Abstract
Childhood and adolescent depression is an increasingly problematic diagnosis for young people due to a lack of effective treatments for this age group. The symptoms of adult depression can be treated effectively with multiple classes of antidepressant drugs which have been developed over the years using animal and human studies. But many of the antidepressants used to treat adult depression cannot be used for pediatric depression because of a lack of efficacy and/or side effects. The reason that children and adolescents respond differently to antidepressant treatment than adults is poorly understood. In order to better understand the etiology of pediatric depression and treatments that are effective for this age group the differences between and adults and children and adolescents needed to be elucidated. Much of the understanding of adult depression has come from studies using adult animals therefore studies using juvenile animals would likely help us to better understand childhood and adolescent depression. Recent studies have shown both neurochemical and behavioral differences between adult and juvenile animals after antidepressant treatment. Juvenile animals have differences compared to adult animals in the maturation of the serotonergic and noradrenergic systems, and in dose of antidepressant drug needed to achieve similar brain levels. Differences after administration of antidepressant drug have also been reported for adrenergic receptor regulation, a physiologic hypothermic response, as well as behavioral differences in two animal models of depression. The differences between adults and juveniles not only in the human response to antidepressants but also with animals studies warrant a specific distinction between the study of pediatric and adult depression and the manner in which new treatments are pursued.
1. Adult depression
Depression describes a transient mood state experienced by virtually all individuals at some time in their life, generally in response to stressful life events as well as a serious clinical disorder. Major depressive disorder (MDD; American Psychiatric Association’s DSM-IV manual) is a debilitating and serious mental illness that affects approximately two to five percent of the population worldwide, with a lifetime prevalence of around 15%. This disorder significantly interferes with the ability of the affected person functionally normally. The symptoms include a persistent sad or “empty” mood and feelings of hopelessness and worthlessness; changes in sleep and appetite; loss of interest in normally pleasurable activities; difficulty concentrating, remembering, making decisions; and thoughts of death or suicide (Fava and Kendler, 2000). Patients formerly hospitalized with depression are at high risk (>10 %) of committing suicide. When left untreated major depressive disorder can reduce the quality of a patient’s life for months if not years at a time. In addition to the emotional and physical pain caused by depression, the economic cost in the United States alone is estimated at $70 billion annually, and The World Health Organization projects that by the year 2020, depression will be second only to heart disease as the leading cause of disability worldwide. In this review, we are concerned with the clinical syndrome, major depressive disorder, not the ubiquitously experienced temporary change in mood.
In adults, depression can be successfully treated with psychotherapeutic methods (i.e., cognitive-behavioral therapy) and electroconvulsive treatment, as well as with several classes of antidepressant drugs, as summarized in Table 1. In general, the various antidepressant drugs have similar efficacies, although there is significant variability in individual responsiveness (Delgado, 2004). The currently available evidence suggests that the initial step in the mechanism of action of these antidepressants is an increase in monoamine levels. In spite of the fact that levels increase 100% – 300% within hours of the initiation of drug treatment, significant therapeutic improvement usually does not occur for two to four weeks (Delgado, 2004). Thus, it appears that other adaptive changes, such as those in G protein-coupled receptors, are necessarily involved in the mechanism of action of antidepressant drugs (Catapano and Manji, 2007).
Table 1.
Antidepressant Drug Classes
| Class | Examples | Mechanism |
|---|---|---|
| Monoamine Oxidase Inhibitors (MAOI) | Phenelzine | Inhibits the metabolism of norepinephrine, serotonin and dopamine |
| Tricyclic antidepressants (TCA) | Desipramnine Imipramine | Blocks the norepinephrine and serotonin transporters |
| Selective serotonin reuptake inhibitors (SSRI) | Fluoxetine Citalopram | Blocks the serotonin transporter |
| Atypical antidepressants | Bupropion Mirtazapine | Alters norepinephrine, serotonin and/or dopamine neurotransmission |
| Selective norepinephrine reuptake inhibitors (NRI) | Reboxetine | Blocks the norepinephrine transporter |
| Serotonin norepinephrine reuptake inhibitors (SNRI) | Venlafaxine | Blocks the norepinephrine and serotonin transporters |
1.1. Animal studies of adult depression
Many animal studies have been conducted over the years related to depression and the action of antidepressant drugs. Two of the major types of these studies are those involving behavior and those involving neurochemical alterations in the brain. These studies have been essential for our current understanding of depression and antidepressant drug action, as well as for the development of new antidepressant treatments.
1.1.1. Behavioral animal models of adult depression and antidepressant drug action
Because human depression is at least in part an abnormal response to stress (Leonard, 2001), many of the animal models that relate to depression and antidepressant drug action are based on the animal’s response to stress (Nestler et al., 2002;Vollmayr and Henn, 2003). Of the available animal models of human depression, including the chronic mild stress model, the olfactory bulbectomized rat, and the Flinders Sensitive Line, the learned helplessness and the forced-swim test are the best replicated and accepted (Kelly and Leonard, 1998).
The forced-swim test, also known as behavioral despair, refers to a paradigm in which rodents are forced to swim in a confined space on two occasions and is widely used to predict antidepressant efficacy (Lucki, 1997;Porsolt et al., 1977). This test consists of placing a rat or a mouse in a cylinder of water from which there is no escape and measuring the animal’s behavior for five minutes. Initially, rodents display escape-oriented behaviors including swimming and vigorous attempts to climb the wall of the cylinder. Eventually this behavior changes to ‘immobility’, which is defined as movements that are just sufficient to keep its head above water. Antidepressants of all major classes, as well as repeated electroconvulsive shock, reduce immobility and increase swimming or climbing behaviors in the forced-swim test. Remarkably, selective serotonin re-uptake inhibitors (SSRIs), which increase extracellular serotonin levels, increase swimming behavior, whereas drugs acting primarily to increase extracellular levels of norepinephrine or dopamine increase climbing behavior (Cryan et al., 2002;Cryan et al., 2005).
Learned helplessness refers to a stress-induced behavioral depression (Seligman and Maier, 1967). Learned helplessness behavior in the rat is normalized by all classes of antidepressant drugs and by electroconvulsive shock after repeated (but not acute) administration, but not by antipsychotic, antianxiety, sedative, or stimulant drugs (Sherman et al., 1982). The learned helplessness model, as originally developed for rodents, is performed in two sessions. In the first session, inescapable shock stress is delivered via an electrified grid floor or via tail electrodes. Levels of shock used are low (1 mA), and when experienced by humans, characterized as unpleasant, rather than painful. The shock is presented in a random pattern, making it unpredictable, during the course of 40 – 120 min. In the second session, the animal is given an opportunity to escape the shock, by pressing a lever or moving across a shuttle box. Naive, non-stressed rats will characteristically learn to escape the aversive shock stimulus efficiently, reliably, and quickly. Rats that have experienced previous inescapable, unpredictable shock demonstrate a range of behavior in the escape test, with some animals performing in a manner similar to naive, non-stressed rats, and some showing varying deficits. Failure to escape, or relatively poor escape performance, is defined as learned helplessness. Although the proportion of rats which demonstrate learned helplessness after inescapable stress varies, depending on factors such as strain, duration of inescapable stress, and the escape test used, this model replicates an important feature of human depression in that similar stress results in some individuals developing depression whereas others do not.
A second important advantage of this model is that the neurochemistry of those animals which exhibit learned helplessness can be compared to those animals which do not. In healthy, non-depressed persons, antidepressant drugs do not have significant effects upon mood. In depressed persons, antidepressant drugs have marked and profound effects upon mood. Thus, in understanding which neurochemical actions of the antidepressant drugs are functionally related to their antidepressant effects, studying drug effects in animals that have developed learned helplessness is likely to yield additional insight over studies of healthy, non-depressed animals.
1.1.2. Effects of antidepressants on G protein-coupled receptors in adult animals
Because antidepressant treatment increases monoamine levels and monoamine G protein-coupled receptors are known to undergo homologous (as well as heterogonous) down-regulation, the regulation of the monoamine receptor systems by antidepressants has been extensively studied (Catapano and Manji, 2007;Donati and Rasenick, 2003). Down-regulation occurs after prolonged agonist treatment, resulting in a decrease in the density of receptor binding sites. Down-regulation displays a relatively long time-course (hours to days) and results in a loss of total receptor binding sites (Hein and Kobilka, 1995). Particular emphasis has been placed on the beta-adrenergic receptor.
Down-regulation of the beta adrenergic receptors appears to be a common and very reproducible effect of most tricyclic and atypical antidepressants, as well as electroconvulsive shock (Duman and Alvaro, 1993;Sulser, 1984). This effect is generally assumed to be secondary to the increased concentration of norepinephrine in the synaptic cleft.
Reports on the effects of antidepressant administration on alpha-1 and alpha-2 receptors are less consistent (Deupree et al., 2007). Several studies have reported down-regulation of the alpha-2 adrenergic receptors following chronic antidepressant administration to adult rats. Down-regulation was found in several brain regions after two weeks of twice daily injections of the tricyclic antidepressant amitriptyline ((Smith et al., 1981)). Treatment with monoamine oxidase inhibitors for two weeks resulted in a time-dependent decrease in alpha-2 adrenergic receptors in the cerebral cortex (Giralt and Garcia-Sevilla, 1989). Forty days of chronic treatment of rats with tricyclic antidepressants produced a down-regulation of both alpha-1 and alpha-2 adrenergic receptors in the cortex, but not in the hippocampus (Subhash et al., 2003)). On the other hand, down-regulation of the alpha-2 receptor in the cortex was not detected following 3 weeks of daily desipramine administration (Tang, et al., 1981), or in the locus coeruleus following 14 days of daily desipramine administration (Sacchetti et al., 2001). A critical variable in these studies is that those studies which find down-regulation tend to use an agonist radioligand, whereas those studies that don’t tend to use an antagonist radioligand. The former is more likely to detect changes in high affinity state of the receptor, whereas the latter measures changes in total receptor population (high and low affinity states).
2. Childhood and adolescent depression
Major depressive disorder not only affects adults but is also commonly diagnosed in the pediatric population. Although the clinical symptoms of adolescent and childhood depression vary with developmental age, overall they are similar to those seen in adults. It is one of the most common mental health disorders in this population, with a prevalence in children of up to 2.5% (Birmaher et al., 1996) and during adolescence somewhere between 4% to 8%, with a 25% prevalence by the end of adolescence (Kessler et al., 2001). Depression in children results in many detrimental effects including loss of social, cognitive and interpersonal skills, social withdrawal, poor school attendance, and irritable family and peer relationships. In addition, there is a significant risk of self-harm and a depressed child may manifest a personality disorder as the depression interferes with the developing personality.
A major difference between adult and pediatric depression is the response to pharmacotherapy. Tricyclic antidepressants do not appear to be clinically effective in children and adolescents, whereas some SSRIs have been shown to be clinically effective (Bridge et al., 2007;Hazell et al., 1995;Kratochvil et al., 2006). Due to the lack of a demonstrable benefit and the possibility of harmful side-effects, the tricyclic antidepressants are not approved for children and adolescents. Only one pharmacologic agent, the SSRI fluoxetine, is currently approved in the United States to treat depression in patients under the age of 18. Because no current medication adequately treats childhood or adolescent depression, there is a need for new treatment modalities (Kessler et al., 2001;Weller and Weller, 2000). A major problem in finding effective treatments for childhood and adolescent depression is that the drugs used to treat depression were first developed for adults and they do not necessarily have efficacy in children. Kids are not just small adults. It is certainly possible that eventually new treatments could be found which are efficacious in childhood and adolescent depression, even if they are not useful in adult depression.
One necessary aspect of the quest to develop more efficacious treatments for childhood and adolescent depression is animal experimentation to better understand both the etiology and treatment of pediatric depression. Unfortunately, there are few published studies of depression in juvenile animals, and no validated juvenile models of depression. Thus, there is a clear need to develop reliable behavioral models of juvenile depression that can be used to test antidepressant effects on juvenile animals. Similarly, we need to understand the differences between juveniles and adults in their brain neurochemistry as it relates to depression and the effects of antidepressant drug administration.
3. Differences between adult and juvenile animals
One approach to address this need of developing better therapies for pediatric depression is to determine the response of juvenile animals to antidepressant administration and compare to the response of adult animals. Because the brains of juvenile animals are not yet mature, it is necessary to have some understanding of the roles that maturation of the various neurotransmitter systems, particularly those for norepinephrine and serotonin, play in the response of juvenile animals to antidepressants as compared to adult animals. In order to appropriately dose juvenile rats for various experimental paradigms, it is first necessary to understand the pharmacokinetic properties of antidepressants in juvenile as compared to adult rats, including the rate of elimination and the presence of any active metabolites.
3.1 Differential maturation of the norepinephrine and serotonin neurotransmitter systems
We have recently reviewed available literature on the development of the mammalian noradrenergic and serotonergic nervous systems, both in terms of neurotransmitter system markers and function (Murrin and Bylund, 2007). We considered several indicators or parameters of maturation including the extent of innervation, the levels of the neurotransmitter and its biosynthetic enzymes, the density of the receptors for the neurotransmitter and the levels of the transporters responsible for the reuptake of the neurotransmitter. Some of these data are summarized in Figure 1. In the rat, the norepinephrine system is not fully developed until sexually maturity is reached at about 5 weeks of age, whereas the parameters for the serotonin system reach adults levels two to three weeks earlier. The difference is even more dramatic in the monkey, with the two available parameters reaching adult levels in two to eight weeks for serotonin, but not until two years for norepinephrine. It is clear from this comparison that the serotonin system reaches maturity much earlier than the norepinephrine system in these two species.
Figure 1.
Indicators of maturation of noradrenergic and serotonergic systems in the mammalian brain. Values are the time (in weeks or years) for the parameter to reach adult levels.
Most of the information available concerning development of neurotransmitter systems is derived from animal studies and data from humans are limited. Because brain development relative to time of birth differs greatly between species, it is admittedly difficult to extrapolate from these data to humans. For example, at birth brain development in rats is equivalent in relative weight to development in humans at the end of the second trimester. Rats become sexually mature at about 5 weeks of age, corresponding roughly to puberty or early adolescence in humans. In contrast, brain development in rhesus monkeys at birth is similar to that found in humans at 10–15 years of age (Dobbing and Sands, 1979). Nevertheless, the basic developmental processes, and the impact of alterations in normal development, appear to be very similar across mammalian species.
Markers for the serotonergic system reach adult stages of development more rapidly than markers for the noradrenergic system, in some cases far more rapidly. If the beneficial effects of antidepressant drugs in the treatment of depression require the neurotransmitter system on which they act predominantly to be fully mature, it would be expected that drugs acting on the serotonergic system would be effective earlier in development than drugs acting primarily on the noradrenergic system. The temporal differences in development of these two neurotransmitter systems may provide at least a partial explanation of the lack of efficacy of antidepressants targeting the noradrenergic system, as compared to the efficacy of those targeting the serotonergic system.
3.2 Appropriate doses of antidepressants for similar brain levels in juveniles and adults
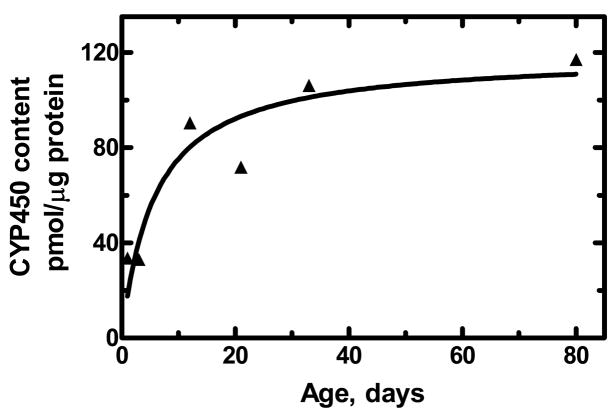
Animal studies comparing the effects of many antidepressant drugs in juvenile and adult animals are complicated by age-related variations in pharmacokinetic parameters such as elimination rates. Similarly in humans, these parameters, such as metabolism and the apparent volume of distribution can differ considerably in children and adolescents as compared with adults (McLeod and Evans, 1992). Many drugs are metabolized primarily by the cytochrome P-450 enzymes in the liver. It has been known for many years that the level of these enzymes is low at birth and then increases over the next four to six weeks (Gillette and Stripp, 1975;MacLeod et al., 1972). For example, as shown in Figure 2, the hepatic microsomal content cytochrome P-450 (in pmol/μg liver protein) in male rats increases markedly during the first 5 weeks of life.
Figure 2.
The hepatic microsomal content cytochrome P-450 (in pmol/μg liver protein) in male rats as a function of age (in days after birth). The data were calculated from data reported in the literature (Atterberry et al., 1997).
Because of these differences in pharmacokinetic parameters dosing regiments may be needed altered in the juvenile to achieve similar brain drug levels as in the adult rats (Kozisek et al., 2007). We recently compared the half-life of desipramine as well as the brain and serum concentrations of desipramine and its active metabolite desmethyldesipramine in juvenile and adult rats after various drug administration paradigms. After acute i.p. administration desipramine is eliminated from the brain more slowly in postnatal day 21 and 28 rats as compared to adults. After chronic i.p. administration (for 4–5 days between postnatal day 9 and 28), lower doses of desipramine are needed with juvenile rats to obtain the same brain desipramine concentrations as adults. For example, using a paradigm that has been used for studying alterations in receptor density (2 i.p. injections per day for 4 days), about 2.4 mg/kg/injection of desipramine to a postnatal day 13 juvenile rat gives approximately the same brain levels as 10 mg/kg/injection given to an adult. Similarly, using a paradigm that has been used for studying behavioral effects (2 ip injections per day for 5.5 days), about 6 mg/kg/injection of desipramine to a postnatal day 28 juvenile rat gives approximately the same brain levels as 10 mg/kg/injection given to an adult. By contrast, two weeks of continuous drug delivery (minipump) to postnatal day 21–35 and adult rats result in similar brain desipramine concentrations. Thus, the pharmacokinetic properties of desipramine vary with the age of the animal and the dose of desipramine used needs to be carefully adjusted in order to have appropriate brain levels of the drug.
3.3 Metabolism and active metabolites
Many antidepressants are metabolized to compounds that have antidepressant activity. For example, citalopram is metabolized to desmethylcitalopram and didesmethylcitalopram. Desmethylcitalopram is 3-fold less selective for the human serotonin transport as compared to citalopram, and 2-fold less selective for the norepinephrine transporter (Tatsumi et al., 1997). Thus, the metabolite is significantly (7-fold) less selective for the serotonin vs the norepinephrine transporter than the parent compound.
In the rat (although not in humans), the major metabolite of desipramine is desmethyldesipramine. Chronic treatment of rats with desmethyldesipramine has been shown to down-regulate the beta adrenergic receptor with a potency similar to desipramine (Argenti and D’Mello, 1994). Whereas desipramine is one of the most selective antidepressants for the norepinephrine transporter as compared to the serotonin transport in both the human and the rat (Owens et al., 1997), desmethyldesipramine has a higher affinity for the rat serotonin transporter (13 nM) than for the rat norepinephrine transporter (153 nM) (Bylund, unpublished data). Treatment of rats with desipramine can result in the concentration of desmethyldesipramine reaching levels similar to that of the parent compound (Kozisek et al., 2007). Thus, desmethyldesipramine could contribute significantly to the antidepressant effect of desipramine under certain conditions.
4. Receptor regulation by antidepressant drugs
Because the adrenergic nervous system is not fully developed until late adolescence, the mechanisms regulating receptor density similarly may not yet be mature in young mammals, and thus the response to antidepressants that increase norepinephrine levels may be different in juveniles as compared to adults. Thus, it is of interest to compare the effects of desipramine treatment on cortical alpha-2 and beta adrenergic receptors in juvenile and adult rats (Deupree et al., 2007). Desipramine was delivered by four days of twice daily injections to post-natal day 9–13 (4 and 7 mg/kg/day) and adult (20 mg/kg/day) rats (Figure 3). These delivery paradigms gave juvenile brain concentrations of desipramine in the juvenile (1.0 and 4.5 μg/g for the lower and higher doses, respectively) similar to those in adult rats (2.3 μg/g). The beta adrenergic receptor was down-regulated to 50% to 60% of control in both juvenile and adult rats. By contrast, in the post-natal day 9–13 rats there was a dose-dependent up-regulation of the alpha-2 adrenergic receptor in the prefrontal cortex, whereas there was no change in density in adult rats. Thus, whereas the regulation of the beta adrenergic receptor appears to the same in the brains of juvenile and adult rats, the regulation of the alpha-2 receptors is strikingly different. This difference in the alpha-2 adrenergic receptor regulation following desipramine treatment suggests that the lack of efficacy of tricyclic antidepressants in treating childhood depression may be related to immature regulatory mechanisms for this receptor.
Figure 3.
Changes in adrenergic receptor density following desipramine administration. Juvenile (postnatal day 13) and adult rats were injected twice a day with 2 or 4 mg/kg/injection (Juvenile 2 and Juvenile 3.5, respectively) or with 10 mg/kg/injection (Adult) of desipramine for four days. Alpha-2 and beta adrenergic receptor density was measured in rat prefrontal cortex and remainder of cortex (Cortex) isolated 12 hours after the last injection. *Significantly different from control.
5. Effect of desipramine administration on hypothermia
Another indication that the adrenergic nervous system is not fully developed until late adolescence is the lack of a hypothermic response of juvenile rats to acute desipramine administration. In adult rats, acute administration of desipramine results in a significant decrease in body (rectal) temperature. This effect may be a result of the increased synaptic norepinephrine activating the alpha-2A adrenergic receptor, because directly stimulating this receptor with an agonist produces hypothermia (Lahdesmaki et al., 2003).
Acute desipramine induced a time-dependent hypothermic response in adult rats, with a significant decrease in rectal temperature at 60 min as compared to time 0 (Figure 4; Bylund, unpublished data). However, a similar decrease in the juvenile was not observed. This suggests for the juvenile animal that somewhere in the signal transduction system starting with the inhibition of the norepinephrine transporter and ending with the hypothermic response, there are one or more immature components. It will be of interest to determine the nature of these components.
Figure 4.
Effect of acute desipramine on hypothermia measurements. A single i.p. injection of desipramine was given to adult (10 mg/kg) and PND 13 (3 mg/kg) rats immediately after measuring their rectal temperatures (0 time). The rectal temperatures were measured again at 30 and 60 min.
6. Animals models of depression and antidepressant drug action
A significant hindrance to basic research related to pediatric depression is the lack of juvenile animal models of study pediatric depression and antidepressant drug action in juveniles. Although there are some studies in which juvenile animals are subjected to various type of manipulations or stressors and then evaluated for depression-like behavior as an adult, studies on juvenile animals themselves are rare indeed. Using the two best accepted adult models, learned helplessness and the forced-swim test as starting points, we have recently determined conditions and procedures that appear to provide valid juvenile animal models for pediatric depression when postnatal day 21 animals are used. Importantly, in both of these juvenile models, the SSRIs are efficacious, whereas the tricyclic antidepressants are not.
6.1. Forced-swim test
Results from the Forced-swim test as modified for juvenile animals are presented in Figure 5. In this model using 21-day old animals, the SSRIs fluoxetine (at 10 mg/kg) and escitalopram (at 10 and 20 mg/kg) increased swimming, but not climbing, behavior just as seen in the adult (Reed et al., 2007). Escitalopram (at 20 mg/kg) also decreased immobility. In stark contrast, imipramine (at 10 mg/kg) and desipramine (at 10 and 20 mg/kg) did not increase climbing behavior, as would occur in adult animals. In fact, these two antidepressant drugs had no significant effect on any of the three behaviors. In the 28-day old animal, however, both fluoxetine and imipramine altered forced-swim behaviors in the same ways as in adults. These data suggest that the juvenile forced-swim test in the 21-day old, but not in the 28-day old rat is a valid model of antidepressant action, and may be useful in developing new treatments for childhood and adolescent depression.
Figure 5.
Effects of SSRI and TCA treatment in the forced-swim test with 21-day old rats. Animals were given i.p. injections of 10 mg/kg fluoxetine (FLX), 10 or 20 mg/kg escitalopram (S-CIT), 10 mg/kg imipramine (IMP), or 10 or 20 mg/kg desipramine (DMI) 23.5, 5, and 1 h before exposure to a 5 min test swim. The time spent immobile, swimming, and climbing during the test was scored for each animal. *Significantly different than control p < 0.05, **Significantly different than control p <0.01.
6.2. Learned Helplessness
Results from the Learned Helplessness model with juvenile animals are shown in Figure 6. Chronic treatment of 21-day old animals in this model with escitalopram (10 mg/kg) showed a marked decrease in the mean escape latency times compared to saline-injected control animals (Reed and Bylund, unpublished data). This decrease in mean escape latency for escitalopram treated animals is indicative of a prevention of learned helplessness, which was present in the saline controls. This prevention of learned helpless behavior is also seen in adult animals chronically treated with escitalopram. In contrast to escitalopram treatment, 21-day old animals that received chronic treatment with 10 or 15 mg/kg of desipramine did not show decreased latency times when compared to saline controls. The juvenile animals treated with desipramine still exhibited learned helpless behavior just like the untreated controls. This contradicts with the response of adult animals to chronic treatment with desipramine in the learned helplessness model, which does show a decrease in escape latency times and prevention of learned helplessness. The 28-day old animals that have been tested in this model after chronic treatment with escitalopram and desipramine show similar responses as the adult animals. These data also suggest that using 21-day old but not 28-day old juvenile rats in the learned helplessness paradigm is a valid model of antidepressant action in pediatric depression. Eventually, this newly-adapted juvenile model of depression may also be used to better study the underlying causes of childhood and adolescent depression.
Figure 6.
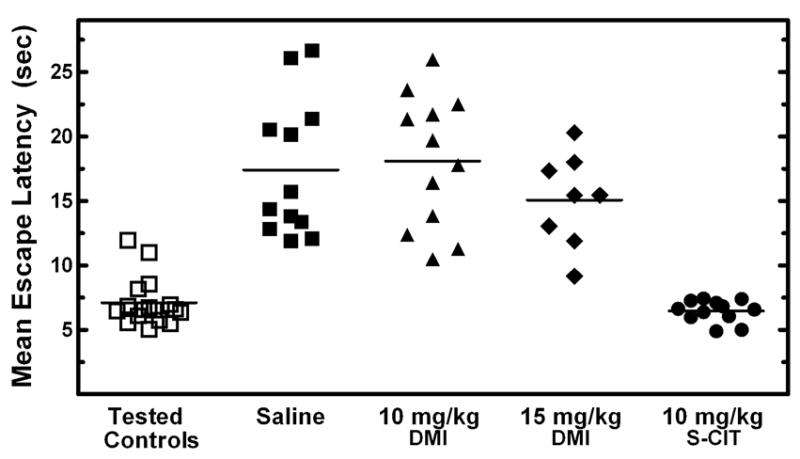
Effects of chronic escitalopram (S-CIT) and desipramine (DMI) treatment in a learned helplessness paradigm modified for use with 21-day old rats. Juvenile animals were injected twice daily for 6.5 days with 10 mg/kg of S-CIT or 10 or 15 mg/kg of DMI. Twenty-four hours after an inescapable foot-shock session the animals mean escape latency times were determined by averaging the time of escape for 25 shuttlebox trials of escapable shock. Tested control animals did not receive a previous inescapable shock before shuttlebox testing occurred but did receive daily injections. Each triangle represents the mean escape latency for one animal. The average of the mean escape latencies for each treatment is indicated by a horizontal black line.
7. Conclusion
Basic research directed toward the understanding of the differences in responses of juvenile and adult animals to drug administration must take into account several factors. First are the developmental aspects of the systems under study, particularly in terms of the signal transduction pathway (pharmacodynamics). Second are the difference in the pharmacokinetics, including differences in dose administered and the half-life of the agent. The antidepressant-like effects of desipramine, but not citalopram, are very different in juvenile as compared to adult rats. These differences mirror those seem in humans, and appear to result, at least in part, from the differences in the rate of maturation of the serotonin and norepinephrine nervous systems.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argenti D, D’Mello AP. The pharmacodynamics of desipramine and desmethyldesipramine in rats. J Pharmacol Exp Ther. 1994;270:512–519. [PubMed] [Google Scholar]
- Atterberry TT, Burnett WT, Chambers JE. Age-related differences in parathion and chlorpyrifos toxicity in male rats: target and nontarget esterase sensitivity and cytochrome P450-mediated metabolism. Toxicol Appl Pharmacol. 1997;147:411–418. doi: 10.1006/taap.1997.8303. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J, Dahl RE, Perel J, Nelson B. Childhood and adolescent depression: a review of the past 10 years. Part I J Am Acad Child Adolesc Psychiatry. 1996;35:1427–1439. doi: 10.1097/00004583-199611000-00011. [DOI] [PubMed] [Google Scholar]
- Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, Ren L, Brent DA. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: A meta-analysis of randomized controlled trials. JAMA. 2007;297:1683–1696. doi: 10.1001/jama.297.15.1683. [DOI] [PubMed] [Google Scholar]
- Catapano LA, Manji HK. G protein-coupled receptors in major psychiatric disorders. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2007;1768:976–993. doi: 10.1016/j.bbamem.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl) 2005:1–10. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- Delgado PL. How antidepressants help depression: mechanisms of action and clinical response. J Clin Psychiatry. 2004;65 Suppl 4:25–30. [PubMed] [Google Scholar]
- Deupree JD, Reed AL, Bylund DB. Differential effects of the tricyclic antidepressant, desipramine, on the density of adrenergic receptors in juvenile and adult rats. J Pharmacol Exp Ther. 2007;321:770–776. doi: 10.1124/jpet.106.118935. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Donati RJ, Rasenick MM. G protein signaling and the molecular basis of antidepressant action. Life Sci. 2003;73:1–17. doi: 10.1016/s0024-3205(03)00249-2. [DOI] [PubMed] [Google Scholar]
- Duman RS, Alvaro JD. Developmental expression of adrenergic receptors in the central nervous system. In: Zagon IS, McLaughlin PJ, editors. Receptors in the Developing Nervous System: Neurotransmitters. Vol. 2. Chapman & Hall; London: 1993. pp. 1–19. [Google Scholar]
- Fava M, Kendler KS. Major depressive disorder. Neuron. 2000;28:335–341. doi: 10.1016/s0896-6273(00)00112-4. [DOI] [PubMed] [Google Scholar]
- Gillette JR, Stripp B. Pre- and postnatal enzyme capacity for drug metabolite production. Fed Proc. 1975;34:172–178. [PubMed] [Google Scholar]
- Giralt MT, Garcia-Sevilla JA. Acute and long-term regulation of brain alpha 2-adrenoceptors after manipulation of noradrenergic transmission in the rat. Eur J Pharmacol. 1989;164:455–466. doi: 10.1016/0014-2999(89)90253-7. [DOI] [PubMed] [Google Scholar]
- Hazell P, O’Connell D, Heathcote D, Robertson J, Henry D. Efficacy of tricyclic drugs in treating child and adolescent depression: a meta-analysis. British Medical Journal. 1995;310:897–901. doi: 10.1136/bmj.310.6984.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein L, Kobilka BK. Adrenergic receptor signal transduction and regulation. Neuropharmacology. 1995;34:357–366. doi: 10.1016/0028-3908(95)00018-2. [DOI] [PubMed] [Google Scholar]
- Kelly JP, Leonard BE. Models and responses in depression and aggression. Biochem Soc Trans. 1998;26:61–65. doi: 10.1042/bst0260061. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Avenevoli S, Ries MK. Mood disorders in children and adolescents: An epidemiologic perspective. Biol Psychiatry. 2001;49:1002–1014. doi: 10.1016/s0006-3223(01)01129-5. [DOI] [PubMed] [Google Scholar]
- Kozisek ME, Deupree JD, Burke WJ, Bylund DB. Appropriate dosing regimens for treating juvenile rats with desipramine for neuropharmacological and behavioral studies. J Neurosci Methods. 2007;163:83–91. doi: 10.1016/j.jneumeth.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratochvil CJ, Vitiello B, Walkup J, Emslie G, Waslick BD, Weller EB, Burke WJ, March JS. Selective serotonin reuptake inhibitors in pediatric depression: Is the balance between benefits and risks favorable? J Child Adolesc Psychopharmacol. 2006;16:11–24. doi: 10.1089/cap.2006.16.11. [DOI] [PubMed] [Google Scholar]
- Lahdesmaki J, Sallinen J, MacDonald E, Sirvio J, Scheinin M. Alpha2-adrenergic drug effects on brain monoamines, locomotion, and body temperature are largely abolished in mice lacking the alpha-2A adrenoceptor subtype. Neuropharmacology. 2003;44:882–892. doi: 10.1016/s0028-3908(03)00080-7. [DOI] [PubMed] [Google Scholar]
- Leonard BE. Stress, norepinephrine and depression. J Psychiatry Neurosci. 2001;26 Suppl:S11–S16. [PMC free article] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- MacLeod SM, Renton KW, Eade NR. Development of hepatic microsomal drug-oxidizing enzymes in immature male and female rats. J Pharmacol Exp Ther. 1972;183:489–498. [PubMed] [Google Scholar]
- McLeod HL, Evans WE. Pediatric pharmacokinetics and therapeutic drug monitoring. Pediatr Rev. 1992;13:413–421. [PubMed] [Google Scholar]
- Murrin LC, Bylund DB. Comparison of the Maturation of the Adrenergic and Serotonergic Neurotransmitter Systems in the Brain: Potential Insights into Childhood and Adolescent Depression. Biochem Pharmacol. 2007;73:1225–1236. doi: 10.1016/j.bcp.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, Hen R, Koester S, Lederhendler I, Meaney M, Robbins T, Winsky L, Zalcman S. Preclinical models: Status of basic research in depression. Biol Psychiatry. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. Neurotransmitter Receptor and Transporter Binding Profile of Antidepressants and Their Metabolites. J Pharmacol Exp Ther. 1997;283:1305–1322. [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Reed AL, Happe HK, Petty F, Bylund DB. Juvenile rats in the forced-swim test model the human response to antidepressant treatment for pediatric depression. Biol Psychiatry. 2007 doi: 10.1007/s00213-007-1052-0. submitted. [DOI] [PubMed] [Google Scholar]
- Sacchetti G, Bernini M, Gobbi M, Parini S, Pirona L, Mennini T, Samanin R. Chronic treatment with desipramine facilitates its effect on extracellular noradrenaline in the rat hippocampus: studies on the role of presynaptic alpha2-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:66–72. doi: 10.1007/s002100000334. [DOI] [PubMed] [Google Scholar]
- Seligman ME, Maier SF. Failure to escape traumatic shock. J Exp Psychol. 1967;74:1–9. doi: 10.1037/h0024514. [DOI] [PubMed] [Google Scholar]
- Sherman AD, Sacquitne JL, Petty F. Specificity of the learned helplessness model of depression. Pharmacol Biochem Behav. 1982;16:449–454. doi: 10.1016/0091-3057(82)90451-8. [DOI] [PubMed] [Google Scholar]
- Smith CB, Garcia-Sevilla JA, Hollingsworth PJ. Alpha 2-adrenoreceptors in rat brain are decreased after long-term tricyclic antidepressant drug treatment. Brain Res. 1981;210:413–418. doi: 10.1016/0006-8993(81)90919-7. [DOI] [PubMed] [Google Scholar]
- Subhash MN, Nagaraja MR, Sharada S, Vinod KY. Cortical alpha-adrenoceptor downregulation by tricyclic antidepressants in the rat brain. Neurochem Int. 2003;43:603–609. doi: 10.1016/s0197-0186(03)00097-4. [DOI] [PubMed] [Google Scholar]
- Sulser F. Regulation and function of noradrenaline receptor systems in brain. Psychopharmacological aspects Neuropharmacology. 1984;23:255–261. doi: 10.1016/0028-3908(84)90067-4. [DOI] [PubMed] [Google Scholar]
- Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340:249–258. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Henn FA. Stress models of depression. Clinical Neuroscience Research. 2003;3:245–251. [Google Scholar]
- Weller EB, Weller RA. Treatment options in the management of adolescent depression. J Affect Disord. 2000;61:S23–S28. doi: 10.1016/s0165-0327(00)00286-x. [DOI] [PubMed] [Google Scholar]