Abstract
Arsenic in drinking water is an established cause of lung, bladder and skin cancers in adults, and may also cause adult kidney and liver cancer. Some evidence for these effects originated from Region II of Chile which had a period of elevated arsenic levels in drinking water, in particular from 1958 to 1970. This unique exposure scenario provides a rare opportunity to investigate the effects of early-life arsenic exposure on childhood mortality; to our knowledge, this is the first study of childhood cancer mortality and high concentrations of arsenic in drinking water. In this paper, we compare cancer mortality rates under the age of 20 in Region II during 1950–2000 with those of unexposed Region V, dividing subjects into those born before, during or after the peak exposure period. Mortality from the most common childhood cancers, leukemia and brain cancer, were not increased in the exposed population. However, we found childhood liver cancer mortality occurred at higher rates than expected; for those exposed as young children liver cancer mortality between ages 0–19 was especially high: the relative risk (RR) for males born during this period was 8.9 (95% CI 1.7–45.8; p=0.009), for females the corresponding RR was 14.1 (95% CI 1.6–126; p=0.018), and for males and females pooled, the RR was 10.6 (95% CI 2.9–39.2; p<0.001). These findings suggest exposure to arsenic in drinking water during early childhood may result in an increase in childhood liver cancer mortality.
Introduction
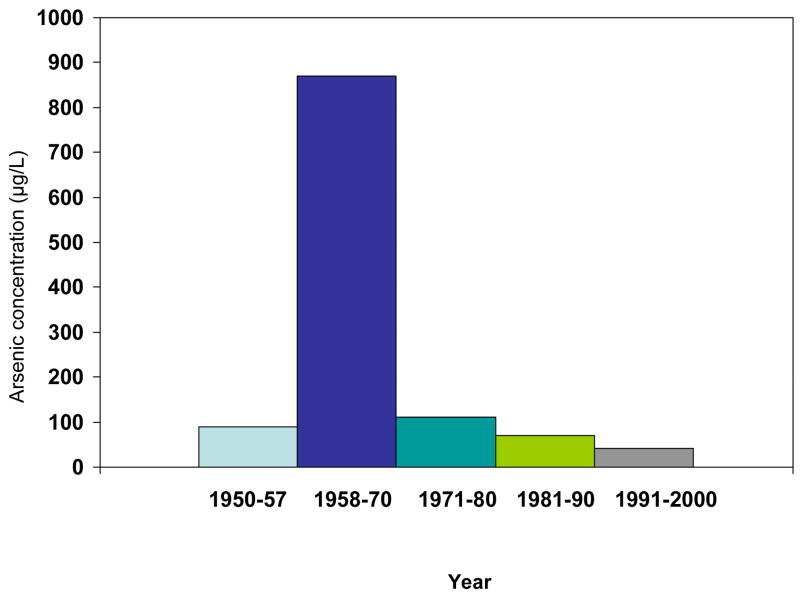
Arsenic has been found in drinking water at high levels in many parts of the world, including Bangladesh, India, Argentina and Chile, and it has been shown to cause numerous health effects, such as skin, bladder and lung cancer (1–5). Antofagasta, the second largest city in Chile, and neighboring city Mejillones, experienced a distinct period of very high arsenic levels in drinking water, when their water supply was supplemented in 1958 with water from rivers that contained arsenic at concentrations near 1000 μg/L. By comparison, the current World Health Organization recommendation for maximum arsenic concentration in drinking water is 10 μg/L. Before 1958, Antofagasta had arsenic concentrations of about 90 μg/L, but from 1958 onwards, levels were on average at 870 μg/L until 1971, when an arsenic removal plant was installed. Arsenic levels in the drinking water thus dropped suddenly to about 110 μg/L and since then have been further reduced (6)(Figure 1). Because the area of Antofagasta and Mejillones is extremely dry, there are few individual water sources, and the whole population drinks from the municipal water source. Until very recently, Chile was divided into 13 Regions, and Antofagasta and Mejillones are located in Region II. Together, these two cities make up more than half of the Region II population. All other major cities and towns in Region II also had high concentrations of arsenic in Region II for varying overlapping periods (2,6). The large population, distinct period of very high exposure, and well-documented exposure history make Region II a highly unique and advantageous area to investigate the health impacts of ingested arsenic. The widespread nature and uniformity of the high exposures in this area, and the presence of a nearby comparable unexposed area, minimize ecologic fallacy and other biases that some researchers commonly associate, sometimes mistakenly, with ecologic studies.
Figure 1.
Arsenic concentrations in Antofagasta/Mejillones water by year. An arsenic removal plant was installed in 1971.
We recently showed that childhood and in utero exposure to arsenic in Antofagasta and Mejillones resulted in markedly increased risks of bronchiectasis and lung cancer in young adults (3). These findings were unexpected, and the magnitude of increased risks from early life exposure are without precedent. Finding adult cancer resulting from early life exposure to arsenic led us to also investigate childhood cancer following early life exposure. The study we report here takes advantage of the unique exposure situation in Region II to assess childhood cancers caused by in utero and/or early life exposure.
Materials and Methods
Computerized mortality data were obtained for Regions II and V of Chile from the Ministry of Health for the period 1980–2000, and from the Chilean National Institute of Statistics (Instituto Nacional Estadisticas) for the period 1971–1979. Due to political unrest in Chile, data from 1976 were unavailable. Computerized mortality data were not available prior to 1971. For the years 1950–1970, death certificates for Region II and a referent Region were photographed and coded by trained nosologists according to the 9th revision of the International Classification of Diseases (ICD-9). The nosologists were kept blind to the Region from which each death certificate originated. Since it was impractical and prohibitively expensive for the study team to code causes of death for all of the death certificates for the entire country of Chile for the years 1950–1970, a smaller referent population was chosen. Region V was used as the referent population because of its sociodemographic similarity to Chile as a whole, its population size, and its low levels of arsenic exposure. Region V has had a population about four times the size of the Region II population over the course of the study period, from 1950 to 2000. Having a referent population that is significantly larger than that of Region II maximizes the statistical precision in the estimation of mortality rate ratios. In preliminary investigations, it was determined that key sociodemographic factors were similar between Region V and the rest of Chile. For example, per capita income in Region V in 1990 was similar to that of the rest of the country (US$2,053 versus US$2,011). Region V has had low exposures to arsenic in drinking water: in data collected from water companies for the period 1990 to 1994, arsenic water levels in the city of Valparaiso (the largest city in Region V) were found to be below the analytical detection limit of 20 μg/L, and there is no evidence to suggest that Valparaiso or any other city or town in Region V had any past exposures to arsenic in drinking water (7). All of the above information indicates that using Region V as a referent population would be a suitable substitute for using all of Chile as a referent population.
Causes of death were coded according to ICD-9 for 1971–1998 and according to the 10th revision for the years 1999–2000, including leukemia (ICD-9 204–208 and ICD-10 C91-C95), brain cancer (ICD-9 191 and ICD-10 C71) and liver cancer (ICD-9 155 and ICD-10 C22). The large majority of death certificates in Region II (89.8%) and Region V (94.5%) were certified by physicians. Annual estimates of the population living in Region II and Region V were obtained for the period 1950–2000 from the National Institute of Statistics (Instituto Nacional de Estadisticas), stratified by age and sex.
We considered childhood cancer deaths in the range from age 0–19. All childhood cancer mortality combined, and the two most common childhood cancers, --leukemia and brain cancer, were examined first along with all “other” cancers combined. The “all other” cancers category displayed unusually elevated relative risks for girls in Region II compared to Region V. The individual cancer types in this category (including liver cancer) were therefore examined separately to see if there were increases in some individual cancer sites.
We used Poisson regression analysis to calculate rate ratios (RRs) between Region II and Region V mortality rates and the associated 95% confidence intervals (CIs) for boys and girls separately and combined, for the age group 0–19 at time of death. Poisson regression analysis was performed using the PROC GENMOD procedure provided in SAS software (version 8.2; SAS Institute, Inc., Cary, North Carolina). RRs were calculated for the groups of persons born in 1950–1957 (before high exposure), 1958–1970 (during high exposure), and 1971–1981 (after high exposure), as the observed number of deaths divided by the expected number of deaths, using Region V as the referent population.
Results
Childhood cancer mortality data for exposed Region II and unexposed Region V for the years 1950–2000 are presented in Table 1, for all childhood cancers combined and for childhood leukemia, brain cancer, and “all other” cancers. Subjects who were born before 1958 had high exposure as young children, those born between 1958 and 1970 had exposure in utero and as young children, while those born after 1970 were never exposed to high levels of arsenic in drinking water.
Table 1.
Leukemia, brain cancer, all other childhood cancer, and all childhood (ages 0–19) cancer mortality rates for 1950–2000, for children born before high exposure (1950–1957), during high exposure (1958–1970) and after high exposure (1971–1981).
| Male | |||||||
|---|---|---|---|---|---|---|---|
| Cause of death | Year-of Birth | Region II Mortality | Region V Mortality | Person-Years Region II | Person-Years Region V | RR (CI) | p-value |
| All male childhood cancers | 1950–1957 | 35 | 124 | 429640 | 1526848 | 1.0 (0.7–1.5) | 0.99 |
| 1958–1970 | 41 | 222 | 805712 | 2900314 | 0.7 (0.5–0.9) | 0.02 | |
| 1971–1981 | 43 | 161 | 833134 | 2689807 | 0.9 (0.6–1.4) | 0.51 | |
| All female childhood cancers | 1950–1957 | 22 | 82 | 433183 | 1528021 | 1.0 (0.6–1.5) | 0.82 |
| 1958–1970 | 48 | 173 | 801664 | 2872533 | 1.0 (0.7–1.4) | 0.97 | |
| 1971–1981 | 36 | 95 | 813999 | 2609758 | 1.2 (0.9–1.6) | 0.20 | |
| Male brain cancer | 1950–1957 | 2 | 7 | 429640 | 1526848 | 1.0 (0.2–4.9) | 0.98 |
| 1958–1970 | 1 | 9 | 805712 | 2900314 | 0.4 (0.1–3.2) | 0.38 | |
| 1971–1981 | 3 | 18 | 833134 | 2689807 | 0.5 (0.1–2.6) | 0.44 | |
| Female brain cancer | 1950–1957 | 0 | 8 | 433183 | 1528021 | 0 | - |
| 1958–1970 | 2 | 9 | 801664 | 2872533 | 0.8 (0.2–3.7) | 0.77 | |
| 1971–1981 | 4 | 9 | 813999 | 2609758 | 1.4 (1.0–2.1) | 0.07 | |
| Male leukemia | 1950–1957 | 22 | 57 | 429640 | 1526848 | 1.4 (0.8–2.2) | 0.21 |
| 1958–1970 | 23 | 102 | 805712 | 2900314 | 0.8 (0.5–1.3) | 0.37 | |
| 1971–1981 | 16 | 71 | 833134 | 2689807 | 0.7 (0.4–1.5) | 0.38 | |
| Female leukemia | 1950–1957 | 8 | 34 | 433183 | 1528021 | 0.8 (0.4–1.8) | 0.64 |
| 1958–1970 | 18 | 90 | 801664 | 2872533 | 0.7 (0.4–1.2) | 0.20 | |
| 1971–1981 | 14 | 50 | 813999 | 2609758 | 0.9 (0.4–2.1) | 0.81 | |
| All other male cancers | 1950–1957 | 10 | 54 | 429640 | 1526848 | 0.7 (0.3–1.3) | 0.22 |
| 1958–1970 | 15 | 93 | 805712 | 2900314 | 0.6 (0.3–1.0) | 0.05 | |
| 1971–1981 | 21 | 61 | 833134 | 2689807 | 1.1 (0.9–1.4) | 0.30 | |
| All other female cancers | 1950–1957 | 14 | 38 | 433183 | 1528021 | 1.3 (0.7–2.4) | 0.40 |
| 1958–1970 | 27 | 69 | 801664 | 2872533 | 1.4 (0.9–2.2) | 0.14 | |
| 1971–1981 | 17 | 35 | 813999 | 2609758 | 1.6 (1.1–2.3) | 0.02 | |
There was little evidence of any increased cancer risks for all childhood cancers combined, nor for the individual sites of brain cancer and leukemia. For leukemia, in males born in 1950–1957 just before high exposure the relative risk estimate (RR) was 1.4 (CI 0.8–2.2) while for those born during high exposure and after high exposure the RRs were 0.8 (CI 0.5–1.3) and 0.7 (CI 0.4–1.5), respectively. As seen in Table 1, RRs for leukemia in females were all near1.0 (1950–1957: 0.8 (CI 0.4–1.8); 1958–1970: 0.7 (CI 0.4–1.2); 1971–1981: 0.9 (CI 0.4–2.1)).
For “all other” cancers combined, the RR among females of Region II was elevated especially for the time period 1971–1981 (RR=1.6 (CI 1.1–2.3)). Because of this unusual finding, we decided to analyze the component cancers for the all “other” cancer group separately. This led to unexpected findings for liver cancer among both boys and girls.
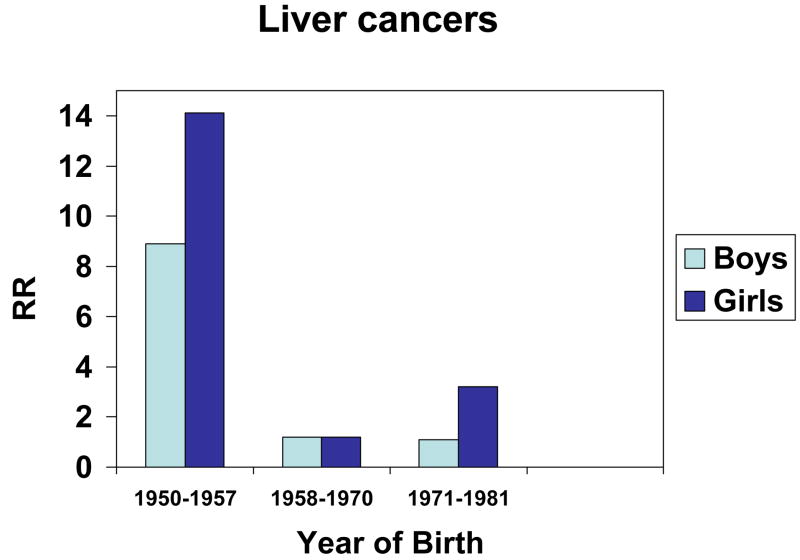
For both males and females, liver cancer deaths occurred at much higher numbers than expected, especially for those born in 1950–1957 just before the high exposure period and who would have been exposed as young children (Table 2 and Figure 2). For males born between 1950 and 1957, the relative risk was 8.9 (CI 1.7–45.8; p=0.009), while for females born between 1950 and 1957, the relative risk was 14.1 (CI 1.6–126.2; p=0.018). The pooled relative risk estimate for boys and girls was 10.6 (CI 2.9–39.3; p<0.001). For those born between 1958 and 1970 (during the high exposure period), the male liver cancer mortality relative risk estimate was 1.2 (CI 0.1–11.5) while the female liver cancer mortality relative risks was 1.2 (CI 0.1–11.5). After the period of high exposure, during the years between 1971 and 1981, the male mortality relative risk estimate was at 1.1 (CI 0.1–10.4), the female mortality relative risk was 3.2 (CI 0.2–51.3) and the pooled relative risk was at 1.6 (CI 0.3–8.8) (Figure 2).
Table 2.
Male, female, and pooled liver cancer mortality rates from 1950 to 2000 for those born before high exposure (1930–1939, 1940–1949, 1950–1957), during high exposure (1958–1970) and after high exposure (1971–1981, 1982–2000), for ages 0–19.
| Cause of death | Year-of Birth | Region II Mortality | Region V Mortality | Person-Years Region II | Person-Years Region V | RR (CI) | p-value |
|---|---|---|---|---|---|---|---|
| Males | 1930–1939 | 2 | 2 | 79145 | 282706 | 3.6 (0.5–25.4) | 0.20 |
| 1940–1949 | 2 | 4 | 204760 | 752514 | 1.8 (0.3–9.8) | 0.50 | |
| 1950–1957 | 5 | 2 | 429640 | 1526848 | 8.9 (1.7–45.8) | 0.009 | |
| 1958–1970 | 1 | 3 | 805712 | 2900314 | 1.2 (0.1–11.5) | 0.87 | |
| 1971–1981 | 1 | 3 | 833134 | 2689807 | 1.1 (0.1–10.4) | 0.95 | |
| 1982–2000 | 1 | 1 | 826691 | 2489933 | 3.0 (0.2–48.2) | 0.44 | |
| Females | 1930–1939 | 0 | 1 | 78833 | 292225 | 0 | - |
| 1940–1949 | 1 | 3 | 313431 | 1123930 | 1.2 (0.1–11.5) | 0.88 | |
| 1950–1957 | 4 | 1 | 433183 | 1528021 | 14.1 (1.6–126.2) | 0.018 | |
| 1958–1970 | 1 | 3 | 801664 | 2872533 | 1.2 (0.1–11.5) | 0.88 | |
| 1971–1981 | 1 | 1 | 813999 | 2609758 | 3.2 (0.2–51.3) | 0.41 | |
| 1982–2000 | 0 | 5 | 794560 | 2389023 | 0 | - | |
| Pooled | 1930–1939 | 2 | 3 | 157978 | 574931 | 2.4 (0.4–14.4) | 0.33 |
| 1940–1949 | 3 | 7 | 623139 | 2232402 | 1.5 (0.4–5.9) | 0.53 | |
| 1950–1957 | 9 | 3 | 862823 | 3054869 | 10.6 (2.9–39.3) | <0.001 | |
| 1958–1970 | 2 | 6 | 1607376 | 5772847 | 1.2 (0.2–5.9) | 0.83 | |
| 1971–1981 | 2 | 4 | 1647133 | 5299565 | 1.6 (0.3–8.8) | 0.58 | |
| 1982–2000 | 1 | 6 | 1621251 | 4878956 | 0.5 (0.1–4.2) | 0.52 |
Figure 2.
Liver cancer relative risks for boys and girls before high exposure period (1950–1957), during high exposure period (1958–1970), and after high exposure period (1971–1981).
All but one of the liver cancer deaths among those born in 1950–57 were aged 10–19 at the time of death. Thus there were 8 liver cancer deaths in Region II aged 10–19, and none in Region V which had a population three times larger. The probability of this occurring by chance is less than 1 in 100,000. Childhood liver cancer mortality is normally extremely rare, and finding no comparable deaths in Region V is not surprising. From 1972 onwards we have mortality data for all of Chile, and there were only 13 liver cancer deaths aged 10–19 during 1972–1982 in the whole of the rest of Chile excluding Region II. Using these data to calculate childhood liver cancer rates for the earlier time period in Region II, we have estimated the rate ratio in 1950–57 to be 27.3 for boys (95%CI 9.3–80, p<0.001) and 53.7 for girls (95% CI 10.8–265, p<0.001).
Further information on the 8 liver cancer deaths in Region II is given in Table 3 including data from death certificates and birth certificates. We could not locate the birth certificates for two cases. The birth certificate of the first case on the list was given as 1949 and not 1950 as calculated from information on the death certificate. We retained this case in some analyses because making corrections based on birth certificates obtained for just five cases could introduce bias in the analyses. However we also estimated relative risk with this case excluded: for males born 1950–1957 the relative risk estimate after the exclusion was = 7.11 (1.30–38.8) p=0.02, and for males and females pooled, relative risk = 9.44 (2.50–35.6) p<0.001. It is likely that the same one year discrepancy in year of birth occurred with others, and making corrections based on birth certificates obtained for five cases would introduce bias in the analyses. Of the eight cases of liver cancer, five were confirmed to have been born in Region II, in the cities of Antofagasta (one), Calama (two), Pedro de Valdivia (one), and Tocopilla (one). One case was born in Region IV, in the city of Barraza but resided in Calama in Region II for an unknown period before her death. In all Region II cities, arsenic levels in drinking water were substantially higher during the years of exposure than the World Health Organization recommended maximum concentration of arsenic in drinking water, 10 μg/L or levels in the rest of the country. In Pedro de Valdivia and Tocopilla, the arsenic concentration in drinking water from 1950–1970 was 250 μg/L, while in Calama, the levels were an average of 150 μg/L (8).
Table 3.
Liver cancer deaths aged 10–19 in Region II among those born between 1950–1957 in Region II of Chile
| Gender | Year of birth from death certificate | Place of birth | Place of death | Year of death | Age of death | Cause of death on death certificate * |
|---|---|---|---|---|---|---|
| M | 1950 # | Tocopilla | Tocopilla | 1969 | 19 | Cancer de higado |
| M | 1954 | Unknown | Antofagasta | 1968 | 14 | Adenocarcinoma hepatocelular |
| M | 1954 | Pedro de Valdivia | Antofagasta | 1972 | 18 | Cancer hepático |
| M | 1955 | Antofagasta | Antofagasta | 1967 | 12 | Neo hepatocelular $ |
| M | 1956 | Calama | Calama | 1970 | 13 | Cáncer primario de higado |
| F | 1953 | Barraza | Antofagasta | 1970 | 18 | Cancer hepatico |
| F | 1955 | Calama | Calama | 1965 | 10 | Cancer de higado, hepatoma |
| F | 1956 | Unknown | Region II city unkown | 1972 | 16 | Previously ICD coded as liver cancer |
“Higado” is Spanish for liver, “hepatico” is Spanish for “of the liver”, “hepatoma” means liver cancer
Year of birth was derived form death certificate data for the study. When the birth certificate was obtained, it was discovered that this boy was actually born in 1949. However identical death certificate data had been used for Region II and Region V, so the boy was included in data analysis.
“Neo” is an abbreviation for neoplasm.
Children born in the period 1958 to 1970 would have also experienced some childhood exposure, as well as in utero exposure. Overall there was no evidence of increased risks for the age range 0–19 (Table 2). However focusing on the age range 10–19, there was one liver cancer death in Region II and one in Region V, giving a rate ratio estimate of 3.60 (95% CI 0.23–57.5). The confidence interval is very wide and no conclusion can be drawn from this risk estimate.
Discussion
This is the first study to find evidence that ingestion of arsenic in drinking water in early childhood might increase the risks of childhood liver cancer mortality. Arsenic in drinking water is established to be a major cause of adult cancer in exposed populations (9) and we previously reported marked increases in lung and bladder cancer in the same study population in Region II of Chile (10), including increased risks of lung cancer in young adults following early childhood exposure (3). In light of these findings it is reassuring not to find increased overall risks for childhood cancer mortality. However, among the subjects exposed as young children born in 1950–1957 based on death certificate data, we found that liver cancer mortality was markedly increased for both boys and girls aged 0–19 (for males, relative risk was 8.9 (CI 1.7 to 45.8); for females, the relative risk was 14.1 (CI 1.6 to 126.2). In the age range of 10–19 years, all 8 liver cancer cases died in Region II, and none in the much larger population of Region V, a finding which had a probability of being due to chance of less than 1 in 100,000. Although the overall number of cases was relatively small, the large exposed and unexposed populations we studied, the high magnitude of the relative risks identified and the low associated p-values along with the consistency of findings in both genders all suggest that these findings are not due to chance or bias.
Arsenic exposure has been suggested to increase hepatic cancer in adults, primarily in studies in Taiwan (11). However we have previously reported that there was no increase in adult liver cancer mortality in Region II of Chile (2). Liver cancer is a rare cancer in children, and usually comprises only about 1% of childhood cancers (12). Childhood hepatic cancers comprise two main types, hepatoblastoma which is the predominant form in children under the age of 5, and hepatocellular carcinoma which predominates in older children. In a recent United States study, among children five years and younger, hepatoblastoma accounted for 91% of cases, while hepatocellular carcinoma accounted for 87% of cases among those 15 to 19 years of age (13). In our study, all but one of the children who died from liver cancer was in the age range 10–19. A limitation of this study is that we do not have pathological reports, although in two cases hepatocellular carcinoma was the diagnosis given on the death certificate. Since hepatitis B is a major risk factor for childhood hepatocellular carcinoma (13–17), we considered the possibility that the increased risks we identified were due to hepatitis B in Region II. However, the pattern of marked increases in liver cancer mortality in both boys and girls coinciding with the very high arsenic exposure period in Region II, with subsequent decreases in risks following the end of this high exposure period suggests that arsenic in water was a more likely explanation. Furthermore, to our knowledge the relationship between hepatitis B and childhood liver cancer has been in populations with chronic hepatitis B infection, rather than occurring in sudden outbreaks.
There is little other information on childhood cancers and exposure to arsenic. We conducted a study of childhood cancer incidence in Nevada and arsenic in drinking water and did not find overall evidence of increased childhood cancer incidence with exposure, and there was only one case of liver cancer in 20 years (18). However, the highest exposure category involved a range from 35–90 μg/L of arsenic in water. We also investigated a remarkable childhood leukemia cluster that occurred in the city of Fallon where water concentrations were about 90 μg/L (19). Eleven cases were diagnosed between 1999 and 2001, resulting in an age-standardized rate ratio in the county of 12.0 (95% CI=6.0–21.4). However, there was no basis for linking this cluster solely to arsenic in the water since the cluster had occurred only relatively recently while arsenic levels in this area had been at the same concentration for about the last 50 years.
Arsenic exposure was assessed in one population-based case-control study of childhood leukemia based in Quebec, Canada (20). The authors reported slightly increased relative risks for childhood acute lymphoblastic leukemia related to postnatal exposure to arsenic (OR=1.94; 95% CI=0.64–5.83) but not for prenatal period exposure. These exposures were very low, at an average water concentration of 5 μg/L — more than 100 times lower than the exposure of children in Region II of Chile in the peak exposure period. Falk et al (1981) reported four female cases of childhood hepatic angiosarcoma, one of whom was exposed to arsenic through multiple sources (21). Her father worked at a mine, and the child was exposed to arsenic via soil around her house, water, and dust on the father’s work clothes. One case of angiosarcoma of the liver has also been reported in Region II of Chile diagnosed in 1971 at the age of 22, who also had arsenic related skin lesions (22). He lived in Pedro de Valdivia like one of the cases we have listed in Table 3. Unfortunately it is not now possible to obtain medical records to search for pathology reports for the liver cancer deaths in Table 3, all of whom died more than 35 years ago, but as we noted above two of the death certificates make specific mention of hepatocellular carcinoma and there is no mention of angiosarcoma.
A limitation of this study is the lack of individual data on exposure. However, the evidence suggests that virtually everyone in Region II was exposed to much higher concentrations of arsenic in drinking water than in Region V. Almost all drinking water in Region II is supplied by a few large municipal water sources, and almost the entire Region II had high concentrations of arsenic in their drinking water during various periods. The population-weighted average arsenic concentration in drinking water for the entire region was about 580 μg/L for around thirteen years from 1958 to 1970 (23). Because almost everyone in Region II was exposed, ecological fallacy which can occur if one cannot be sure if those with the outcome were actually exposed, is highly unlikely in this study. In addition, the increases in relative risks are too large to result from factors such as migration. In fact, any in-migration of people from other regions of Chile would most probably have diluted the relative risk effects caused by arsenic in water.
In conclusion, we found marked increases in liver cancer mortality in the age range 10–19 among children who had exposure to high concentrations of arsenic in drinking water starting soon after birth. We did not find overall increases in childhood cancer mortality in Region II of Chile. The importance of these findings is in part due to Region II of Chile having the largest population in the world uniformly exposed to very high concentrations of arsenic in drinking water. This gives reassurance that arsenic in water probably does not increase overall childhood cancer mortality. However, the uniqueness of the exposure scenario in Region II also means that it will be difficult to identify other large enough populations with high, well-documented arsenic exposure to confirm our findings concerning childhood liver cancer.
References
- 1.Steinmaus CM, Moore LE, Hopenhayn-Rich C, Biggs ML, Smith AH. Arsenic in drinking water and bladder cancer. Cancer Invest. 2000;18:174–82. doi: 10.3109/07357900009038249. [DOI] [PubMed] [Google Scholar]
- 2.Smith AH, Goycolea M, Haque R, Biggs ML. Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am J Epidemiol. 1998;147:660–9. doi: 10.1093/oxfordjournals.aje.a009507. [DOI] [PubMed] [Google Scholar]
- 3.Smith AH, Marshall G, Yuan Y, et al. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ Health Perspect. 2006;114:1293–6. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith AH, Arroyo AP, Mazumder DN, et al. Arsenic-induced skin lesions among Atacameno people in Northern Chile despite good nutrition and centuries of exposure. Environ Health Perspect. 2000;108:617–20. doi: 10.1289/ehp.00108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tondel M, Rahman M, Magnuson A, Chowdhury IA, Faruquee MH, Ahmad SA. The relationship of arsenic levels in drinking water and the prevalence rate of skin lesions in Bangladesh. Environ Health Perspect. 1999;107:727–9. doi: 10.1289/ehp.99107727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferreccio C, Gonzalez CA, Milosavjlevic V, Marshall G, Sancha AM, Smith AH. Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology. 2000;11:673–9. doi: 10.1097/00001648-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Hopenhayn-Rich C, Browning SR, Hertz-Picciotto I, Ferreccio C, Peralta C, Gibb H. Chronic arsenic exposure and risk of infant mortality in two areas of Chile. Environ Health Perspect. 2000;108:667–73. doi: 10.1289/ehp.00108667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivara MI, Cebrian ME, Corey G, Hernandez M, Romieu I. Cancer risk in an arsenic-contaminated area of Chile. Toxicol Ind Health. 1997;13:321–38. doi: 10.1177/074823379701300217. [DOI] [PubMed] [Google Scholar]
- 9.International Agency for Research on Cancer. Some drinking-water disinfectants and contaminants, including arsenic. Vol. 84. Lyon, France: World Health Organization; 2004. [Google Scholar]
- 10.Marshall G, Ferreccio C, Yuan Y, et al. Fifty-year study of lung and bladder cancer mortality in Chile related to arsenic in drinking water. J Natl Cancer Inst. 2007;99:920–8. doi: 10.1093/jnci/djm004. [DOI] [PubMed] [Google Scholar]
- 11.Chiu HF, Ho SC, Wang LY, Wu TN, Yang CY. Does arsenic exposure increase the risk for liver cancer? J Toxicol Environ Health A. 2004;67:1491–500. doi: 10.1080/15287390490486806. [DOI] [PubMed] [Google Scholar]
- 12.IARC. International incidence of childhood cancer. IARC Sci Publ. 1998;2 [PubMed] [Google Scholar]
- 13.Darbari A, Sabin KM, Shapiro CN, Schwarz KB. Epidemiology of primary hepatic malignancies in U.S. children. Hepatology. 2003;38:560–6. doi: 10.1053/jhep.2003.50375. [DOI] [PubMed] [Google Scholar]
- 14.Chang MH, Shau WY, Chen CJ, et al. Hepatitis B vaccination and hepatocellular carcinoma rates in boys and girls. Jama. 2000;284:3040–2. doi: 10.1001/jama.284.23.3040. [DOI] [PubMed] [Google Scholar]
- 15.Stiller CA, Pritchard J, Steliarova-Foucher E. Liver cancer in European children: incidence and survival, 1978–1997. Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2115–23. doi: 10.1016/j.ejca.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Chen JC, Chang ML, Lin JN, et al. Comparison of childhood hepatic malignancies in a hepatitis B hyper-endemic area. World J Gastroenterol. 2005;11:5289–94. doi: 10.3748/wjg.v11.i34.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang MH, Chen TH, Hsu HM, et al. Prevention of hepatocellular carcinoma by universal vaccination against hepatitis B virus: the effect and problems. Clin Cancer Res. 2005;11:7953–7. doi: 10.1158/1078-0432.CCR-05-1095. [DOI] [PubMed] [Google Scholar]
- 18.Moore LE, Lu ML, Smith AH. Childhood cancer incidence and arsenic exposure in drinking water in Nevada. Arch Environ Health. 2002;57:201–6. doi: 10.1080/00039890209602937. [DOI] [PubMed] [Google Scholar]
- 19.Steinmaus CM, Lu ML, Todd RL, Smith AH. Probability estimates for the unique childhood leukemia cluster in Fallon, Nevada, and risks near other U.S. military aviation facilities. Environ Health Perspect. 2004;112:766–71. doi: 10.1289/ehp.6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Infante-Rivard C, Olson E, Jacques L, Ayotte P. Drinking water contaminants and childhood leukemia. Epidemiology. 2001;12:13–9. doi: 10.1097/00001648-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Falk HL, Herbert JT, Edmonds L, Heath CW, Jr, Thomas LB, Popper H. Review of four cases of childhood hepatic angiosarcoma--elevated environmental arsenic exposure in one case. Cancer. 1981;47:382–91. doi: 10.1002/1097-0142(19810115)47:2<382::aid-cncr2820470228>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 22.Rennke H, Prat G, Etcheverry R, et al. Hemangioendothelioma maligno del higato y arsenicismo cronico. (In Spanish) Rev Med Chil. 1971;99:664–8. [PubMed] [Google Scholar]
- 23.Yuan Y, Marshall G, Ferreccio C, et al. Acute myocardial infarction mortality in comparison with lung and bladder cancer mortality in arsenic-exposed Region II of Chile from 1950 to 2000. Am J Epidemiol. 2007;166:1381–91. doi: 10.1093/aje/kwm238. [DOI] [PubMed] [Google Scholar]