Abstract
Abstract Melanocortins and the melanocortin-4 receptor (MC4-R) are enriched in the nucleus accumbens, a brain region that has been implicated in the rewarding action of cocaine and other drugs of abuse. In the present study we use a number of rat behavioral models to show that infusion of a melanocortin peptide antagonist into the nucleus accumbens blocks the reinforcing, incentive motivational, and locomotor sensitizing effects of cocaine. We also show that locomotor responses to repeated cocaine exposure are completely blocked in MC4-R null mutant mice and reduced in Agouti mice that overexpress an endogenous inhibitor of melanocortins in the brain. The results also demonstrate that cocaine administration increases the expression of MC4-R in the nucleus accumbens and striatum, and that MC4-R is co-localized with prodynorphin in medium spiny neurons in the nucleus accumbens. Together, these findings indicate that the behavioral actions of cocaine are dependent on activation of MC4-R, and suggest that upregulation of this receptor by drug exposure may contribute to sensitization of these behavioral responses. Modulation of cocaine reward is a novel action of the melanocortin–MC4-R system and could be targeted for the development of new medications for cocaine addiction.
Keywords: dopamine, drugs of abuse, motivation, nucleus accumbens, rodent
Introduction
The melanocortin neuropeptides α, β and γ melanocyte-stimulating hormone (MSH), which are derived from proopiomelanocortin (POMC)-expressing neurons in the arcuate nucleus of the hypothalamus have been implicated in a number of behavioral and neuroendocrine responses, including grooming, thermoregulation and learning (Spruijt et al., 1992; Alvaro et al., 1997). In addition, the melanocortin system is involved in the control of feeding behavior via regulation of the melanocortin-4 receptor (MC4-R) in the hypothalamus (Fan et al., 1997; Huszar et al., 1997). Melanocortin neuropeptides have also been implicated in the actions of opiates, including modulation of opiate tolerance, dependence and withdrawal (Szekaly et al., 1979; Contreras & Takemori, 1984; Alvaro et al., 1997), and we have reported that the expression of the MC4-R is regulated by repeated opiate administration (Alvaro et al., 1996, 2003).
The role of melanocortins in drug-seeking and -taking behavior has not received as much attention. This is surprising given the amount of evidence demonstrating an interaction between the melanocortin system and dopaminergic neurotransmission in regions known to regulate drug reinforcement and motivational processes. Previous studies have demonstrated an enrichment of melanocortin neuropeptides and MC4-R in dopamine-rich brain regions, including nucleus accumbens and dorsal striatum (Jacobowitz & O’Donohue, 1978; Eskay et al., 1979; Mountjoy et al., 1994; Alvaro et al., 1996; Adan & Gispen, 1997). Moreover, functional interactions between melanocortins and dopaminergic neurotransmission have been demonstrated in these brain regions. For example, infusions of a melanocortin agonist into the lateral ventricle or ventral tegmental area increase dopamine release in the striatum and nucleus accumbens (Florijn et al., 1993a; Lindblom et al., 2001). Melanocortin agonist infusions into the nucleus accumbens also increase dopaminergic activity that is associated with increased stereotypic behaviors, such as grooming (Ryan & Isaacson, 1983; Torre & Celis, 1986; Spruijt et al., 1992; Florijn et al., 1993a; Lezcano et al., 1995). In addition, in vitro studies demonstrate that α-MSH stimulates cAMP production via interactions with dopamine D1 receptors (Lezcano et al., 1995; Cremer et al., 2000). Activation of dopamine neurotransmission, by administration of either a directacting dopamine agonist or an indirect agonist like cocaine, is also reported to upregulate POMC expression in the arcuate nucleus and melanocortin neuropeptide levels in nucleus accumbens (Sarnyai et al., 1992; Tong & Pelletier, 1992). Finally, one recent study demonstrated that infusion of a melanocortin agonist into the lateral ventricle enhances the effects of amphetamine on lateral hypothalamic self-stimulation (Cabeza de Vaca et al., 2002). Together, these studies indicate that melanocortins enhance dopaminergic neurotransmission, and that dopamine and psychostimulants increase melanocortin function.
Given the localization of melanocortins and MC4-R in brain reward regions and functional interactions with dopamine, the current study was undertaken to test the hypothesis that the melanocortin signaling system is necessary for the reinforcing and locomotor activating effects of cocaine. Using a combination of pharmacological and mutant mouse approaches to modulate melanocortin activity, the results of behavioral studies demonstrate that blockade of melanocortins or MC4-R inhibits reinforcing, incentive motivational and locomotor activating responses to cocaine.
Materials and methods
Cocaine self-administration
Rats were used for all behavioral studies, with the exception of the locomotor studies conducted in Agouti with MC4-R null mutant mice as indicated. Intra-accumbens infusions of SHU-9119 or saline vehicle were given via a bilateral indwelling cannulae (23 gauge) using standard stereotaxic procedures. Cannulae placements were confirmed at the conclusion of the experiments by examining the cannulae tracts in collected brains. All animal procedures were in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Yale Animal Care and Use Committee. Cocaine self-administration experiments were conducted according to a standard protocol (Piazza et al., 2000). Male Sprague—Dawley rats (300–400 g) were first trained for food self-administration on a fixed-ratio 1 (FR1) schedule until all animals were self-administering 100 pellets of food per session for at least three consecutive days. Animals were administered Equithesin (4.5 mg/kg, i.p.) anesthesia. Cannulae were then surgically implanted bilaterally in the nucleus accumbens for infusions of a SHU-9119, a selective melanocortin receptor antagonist (Hruby et al., 1995), and a catheter was placed in a jugular vein for administration of cocaine. After a 2-week recovery period, rats were trained to lever press for cocaine (0.50 mg/kg/injection) on a FR1 schedule until stable, defined as lever pressing with less than 10% variability on three consecutive days. Rats self-administered an average of 25 mg cocaine, with no subject exhibiting less than 28 lever presses per training session. In the first experiment, the influence of vehicle or different doses of SHU-9119 (0.125, 0.25, 0.50 and 1.00 lg in 0.5 lL per side) infused into the nucleus accumbens 20 min prior to the start of the self-administration session on lever pressing for cocaine was determined. The stereotaxic coordinates for the nucleus accumbens cannula placements were: AP +1.7 from bregma, ML ± 1.5 from midline, DV -6.0 from the skull. Each animal was infused with a different dose of SHU-9119 in a randomized order for effects on lever pressing for cocaine. Animals received another dose of SHU-9119, but not until they were returned to stable lever pressing (minimum of 25 lever presses per session) for cocaine for at least 3 days and not more than 5 days. Each animal received a minimum of two infusions but not more than five infusions. In addition, the first three doses (0.125, 0.25 and 0.50 μg) were tested first in randomized order because these doses were determined to be in the effective dose range from preliminary studies. In the second experiment, another group of rats were again trained until stable on 0.50 mg/kg/injection of cocaine. On test day 1 the animals were allowed to self-administer one of three different doses of cocaine (0.125, 0.25, 0.50 mg/kg/injection). On test day 2 the animals were infused with a fixed dose of SHU-9119 (0.25 μg per side), and lever pressing for the same dose of cocaine tested on the previous day was determined. The 0.25-μg dose of SHU-9119 was chosen because the higher doses (0.50 and 1.0 μg) completely blocked lever pressing for cocaine, even at the highest dose of cocaine tested (0.50 mg/kg). Animals were returned to stable lever pressing for cocaine at 0.5 mg/kg/injection of cocaine for at least 3 days and not more than 15 days before they were infused with the same dose of SHU-9119 (0.25 μg) but a different dose of cocaine. Each animal received a total of three infusions of SHU-9119 and was tested on the three different doses of cocaine lever pressing.
Cocaine place conditioning
In this test, animals (rats for this study) repeatedly exposed to cocaine in a distinctive environment will come to spend more time in that environment in the absence of cocaine. This type of drug-induced place conditioning is considered a measure of the rewarding effects of cocaine (Van der Kooy, 1987). Place conditioning was conducted using a three-compartment test as described previously (Carlezon et al., 1998). Pre-test studies demonstrated that there was no baseline bias for a particular chamber. Three different procedures were used to determine the influence of SHU-9119 on conditioning to cocaine when given on each training day or on the test day only, in the absence of cocaine in animals that have been previously paired or not paired with cocaine. For the first procedure, subjects either received an intra-accumbens infusion of SHU-9119 (1.0 μg/0.5 μL) or saline 30 min before each cocaine or saline injection on each of six training days (3 days pairing with cocaine and 3 days pairing with saline), but not on the test day when there was no cocaine administered. In the second procedure subjects were paired with cocaine or saline exactly as described for the first procedure but did not receive infusions of SHU-9119; SHU-9119 was then infused 30 min prior to testing on the test day only, in the absence of cocaine. In the third paradigm, saline or SHU-9119 was infused into the nucleus accumbens prior to pairing with saline for 6 days to determine if the antagonist alone influenced preference on the test day. In all cases conditioning was established on six consecutive days by administering cocaine (15 mg/kg, i.p.) or saline given 5 min before confining the animals to one of the two outside conditioning compartments of the three-compartment chamber for 30 min. On alternative conditioning sessions, subjects were given the other treatment and confined to the opposite compartment after a saline or SHU-9119 infusion. On the test day, subjects were infused with saline, placed in the middle compartment and were subsequently allowed 30 min to freely explore all three compartments of the apparatus.
Responding for conditioned reinforcement: effect of cocaine
Enhanced responding for conditioned reinforcement (CR) is increased by exposure to psychomotor stimulant drugs, effects dependent on dopamine in the nucleus accumbens (Taylor & Robbins, 1984, 1986). The ability of prior cocaine exposure to enhance responding for conditioned reinforcers also has been demonstrated and has been hypothesized to contribute to increases in the incentive salience of reward-related stimuli in addiction (Robinson & Berridge, 1993; Jentsch & Taylor, 1999; Taylor & Horger, 1999). Responding for CR was conducted as previously described (Horger et al., 1999). Thirsty rats were trained to associate a compound (tone + light) stimulus with water reward for 15 days. Animals were otherwise restricted to 30 min access to water in the home cage after the training or test sessions (except during surgery or recovery). Food was available ad libitum. On two subsequent test sessions separated by 2 days, they were given cocaine (15 mg/kg) or saline injections 20 min after an intra-accumbens infusion of SHU-9119 (1.0 μg/0.5 μL) and responding for conditioned reinforcement was measured. Responding on one lever resulted in the delivery of the conditioned stimulus (conditioned reinforcer or CR lever), but without the water reinforcement, whereas responding on the other lever resulted in no CR stimulus presentation (NCR lever).
Locomotor sensitization to cocaine
Locomotor sensitization in rats was conducted as previously described (Carlezon et al., 1998). Subjects were habituated to the test apparatus over three consecutive days. Horizontal locomotor activity was quantified using automated beam crossing. Rats were used for the SHU-9119 infusion studies. Thirty minutes after intra-accumbens SHU-9119 infusions (1.0 μg/0.5 μL) or saline, rats were injected with cocaine (15 mg/kg, i.p.) and placed in the apparatus. This procedure was repeated daily over four or five consecutive days. Locomotor sensitization in mice was conducted as previously described (Hiroi et al., 1997). Two mutant mouse models, Agouti mice and MC4-R null mutant mice, were used to further test the influence of the melanocortin system in the actions of cocaine. Agouti mice display ectopic expression of the agouti peptide, which acts as an antagonist of melanocortin receptors in brain and other tissues, and not just skin where it is normally expressed. Agouti mice (C57BL/6J-Ay, #00021) and C57Bl/6J controls (#000664), both from Jackson Laboratories (Bar Harbor, MA, USA), of the same age (10–12 weeks) and weight (30–35 g) were used for these studies. It is possible that behavioral differences between the Agouti and C57Bl/6J are also influenced by genetic background differences, which are not identical in these two lines. MC4-R is one of the most prevalent melanocortin receptor subtypes expressed in brain, and previous studies have demonstrated that this receptor is expressed in the striatum and nucleus accumbens and is regulated by chronic administration of morphine (Alvaro et al., 1996). MC4-R null mutant mice, on a C57BL/6J background, were kindly provided by the laboratory of Dr Dennis Huszar (Millenium Pharmaceuticals, Boston, MA, USA). Wild type (+/+), heterozygous (+/–) and homozygous (–/–) littermates (10–12 weeks old) were used for locomotor sensitization experiments. Mice were habituated to the test apparatus over three consecutive days, and then received cocaine (10 mg/kg, i.p.) or saline injections for six consecutive days. Horizontal locomotor activity was quantified by automated beam crossing for 60 min each day.
RNAse protection and in situ hybridization analysis
Rats were treated with saline or cocaine (15 mg/kg, twice per day, i.p.) for 14 days, killed by decapitation, and brains were collected 3 h after the last injection. The dorsal and nucleus accumbens were dissected together to provide sufficient tissue and total RNA for the RNase protection assay of MC4-R as described (Alvaro et al., 1996). Briefly, total RNA extracted (30 μg) from dissected tissues is hybridized with a 32P-labeled antisense MC4-R riboprobe in solution overnight. After RNase treatment, the double-stranded ‘protected’ RNA was isolated by polyacrylamide gel electrophoresis and quantified by densitometry of film autoradiograms. Double in situ hybridization was conducted according to a published protocol using a 33P-labeled MC4-R riboprobe and digoxigenin-labeled prodynorphin or proenkephalin riboprobes according to standard procedures (Stone et al., 1999). Color reactions were carried out on the hybridized sections, which were then exposed to Kodak emulsion for 14 days, and developed.
Results
Infusion of a melanocortin antagonist blocks the rewarding and locomotor-activating effects of cocaine in rat
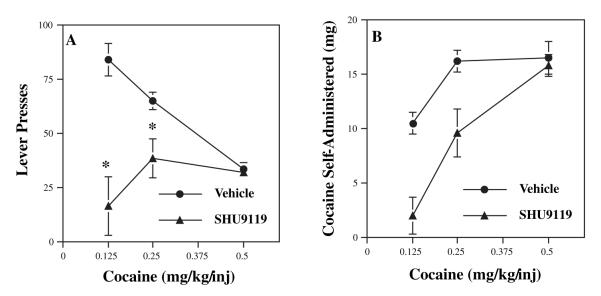
The primary reinforcing effects of cocaine were examined by determining the influence of intra-accumbens infusions of the melanocortin antagonist, SHU-9119, on cocaine self-administration. This synthetic peptide antagonist has approximately 10-fold greater affinity for MC4-R than for MC3-R, the other major melanocortin receptor subtype found in brain (Hruby et al., 1995). Cannulae were surgically placed in the nucleus accumbens and then rats were trained to lever press for cocaine as described in the Materials and methods. Cannula placement was verified by sectioning the brains after the completion of behavioral testing. We found that infusion of SHU-9119 at doses of 0.50 and 1.00 μg per side (in 0.5 μL) into nucleus accumbens completely blocked cocaine self-administration, while lower doses (0.125 and 0.25 μg per side) had no effect (data not shown). Next, we determined the influence of a fixed dose of SHU-9119, 0.25 μg per side, over a dose range of cocaine (0.125, 0.25 and 0.50 mg/kg/injection). This dose of SHU-9119 was chosen so that a dose—response to cocaine could be examined, as preliminary data demonstrated higher doses of SHU-9119 (0.50 and 1.0 μg per side) completely blocked self-administration of 0.50 mg/kg cocaine. SHU-9119 completely blocked level pressing for the lowest dose of cocaine (0.125 mg/kg/injection) and partially, but significantly, blocked the intermediate dose of cocaine (0.25 mg/kg/injection) (Fig. 1A). In contrast, this low dose of SHU-9119 had no effect on lever pressing for the higher cocaine dose (0.5 mg/kg/injection). These data were also plotted as the total amount of cocaine self-administered (Fig. 1B).
Fig. 1.
ocaine self-administration is blocked by intra-accumbens infusion of a melanocortin antagonist. The effect of direct infusions of SHU-9119 into the nucleus accumbens on lever pressing for cocaine was determined. (A) Rats were trained to lever press for cocaine (0.50 mg/kg/injection) and tested with different doses of cocaine on test day 1 as indicated. Subjects were then tested in the presence of a single dose of SHU-9119 (0.25 μg/side) on test day 2 at the same dose of cocaine from the previous day. The results are expressed as the number of lever presses and are the means ± SEM (n = 3 for the 0.125 dose of cocaine and n = 6 animals for each of the two higher doses of cocaine, 0.25 and 0.50). Repeated-measures anova shows a significant interaction between treatment groups (vehicle vs. SHU-9119) and dose of cocaine (F2,12 = 13.99). Post-hoc analysis demonstrates that there are significant differences at the two lower doses of cocaine (*P < 0.01). (B) The results are expressed as the total amount of cocaine self-administered at each dose in the absence or presence of SHU-9119.
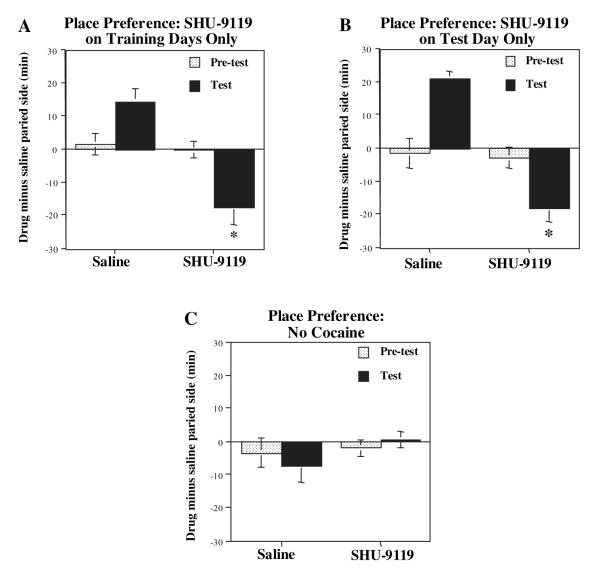
To examine the role of the melanocortin system on the conditioned rewarding properties of cocaine, the influence of intra-accumbens infusion of SHU-9119 on cocaine place preference was examined as described in Materials and methods. Three different SHU-9119 infusion schedules were tested to determine the influence of the melanocortin antagonist on conditioning to cocaine during each paired session and on the test day only in the absence of cocaine either with or without prior cocaine pairing (Carlezon et al., 1998). First, SHU-9119 was infused into the nucleus accumbens each day 30 min prior to cocaine (15 mg/kg) pairing with a particular compartment, but not on the test day. Having established an effective dose range for SHU-9119 in the self-administration paradigm, we chose a dose of 1.0 μg/side in 0.5 μL for these studies. This dose of SHU9119 does not influence locomotor activity of rats compared with saline infusions (see locomotor sensitization experiments). Under these conditions, a significant increase in cocaine place conditioning was observed in the vehicle-infused animals (Fig. 2A). In contrast, this rewarding effect of cocaine was changed to conditioned avoidance with infusion of SHU-9119. This suggests that cocaine was made aversive by pre-treatment with the melanocortin receptor antagonist. In the second paradigm, SHU-9119 was infused on the test day only, and not on the previous days when cocaine was administered (Fig. 2B). Infusion of SHU-9119 on the test day also produced conditioned avoidance as was observed in the previous paradigm. Finally, SHU-9119 was infused each day before saline administration (Fig. 2C). Under these conditions, without exposure to cocaine, SHU-9119 did not produce either avoidance or preference to the saline-paired side.
Fig. 2.
Cocaine-induced place conditioning is blocked by intra-accumbens infusion of SHU-9119. Rats received intra-accumbens infusions of SHU-9119 (1 μg/0.5 μL) or vehicle according to three different schedules. (A) SHU-9119 was infused each day 30 min before cocaine administration, but not on the test day. (B) SHU-9119 was infused on the test day only. (C) SHU-9119 was infused alone in the absence of cocaine. The results are presented as time spent in the drug-paired side minus time spent in the saline-paired side, and are the mean ± SEM of eight—nine animals per group (A and B) or six—seven per group (C). *P < 0.05 compared with saline-infused control (anova and Fisher’s post-hoc test).
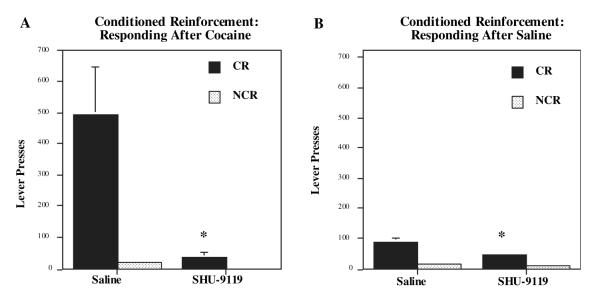
The influence of the melanocortin receptor antagonist on responding for non-drug (i.e. water) reward-related CR was also examined to assess incentive motivation. Intra-accumbens SHU-9119 (1.0 μg per side in 0.5 μL) significantly blocked the ability of cocaine (15 mg/kg) to enhance responding for CR, but did not effect responding on the control or NCR that did not result in presentation of the CR stimulus (Fig. 3A) This suggests that melanocortin receptor antagonism could selectively produce alterations in dopamine-dependent CR mechanisms rather than produce changes related to non-specific motor activation. After saline challenge, intra-accumbens SHU-9119 also significantly attenuated responding on the CR, but not on the control (NCR) lever (Fig. 3B), suggesting that under baseline conditions intra-accumbens SHU-9119 infusions selectively attenuated the ability of the stimulus to act as a CR.
Fig. 3.
Cocaine-enhanced conditioned reward is blocked by intra-accumbens infusion of SHU-9119. Rats were trained to associate a compound stimulus (tone + light) with water reward for 15 days. Responding for the conditioned reinforcer (CR lever) relative to the no CR stimulus (NCR lever) was measured after (A) cocaine (15 mg/kg) or (B) saline injections. Intra-accumbens SHU-9119 significantly blocked cocaine-enhanced responding for CR compared with the saline infusions. After saline, animals receiving intra-accumbens infusions of SHU-9119 (1 μg/0.5 μL) also made significantly fewer CR responses than subjects given intra-nucleus accumbens saline infusions. Bars represent the mean number of lever responses ± SEM, n = 6,7 for (A) and n = 14 for (B). *P < 0.01 SHU-9119 compared with saline-infused controls (ANOVA and Fisher’s post-hoc test).
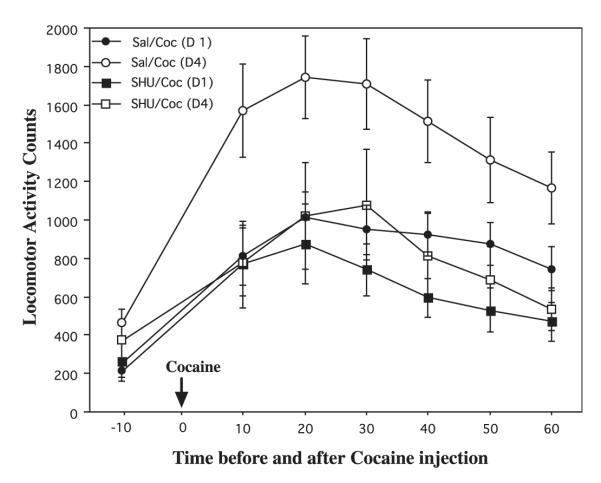
The influence of SHU-9119 on the psychostimulant or locomotor-activating effects of cocaine was also determined. Figure 4 demonstrates the locomotor activity counts 10 min before and for the 60-min period after cocaine administration. Animals received intra-accumbens saline or SHU-9119 infusions 30 min prior to cocaine administration. The responses on day 1 and 4 of cocaine exposure are shown. There were significant differences between the groups that were observed over the 60-min post-cocaine injection period. Administration of cocaine for 4 days resulted in a significant increase in levels of locomotor activity relative to cocaine for 1 day demonstrating the locomotor-activating effects of repeated cocaine. Intra-accumbens infusion of SHU-9119 completely blocked the effects of repeated cocaine administration. SHU-9119 infusion did not influence baseline locomotor activity during the 10-min interval prior to cocaine administration (Fig. 4). In contrast to this antagonist effect, infusion of a melanocortin agonist, α-melanocyte-stimulating hormone (α-MSH), into nucleus accumbens (30 min before each cocaine treatment) significantly increased cocaine responsiveness [activity counts on day 5: vehicle = 1243 ± 172, α-MSH = 2204 ± 243, mean ± SEM (n = 6), p < 0.05 (Student’s t-test)].
Fig. 4.
Cocaine-induced locomotor activation is blocked by intra-accumbens infusion of SHU-9119. Rats received bilateral intra-nucleus accumbens infusions of SHU-9119 (1 μg/0.5 μL) or saline 30 min before administration of cocaine (15 mg/kg) for four consecutive days. Locomotor activity is shown 10 min prior to cocaine and for 10-min intervals after cocaine administration as indicated. The locomotor activity counts on day 1 (D1) and day 4 (D4) of cocaine administration are shown. The results are presented as mean activity counts ± SEM, n = 13 for saline + cocaine, and n = 11 for SHU-9119 + cocaine. Post-hoc comparisons revealed significant reductions in the SHU-9119 animals compared with saline animals at 10, 20, 40, 50 and 60 min (P < 0.05), and a trend at the 30 min time point after cocaine on day 4. No differences were observed between the groups on day 1 or before the cocaine infusions on day 1 or 4. P < 0.05 compared with vehicle-infused control (ANOVA and Fisher’s post-hoc test).
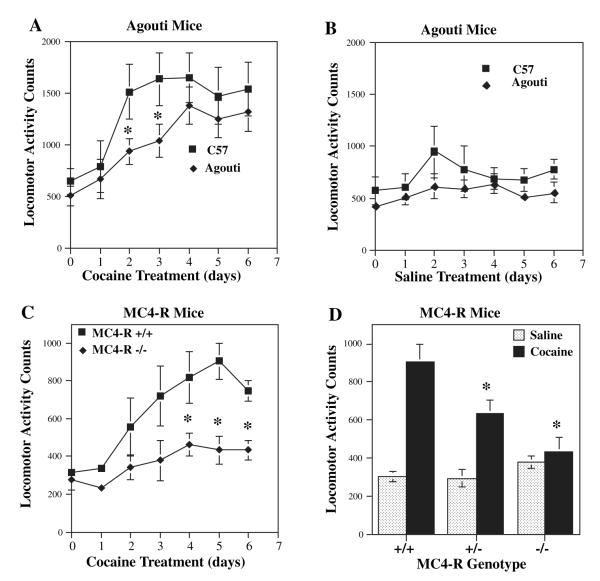
Locomotor-activating effects of cocaine are blocked in Agouti and MC4-R mutant mice
A role for the melanocortin system was further examined in Agouti mice, and the role of MC4-R was directly tested in MC4-R null mutant mice. In Agouti mice, ectopic expression of the agouti peptide results in blockade of melanocortin receptors in tissues other than skin, where the neuropeptide is normally found (Lu et al., 1994). Both of these mutant models have been used to demonstrate that blockade of melanocortins and MC4-R increases food consumption and results in obesity via regulation of hypothalamic melanocortin receptors (Fan et al., 1997; Huszar et al., 1997). In control mice repeated administration of a low dose of cocaine (10 mg/kg), which did not significantly increase locomotor activity after a single dose, increased the locomotor-activating effects of cocaine approximately threefold by day 3 of cocaine treatment (Fig. 5A) (Hiroi et al., 1997). Locomotor activation induced by cocaine was attenuated in the Agouti mice relative to control mice (Fig. 5A). This effect was significant only at the earlier time points studied (days 2 and 3), possibly due to incomplete inhibition of the melanocortin system by agouti protein. There was no significant difference in the response to saline between the Agouti mice and controls (Fig. 5B). A similar but more robust effect was observed in the MC4-R null mutant mice (–/–) relative to their wild-type littermates (+/+). In the homozygous MC4-R –/– mice, cocaine-induced locomotor activation was completely abolished relative to +/+ mice (Fig. 5C), and an intermediate reduction in the cocaine response was observed in MC4-R +/– mice (Fig. 5D). In contrast, locomotor activity in response to saline administration was indistinguishable among wild-type littermates and MC4-R +/– and –/– mice (Fig. 4D).
Fig. 5.
Cocaine-induced locomotor activity is blocked in Agouti and MC4-R null mutant mice. (A, B) Agouti mice and C57BL/6 control mice were given cocaine (10 mg/kg) or saline on days 1–6, and levels of locomotor activity were measured. Day 0 reflects locomotor activity after 3 days of habituation to the chambers. Cocaine-induced locomotor activation was significantly reduced in the Agouti mice (A), but the response to saline administration (B) was not significantly different between Agouti and C57BL/6 mice. The results are presented as locomotor activity counts and are the mean ± SEM. The numbers of animals per group were: C57BL/6 + cocaine = 8; Agouti + cocaine = 9; C57BL/6 + saline = 6; Agouti + saline = 5. (C, D) MC4-R wild-type (+/+), heterozygous (+/–) and homozygous null mutant (–/–) littermates were also tested for cocaine-induced locomotor behavior. The response to cocaine on each day was significantly decreased in MC4-R –/– mice (C). (D) The response to either cocaine or saline treatment on day 5 only, demonstrating a significant, but intermediate, reduction in the MC4-R +/– mice, as well as complete inhibition of the cocaine response in the MC4-R –/– mice. The locomotor activity counts in response to cocaine in homozygous null mutant mice demonstrated no significant difference from locomotor activity counts in wild-type littermates receiving saline in all the days tested (data not shown). In addition, (D) demonstrates that there is no significant difference in baseline locomotor activity, in the absence of cocaine, between the MC4-R +/+, +/– and –/– littermates. The results shown are the first 10 min after cocaine administration and are presented as the mean locomotor activity counts ± SEM. The numbers of animals per group for cocaine treatment were: +/+ = 5; +/– = 7; and –/– = 10; and for saline treatment: +/+ = 4; +/- =7; and –/– = 9. *P < 0.05 compared with the corresponding control (ANOVA and Fisher’s post-hoc test).
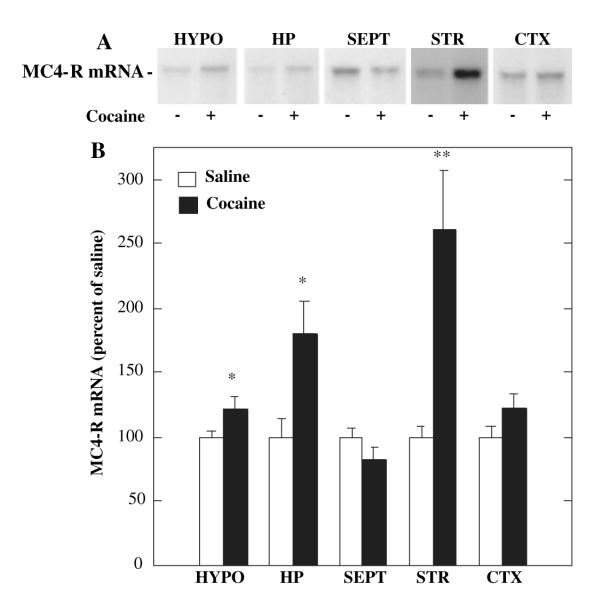
MC4-R expression is upregulated by cocaine
The molecular and cellular interactions between the melanocortin and mesolimbic dopamine systems were examined to elucidate the mechanisms underlying the effects of melanocortin blockade on cocaine reward. Because MC4-R is one of the major melanocortin receptors expressed in the striatum (Alvaro et al., 1996, 2003), and based on the results obtained in the MC4-R null mutant mice, efforts were focused on this receptor subtype. We first examined the influence of cocaine administration on expression of MC4-R mRNA by RNase protection analysis of dissected brain regions containing both the dorsal and ventral (nucleus accumbens) striatum. The entire striatum was used because more selective dissections of the nucleus accumbens alone did not provide sufficient amounts of mRNA for the RNase protection analysis. The results demonstrate that chronic cocaine administration results in a two- to threefold induction of MC4-R mRNA in striatum and smaller increases in the hypothalamus and hippocampus (Fig. 6A and B). There was no significant effect of cocaine treatment on expression of MC4-R mRNA in the septum or cerebral cortex (Fig. 6A and B). We have also found that this effect is dependent on chronic cocaine administration (Alvaro et al., 2003).
Fig. 6.
Regulation of MC4-R mRNA by administration of cocaine. (A, B) Rats were given cocaine (15 mg/kg, twice daily for 14 days) and MC4-R mRNA was determined by RNase protection analysis. MC4-R mRNA was measured in hypothalamus (HYPO), hippocampus (HP), septum (SEPT), striatum (STR), and cerebral cortex (CTX). (A) Representative autoradiograms for each of the brain regions examined. (B) MC4-R mRNA was quantified by densitometry and the results are expressed as percent of control (saline). Each bar represents the mean ± SEM, n = 6. *P < 0.05 or **P < 0.005, compared with control (Student’s t-test).
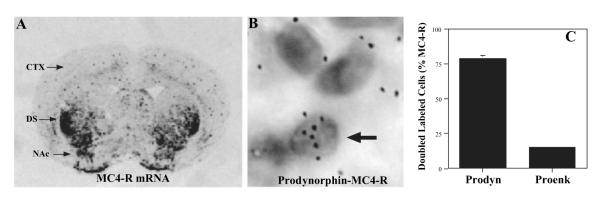
The results of our behavioral studies indicate that the melanocortin—MC4-R system in nucleus accumbens contributes to both the primary and conditioned rewarding, as well as the locomotor-activating effects of cocaine, and that blockade of this neuropeptide system inhibits these behavioral responses to cocaine. To examine the cellular basis for the actions of the melanocortin system on cocaine-mediated behaviors, co-localization of MC4-R in prodynorphin- as well as in proenkephalin-containing neurons in the nucleus accumbens was examined. In situ hybridization analysis demonstrates the enrichment of MC4-R mRNA in dorsal striatum and nucleus accumbens (Fig. 7A). Double in situ hybridization analysis demonstrates that MC4-R is primarily co-localized with prodynorphin (Fig. 7B), and is expressed with proenkephalin at a much lower rate. Quantitative analysis revealed that in the nucleus accumbens 79.1 ± 1.2% of cells positive for MC4-R mRNA also stained positive for prodynorphin (100 cells counted per rat, n = 6 rats, for a total of 600 cells), while only 15.3 ± 0.4% of cells positive for MC4-R mRNA also stained positive for proenkephalin (100 cells counted/rat, n = 6, for a total of 600 cells).
Fig. 7.
Co-localization of MC4-R in prodynorphin-expressing neurons. (A) In situ hybridization analysis of MC4-R mRNA using a 35S-labeled riboprobe demonstrates enrichment of MC4-R expression in the striatum, including the dorsal striatum (DS) and nucleus accumbens (NAc). Co-localization of 35S-labeled MC4-R riboprobe and a digoxigenin-labeled prodynorphin riboprobe (B) in the nucleus accumbens was conducted according to standard procedures. Representative micrographs demonstrate 35S-labeled MC4-R grains over (B) prodynorphin-expressing cells in the nucleus accumbens. (C) The number of MC4-R-positive cells that also express either prodynorphin or proenkepalin was determined. Co-localization of MC4-R with proenkephalin was also determined (not shown). A total of 600 MC4-R-positive cells was identified for each double-labeling condition (100 cells per rat, n = 6). The results are expressed as percent of MC4-R-positive cells that are also positive for either prodynorphin or proenkephalin.
Discussion
Previous studies have demonstrated anatomical and functional interactions between the melanocortin- and dopamine-regulated signaling systems. The results of the present study provide the first direct evidence that the rewarding and locomotor-activating effects of cocaine are critically dependent on the activity of the melanocortin system in the nucleus accumbens. The requirement for melanocortins was demonstrated in three different behavioral models that are sensitive to the effects of cocaine on reinforcement processes, as well as analysis of the locomotor-activating effects of cocaine. Self-administration is regarded to be one of the most relevant behavioral models for assessing the reinforcing actions of cocaine (i.e. drug taking), and microinfusions of a melanocortin antagonist into the nucleus accumbens attenuated cocaine self-administration. Administration of SHU-9119 resulted in a downward shift in the number of lever presses in the dose—response to cocaine, as well as a rightward shift when expressed as total amount of cocaine self-administered. This suggests that SHU-9119 produces both a rate-decreasing effect as well as a competitive shift in responding that is overcome by higher doses of cocaine. Together, the results indicate that the primary reinforcing effects of cocaine are dependent on the melanocortin system in the nucleus accumbens. However, we cannot exclude the possibility that infusions of SHU-9119 also reached the dorsal striatum by traveling up the cannula track. Further studies must be conducted to test the effects of SHU-9119 in dorsal striatum and other brain regions.
The results of the current study are also consistent with a recent report demonstrating that infusion of a melanocortin agonist increases the acute reinforcing effects of amphetamine measured by self-stimulation of the lateral hypothalamus (Cabeza de Vaca et al., 2002). However, there were several differences between the latter and present studies. First, for the hypothalamic self-stimulation study the melanocortin agonist infusions were made into the lateral ventricle, and it was not possible to determine the brain region that underlies the effects of the melanocortin system on psychostimulant responsiveness. Second, the effects of the melanocortin agonist on acute, but not repeated, amphetamine enhancement of self-stimulation was examined. This could also account for the lack of effect of SHU-9119 on acute amphetamine enhancement of self-stimulation of lateral hypothalamus (Cabeza de Vaca et al., 2002) and is consistent with the lack of effect of SHU-9119 on the acute locomotor response to cocaine in the present study (i.e. day 1). All of the drug reward models of the current study used repeated administration of cocaine that results in sensitization of the behavioral actions of cocaine. Intra-accumbens infusions of SHU-9119 blocked cocaine-induced self-administration and locomotor sensitization. Taken together, the results of the current study demonstrate that upregulation of the nucleus accumbens melanocortin signaling is necessary for the rewarding effects of cocaine that are believed to contribute to aspects of drug addiction (Everitt & Wolf, 2002).
Similar results were obtained with the conditioned place preference and conditioned reinforcement paradigms that model the conditioned reinforcing and incentive motivational effects of drugs of abuse. Infusions of the melanocortin antagonist into the nucleus accumbens blocked cocaine-induced place preference and cocaine-enhanced responding for conditioned reinforcement, as well as the ability of the conditioned stimulus to act as a reinforcer. Inhibition of cocaine-induced place conditioning was observed whether the antagonist was infused before each pairing of a compartment with cocaine administration or only infused on the test day in the absence of cocaine. Moreover, the place conditioning studies indicate that infusion of the melanocortin antagonist causes avoidance to cocaine, although SHU-9119 infusions do not produce avoidance when administered in the absence of cocaine. The selective avoidance produced by the melanocortin antagonist on the test day could be due to an attenuation of cocaine’s positive conditioned effect, thereby unmasking possible conditioned aversive actions of the psychostimulant (Carlezon et al., 1998). Together these findings indicate that the heightened incentive qualities of drugs and drug-associated stimuli (i.e. conditioned reinforcing properties) are dramatically attenuated by blockade of nucleus accumbens melanocortin signaling, in addition to blockade of the primary reinforcing (i.e. unconditioned) effects of cocaine demonstrated in the self-administration studies. Moreover, the results suggest that blockade of the melanocortin system may attenuate the conditioned reinforcing properties of drugs of abuse that contribute to certain aspects of addiction.
The results also demonstrate that the melanocortin system is necessary for the development of cocaine-induced locomotor activation. Infusions of the melanocortin antagonist into nucleus accumbens completely blocked locomotor sensitization to cocaine. In contrast, SHU-9119 did not influence locomotor activity prior to administration of cocaine. Although the latter result suggests that SHU-9119 does not produce generalized sedation, we cannot rule out the possibility that rate-decreasing or sedation contribute to the effects of SHU-9119 in the operant or conditioned reinforcement paradigms. Similar effects were also observed in Agouti mice, in which ectopic expression of agouti protein blocks melanocortin receptors in brain and other tissues (Lu et al., 1994). Locomotor activation in response to repeated cocaine administration was significantly decreased in the Agouti mice relative to controls, but there was no significant difference in baseline locomotor activity between Agouti and control mice after administration of saline. This demonstrates, by a complementary approach, that inhibition of the melanocortin system blocks behavioral responses to cocaine. The melanocortin antagonist, SHU-9119, used for the behavioral studies has affinity for both MC4-R as well as MC3-R (Hruby et al., 1995), the other melanocortin receptor subtype expressed in the brain, although MC4-R is the predominant subtype expressed in the striatum and nucleus accumbens (Alvaro et al., 1996). The role of the MC4-R subtype was directly demonstrated by studies showing that the locomotor-activating effects of cocaine were completely blocked in MC4-R homozygous null mutant mice and partially blocked in heterozygous null mutants. The effect of MC4-R mutation was specific to the cocaine response because there was no difference in locomotor activity following saline injections. These results provide strong evidence that MC4-R is the receptor subtype that mediates the actions of the melanocortin system on cocaine-induced locomotor sensitization. Future studies will examine the influence of the melanocortin system on responding to saline or cocaine challenge to examine the expression of locomotor sensitization in the absence of the melanocortin antagonist.
The molecular and cellular mechanisms underlying the actions of the melanocortin system in the responses to cocaine were also examined. We found that repeated administration of cocaine increases the expression of MC4-R mRNA, determined by RNase protection analysis. In a recent study we have found that administration of a low dose of morphine also upregulates the expression of MC4-R mRNA in striatum (Alvaro et al., 2003). The observed upregulation of MC4-R in the striatum would be expected to enhance the behavioral effects of cocaine and could therefore be one mechanism contributing to the development of drug-induced behavioral sensitization. Previous studies have demonstrated that agonist-activation of the melanocortin system increases dopaminergic activity (Ryan & Isaacson, 1983; Torre et al., 1986; Spruijt et al., 1992; Lezcano et al., 1995; Lindblom et al., 2001; Florijn et al., 1993a,b). Based on these reports, upregulation of MC4-R in the nucleus accumbens by cocaine treatment would be expected to increase dopaminergic-regulated neurotransmission and intracellular signaling, resulting in increased sensitivity to cocaine, which could be one mechanism contributing to drug-induced sensitization. Upregulation of MC4-R by repeated cocaine also provides indirect support for the hypothesis that this receptor subtype mediates the actions of the melanocortin antagonist on cocaine-induced behaviors. Because of the requirement for relatively large amounts of tissue and RNA for the RNase protection assay, expression of MC4-R mRNA was determined in the entire striatum. Additional studies will be required to examine the regulation of MC4-R mRNA in the nucleus accumbens to further test this hypothesis.
Previous reports of positive interactions between melanocortins and dopaminergic neurotransmission suggest a possible mechanism by which the melanocortin—MC4-R system could be involved in the actions of cocaine (Ryan & Isaacson, 1983; Torre et al., 1986; Spruijt et al., 1992; Florijn et al., 1993a; Lezcano et al., 1995). MC4-R, like the dopamine-D1 receptor, is positively coupled to the cAMP second messenger cascade (Gantz et al., 1993; Mountjoy et al., 1994). In addition, D1 receptor antagonist administration blocks cocaine-induced place preference and responding for conditioned reinforcement (Wolterink et al., 1993; Shippenberg & Heidbreder, 1995). These findings suggest that activation of the MC4-R-coupled cAMP cascade could enhance D1 receptor function. In support of this hypothesis, the results of the double in situ hybridization studies demonstrate that MC4-R is co-localized with prodynorphin mRNA in nucleus accumbens medium spiny neurons. Given the high degree of D1 receptor localization with prodynorphin, this suggests that MC4-R is expressed in the same population of neurons that express the D1 receptor in nucleus accumbens. Thus, the shared post-receptor signaling pathways for MC4-R and D1 receptors, activation of the cAMP cascade, could account for their positive functional interactions. An alternative explanation is provided by studies demonstrating that α-MSH acts as an allosteric inhibitor of D1 receptors (Lezcano et al., 1995), and upregulation of MC4-R and subsequent binding of α-MSH could reduce the availability of this neuropeptide for this allosteric site.
The molecular and cellular mechanisms underlying cocaine-induced neural plasticity in the nucleus accumbens are reported to be critically dependent on adaptations of the dopamine and glutamate neurotransmitter systems (Hyman & Malenka, 2001; Nestler, 2001; Everitt & Wolf, 2002), and the results of the present study demonstrate a novel and important role for adaptation of the melanocortin neuropeptide system. The results demonstrating that the melanocortin antagonist blocks not only the primary reinforcing, but also the conditioned reinforcing, effects of cocaine raise the possibility that the melanocortin system may be involved in the ability of reward-related stimuli to induce compulsive drug-seeking behavior. Moreover, the results of this study support the possibility that an MC4-R antagonist could be used for the treatment of cocaine addiction by blocking the adaptive processes associated with addiction (Hyman & Malenka, 2001; Nestler, 2001; Everitt & Wolf, 2002). Future studies will examine the influence of small molecule antagonists that can be administered peripherally to block central melanocortin receptors to directly test this hypothesis. The discovery that melanocortins and MC4-R regulate eating behavior (Fan et al., 1997; Huszar et al., 1997) has stimulated the development of such drugs. It is also interesting to speculate that melanocortin regulation of feeding may be mediated, in part, via similar effects on brain reward systems that include the nucleus accumbens (Kelley & Berridge, 2002), in addition to actions on hypothalamic nuclei known to control eating homeostasis (Cowley et al., 1999). Thus, drugs acting at MC4-R could prove useful for a wide array of disorders related to appetitive-motivated behavior.
Acknowledgements
Supported by grants (to R.S.D., E.J.N. and J.R.T.) from the National Institute on Drug Abuse, and a grant (to V.J.H.) from the US Public Health Service. We would also like to acknowledge Millenium Pharmaceuticals (Cambridge, MA, USA) for providing the MC4-R null mutant mice and Valyphone Phantharangsy for excellent technical support.
Abbreviations
- CR
conditioned reinforcement
- FR-1
fixed-ratio 1
- MC4-R
melanocortin-4 receptor
- MSH
melanocyte-stimulating hormone
- NCR
non-conditioned reinforcement
- POMC
proopiomelanocortin.
References
- Adan R, Gispen WH. Brain melanocortin receptors: from cloning to function. Peptides. 1997;18:1279–1287. doi: 10.1016/s0196-9781(97)00078-8. [DOI] [PubMed] [Google Scholar]
- Alvaro JD, Hsu R, Duman RS. Chronic cocaine administration increases the expression of MC4-R in rat neostriatum. J. Pharmacol. Exp. Ther. 2003;304:391–399. doi: 10.1124/jpet.102.040311. [DOI] [PubMed] [Google Scholar]
- Alvaro J, Tatro JB, Duman RS. Melanocortins and opiate addiction. Life Sci. 1997;61:1–9. doi: 10.1016/s0024-3205(97)00029-5. [DOI] [PubMed] [Google Scholar]
- Alvaro J, Tatro JB, Quillan JM, Fogliano M, Eisenhard M, Lerner MR, Nestler EJ, Duman RS. Morphine down-regulates melanocortin-4 receptor expression in brain regions that mediate opiate addiction. Mol. Pharmacol. 1996;50:583–591. [PubMed] [Google Scholar]
- de Vaca S. Cabeza, Kim G-Y, Carr KD. The melanocortin receptor agonist MTII augments the rewarding effect of amphetamine ad-libitum-fed and food-restricted rats. Psychopharmacol. 2002;161:77–85. doi: 10.1007/s00213-002-0998-1. [DOI] [PubMed] [Google Scholar]
- Carlezon WJ, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Contreras P, Takemori AE. Antagonism of morphine-induced analgesia tolerance and dependence by α-melanocyte-stimulation hormone. JPET. 1984;229:21–26. [PubMed] [Google Scholar]
- Cowley M, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY, AGRP, and melanocortin signals in the hypothalalmic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–163. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- Cremer M, Sanchez MS, Celis ME. Structure-activity studies of α-melanotropin fragments on cAMP production in striatal slices. Peptides. 2000;21:803–806. doi: 10.1016/s0196-9781(00)00211-4. [DOI] [PubMed] [Google Scholar]
- Eskay R, Giraud P, Oliver C, Brown-Stein MJ. Distribution of α-melanocyte-stimulating hormone in the rat brain: evidence that α-MSH-containing cells in the arcuate region send projections to extrahypothalamic areas. Brain Res. 1979;178:55–67. doi: 10.1016/0006-8993(79)90087-8. [DOI] [PubMed] [Google Scholar]
- Everitt B, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J. Neurosci. 2002;22:3312–3320. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- Florijn W, Holtmaat AJ, de Lang H, Spierenburg H, Gispen WH, Versteeg DH. Peptide-induced grooming behavior and caudate nucleus dopamine release. Brain Res. 1993a;625:169–172. doi: 10.1016/0006-8993(93)90151-c. [DOI] [PubMed] [Google Scholar]
- Florijn W, Mulder AH, Versteeg DH, Gispen WH. Adrenocorti-cotropin/alpha-melanocyte-stimulation hormone (ACTH/MSH)-like peptides modulate adenylate cyclase activity in rat brain slices: evidence for an ACTH/MSH receptor-coupled mechanism. J. Neurochem. 1993b;60:2204–2211. doi: 10.1111/j.1471-4159.1993.tb03506.x. [DOI] [PubMed] [Google Scholar]
- Gantz I, Konda Y, Tashiro T, Shimoto H, Miwa H, Munsert G, De Watson SJI, Valle J, Yamada T. Molecular cloning of a novel melanocortin receptor. J. Biol. Chem. 1993;268:8246–8250. [PubMed] [Google Scholar]
- Hiroi N, Brown JR, Haile CN, Ye H, Greenberg ME, Nestler EJ. FosB mutant mice: loss of chronic cocaine induction of Fos-related proteins and heightened sensitivity to cocaine’s psychomotor and rewarding effects. PNAS. 1997;94:10397–10402. doi: 10.1073/pnas.94.19.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horger B, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J. Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby V, Lu D, Sharma SD, Castrucci AL, Kesterson RA, al-Obeidi FA, Hadley ME, Cone RD. Cyclic lactam alpha-melanotropin analogues of Ac-Nle4-cyclo [Asp5, D-Phe7, Lys10] alpha-melanocyte-stimulating hormone-(4–10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. J. Med. Chem. 1995;38:3454–3461. doi: 10.1021/jm00018a005. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of themelanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- Hyman S, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat. Rev. Neuro. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Jacobowitz D, O’Donohue TL. g-Melanocyte stimulating hormone: immunohistochemical identification and mapping in neurons in brain. PNAS. 1978;75:6300–6304. doi: 10.1073/pnas.75.12.6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JP, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control over behavior by reward-related stimuli. Psychopharmacol. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J. Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezcano N, DeBarioglio SR, Celis ME. α-MSH changes cyclic AMP levels in rat brain slices by an interaction with the D1 dopamine receptor. Peptides. 1995;16:133–137. doi: 10.1016/0196-9781(94)00157-2. [DOI] [PubMed] [Google Scholar]
- Lezcano N, Salvatierra NA, Celis ME. α-Melanotropin hormone inhibits the binding of [3H]SCH 23390 to the dopamine D1 receptor in vitro. Eur. J. Pharmacol. 1995;363:211–215. doi: 10.1016/s0014-2999(98)00772-9. [DOI] [PubMed] [Google Scholar]
- Lindblom J, Opmane B, Mutulis F, Mutule I, Petrovska R, Klusa V, Bergstrom L, Wikberg JE. The MC4 receptor mediates alpha-MSH induced release of nucleus accumbens dopamine. Neuroreport. 2001;12:2155–2158. doi: 10.1097/00001756-200107200-00022. [DOI] [PubMed] [Google Scholar]
- Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik RP, Wilkison WO, Cone RD. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. 1994;371:799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- Mountjoy K, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol. Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- Nestler E. Total recall — the memory of addiction. Science. 2001;292:2266–2267. doi: 10.1126/science.1063024. [DOI] [PubMed] [Google Scholar]
- Piazza P, Deroche-Gamonent V, Rouge-Pont F, LeMoal M. Vertical shifts in self-administration dose—response functions predict a drug-vulnerable phenotype predisposed to addiction. J. Neurosci. 2000;11:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Ryan J, Isaacson RL. Intra-accumbens injections of ACTH induce excessive grooming in rats. Physiol. Psychol. 1983;11:54–58. [Google Scholar]
- Sarnyai Z, Vecsernyes M, Julesz J, Szabo G, Telegdy G. Effects of cocaine and pimozide on plasma and brain alpha-melanocyte-stimulating hormone levels in rats. Neuroendocrinology. 1992;55:9–13. doi: 10.1159/000126090. [DOI] [PubMed] [Google Scholar]
- Shippenberg T, Heidbreder C. Sensitization to the conditioned rewarding effects of cocaine: pharmacological and temporal characteristics. JPET. 1995;273:808–815. [PubMed] [Google Scholar]
- Spruijt B, VanHooff JA, Gispen WH. Ethology and neurobiology of grooming behavior. Physiol. Rev. 1992;72:825–852. doi: 10.1152/physrev.1992.72.3.825. [DOI] [PubMed] [Google Scholar]
- Stone D, Walsh J, Benes FM. Localization of cells preferentially expressing GAD (67) with negligible GAD (65) transcripts in the rat hippocampus. A double in situ hybridization study. Mol. Brain Res. 1999;71:201–209. doi: 10.1016/s0169-328x(99)00185-0. [DOI] [PubMed] [Google Scholar]
- Szekaly J, Miglecz E, Dunai-Kovacs Z, Tarnawa I, Ronai AZ, Graf L, Bajusz S. Attenuation of morphine tolerance and dependence by a-melanocyte stimulating hormone (α-MSH) Life Sci. 1979;24:1931–1938. doi: 10.1016/0024-3205(79)90302-3. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Horger BA. Enhanced responding for conditioned reward produced by intra-accumbens amphetamine is potentiated after cocaine sensitization. Psychopharmacol. 1999;142:31–40. doi: 10.1007/s002130050859. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacol. 1984;84:405–412. doi: 10.1007/BF00555222. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. 6-Hydroxydopamine lesions of the nucleus accumbens, but not of the caudate nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacol. 1986;90:390–397. doi: 10.1007/BF00179197. [DOI] [PubMed] [Google Scholar]
- Tong Y, Pelletier G. Role of dopamine in the regulation of pro-opiomelanocortin (POMC) mRNA levels in the arcuate nucleus and pituitary gland of the female rat as studied by in situ hybridization. Mol. Brain Res. 1992;15:27–32. doi: 10.1016/0169-328x(92)90147-4. [DOI] [PubMed] [Google Scholar]
- Torre E, Celis ME. Alpha-MSH injected into the substantia nigra or intraventricularly alters behavior and the striatal dopaminergic activity. Neurochem. Int. 1986;9:85–89. doi: 10.1016/0197-0186(86)90035-5. [DOI] [PubMed] [Google Scholar]
- Van der Kooy D. Place Conditioning: a Simple and Effective Method for Assessing the Motivational Properties of Abused Drugs. Springer; New York: 1987. [Google Scholar]
- Wolterink G, Phillips G, Cador M, Donselaar-Wolterink I, Robbins TW, Everitt BJ. Relative roles of ventral striatal D1 and D2 dopamine receptors in responding with conditioned reinforcement. Psychopharmacol. 1993;110:355–364. doi: 10.1007/BF02251293. [DOI] [PubMed] [Google Scholar]