Abstract
We have previously shown that human intestinal epithelial cell monolayers (Caco-2) subjected to hypoxia and re-oxygenation release soluble factors into the apical media that activate the virulence of the opportunistic pathogen Pseudomonas aeruginosa to express the potent barrier dysregulating protein, the PA-I lectin/adhesin. Here we defined the role of HIF-1α in this response. We tested the ability of media from Caco-2 cells with forced expression of HIF-1α to increase PA-I expression in P. aeruginosa, and found that media from Caco-2 cells overexpressing HIF-1α increased PA-I expression compared to media from control cells (P<0.001 ANOVA). To identify the components responsible for this response, media was fractionated by molecular weight and subjected to mass spectroscopy which identified adenosine as the possible mediator. Both adenosine, and its immediate downstream metabolite inosine, induced PA-I expression in P. aeruginosa in a dose-dependent fashion. Because inosine was not detectable in the media of Caco-2 cells exposed to hypoxia or overexpressing HIF-1α, we hypothesized that P. aeruginosa itself might metabolize adenosine to inosine. Using mutant and parental strains of P. aeruginosa, we demonstrated that P. aeruginosa metabolized adenosine to inosine via adenosine deaminase and that the conditioned media enhanced the extracellular accumulation of inosine. Taken together these results provide evidence that P. aeruginosa can recognize and respond to extracellular end-products of intestinal hypoxia that are released following activation of HIF-1α. The ability of P. aeruginosa to metabolize adenosine to inosine may represent a subversive microbial virulence strategy that deprives the epithelium of the cytoprotective actions of adenosine.
INTRODUCTION
There is now ample evidence to show that opportunistic pathogens that colonize the intestinal epithelial surface are fully capable of recognizing and responding to a variety of host derived compounds encompassing multiple host cell functions such as immune activation (22), cellular cytoprotection (7), and mucosal defense function (1). The notion that microbial organisms have evolved a “sense and respond” system to recognize elements within their hosts is logical, not only as a means to develop an effective countermeasure to an attack by the host immune system, but also because such a contingency based system allows bacteria to be more cost effective with regard to virulence gene activation. Multiple lines of evidence are beginning to uncover the mechanistic details of this molecular dialogue in the direction between the host and pathogen (4, 7, 11, 14, 20, 22), and there are likely additional, yet to be identified, cell-to-cell communication molecules that allow bidirectional communication. Elucidation of the various compounds and pathways involved in this complex molecular interaction has the potential to provide a unique opportunity to interdict in the infectious process at its most proximate point.
In this regard, we have previously shown that the human opportunistic pathogen, Pseudomonas aerugionsa, is able to activate its virulence circuitry in response to soluble elements of epithelial hypoxia and reoxygenation (7). Using the human intestinal epithelial cell line Caco-2BBe, we demonstrated that soluble compounds released into the apical but not basolateral media by hypoxic/reoxygenated cultured intestinal epithelial cells, induced the expression of a key virulence protein in P. aeruginosa, the PA-I lectin/adhesin (lecA) (7). We have previously shown that the PA-I lectin of P. aeruginosa induces a defect in intestinal barrier function that allows exotoxin A to cross the epithelium, resulting in lethal gut-derived sepsis in mice (9). The PA-I is regulated by the well described quorum sensing signaling system (QS), a hierarchical system of virulence regulation in P. aeruginosa and other bacteria (21). Activation of QS dependent virulence factors in P. aeruginosa in response to media from hypoxic Caco-2BBe cells provides a novel example by which bacterial cell-cell communication networks can recognize eukaryotic paracrine signals. The precise signals released into the apical media of Caco-2 cells in response to hypoxia and reoxygenation that activate P. aeruginosa to express a virulent phenotype are unknown. Furthermore, the pathways within intestinal epithelial cells that regulate the release of host-derived bacterial signaling compounds are also unknown. In the present study, we sought to define the role of hypoxia-inducible factor 1 alpha (HIF-1α), a molecule central to hypoxic signaling, on the ability of epithelial cells to release soluble compounds that activate P. aeruginosa virulence. We used the PA-I as a biologically and clinically relevant marker of virulence activation. In addition, we sought to define the role of adenosine in this response since adenosine is released by hypoxic epithelial cells (16, 17), and we previously identified adenosine as a compound with PA-I inducing activity (7).
MATERIALS AND METHODS
Reagents
Adenosine, inosine, hypoxanthine, AMP, ADP, and ATP, were purchased from Sigma Aldrich (St. Louis, MO). Adenosine deaminase obtained from bovine spleen was purchased from Sigma Aldrich (St. Louis, MO).
Bacterial and human epithelial cells
Four bacterial strains of Pseudomonas aeruginosa were used in these studies. Strain PA27853 is a prototype clinical strain used in our previous studies and was obtained from the American Type Culture Collection (Manassas, VA) (23). Strain PAO1 is a completely genomically sequenced strain with a comprehensive transposon mutant library available from the University of Washington Genome Center and previously used in our lab (22). An adenosine deaminase mutant derivative of the PAO1 strain was obtained from the transposon mutant library at the University of Washington. Strain PA27853/PLL-EGFP is a PA-I GFP reporter strain previously constructed and validated in our laboratory (7). The high resistance human intestinal epithelial cell line, Caco-2BBe, and a stably-tansfected Caco-2 BBe cell line overexpressing HIF-1α, were constructed as described previously (3). Verification of HIF-1α overexpression in the HIF-1α transfected cells was confirmed by western blot analysis (data not shown). Caco-2BBe cells were maintained in HDMEM (high-glucose DMEM) with 10% fetal calf serum, 15 mM HEPES, pH 7.4, and 0.25 mg/ml geneticin, as previously described (19). Cells were plated on 6-well plates (Corning-Costar, Acton, MA) with identical media without geneticin and grown to confluence.
Mouse model of segmental intestinal ischemia/reperfusion
In order to determine if soluble factors released into the intestinal lumen during ischemia and reperfusion could induce PA-I expression in P. aeruginosa, intestinal contents were collected from segments of small bowel exposed to segmental vascular occlusion and reperfusion. Balb/c mice weighing between 25–35 grams were used for all studies. Animals were kept non per os except for water twenty four hours prior to the procedure. All studies were approved by the Animal Care and Use Committee at the University of Chicago. Mice were anesthetized using xylazine (10 mg/kg) and ketammine (80 mg/kg). A midline laparotomy was made and a constant 10 cm segment of mid small bowel isolated. The proximal end of the 10 cm segment was ligated with 3-0 silk suture and the lumen cannulated with silastic tubing and flushed with Ringers solution to evacuate all luminal contents. Following flushing, the distal end of the 10 cm segment was ligated with a silk suture and the feeding mesenteric vessels identified and isolated. At time 0, the lumen was flushed with 1 ml of Ringers solution, and 1 minute later collected via the tubing from the distal end. Luminal flushes and collections were then repeated after 10 minutes of mesenteric vessel occlusion with a vascular clamp, and again repeated following 10 minutes of reperfusion. Luminal flushes were then filtered through a 0.22 micron filter, placed on ice, and immediately used in PA-I expression assays.
Role of HIF-1α on the extracellular release of soluble mediators from Caco-2BBe cells that induce PA-I expression in P. aeruginosa
Media from HIF-1α overexpressing (experimental) and parental Caco-2BBe cells (control) was passed through 0.22μM filters and tested for its ability to induce PA-I expression in the PA-I GFP reporter strain of P. aeruginosa, PA27853/PLL-EGFP. To identify potential components released by HIF-1α overexpressing cells responsible for the PA-I inducing effect, media from control and experimental groups was collected, filtered, and fractionated by molecular weight using Millipore centrifugal filter units. In parallel, Caco-2BBe parental cells were subjected to 2 hours of hypoxia (<0.3% O2) as previously described (7), and the apical media from the hypoxic cells (experimental) was collected and subjected to filtration and fractionation as above.
GFP fluorescence assay to detect PA-I expression in P. aeruginosa
The PA27853/PLL-EGFP reporter strain was used to measure the expression of PA-I lectin as previously described with modifications (7). The PA27853/PLL-EGFP was cultured overnight in LB media containing 50μg/mL gentamicin at 37°C under shaking conditions. 20μL of the bacterial suspension was added to the 96-well plates containing 180μL of the experimental or control extracellular fractions from epithelial cells. GFP fluorescence (485/528 nm) and optical density (600 nm) measurements were recorded using a 96-well microplate fluorometer (Synergy HT, Biotek Inc., Winooski, VT) immediately following bacterial inoculation and then hourly thereafter for 7 hours. Between measurements, plates were maintained in an incubator-shaker set at 37°C and 100 rpm. The Relative Florescence Unit (RFU) of the reporter strain for each time point was divided by its corresponding optical density to control for small variations in bacterial cell density. Fluorescence values were calculated as follows, where RFUx refers to experimental and RFUc refers to control at time n: % of control = 100*(RFUxt=n-RFUxt=0)/RFUct=n-RFUct=0).
Measurement of adenosine concentration by LC/MS/MS
In preliminary experiments, molecular weight fractions of <3kDa induced a significant increase in PA-I expression. Based on data base searches and our previously published data showing adenosine to be a potent inducer of PA-I expression (7), extracellular adenosine concentration was measured in the <3kDa filtered media samples obtained from parental Caco-2BBe cells, HIF-1α overexpressing cells, and parental Caco-2BBe cells exposed to hypoxia (<0.3% O2, 2h). Adenosine was measured using LC/MS/MS. Briefly, adenosine was quantified using LC/MS/MS (Aglient 1100 Series LC/MSD Trap XCT). Samples were chromatographed on a 3.0×150mm column packed with 5μM particles of C18 (XTerra MS C18 5μM). The mobile phase consisted of solution A (97.5% H20, 2.5% methanol, 0.1% formic acid) and solution B (99.9% ACN, 0.1% formic acid). Adenosine was eluted with a solvent B gradient from 0 to 60% from 9–23 minutes. The solvent flow rate was 400μL/min. The data dependent MS/MS-MS was run with the MS-MS trigger for any ion >10,000 intensity. The scan range was 90–400 daltons. Adenosine was identified and quantified by comparison of the MS-MS fragmentation of the samples to adenosine standards. Adenosine was measured at various time periods of hypoxia (0–5 hours) and its concentration found to be greatest at the 2 hour time point (data not shown). Inosine was also quantified using the above method.
Role of adenosine and its precursors and metabolites on PA-I expression in P. aeruginosa
Adenosine alone was tested for its ability to induce PA-I expression. A 100 mM stock solution of adenosine was prepared in HDMEM media acidified with HCl to dissolve the adenosine. An equal amount of HCl was added to the HDMEM alone to control for the effect of the acid. The 100 mM adenosine solution was serially diluted in HDMEM to make 10,5,1, and 0.5 mM concentrations.
AMP, ADP, and ATP, and inosine were dissolved in HDMEM media to make 10, 5, 1, and 0.5 mM concentrations. All of the above compounds were tested for their effect on PA-I expression in P. aeruginosa using the GFP fluorescence assay.
Depletion of Caco-2 BBe cell media of adenosine by treatment with adenosine deaminase
In order to determine the putative role of adenosine in PA-I expression within the media of HIF-1α overexpressing cells, media was treated with the adenosine deaminase to deplete adenosine. Briefly, 25 units of adenosine deaminase was added to media from HIF-1α overexpressing cells, and, as a control, to a solution 10 mM of adenosine. An equal concentration of adenosine deaminase was also added to the parental Caco-2 BBe cell media and HDMEM media free of adenosine to control for potential impurities in the enzyme solution that could affect PA-I expression. Preliminary data demonstrated that the addition of adenosine deaminase to either media from Caco-2 BBe cells overexpressing HIF-1α or to stock solutions of adenosine increased PA-I expression. We therefore tested the ability of inosine, the immediate downstream metabolite of adenosine, to induce PA-I expression using the GFP fluorescence assay. Preliminary experiments showed that inosine was a potent inducer of PA-I expression, therefore we then sought to determine if the downstream metabolite of inosine, hypoxanthine, could induce PA-I expression. A 100 mM stock solution of hypoxanthine was prepared in HDMEM media and NaOH to dissolve the hypoxanthine. An equal amount of NaOH was added to the HDMEM alone to control for the possible pH effect. The 100 mM hypoxanthine solution was serially diluted in HDMEM to make 10, 5, 1, and 0.5 mM concentrations, and tested in the GFP fluorescence assay.
Determination of inosine concentration in adenosine solution treated with P. aeruginosa
Preliminary experiments indicated no detectable levels of inosine in HIF-1α overexpressing and hypoxic cell media as measured by LC/MS/MS (data not shown). We therefore speculated that P. aeruginosa may convert adenosine to inosine. Based on the annotation of the PAO1 genome sequence (15), PA0148 was found to have a 46% similarity to adenosine deaminase from Escherichia coli and 62% similarity to adenosine deaminase (putative) from Saccharomyces cerevisiae, and therefore was characterized as probable adenosine deaminase (http://www.pseudomonas.com/). To test the ability of P. aeruginosa to metabolize adenosine to inosine, the PAO1 strain of P. aeruginosa was cultured overnight in TSB media at 37°C under shaking conditions. The PAO1 strain was then added in a 1:10 ratio to TSB media and 10 mM adenosine solution. After bacterial inoculation, samples were placed at 37°C and 300 rpm for six hours. The four groups, (1) TSB, (2) TSB with PAO1, (3) 10 mM adenosine, and (4) 10 mM adenosine with PAO1, were analyzed via LC/MS/MS and thin-layer chromatography (TLC) for adenosine and inosine.
Determination that P. aeruginosa metabolizes adenosine to inosine via its potential adenosine deaminase
To further confirm that adenosine deaminase originating from P. aeruginosa was responsible for the conversion of adenosine to inosine, we used wild type PAO1 and its derivative strain, ID35276, with an adenosine deaminase (PA0148) knockout mutation (5). The derivative strain was confirmed to be a knockout using PCR analysis (data not shown). PAO1 and its adenosine deaminase mutant strain of P. Aeruginosa were cultured overnight in TSB media at 37°C under shaking conditions. Mutant and PAO1 strains were added in a 1:10 ratio to 10 mM adenosine solution. After bacterial inoculation, samples were placed at 37°C and 300 rpm for twelve hours. Samples were then centrifuged at 5000 rpm for 3 minutes to separate the bacteria, and the supernatant was collected. The two groups, (1) 10 mM adenosine with PAO1, and (2) 10 mM adenosine with adenosine deaminase mutant strain of P. aeruginosa, were analyzed via TLC for adenosine and inosine.
Does P. aeruginosa change its metabolism of adenosine in the presence of media from HIF-1α overexpressing or hypoxic Caco-2BBe cells?
Next we sought to determine if the dynamics by which P. aeruginosa metabolizes adenosine are altered by the media from Caco-2BBe cells overexpressing HIF-1α or Caco-2 BBe cells subjected to hypoxia, speculating that conditioned media might itself change the kinetics of P. aeruginosa adenosine deaminase. The PAO1 strain of P. aeruginosa was grown overnight in TSB at 37°C under shaking conditions. The overnight culture was added in a 1:10 ratio to samples containing (1) HDMEM (control) media, (2) media from parental Caco-2 BBe cells, (3) media from Caco-2 BBe cells overexpressing HIF-1α, and (4) media from parental Caco-2 BBe cells subjected to 2 hours of hypoxia. All samples were placed at 37°C under shaking conditions for eight hours. After 8 hours, all samples were inoculated with adenosine. Samples were kept at 37°C under shaking conditions, and evaluated for adenosine and inosine via thin-layer chromatography at times 0, 5, and 15 hours.
Western blot analysis
In selected experiments, PA-I expression was confirmed using western blot analyses as previously described (23).
Statistical Analysis
Data analysis and statistical significance calculations were performed using Prism 4.0 (GraphPad Software, San Diego, CA). Statistical significance was defined as p<0.05 by two-way ANOVA, or student t-test as appropriate.
RESULTS
Soluble factors from the lumen of mouse intestine subjected to segmental ischemia and reperfusion induce PA-I expression in P. aeruginosa
As seen in figure 1, filtered perfusates from the lumen of mouse intestine subjected to 10 minutes of vascular occlusion induced the expression of PA-I in P. aeruginosa strain PA27853/PLL-EGFP. (P<0.01, ANOVA, n=6). This finding was also observed following exposure of PA27853/PLL-EGFP to luminal perfusates following 10 minutes of reperfusion (Fig 1). In contrast, blood components did not induce PA-I (P>0.05, ANOVA, n=6), raising the possibility that the factors responsible for PA-I expression are released from the intestinal tissue itself. To rule out the possibility that the in vivo expression of the PA-I expression was not due to secondary effects of surgical stress such as physico-chemical changes in the local microenvironment, stock strains PA27853 and reporter strains PA27853/PLL-EGFP were exposed to ambient hypoxia (0.3% O2), pH changes (6–8), and 80% CO2. None of these conditions induced PA-I expression (data not shown).
Figure 1.
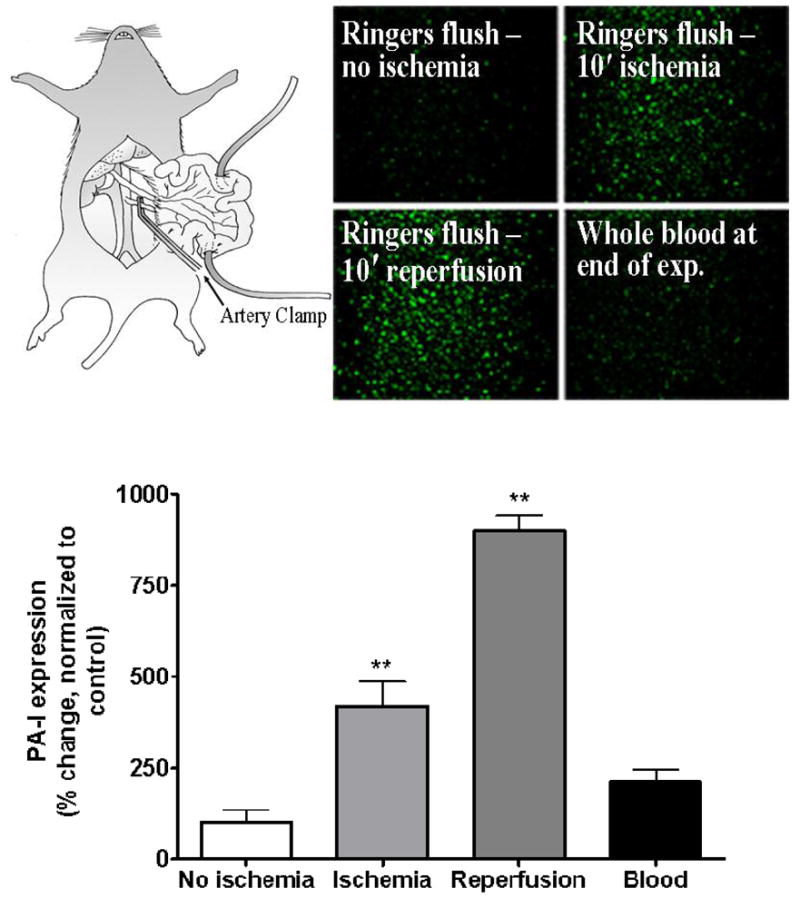
Mouse model of segmental intestinal ischemia reperfusion injury (I/R). Soluble factors from the lumen of mouse intestine subjected to I/R injury induce the expression of the PA-I lectin in P. aeruginosa. Following 10 min of vascular occlusion of a 10 cm portion of small bowel, or following 10 min of reperfusion, filtered perfusates of the intestinal lumen induced a significant increase in PA-I expression (**=P<0.01 ANOVA). Systemic blood collected immediately following the period of I/R did not induce PA-I expression in P. aeruginosa (p=NS)
Media from Caco-2BBe cells overexpressing HIF-1α induces PA-I expression in P. aeruginosa
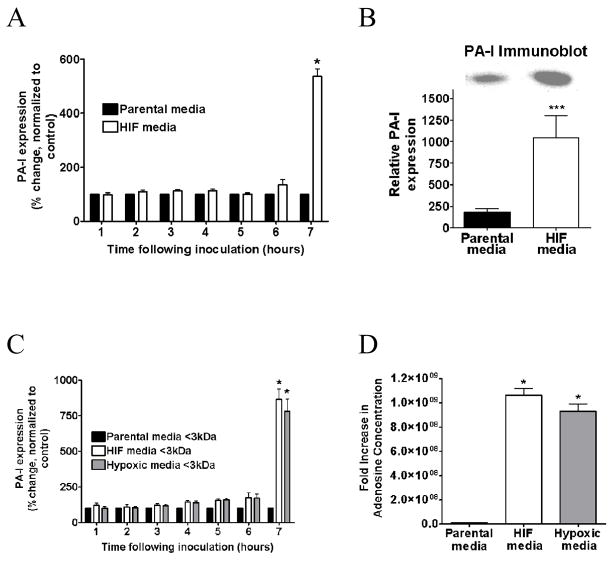
The PA27853/PLL-EGFP reporter strain of P. aeruginosa exposed to media from HIF-1α overexpressing cells demonstrated significant time dependent induction of PA-I as measured by fluorescence (P<0.001, ANOVA, n=6) (Fig. 2A). Results were confirmed by western blot analysis (P<0.05, student t-test, n=3) (Fig. 2B).
Figure 2.
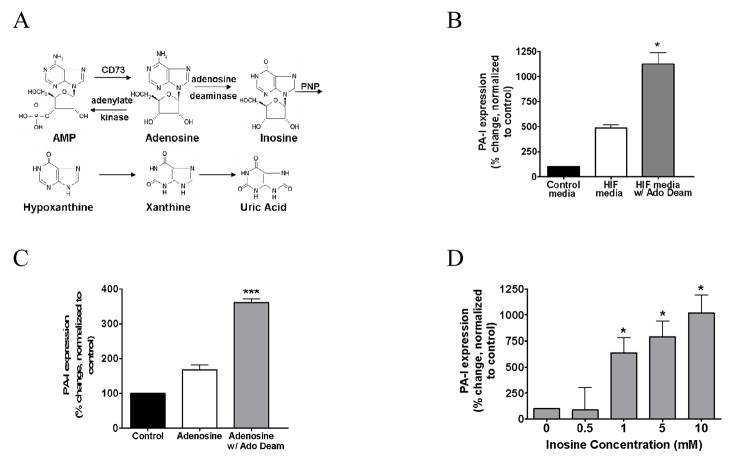
A. Effect of media from Caco-2 cells overexpressing HIF-1α and their parental controls on PA-I expression in P. aeruginosa. Results demonstrate a time-dependent increase in PA-I expression in PA27853/PLL-EGFP reporter strains exposed to media from Caco-2 cells overexpressing HIF-1α (*=P<0.001 ANOVA). B: Results were confirmed by Western blot. C: Effect of < 3kDa media fraction on PA-I expression P. aeruginosa from 1.) Parental Caco-2 cells exposed to normoxia (21% for 2 h), 2.) Caco-2 cells overexpressing HIF-1α, and 3.) Parental Caco-2 cells exposed to 2 hours of hypoxia (<0.3% 02). Results demonstrate a significant increase in PA-I expression in Caco-2 cells exposed to hypoxia and Caco-2 cells overexpressing HIF-1α (*=P<0.001 ANOVA). D: Extracellular concentration of adenosine in Caco-2 cells overexpressing HIF-1α and Caco-2 cells exposed to hypoxia for 2 hours (<0.3% O2). A significant increase in extracellular adenosine concentration is seen in Caco-2 cells overexpressing HIF-1α and in Caco-2 cells exposed to hypoxia compared to control (*=P<0.001 student t-test).
Media fractions <3kDa induce PA-I expression in P. aeruginosa
In order to identify specific molecular weight fractions from the media of Caco-2 BBe cells that induce PA-I expression, media from parental Caco-2 BBe cells, Caco-2 BBe cells exposed to hypoxia (2 hours <0.3% O2), and Caco-2 BBe cells with forced expression of HIF-1α was fractionated into four molecular weight fractions and tested for their ability to induce PA-I expression using the GFP fluorescence assay. Results demonstrated that media fractions with a molecular weight of <3kDa in both HIF-1α overexpressing and hypoxic cell media, significantly induced PA-I expression (P<0.001, ANOVA, n=6) (Fig. 2C). The remaining fractions had no effect on PA-I expression (data not shown).
Extracellular adenosine concentration is ~ 109 fold increased in Caco-2BBe cells overexpressing HIF-1α or following exposure to hypoxia
Potential candidate compounds in the <3kDa range that could induce PA-I expression in P. aeruginosa included adenosine, a nucleoside known to extracellularly accumulate in high concentration following intestinal epithelial cell hypoxia and HIF-1α activation. To confirm the presence of extracellular adenosine in the <3kDa molecular weight range fraction of Caco-2BBe cells, adenosine concentration was measured in the <3kDa fraction from parental Caco-2 BBe cells, Caco-2 BBe cells exposed to hypoxia (2 hours <0.3% O2), and Caco-2 BBe cells overexpressing of HIF-1α by the LC/MS/MS assay. Adenosine concentration was found to be significantly higher in the media from HIF-1α overexpressing and hypoxic cells compared to the media from parental Caco-2BBe cells under normal conditions (P<0.001, student t-test, n=3) (Fig. 2D).
Adenosine alone induces PA-I expression in P. aeruginosa
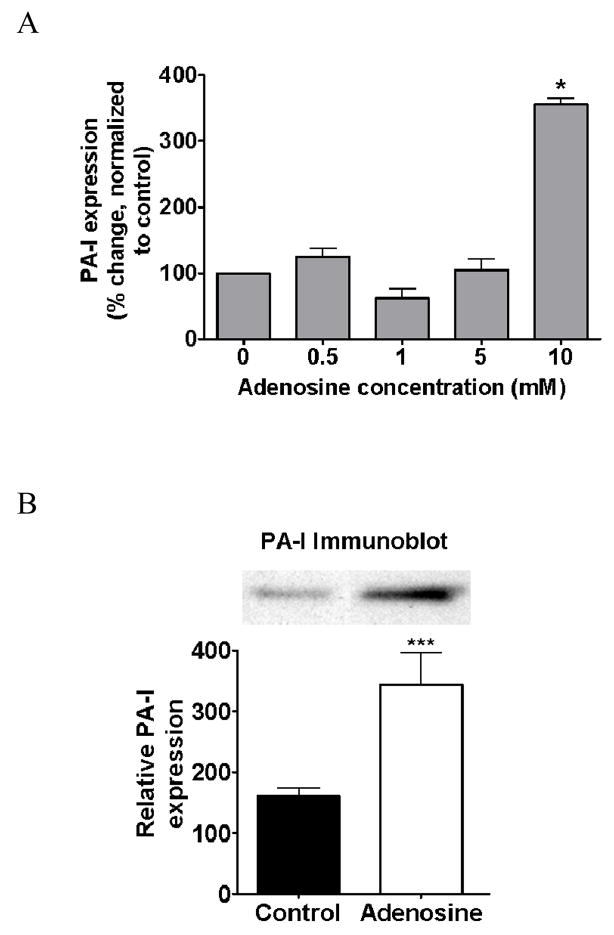
In order to determine if adenosine alone could induce PA-I expression in P. aeruginosa, to varying concentrations of adenosine were tested in our GFP fluorescence assay. Results demonstrated that PA27853/PLL-EGFP responded to 10 mM adenosine with a significant increase in PA-I expression (P<0.001, ANOVA, n=6) (Fig. 3A). Results were confirmed by western blot (P<0.05, student t-test, n=3) (3B). The effect of ATP, ADP, and AMP at similar concentrations were also tested, however, no inducing effect was found (data not shown).
Figure 3.
A. Effect of adenosine on PA-I expression in P. aeruginosa. PA-I expression was significantly increased in the PA27853/PLL-EGFP reporter strain in response to 10 mM concentration of adenosine (*=P < 0.001 ANOVA), B: and was confirmed by Western blot analysis (***=P<0.05 student t-test).
Depletion of adenosine in the cell media from Caco-2 BBe cells overexpressing HIF-1α with adenosine deaminase significantly increases PA-I expression
In order to determine if adenosine was the putative component within the media of HIF-1α overexpressing cells that induces the expression of PA-I, the enzyme adenosine deaminase was added to deplete the media of adenosine. Remarkably, the addition of adenosine deaminase to the media resulted in a significant increase in PA-I expression (P<0.001, ANOVA, n=6) (4B). Adenosine alone treated with adenosine deaminase also resulted in a significant increase in PA-I expression (P<0.05, ANOVA, n=6) (4C), raising the possibility that inosine, the immediate downstream metabolite of adenosine, might play a role in PA-I expression. We next determined if inosine alone could induce PA-I expression by testing varying concentrations of inosine in our GFP fluorescence assay, and found that inosine significantly induced PA-I expression at a 1 mM concentration (P<0.001, ANOVA, n=12), a concentration of 10 fold less than that of adenosine (4D). Next we determined if the next downstream metabolite of inosine, hypoxanthine, could also induce PA-I expression using the GFP fluorescence assay. Hypoxanthine significantly induced PA-I expression at a 10 mM concentration (P<0.01, ANOVA, n=8), the same concentration required for adenosine to induce PA-I (Fig. 5).
Figure 5.
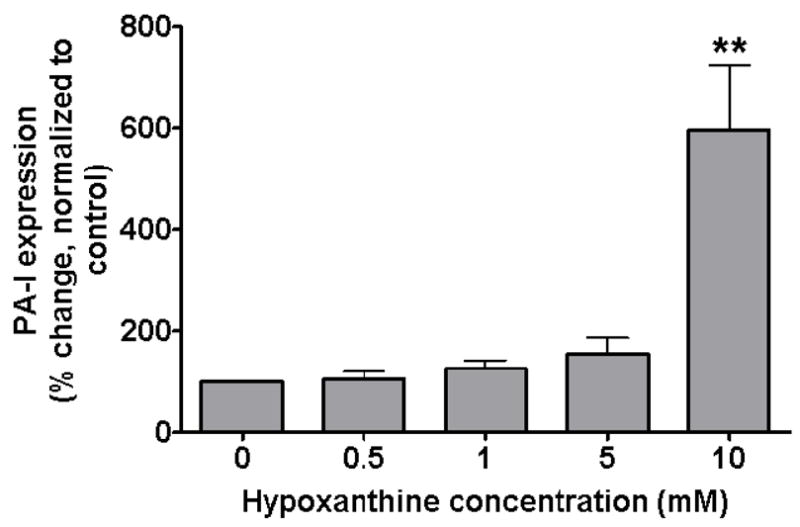
Effect of hypoxanthine, the next downstream metabolite of inosine, on PA-I expression in P. aeruginosa. PA-I expression was significantly increased in the PA27853/PLL-EGFP reporter strain in response to a 10 mM concentration of hypoxanthine (**=P < 0.01 ANOVA).
P. aeruginosa metabolizes adenosine to inosine via its adenosine deaminase, PA0148
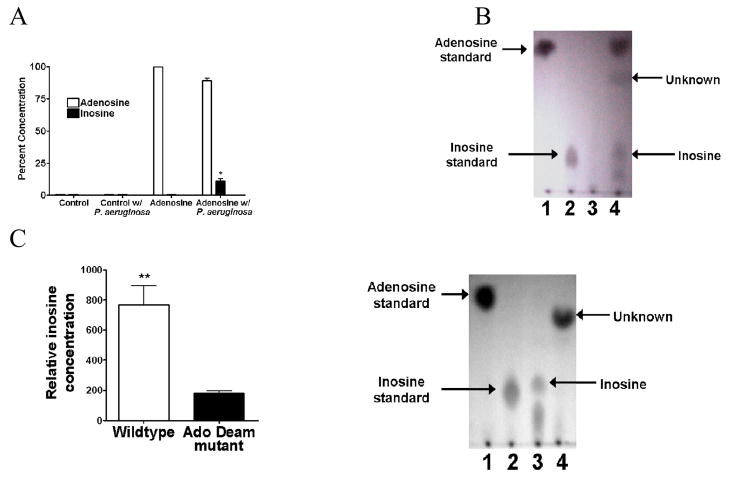
Given that inosine is a more potent inducer of PA-I expression than adenosine, its concentration was measured in HIF-1α overexpressing and hypoxic cell media via LC/MS/MS. No detectable levels of inosine were found (data not shown). Therefore we speculated that P. aeruginosa itself might convert adenosine to inosine. When adenosine solutions were inoculated with overnight cultures of wildtype PAO1, significant levels of inosine were detected by LC/MS/MS (P<0.001, student t-test, n=3) (Fig. 6A). Results were confirmed via thin-layer chromatography (Fig. 6B). To further confirm that adenosine deaminase originating from P. aeruginosa was responsible for the conversion of adenosine to inosine in the previous experiments, the wildtype parent strain PAO1 and its adenosine deaminase mutant, ID 35276, were cultured in the presence of adenosine and the conversion of adenosine to inosine measured. Samples of adenosine inoculated with P. aeruginosa in overnight culture resulted in a significantly greater conversion of adenosine to inosine than adenosine inoculated with the adenosine deaminase mutant strain of P. aeruginosa (P<0.01 student t-test, n=3) (Fig. 6C), demonstrating that P. aeruginosa has the capacity to convert adenosine to inosine via its adenosine deaminase.
Figure 6.
A. Ability of P. aeruginosa to metabolize adenosine to inosine. Overnight growth of P. aeruginosa in culture media containing adenosine resulted in significant conversion of adenosine to inosine (*=P<0.001 student t-test) as measured by LC/MS/MS. B: Results were confirmed via thin layer chromotography (TLC) (lane 1-Adenosine, lane 2-Inosine, lane 3- P. aeruginosa in the presence of TSB media, 4-P. aeruginosa in the presence of adenosine). C: Overnight cultures of mutant strains of P. aeruginosa lacking adenosine deaminase grown in the presence of adenosine failed to convert adenosine to inosine (*=P<0.001 student t-test) as measured by TLC. (lane 1-Adenosine, lane 2-Inosine, lane 3-P. aeruginosa in the presence of adenosine, lane 4-Adenosine deaminase mutant strain of P. aeruginosa in the presence of adenosine).
Extracellularinosine accumulates when P. aeruginosa is cultured in the presence of cell media from Caco-2 BBe cells overexpressing HIF-1α or in the presence of media from Caco-2 BBe cells exposed to hypoxia
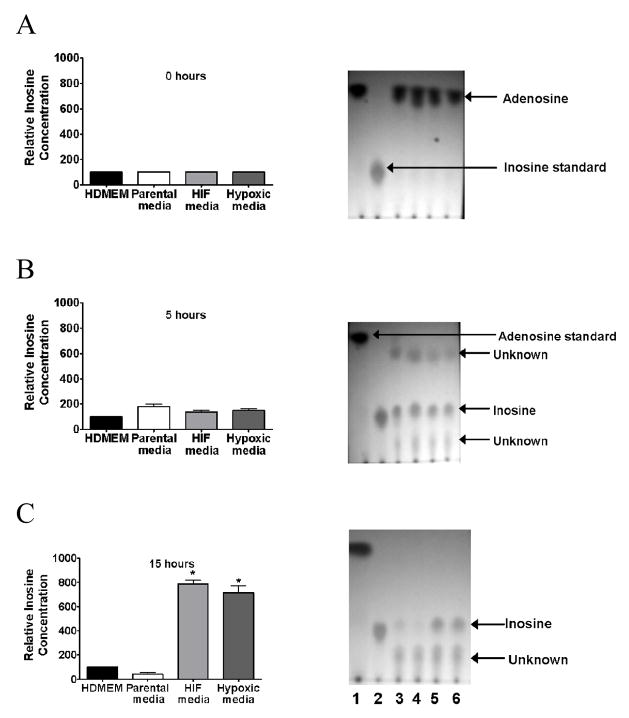
In order to determine if media from Caco-2 BBe cells overexpressing HIF-1α or media from Caco-2 BBe cells exposed to hypoxia could shift the metabolism of P. aeruginosa to convert adenosine to inosine, P. aeruginosa was cultured in the presence of HDMEM media, (control), media from parental Caco-2 BBe cells, media from HIF-1α overexpressing Caco-2 BBe cells, and media from Caco-2 BBe cells exposed to hypoxia. Following eight hours of growth, all samples were inoculated with adenosine and assayed for adenosine and inosine using TLC for 0, 5 hours, and 15 hours. Results demonstrated that all conditioned media converted adenosine to inosine (Fig. 7B), however at 15 hours, significant accumulation of inosine was seen only in P. aeruginosa exposed to media from either HIF-1α overexpressing Caco-2 BBe cells or Caco-2 BBe cells exposed to hypoxia. (P<0.01, n=3) (Fig. 7C) suggesting the possibility of that inosine metabolism in P. aeruginosa colonizing intestinal epithelium under ischemic stress is inhibited.
Figure 7.
Inosine accumulates in the media of P. aeruginosa cultures when exposed to media from Caco-2 cells overexpressing HIF-1α or media from Caco-2 cells exposed to hypoxia. B: Results demonstrate that all conditioned media (i.e. overexpressing HIF-1α media or media from Caco-2 cells exposed to hypoxia) converted adenosine to inosine. C: However at 15 hours, significant accumulation of inosine was seen only in P. aeruginosa exposed to media from either HIF-1α overexpressing Caco-2 cells or Caco-2 cells exposed to hypoxia (*=P<0.001 student t-test) (lane 1-Adenosine, lane 2- inosine, lane 3- P. aeruginosa cultured in the presence of HDMEM media (control), lane 4- P. aeruginosa cultured in the presence of parental control Caco-2 cell media, lane 5- P. aeruginosa cultured in the presence of media from Caco-2 cells overexpressing HIF-1α, lane 6- P. aeruginosa cultured in the presence of Caco-2 cells exposed to hypoxia).
DISCUSSION
Data from the present study add to the small but growing body of evidence that demonstrates that certain bacteria are full capable of recognizing and responding to host-derived elements released during physiologic stress. Under conditions of physiologic stress or immune activation, soluble compounds released by the host, such as epinephrine and INFγ, have been shown to activate the virulence of important intestinal bacteria such as E. coli and P. aeruginosa (14, 22). That intestinal bacteria are signaled to up-regulate their virulence by host derived compounds released during physiologic stress may have important implications in the pathogenesis by which intestinal bacteria cause sepsis in critically ill patients. This may be particularly relevant for the human opportunistic pathogen P. aeruginosa whose prevalence within the intestinal tract of critically ill patients approaches 50% and whose mere presence in this site is associated with a fourfold increase in mortality (10).
Intestinal ischemia and hypoxia are physiologic disturbances that invariably complicate the course of critically ill patients as blood flow is re-distributed away from the intestinal tract to more vital organs. As a compensatory response, HIF-1α, a highly conserved global transcriptional regulator, is activated in direct response to both hypoxia and inflammation. As has been previously reported, hypoxia/HIF-1α expression results in the extracellular accumulation of the cytoprotective compound adenosine that develops as a result of : 1.) upregulation of 5′ ectonucleasidase (CD73) which accelerates the conversion of AMP to adenosine, 2.) down-regulation of adenosine deaminase which prevents adenosine metabolism to inosine, and 3.) down-regulation of adenosine kinase which prevents recycling of adenosine back to AMP (6, 17). Up-regulation of CD73 with resultant accumulation of adenosine has been shown to be cytoprotective by enhancing tight junctional barrier function via mechanisms that involve adenosine activation of the AR 28 receptor (2, 13, 18). We have previously reported that hypoxic intestinal epithelial cells remain resistant to the barrier dyrsegulating effect of P. aeruginosa, whereas in the absence of hypoxia, P. aeruginosa induces a profound and rapid effect on the barrier function of cultured intestinal epithelial cells (Caco-2BBe) via expression of the PA-I lectin (7). Yet over time, the media of hypoxic intestinal epithelial cells directly up-regulates the expression of the potent barrier dysregulating PA-I protein in P. aeruginosa, eventually leading to a decrease in transepithelial electrical resistance of cultured intestinal epithelial cell monolayers (7). Taken together, data from the present study, in conjunction with our previous data and data from others, suggest that during intestinal epithelial hypoxia, eukaryotic cells activate a cytoprotective barrier enhancing response to invading pathogens in association with HIF-1α expression and extracellular adenosine release. Yet at the same time prokaryotic cells (P. aeruginosa) can intercept and use these signals to develop a countermeasure to this response by expressing potent barrier dysregulating proteins such as the PA-I lectin.
Data from the present study suggest that P. aeruginosa may have developed a system to not only recognize and respond to end-products of intestinal epithelial hypoxia, but also to metabolize these products into molecules that can participate in bacterial cell-cell communication networks such as the quorum sensing signaling system (QS). The observation in the present study that media from HIF-1α expressing Caco-2 cells, or media from hypoxic Caco-2 cells alters the metabolism of P. aeruginosa such that inosine accumulates, may provide an example whereby P. aeruginosa itself uses eukaryotic signals as its own quorum sensing signaling molecules. Whether inosine can act as a surrogate QS molecule in P. aeruginosa similar to that described for epinephrine in E. col, remains to be clarified (14). The precise mechanisms by which adenosine and inosine activate P. aeruginosa to express the quorum sensing dependent virulence factor PA-I, will require further studies.
It has been recently shown that apical exposure of Caco-2 cells to P. aeruginosa results in HIF-1α expression (8). When Caco-2 cells were co-cultured with P. aeruginosa, even during the re-oxygenation phase, HIF-1α protein levels remained elevated, unlike cells exposed to hypoxia/re-oxygenation in the absence of P. aeruginosa whereby HIF-1α expression is rapidly degraded (8). That the HIF-1 response is potentiated by P. aeruginosa is interesting, given that results of the present study, providing further evidence that the molecular dialogue between intestinal pathogens and the intestinal epithelium is bidirectional and highly dynamic. During severe critical illness where intestinal ischemia is often present and where intestinal colonization with P. aeruginosa is highly prevalent, the final interplay of this dynamic interaction may be highly predictive of the development of severe sepsis and a systemic inflammatory response (12). The observation that intestinal ischemia is lethal when accompanied by intestinal colonization with P. aeruginosa could be explained in part by the findings in the present study that show that the virulence circuitry of this pathogen has evolved to recognize and respond to end-products of epithelial hypoxia (24). That the enzyme of P. aeruginosa that metabolize adenosine (adenosine deaminase) is up-regulated in response to media from hypoxic epithelial cells also suggests that P. aeruginosa may have evolved a very clever virulence tactic to deplete epithelial cells of a major cytoprotective compound rendering them all the more vulnerable to the effects of this highly feared pathogen. In summary, data from the present study suggest that the co-evolution of bacteria and epithelial cells that is unique to the intestinal tract environment may have resulted in a bacterial-epithelial molecular dialogue that is much more complex than previously appreciated.
Figure 4.
A. Metabolites and precursors of adenosine and their rate limiting enzymes. B: Effect of media from Caco-2 cells overexpressing HIF-1α treated with adenosine deaminase to deplete samples of adenosine. Treatment of samples with adenosine deaminase resulted in a significant increase in PA-I expression compared to media from HIF-1α overexpressing cells alone (*=P<0.001 ANOVA). C: Effect of adenosine plus adenosine deaminase on PA-I expression in P. aeruginosa. Similar to results in B, the addition of adenosine deaminase to samples with adenosine induced an increase in PA-I expression greater than that of adenosine alone (***=P<0.05 ANOVA), raising the possibility that metabolism of adenosine to inosine induces PA-I expression. D: The effect of inosine on PA-I expression. Exposure of P. aeruginosa to inosine resulted in a dose dependent increase PA-I expression at a concentration 10 fold less than adenosine (*=P<0.001 ANOVA).
References
- 1.Bader MW, Sanowar S, Daley ME, Schneider AR, Cho U, Xu W, Klevit RE, Le Moual H, Miller SI. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell. 2005;122:461–472. doi: 10.1016/j.cell.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 2.Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, Robson SC, Colgan SP. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- 3.Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooi DS, Bycroft BW, Chhabra SR, Williams P, Pritchard DI. Differential immune modulatory activity of Pseudomonas aeruginosa quorum-sensing signal molecules. Infect Immun. 2004;72:6463–6470. doi: 10.1128/IAI.72.11.6463-6470.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S, Will O, Kaul R, Raymond C, Levy R, Chun-Rong L, Guenthner D, Bovee D, Olson MV, Manoil C. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2003;100:14339–14344. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi S, Zimmermann H, Millhorn DE. Chronic hypoxia enhances adenosine release in rat PC12 cells by altering adenosine metabolism and membrane transport. J Neurochem. 2000;74:621–632. doi: 10.1046/j.1471-4159.2000.740621.x. [DOI] [PubMed] [Google Scholar]
- 7.Kohler JE, Zaborina O, Wu L, Wang Y, Bethel C, Chen Y, Shapiro J, Turner JR, Alverdy JC. Components of intestinal epithelial hypoxia activate the virulence circuitry of Pseudomonas. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1048–1054. doi: 10.1152/ajpgi.00241.2004. [DOI] [PubMed] [Google Scholar]
- 8.Koury J, Deitch EA, Homma H, Abungu B, Gangurde P, Condon MR, Lu Q, Xu DZ, Feinman R. Persistent HIF-1alpha activation in gut ischemia/reperfusion injury: potential role of bacteria and lipopolysaccharide. Shock. 2004;22:270–277. doi: 10.1097/01.shk.0000135256.67441.3f. [DOI] [PubMed] [Google Scholar]
- 9.Laughlin RS, Musch MW, Hollbrook CJ, Rocha FM, Chang EB, Alverdy JC. The key role of Pseudomonas aeruginosa PA-I lectin on experimental gut-derived sepsis. Ann Surg. 2000;232:133–142. doi: 10.1097/00000658-200007000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall JC, Christou NV, Meakins JL. The gastrointestinal tract. The “undrained abscess” of multiple organ failure. Ann Surg. 1993;218:111–119. doi: 10.1097/00000658-199308000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritchie AJ, Yam AO, Tanabe KM, Rice SA, Cooley MA. Modification of in vivo and in vitro T- and B-cell-mediated immune responses by the Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl)-L-homoserine lactone. Infect Immun. 2003;71:4421–4431. doi: 10.1128/IAI.71.8.4421-4431.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu K, Ogura H, Goto M, Asahara T, Nomoto K, Morotomi M, Yoshiya K, Matsushima A, Sumi Y, Kuwagata Y, Tanaka H, Shimazu T, Sugimoto H. Altered gut flora and environment in patients with severe SIRS. J Trauma. 2006;60:126–133. doi: 10.1097/01.ta.0000197374.99755.fe. [DOI] [PubMed] [Google Scholar]
- 13.Sitkovsky MV, Ohta A. The ‘danger’ sensors that STOP the immune response: the A2 adenosine receptors? Trends Immunol. 2005;26:299–304. doi: 10.1016/j.it.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Sperandio V, Torres AG, Jarvis B, Nataro JP, Kaper JB. Bacteria-host communication: the language of hormones. Proc Natl Acad Sci U S A. 2003;100:8951–8956. doi: 10.1073/pnas.1537100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 16.Strohmeier GR, Lencer WI, Patapoff TW, Thompson LF, Carlson SL, Moe SJ, Carnes DK, Mrsny RJ, Madara JL. Surface expression, polarization, and functional significance of CD73 in human intestinal epithelia. J Clin Invest. 1997;99:2588–2601. doi: 10.1172/JCI119447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner JR, Rill BK, Carlson SL, Carnes D, Kerner R, Mrsny RJ, Madara JL. Physiological regulation of epithelial tight junctions is associated with myosin light-chain phosphorylation. Am J Physiol. 1997;273:C1378–1385. doi: 10.1152/ajpcell.1997.273.4.C1378. [DOI] [PubMed] [Google Scholar]
- 20.Walters M, Sperandio V. Quorum sensing in Escherichia coli and Salmonella. Int J Med Microbiol. 2006;296:125–131. doi: 10.1016/j.ijmm.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 21.Winzer K, Falconer C, Garber NC, Diggle SP, Camara M, Williams P. The Pseudomonas aeruginosa lectins PA-IL and PA-IIL are controlled by quorum sensing and by RpoS. J Bacteriol. 2000;182:6401–6411. doi: 10.1128/jb.182.22.6401-6411.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu L, Estrada O, Zaborina O, Bains M, Shen L, Kohler JE, Patel N, Musch MW, Chang EB, Fu YX, Jacobs MA, Nishimura MI, Hancock RE, Turner JR, Alverdy JC. Recognition of host immune activation by Pseudomonas aeruginosa. Science. 2005;309:774–777. doi: 10.1126/science.1112422. [DOI] [PubMed] [Google Scholar]
- 23.Wu L, Holbrook C, Zaborina O, Ploplys E, Rocha F, Pelham D, Chang E, Musch M, Alverdy J. Pseudomonas aeruginosa expresses a lethal virulence determinant, the PA-I lectin/adhesin, in the intestinal tract of a stressed host: the role of epithelia cell contact and molecules of the Quorum Sensing Signaling System. Ann Surg. 2003;238:754–764. doi: 10.1097/01.sla.0000094551.88143.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yale CE, Balish E. The importance of six common bacteria in intestinal strangulation. Arch Surg. 1972;104:438–442. doi: 10.1001/archsurg.1972.04180040052009. [DOI] [PubMed] [Google Scholar]