral blood cells, and sperm fluids from healthy individuals. Cancer Res. 1996;56(20):4820–5. [PubMed] [Google Scholar]
- 118.Delbue S, Pagani E, Guerini FR, Agliardi C, Mancuso R, Borghi E, Rossi F, Boldorini R, Veggiani C, Car PG, et al. Distribution, characterization and significance of polyomavirus genomic sequences in tumors of the brain and its covering. J Med Virol. 2005;77(3):447–54. doi: 10.1002/jmv.20474. [DOI] [PubMed] [Google Scholar]
- 119.Arthur RR, Grossman SA, Ronnett BM, Bigner SH, Vogelstein B, Shah KV. Lack of association of human polyomaviruses with human brain tumors. J Neurooncol. 1994;20(1):55–8. doi: 10.1007/BF01057961. [DOI] [PubMed] [Google Scholar]
- 120.Greenlee JE, Becker LE, Narayan O, Johnson RT. Failure to demonstrate papovavirus tumor antigen in human cerebral neoplasms. Ann Neurol. 1978;3(6):479–81. doi: 10.1002/ana.410030604. [DOI] [PubMed] [Google Scholar]
- 121.Weggen S, Bayer TA, von Deimling A, Reifenberger G, von Schweinitz D, Wiestler OD, Pietsch T. Low frequency of SV40, JC and BK polyomavirus sequences in human medulloblastomas, meningiomas and ependymomas. Brain Pathol. 2000;10(1):85–92. doi: 10.1111/j.1750-3639.2000.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stolt A, Kjellin M, Sasnauskas K, Luostarinen T, Koskela P, Lehtinen M, Dillner J. Maternal human polyomavirus infection and risk of neuroblastoma in the child. Int J Cancer. 2005;113(3):393–6. doi: 10.1002/ijc.20573. [DOI] [PubMed] [Google Scholar]
- 123.Rollison DE, Utaipat U, Ryschkewitsch C, Hou J, Goldthwaite P, Daniel R, Helzlsouer KJ, Burger PC, Shah KV, Major EO. Investigation of human brain tumors for the presence of polyomavirus genome sequences by two independent laboratories. Int J Cancer. 2005;113(5):769–74. doi: 10.1002/ijc.20641. [DOI] [PubMed] [Google Scholar]
- 124.Caputo A, Corallini A, Grossi MP, Carra L, Balboni PG, Negrini M, Milanesi G, Federspil G, Barbanti-Brodano G. Episomal DNA of a BK virus variant in a human insulinoma. J Med Virol. 1983;12(1):37–49. doi: 10.1002/jmv.1890120105. [DOI] [PubMed] [Google Scholar]
- 125.Corallini A, Pagnani M, Viadana P, Silini E, Mottes M, Milanesi G, Gerna G, Vettor R, Trapella G, Silvani V, et al. Association of BK virus with human brain tumors and tumors of pancreatic islets. Int J Cancer. 1987;39(1):60–7. doi: 10.1002/ijc.2910390111. [DOI] [PubMed] [Google Scholar]
- 126.Pagnani M, Negrini M, Reschiglian P, Corallini A, Balboni PG, Scherneck S, Macino G, Milanesi G, Barbanti-Brodano G. Molecular and biological properties of BK virus-IR, a BK virus variant isolated from a human tumor. J Virol. 1986;59(2):500–5. doi: 10.1128/jvi.59.2.500-505.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Calos MP, Miller JH. Transposable elements. Cell. 1980;20(3):579–95. doi: 10.1016/0092-8674(80)90305-0. [DOI] [PubMed] [Google Scholar]
- 128.Casini B, Borgese L, Del Nonno F, Galati G, Izzo L, Caputo M, Perrone Donnorso R, Castelli M, Risuleo G, Visca P. Presence and incidence of DNA sequences of human polyomaviruses BKV and JCV in colorectal tumor tissues. Anticancer Res. 2005;25(2A):1079–85. [PubMed] [Google Scholar]
- 129.Hernandez-Losa J, Fedele CG, Pozo F, Tenorio A, Fernandez V, Castellvi J, Parada C, Ramon y Cajal S. Lack of association of polyomavirus and herpesvirus types 6 and 7 in human lymphomas. Cancer. 2005;103(2):293–8. doi: 10.1002/cncr.20801. [DOI] [PubMed] [Google Scholar]
- 130.Barzon L, Trevisan M, Masi G, Pacenti M, Sinigaglia A, Macchi V, Porzionato A, De Caro R, Favia G, Iacobone M, et al. Detection of polyomaviruses and herpesviruses in human adrenal tumors. Oncogene. 2008;27(6):857–64. doi: 10.1038/sj.onc.1210699. [DOI] [PubMed] [Google Scholar]
- 131.Moens U, Subramaniam N, Johansen B, Johansen T, Traavik T. A steroid hormone response unit in the late leader of the noncoding control region of the human polyomavirus BK confers enhanced host cell permissivity. J Virol. 1994;68(4):2398–408. doi: 10.1128/jvi.68.4.2398-2408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Giraud G, Ramqvist T, Ragnarsson-Olding B, Dalianis T. DNA from BK virus and JC virus and from KI, WU, and MC polyomaviruses as well as from simian virus 40 is not detected in non-UV-light-associated primary malignant melanomas of mucous membranes. J Clin Microbiol. 2008;46(11):3595–8. doi: 10.1128/JCM.01635-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Barbanti-Brodano G, Pagnani M, Viadana P, Beth-Giraldo E, Giraldo G, Corallini A. BK virus DNA in Kaposi’s sarcoma. Antibiot Chemother. 1987;38:113–20. [PubMed] [Google Scholar]
- 134.Negrini M, Rimessi P, Mantovani C, Sabbioni S, Corallini A, Gerosa MA, Barbanti-Brodano G. Characterization of BK virus variants rescued from human tumours and tumour cell lines. J Gen Virol. 1990;71( Pt 11):2731–6. doi: 10.1099/0022-1317-71-11-2731. [DOI] [PubMed] [Google Scholar]
- 135.Monini P, Rotola A, de Lellis L, Corallini A, Secchiero P, Albini A, Benelli R, Parravicini C, Barbanti-Brodano G, Cassai E. Latent BK virus infection and Kaposi’s sarcoma pathogenesis. Int J Cancer. 1996;66(6):717–22. doi: 10.1002/(SICI)1097-0215(19960611)66:6<717::AID-IJC1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 136.Engels EA, Rollison DE, Hartge P, Baris D, Cerhan JR, Severson RK, Cozen W, Davis S, Biggar RJ, Goedert JJ, et al. Antibodies to JC and BK viruses among persons with non-Hodgkin lymphoma. Int J Cancer. 2005;117(6):1013–9. doi: 10.1002/ijc.21277. [DOI] [PubMed] [Google Scholar]
- 137.Newton R, Ribeiro T, Alvarez E, Ziegler J, Casabonne D, Carpenter L, Beral V, Mbidde E, Parkin DM, Wabinga H, et al. BK virus and cancer in Uganda. Eur J Cancer Prev. 2006;15(4):285–9. doi: 10.1097/00008469-200608000-00002. [DOI] [PubMed] [Google Scholar]
- 138.Newton R, Ribeiro T, Casabonne D, Alvarez E, Touze A, Key T, Coursaget P. Antibody levels against BK virus and prostate, kidney and bladder cancers in the EPIC-Oxford cohort. Br J Cancer. 2005;93(11):1305–6. doi: 10.1038/sj.bjc.6602869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lundstig A, Stattin P, Persson K, Sasnauskas K, Viscidi RP, Gislefoss RE, Dillner J. No excess risk for colorectal cancer among subjects seropositive for the JC polyomavirus. Int J Cancer. 2007;121(5):1098–102. doi: 10.1002/ijc.22770. [DOI] [PubMed] [Google Scholar]
- 140.Cacciotti P, Libener R, Betta P, Martini F, Porta C, Procopio A, Strizzi L, Penengo L, Tognon M, Mutti L, et al. SV40 replication in human mesothelial cells induces HGF/Met receptor activation: a model for viral-related carcinogenesis of human malignant mesothelioma. Proc Natl Acad Sci U S A. 2001;98(21):12032–7. doi: 10.1073/pnas.211026798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cacciotti P, Strizzi L, Vianale G, Iaccheri L, Libener R, Porta C, Tognon M, Gaudino G, Mutti L. The presence of simian-virus 40 sequences in mesothelioma and mesothelial cells is associated with high levels of vascular endothelial growth factor. Am J Respir Cell Mol Biol. 2002;26(2):189–93. doi: 10.1165/ajrcmb.26.2.4673. [DOI] [PubMed] [Google Scholar]
- 142.Catalano A, Romano M, Martinotti S, Procopio A. Enhanced expression of vascular endothelial growth factor (VEGF) plays a critical role in the tumor progression potential induced by simian virus 40 large T antigen. Oncogene. 2002;21(18):2896–900. doi: 10.1038/sj.onc.1205382. [DOI] [PubMed] [Google Scholar]
- 143.Waheed I, Guo ZS, Chen GA, Weiser TS, Nguyen DM, Schrump DS. Antisense to SV40 early gene region induces growth arrest and apoptosis in T-antigen-positive human pleural mesothelioma cells. Cancer Res. 1999;59(24):6068–73. [PubMed] [Google Scholar]
- 144.Galloway DA, McDougall JK. The oncogenic potential of herpes simplex viruses: evidence for a ‘hit-and-run’ mechanism. Nature. 1983;302(5903):21–4. doi: 10.1038/302021a0. [DOI] [PubMed] [Google Scholar]
- 145.Madeley CR. “Is it the cause?” --Robert Koch and viruses in the 21st century. J Clin Virol. 2008;43(1):9–12. doi: 10.1016/j.jcv.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 146.zur Hausen H. Molecular pathogenesis of cancer of the cervix and its causation by specific human papillomavirus types. Curr Top Microbiol Immunol. 1994;186:131–56. doi: 10.1007/978-3-642-78487-3_8. [DOI] [PubMed] [Google Scholar]
- 147.zur Hausen H. Viruses in human tumors--reminiscences and perspectives. Adv Cancer Res. 1996;68:1–22. [PubMed] [Google Scholar]
- 148.zur Hausen H. Viruses in human cancers. Eur J Cancer. 1999;35(14):1878–85. doi: 10.1016/s0959-8049(99)00291-9. [DOI] [PubMed] [Google Scholar]
- 149.zur Hausen H. Oncogenic DNA viruses. Oncogene. 2001;20(54):7820–3. doi: 10.1038/sj.onc.1204958. [DOI] [PubMed] [Google Scholar]
- 150.Barbanti-Brodano G, Martini F, De Mattei M, Lazzarin L, Corallini A, Tognon M. BK and JC human polyomaviruses and simian virus 40: natural history of infection in humans, experimental oncogenicity, and association with human tumors. Adv Virus Res. 1998;50:69–99. doi: 10.1016/s0065-3527(08)60806-4. [DOI] [PubMed] [Google Scholar]
- 151.Lednicky JA, Butel JS. Polyomaviruses and human tumors: a brief review of current concepts and interpretations. Front Biosci. 1999;4:D153–64. doi: 10.2741/lednicky. [DOI] [PubMed] [Google Scholar]
- 152.Kim JY, Koralnik IJ, LeFave M, Segal RA, Pfister LA, Pomeroy SL. Medulloblastomas and primitive neuroectodermal tumors rarely contain polyomavirus DNA sequences. Neuro Oncol. 2002;4(3):165–70. doi: 10.1093/neuonc/4.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Volter C, Hausen H, Alber D, de Villiers EM. Screening human tumor samples with a broad-spectrum polymerase chain reaction method for the detection of polyomaviruses. Virology. 1997;237(2):389–96. doi: 10.1006/viro.1997.8772. [DOI] [PubMed] [Google Scholar]
- 154.Zambrano A, Kalantari M, Simoneau A, Jensen JL, Villarreal LP. Detection of human polyomaviruses and papillomaviruses in prostatic tissue reveals the prostate as a habitat for multiple viral infections. Prostate. 2002;53(4):263–76. doi: 10.1002/pros.10157. [DOI] [PubMed] [Google Scholar]
- 155.Tognon M, Casalone R, Martini F, De Mattei M, Granata P, Minelli E, Arcuri C, Collini P, Bocchini V. Large T antigen coding sequences of two DNA tumor viruses, BK and SV40, and nonrandom chromosome changes in two glioblastoma cell lines. Cancer Genet Cytogenet. 1996;90(1):17–23. doi: 10.1016/0165-4608(96)00067-2. [DOI] [PubMed] [Google Scholar]