Abstract
Background
The expression of Type III secretion system (TTSS) in Shigella is regulated in response to changes in environmental osmolarity and temperature. Temperature-dependent regulation of virF, the master regulator of TTSS synthesis, is believed to occur at the transcriptional level. We recently demonstrated, however, that TTSS synthesis also involves post-transcriptional regulation of the synthesis of InvE, a target of virF and key regulator of TTSS synthesis. The mRNA levels of invE (virB) are stable at 37°C, but mRNA stability markedly decreases at low temperatures where the TTSS synthesis is tightly repressed. Deletion of hfq, which encodes an RNA chaperone in Gram-negative bacteria, results in the restoration of expression of invE and other TTSS genes at low temperature due to an increase in the stability of invE mRNA. To date, the molecular details of the regulation of TTSS expression in response to osmotic pressure are not known. In the current study, we investigated the mechanism of regulation of TTSS by osmotic pressure.
Results
Transcription of virF, which encodes the master regulator of TTSS expression, was partially repressed under low osmotic conditions. Several lines of evidence indicated that osmolarity-dependent changes in TTSS synthesis are controlled at the post-transcriptional level, through the regulation of InvE synthesis. First, the expression InvE protein was tightly repressed under low osmotic growth conditions, even though invE mRNA transcripts were readily detectable. Second, under low osmotic conditions, invE mRNA was rapidly degraded, whereas deletion of hfq, which encodes an RNA chaperone, resulted in increased invE mRNA stability and the production of InvE protein. Third, the binding of purified Hfq in vitro to invE RNA was stronger in low-salt buffer, as assessed by gel-shift analysis and surface plasmon resonance (Biacore analysis).
Conclusion
Osmolarity-dependent changes in TTSS synthesis in Shigella involve the post-transcriptional regulation of InvE expression, in addition to partial transcriptional activation by virF. The stability of invE mRNA is reduced under low osmotic conditions, similar to the effect of temperature. Deletion of an RNA chaperone gene (hfq) abolished the repression of TTSS synthesis at low osmolarity through a mechanism that involved increased stability of invE mRNA. We propose that the expression of Shigella virulence genes in response to both osmolarity and temperature involves the post-transcriptional regulation of expression of InvE, a critical regulator of TTSS synthesis.
Background
TTSS plays a major role in virulence determination in pathogenic Shigella. The expression of TTSS is regulated in response to environmental stimuli, such as changes in salt concentration [1] and growth temperature [2,3]. This response to environmental factors is appropriate for the life cycle of Shigella, in which the expression of virulence genes is required for invasion and propagation in the host intestinal tract, but might be a potential burden for survival in the natural environment.
The genes that encode the components of TTSS in Shigella are located on the virulence plasmid, and are controlled by two regulator proteins, VirF and InvE (VirB) [4,5]. VirF, an AraC-type transcriptional regulator, activates the transcription of invE (virB) [4,6-8]. InvE is a homologue of a plasmid-partitioning factor, ParB [7], and possesses DNA binding activity [9]. InvE activates the transcription of the mxi-spa and ipa genes, which encode the components of TTSS, through competition with the global repressor H-NS, a histone-like DNA binding protein [10].
Recently, we reported that the temperature-dependent expression of TTSS is controlled at the post-transcriptional level, through the regulation of InvE synthesis [11]. The mRNA of invE is highly stable at 37°C, but stability decreases significantly at 30°C where the TTSS synthesis is tightly repressed. Deletion mutants of hfq, which encodes an RNA-binding protein in Gram-negative bacteria, restores the expression of invE and other TTSS genes at low temperature due to the increased stability of the invE mRNA.
To date, a detailed mechanism of osmolarity-dependent regulation of TTSS expression has yet to be elucidated. In the current study, we examined whether osmotic-dependent changes in TTSS expression involved post-transcriptional regulation. We present several lines of evidence that invE expression is regulated at the post-transcriptional level during TTSS synthesis in Shigella, and that the RNA chaperone Hfq plays a key role in regulating invE mRNA stability.
Results
Osmolarity and TTSS expression
The expression of TTSS in Shigella is markedly reduced in low-salt LB medium [1]. However, it is not clear whether the critical factor for the decreased expression of TTSS in LB medium is low osmolarity or low-salt concentration. We analysed the expression of TTSS in the presence of several different osmolytes, but similar osmotic pressures. There was a difference in the growth rate of S. sonnei in LB medium in the absence (doubling time, 42.1 minutes) and presence (doubling time, 30.6 minutes) of 150 mM NaCl. To control for differences in growth rate in LB medium, we used yeast extract and nutrient broth (YENB) medium [12], since growth rate in YENB in the absence (doubling time, 32.2 minutes) and presence (doubling time, 31.4 minutes) of 150 mM NaCl was similar at 37°C. The osmotic pressure of YENB medium without and with 150 mM NaCl was 96 ± 3 and 397 ± 3 mOsm/kg• H2O, respectively. When 150 mM NaCl was replaced with 155 mM KCl, the osmotic pressure was 391 ± 2 mOsm/kg• H2O, whereas when NaCl was replaced with 260 mM sorbitol, osmotic pressure was 384 ± 1 mOsm/kg• H2O.
To monitor the expression of TTSS, we measured the expression of the effector protein IpaB and the regulatory molecule InvE. The expression of IpaB and InvE was tightly repressed in low osmotic conditions, whereas in the presence of either 150 mM NaCl or 155 mM KCl, the level of both proteins increased to a similar extent (Fig. 1A). A linear relationship was observed between salt concentration and the levels of InvE and IpaB (data not shown), which indicated that there is no threshold for the effective induction of TTSS synthesis. In the presence of 260 mM sorbitol, the levels of both InvE and IpaB were approximately 50% lower than in the presence of NaCl and KCl (Fig. 1A). When the concentration of sorbitol was increased to 520 mM, InvE and IpaB levels increased to the level of the NaCl and KCl growth conditions. These results indicated that in addition to salt concentration, osmolarity regulates the expression of TTSS, although the optimum concentration for maximum induction differed among osmolytes (see discussion).
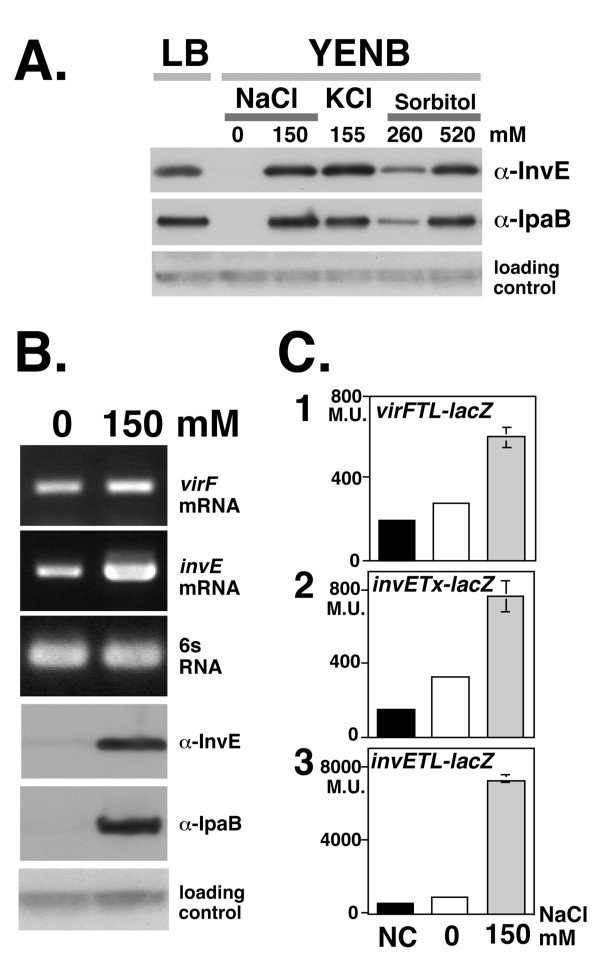
Figure 1.
A. InvE and IpaB expression in different osmotic conditions. An overnight culture of strain MS390 at 30°C was inoculated into fresh YENB medium with or without osmolytes and then incubated at 37°C until mid-log phase (A600 = 0.8). Medium, osmolyte, and concentration are indicated at the top of the panel. Antibodies used for detection are indicated on the right of the panels. A cross-reactive unknown protein detected by the anti-InvE antiserum was used as a loading control for InvE Western blot analysis throughout this study. B. Expression of >invE and virF mRNA and InvE and IpaB protein expression in S. Sonnei. Total RNA (100 ng) and 10 μl of the indicate culture were subjected to analysis of mRNA and protein levels, respectively. The 6S RNA ssrS gene was used as control for RT-PCR. Primers and antibodies are indicated on the right side of the panels. Concentration of NaCl in the medium is indicated at top of the panel. C. Expression of invE and virF >promoter-driven reporter genes. Wild-type S. sonnei strain MS390 carrying the indicated reporter plasmids were subjected to a β-galactosidase assay: Graph 1, virFTL-lacZ translational fusion plasmid pHW848; Graph 2, invETx-lacZ transcriptional fusion plasmid pJM4320; Graph 3, invETL-lacZ translational fusion plasmid pJM4321. Concentration of NaCl is indicated at the bottom of the graphs. Details of the control experiments, indicated by black bars (NC)are described in methods.
Transcription of virF and invE under low osmotic conditions
Both ipaB and invE are under the control of the upstream transcriptional regulator VirF [4,6-8]. To identify the level at which IpaB and InvE expression was regulated in response to changes in osmolarity, we analyzed the expression of virF. In the absence of salt, virF mRNA was detectable by RT-PCR (Fig. 1B, virF mRNA), although the level of mRNA expression was approximately 29.0 ± 4.6% of the maximum level observed in the presence of 150 mM NaCl. In an attempt to determine the mechanism of regulation of virF transcription, we performed a reporter gene assay in which the expression of lacZ was driven by the virF promoter [8]. In wild-type S. sonnei carrying the virF-lacZ reporter gene, the level of β-galactosidase activity in the absence of salt was 20.6% of that in the presence of 150 mM NaCl (Fig. 1C, Graph 1), which indicated that the virF promoter is partially active even in the absence of NaCl.
We examined VirF-dependent expression of invE by Western blot and RT-PCR. The production of InvE protein was almost completely repressed under conditions of low osmolarity (Fig. 1B, α-InvE), whereas under the same conditions, there was a significant level of invE mRNA detectable by RT-PCR (Fig. 1B, invE mRNA). Real-time RT-PCR analysis indicated that the amount of invE mRNA in the absence of NaCl was 9.5 ± 1.6% of the level in the presence of 150 mM NaCl. We carried out a reporter gene assay to examine the expression of invE at both the transcriptional and translational levels [13]. In low osmolarity, β-galactosidase activity in wild-type S. sonnei that expressed the transcriptional fusion gene invETx-lacZ was moderately decreased, to 28.9% of that seen in the presence of 150 mM NaCl (Fig. 1C, Graph 2). In contrast, β-galactosidase activity in cells that expressed the translational fusion gene invETL-lacZ was 7.3% of the level in the presence of 150 mM NaCl (Fig. 1C, Graph 3). These results indicated that the expression of InvE protein is repressed in the absence of salt, a condition under which genes for at least two regulatory proteins are still transcribed, albeit at reduced levels. Thus, the repression of InvE synthesis occurs primarily at the post-transcriptional level.
Post-transcriptional regulation of invE
To examine the mechanism of post-transcriptional regulation of invE expression more directly, we replaced the native invE promoter with a promoter cassette containing the E. coli araC repressor and the araBAD promoter region [14]. In this system, we were able to examine VirF-independent expression of InvE under the control of the AraC-dependent araBAD promoter. Strain MS5512 carrying ΔpinvE::paraBAD [11] was cultured in the presence or absence of 150 mM NaCl, and the synthesis of InvE protein was induced by increasing the concentration of arabinose. Similar levels of invE mRNA were detected in the presence of 0.2 and 1.0 mM arabinose, independently of the presence or absence of NaCl (Fig. 2A, invE mRNA). However, the synthesis of InvE protein was significantly decreased in the absence of NaCl (Fig. 2A, α-InvE), as was InvE-dependent synthesis of the TTSS effector protein IpaB (Fig. 2A, α-IpaB).
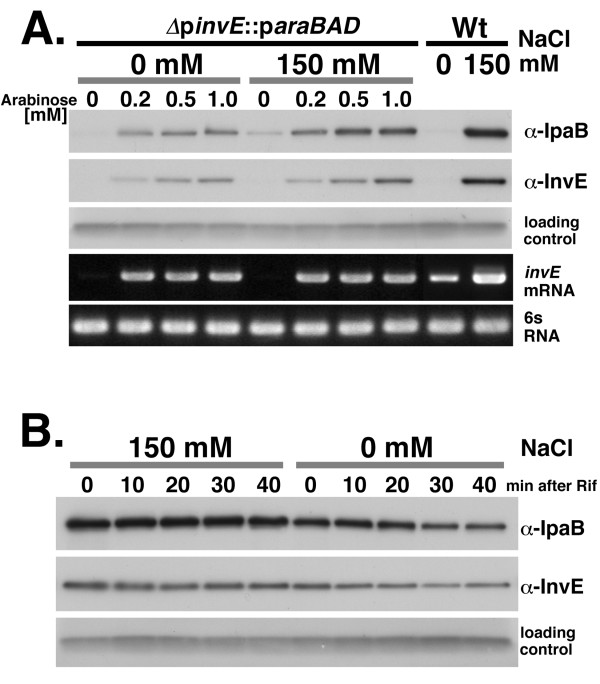
Figure 2.
A. InvE expression in ΔpinvE::paraBAD strain MS5512. ΔpinvE::paraBAD strain MS5512 and wild-type strain MS390 were grown overnight in LB medium containing chloramphenicol and 50 μM arabinose, washed twice with fresh LB medium, and then inoculated into YENB media containing increasing concentrations of arabinose and cultured at 37°C with or without 150 mM NaCl, as indicated. Strains (ΔpinvE::paraBAD, MS5512; Wt, wild-type strain MS390), concentration of NaCl (0 mM or 150 mM) and concentration of arabinose (0, 0.2, 0.5, 1.0 mM) are indicated above the panels. Primers and antibodies used in the experiments are indicated on the right side of the panels. B. Stability of InvE protein. ΔinvE strain MS1632 carrying the expression plasmid pBAD-invE was grown in YENB media containing ampicillin and 100 μM arabinose, with or without 150 mM NaCl, at 37°C. When cultures reached an A600 of 0.8, rifampicin was added. Cells were harvested at 10 min intervals for a period of 40 min. Whole cell cultures (10 μl) were analysed by Western blot using anti-InvE and -IpaB antibodies.
To determine whether the low level of InvE protein synthesis under conditions of low NaCl was due to decreased protein stability, we examined the metabolic stability of InvE in an invE deletion mutant strain (strain MS1632) carrying an expression plasmid for InvE (pBAD-invE) [11] at various times after treatment with rifampicin. The levels of InvE and IpaB were slightly lower in the absence of NaCl than in the presence of NaCl. Both proteins gradually degraded over time after rifampicin treatment, but the rate of degradation was essentially the same in the presence or absence of NaCl (Fig. 2B). By comparison, invE mRNA decayed within 10 minutes (min) after rifampicin treatment, and the rate of decay was much faster in low NaCl than in 150 mM NaCl (see below). These results indicated that InvE protein is metabolically stable once it is synthesized.
Involvement of Hfq in the post-transcriptional regulation of InvE synthesis
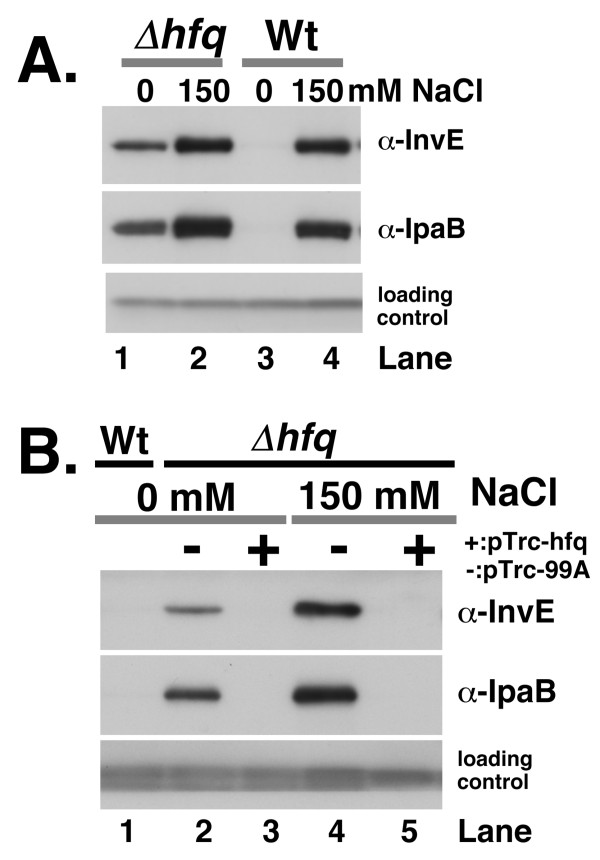
Previously, we showed that the RNA-binding protein Hfq [15,16] is involved in the temperature-dependent regulation of invE expression, and that this regulation occurs at the post-transcriptional level [11]. We next examined the expression of InvE in an hfq deletion mutant strain of S. sonnei (strain MS4831) under low osmotic conditions. As in the case of temperature-dependent regulation, the level of expression of InvE and IpaB in an hfq mutant strain in the absence of NaCl was approximately 33% of that seen in the presence of 150 mM NaCl (Fig. 3A lane 1), which suggested that Hfq is involved in the osmolarity-dependent post-transcriptional regulation of InvE and IpaB synthesis. Real-time analysis of virF mRNA in the hfq mutant in the absence of NaCl indicated that the level of expression of virF was 36.5 ± 4.5% of that seen in the wild-type strain in the presence of 150 mM NaCl, which suggested that the level of virF transcription in the hfq mutant parallels the level of InvE protein synthesis. Thus, in the absence of Hfq, the level of InvE protein in low osmotic conditions correlated with the level of virF and invE transcription (Fig. 1C, graph 1 and 2). To confirm these results, we introduced an Hfq expression plasmid, pTrc-hfq, into the hfq deletion mutant. Ectopic expression of Hfq in the mutant strain resulted in the repression of InvE expression in low osmotic conditions (Fig. 3B, lane 3), and abolished the expression of InvE and IpaB even in physiological osmotic conditions (Fig. 3B, lane 5).
Figure 3.
A. InvE and IpaB expression in the hfq deletion mutant. Wild-type strain MS390 and the hfq mutant strain MS4831 were cultured in YENB media with or without NaCl, and then subjected to Western blot analysis. Strains and concentration of NaCl are indicated above the panels. Antibodies used in the experiment are indicated on the right side of the panels. B. Effect of ectopic Hfq expression on InvE and IpaB in the hfq mutant. hfq deletion mutants carrying an Hfq expression plasmid or a control plasmid were subjected to Western blot analysis. Strains were grown in YENB medium containing ampicillin and IPTG, or YENB medium containing ampicillin, IPTG and 150 mM NaCl at 37°C, and then harvested. Strains, concentration of NaCl and plasmids (minus, pTrc99A; plus, pTrc-hfq) are indicated above the panel. Lane 1, wild-type strain MS390 grown in YENB medium; Lane 2, Δhfq (pTrc99A) grown in YENB plus 0.1 mM IPTG; Lane 3, Δhfq (pTrc-hfq) grown in YENB plus 0.1 mM IPTG; Lane 4, Δhfq (pTrc99A) grown in YENB with 150 mM NaCl plus 1 mM IPTG; Lane 5, Δhfq (pTrc-hfq) grown in YENB with 150 mM NaCl plus 1 mM IPTG.
Stability of invE mRNA
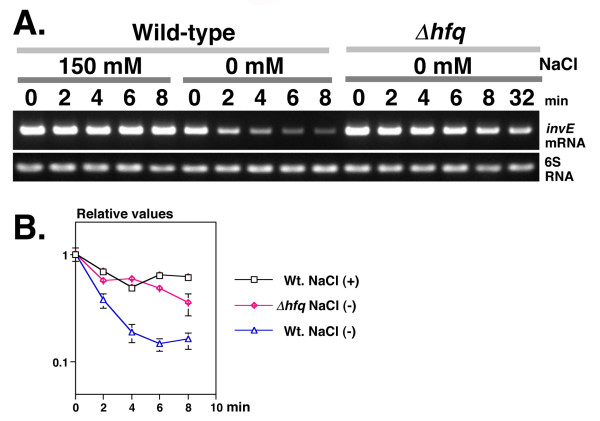
We examined the stability of invE mRNA in the hfq mutant by RT-PCR and real-time PCR analysis. Under physiological osmotic conditions, invE mRNA levels in the wild-type strain were high, and remained stable for at least 8 min after rifampicin treatment (T1/2 = 8.05 min). Under low osmotic conditions, invE mRNA levels were low (10 ± 2% of that seen under physiological osmotic conditions), and invE mRNA was rapidly degraded within the first 4 min after rifampicin treatment (T1/2 = 2.46 min). By comparison, the stability of invE mRNA was markedly increased in the hfq deletion mutant even under low osmotic conditions (T1/2 = 5.70 min) (Fig. 4A and 4B). This increase in invE mRNA stability correlated with increased InvE protein levels in cells. These results further support the prediction that the stability of invE mRNA is intimately coupled with the expression of InvE protein.
Figure 4.
A. Stability of invE mRNA in low osmotic growth conditions. Pre-cultures were inoculated into 35 ml of fresh YENB media and then grown at 37°C with shaking. When cultures reached an A600 of 0.8, rifampicin was added, then cells were harvested at 2 min intervals. Total RNA (100 ng) was used for RT-PCR analysis, and 10 μl of the amplified product was subjected to agarose gel electrophoresis. NaCl concentration (150 mM, 0 mM), strains (Wild-type strain MS390; Δhfq, MS4831) and time after rifampicin treatment (0, 2, 4, 6, 8, or 32 min) are indicated above the panels. Primers used in the experiments are indicated on the right side of the panels. B. Decay curves of invE mRNAs. Total RNA (100 ng) was subjected to real-time PCR analysis. The amount of RNA was normalized to an internal control (6S RNA) and expression was expressed relative to expression at time 0, which was set as 1.0. The X-axis indicates time after rifampicin treatment (0 to 8 min). Presence or absence of 150 mM NaCl (plus, minus) and strains (Wt, wild-type strain MS390; Δhfq, MS4831) are indicated on the right side of the graph.
Hfq-invE mRNA interaction in vitro under low-salt conditions
In low osmotic conditions, bacteria maintain intracellular osmotic homeostasis through the rapid release of small intracellular molecules, such as ions and amino acids [17]. Since potassium ion is a major cation in bacteria [18], we measured intracellular K+ concentrations in S. sonnei under low osmotic conditions. In S. sonnei strain MS506 grown in the absence and presence of 150 mM NaCl, the intracellular K+ concentration was 131 ± 4 mmoles/mg cell and 316 ± 0 mmoles/mg cell, respectively. These results indicated that K+ concentration under low osmotic conditions decreases to nearly 40% of that seen under physiological osmotic conditions.
Since interactions between proteins and nucleic acids are influenced by salt concentration, we examined the effect of salt concentration on the interaction of Hfq and invE RNA in vitro, using an RNA gel-shift assay and surface plasmon resonance (Biacore analysis). Hfq-invE RNA complex formation was examined by gel-shift assay using a binding buffer that contained 100 mM NH4Cl [19]. To control for the decrease in intracellular K+ concentration in the absence of physiological concentrations of NaCl, we also performed the gel-shift assay in buffer that contained 40 mM NH4Cl. The RNA probe (2 nM) was mixed with increasing concentrations of purified Hfq hexamer complex (from 1–16 nM) at 37°C for 10 min. In the presence of 40 mM NH4Cl, we observed an initial shift of the RNA probe upon the addition of 1 nM Hfq hexamer (Fig. 5A, lane 1), whereas the corresponding shift in the presence of 100 mM NH4Cl required 8 nM hexamer (Fig. 5A, lane 11). The apparent binding constant, as determined by the disappearance of half of the free RNA probe, was 1.7 nM Hfq in the presence of 40 mM NH4Cl and 6.2 nM in the presence of 100 mM NH4Cl.
Figure 5.
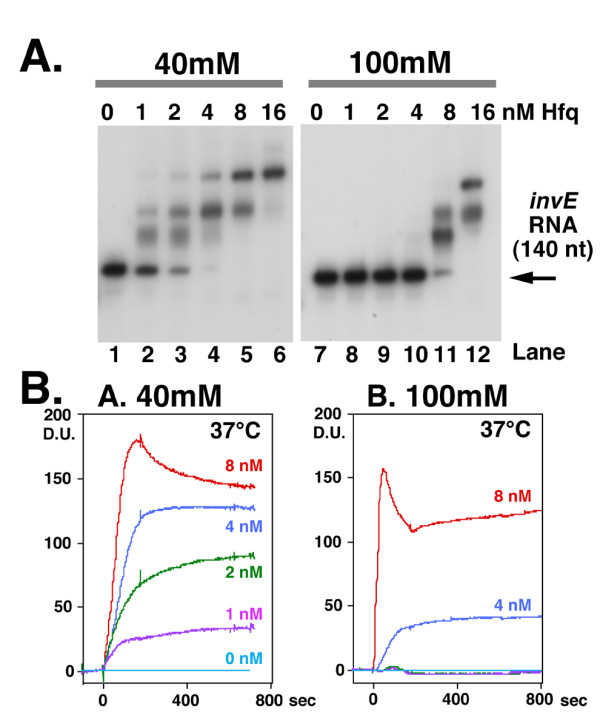
A. Gel-shift analysis in the presence of 40 mM or 100 mM NH4Cl. A 5'-end labelled invE RNA probe (2 nM) was mixed with Hfq protein and then incubated at 37°C for 10 min. Electrophoresis was carried out at 37°C. Concentration of NH4Cl (40 mM, 100 mM) and Hfq protein are indicated above the panels. The final concentration of Hfq hexamer was as follows: lanes 1 and 7, 0; lanes 2 and 8, 1 nM; lanes 3 and 9, 2 nM; lanes 4 and 10, 4 nM; lanes 5 and 11, 8 nM; lanes 6 and 12, 16 nM. B. Analysis of the interaction of Hfq and invE RNA by surface plasmon resonance. The invE RNA probe was immobilized onto a sensor chip and binding assays were carried out using a Biacore 2000 optical sensor device. Experiments were performed in 40 mM (Graph A) and 100 mM (Graph B) NH4Cl at 37°C. Hfq was diluted in the indicated RNA binding buffer (0, 1, 2, 4 or 8 nM, as indicated on the right side of the graph), and then injected for 180 seconds at a flow rate of 20 ml/min. The results are expressed as difference units (D.U.).
We also examined the interaction between Hfq and invE RNA by surface plasmon resonance (Biacore analysis). Similar to the gel-shift assay, we examined the interaction in the presence of either 40 mM or 100 mM NH4Cl at 37°C. The 140 nucleotide invE RNA probe that was used for the gel-shift assay was immobilized onto a sensor chip, and then increasing amounts of Hfq protein were added. The binding of Hfq hexamer to invE RNA reached a plateau at a concentration of nearly 8 nM Hfq under both buffer conditions (Fig. 5B) when the Hfq protein was used up to 32 nM (data not shown). Thus, the apparent binding affinity based on surface plasmon resonance was higher than that (16 nM) determined by gel-shift analysis. Distinct differences in the RNA binding properties of Hfq were observed in the presence of 40 mM and 100 mM NH4Cl. The minimum concentration of Hfq required for initial binding was 1 nM in the presence of 40 mM NH4Cl and 4 nM in the presence of 100 mM NH4Cl. In the presence of 40 mM NH4Cl, sequential binding of Hfq complexes was observed in an Hfq concentration-dependent manner, whereas in the presence of 100 mM NH4Cl, there was a sudden increase in Hfq binding at a concentration of 4 nM Hfq. These results confirmed the results of the gel-shift assay, and indicated that the binding of Hfq to invE RNA is influenced by salt concentration.
Effect of hfq mutation on invasion and virulence in vivo
To determine whether the repression of TTSS expression in low osmotic conditions influenced invasion by S. sonnei, we performed an invasion assay using S. sonnei strains that were grown in the absence of NaCl. When grown in low-salt conditions, the ability of the wild-type strain to invade HeLa cells was tightly repressed. The hfq mutant strain MS4831 was highly invasive, and invasion was markedly repressed by the addition of IPTG, which induced the expression of Hfq (Table 1). These results indicated that Hfq is intimately involved in synthesis of TTSS-associated genes in S. sonnei.
Table 1.
Invasion efficiency of bacteria grown in low-salt conditions
| Bacterial strain | Rate of invasion |
|---|---|
| HS506 | 1 ± 1 |
| MS390 | 2 ± 1 |
| MS4831 (pTrc99A) | 100 ± 29 |
| MS4831 (pTrc-hfq) | 0 |
| MS390 (YENB+150 mM NaCl) | 11 ± 3 |
In the case of Shigella, hfq mutation has been shown to increase invasion efficiency in cultured cell lines [11]. However, hfq mutations have also been shown to reduce the virulence of other Gram-negative bacteria in a variety of animal models [20-25] through the regulation of expression of stress response genes [25]. To investigate the role of Hfq in Shigella virulence in vivo, we performed a Sereny test, in which we monitored the development of keratoconjunctivitis in guinea pigs following inoculation with wild-type and hfq mutant strains of Shigella.
Guinea pigs infected with either the wild-type or hfq mutant strain developed keratoconjunctivitis within three days of infection. The symptoms, including swelling of the cornea, development of conjunctivitis and excretion of pus, appeared to be more severe in animals infected with the wild-type strain (Fig. 6A). The recovery period for animals infected with the wild-type strain was significantly longer on average than for animals infected with the hfq mutant strain (8 days versus 5 days, respectively). The production of serum antibodies against TTSS-associated secretary effector molecules was significantly higher in animals that were infected with the wild-type strain (Fig. 6B). Similar results were also observed when using an hfq mutant of S. flexneri MF4835 (data not shown). Thus, hfq mutation appeared to diminish the virulence of S. sonnei in vivo, independently of TTSS-associated gene expression.
Figure 6.
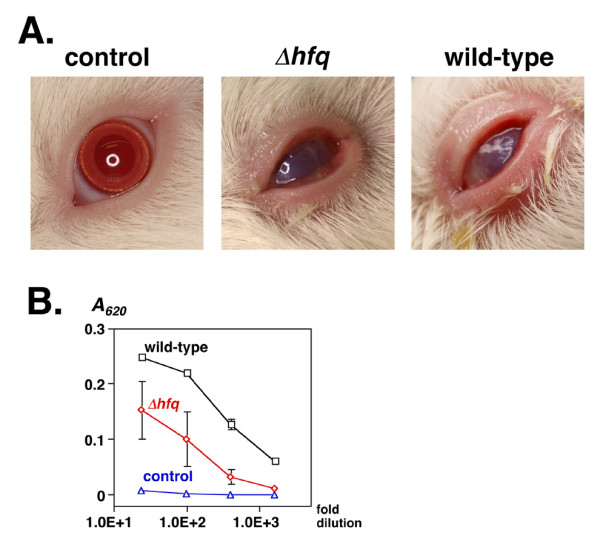
A. Development of experimental keratoconjunctivitis. Photograph of the left eyes of guinea pigs 4 days after infection. A bacterial cell suspension (5 × 108 cells) was dropped into the conjunctival sacs of male Hartley guinea pigs, and the animals were observed for four consecutive days. Left panel, control animal infected with LB medium alone; middle panel, animal infected with Δhfq strain MS4831; right panel, animal infected with wild-type strain MS390. B. Serum antibodies against effector molecules of TTSS. Sera were obtained from three animals two weeks after infection. Serial 25-, 100-, 400-, and 1600-fold dilutions were added to immobilized soluble effector molecules (see Methods) on a microtiter plate. Antibodies were detected using peroxidase-conjugated anti-guinea pig IgG. The absorbance at 620 nm (A620) of each well was monitored after the addition of ABTS using a microplate reader. Black squares, animals infected with wild-type strain MS390; red diamonds, animals infected with Δhfq strain MS4831; blue circles, control animals that received LB medium. Data represents the means and standard deviation of 2 samples.
Effect of H-NS on virF expression in low osmotic conditions
The nucleoid protein H-NS is involved in the expression of TTSS through its ability to regulate virF expression [26,27]. The effect of H-NS on virF expression in low osmotic conditions was examined using the β-galactosidase reporter gene assay. Although the hns mutation of Shigella has been reported as transposon insertion, deletion of the full-length hns gene resulted in the loss of the virulence plasmid in our experiment using S. sonnei. Since the transcription of virF is regulated by chromosomal factors, the effect of H-NS on virF transcription was examined in the hns deletion mutant strain MS4841. In hns mutants carrying the virF-lacZ reporter gene [8], the β-galactosidase activity under low osmotic conditions was 60.6% of that under physiological osmotic conditions (Fig. 7A). In the S. sonnei wild-type strain, it was 20.6% (see Fig. 1C, Graph 1). These results indicated that the nucleoid protein H-NS is involved, at least in part, in the osmolarity-dependent regulation of virF expression. The level of H-NS protein and that of the two-component regulator CpxR, which is a critical activator of virF transcription [28], were similar under both low and physiological osmotic conditions at 30°C and 37°C (Fig. 7B).
Figure 7.
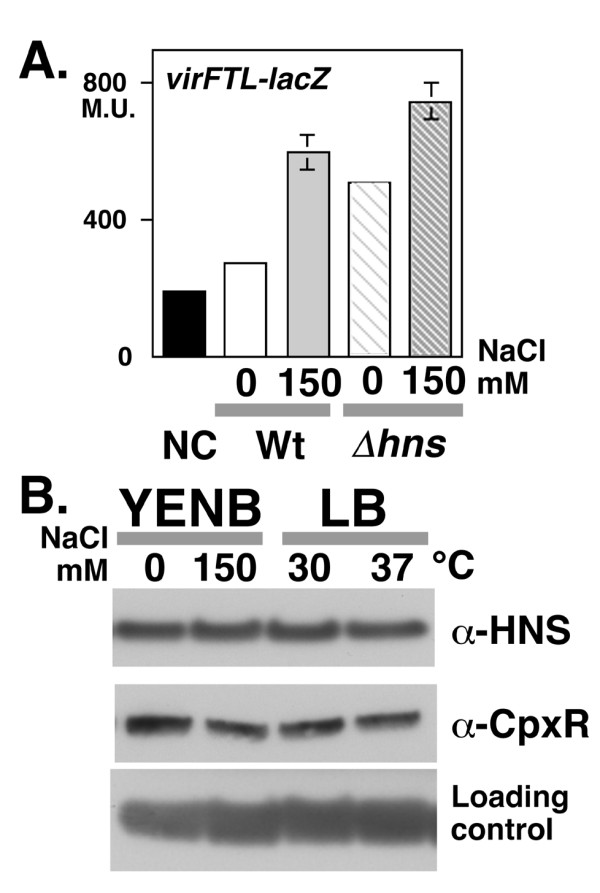
A. Reporter assay of virF promoter activity in an hns mutant. An hns deletion mutant of S. sonnei strain MS4841 carrying virFTL-lacZ (striped bars) was grown in YENB media with or without 150 mM NaCl were subjected to the β-galactosidase assay. For a comparison of activities, the data from Figure 1C, Graph 1, which was derived from simultaneous assays, is indicated by three solid bars on the left side of the graph. Strain and concentration of NaCl are indicated at the bottom of the graph as follows: Wt, wild-type strain (solid bars); hns, hns deletion mutant (striped bars); YENB medium, 0 (white bars); YENB medium with 150 mM NaCl, 150 mM (gray bars). B. Western blot analysis of H-NS and CpxR expression. An overnight LB culture of MS390 at 30°C was inoculated into fresh media and then the cells were cultured until they reached mid-log phase (A600 = 0.8). Media, temperature (YENB at 37°C; LB at 30°C and 37°C) and the concentration of NaCl are indicated on the top of the panel. Antibodies used for detection are indicated on the right side of the panels. A cross-reactive unknown protein detected by the anti-H-NS antiserum was used as a loding control.
Discussion
Virulence genes in Shigella are expressed in response to increases in temperature and/or osmolarity. Previously, we demonstrated that the temperature-dependent expression of virulence-related genes is regulated mainly at the post-transcriptional level, and that the RNA chaperone Hfq is involved in the translational control of virulence gene mRNA expression [11]. At that time, however, precise details on the mechanism of osmolarity-dependent regulation of virulence gene expression in Shigella were unavailable.
The expression and synthesis of TTSS is controlled by the VirF-InvE regulator cascade. The expression of TTSS is markedly reduced by low osmolarity due to the repression of InvE synthesis. In the current study, several lines of evidence indicated that the repression of InvE occurs mainly at the post-transcriptional level: 1) there were significant, albeit low levels of invE mRNA in cells under low osmotic conditions, whereas InvE protein was barely detectable (Fig. 1BinvE mRNA); 2) expression of the translational fusion gene invE-lacZ was fully repressed under low osmotic conditions, whereas expression of the corresponding transcriptional fusion gene was only partially repressed (Fig. 1C, Graphs 2 and 3); 3) in an arabinose-inducible promoter system, production of InvE protein decreased under low osmotic conditions even in the presence of sufficient amounts of invE mRNA (Fig. 2A); 4) in the absence of the RNA chaperone Hfq, the amount of InvE protein correlated with the level of virF transcription, even in low osmotic conditions (Fig. 3A); 5) InvE production was reduced upon over-expression of Hfq protein, even in physiological osmotic conditions (Fig. 3B); and 6) the stability of invE mRNA decreased under low osmotic conditions in the wild-type strain, but was increased in the hfq mutant (Fig. 4).
The synthesis of TTSS is induced in response to changes in osmolarity. While several osmolytes were able to induce TTSS synthesis, the response was weaker with the non-salt osmolyte sorbitol. Differences in TTSS synthesis in response to different osmolytes might be due to differences in permeability or influx through the bacterial membrane. Under physiological conditions, the contribution of non-salt osmolytes is likely to less relevant, because carbohydrates are almost completely absorbed in the ileum before reaching the colon, where infection and propagation of Shigella takes place. In the colon, Na+ ions and water are actively absorbed, and K+ ions are passively secreted, leading to an induction of TTSS synthesis. However, we did not observe significant differences in the expression of TTSS (Fig. 1A) and invasion (data not shown) in the presence of the two ions, which indicates that the trigger for TTSS induction is ionic strength, and not the nature of the ionic species.
In prokaryotes, the regulation of gene expression takes place mainly at the level of transcription. In the expression of a set of genes, however, regulation takes place at any one of several post-transcriptional stages, including the regulation of mRNA stability and translation, through a variety of mechanisms. We propose a model for the post-transcriptional repression of InvE expression in which the association of invE mRNA with the RNA chaperone Hfq controls mRNA stability. Recently, it was suggested that an iron-regulated small RNA, RyhB [29], plays a regulatory role in invE expression [30]. At present, we cannot rule out the possibility that an interaction between invE mRNA and an as-yet unidentified RNA is involved in the temperature- and osmotic pressure-dependent activation of InvE synthesis. To date, various mechanisms have been proposed for the regulation of translation initiation through the modulation of RNA structure, including the structure of the initiation codon [31]. For example, the temperature-dependent formation of a secondary structure within the 5'-untranslated element of the heat-shock operon mRNA of the plant bacterium Bradyrhizobium japonicum has been shown to regulate the level of translation of that mRNA [32-34]. In case of invE mRNA, a change of the signal that represents thermodynamic alteration of the structure was actually detected in circular dichroism spectroscopy [34] for the 140 nucleotides invE RNA [11]. Furthermore, the characteristics of the binding of invE mRNA to Hfq in low-salt (Fig. 5) and low-temperature [11] conditions are consistent with an opening of the secondary structure of the RNA through the binding of multiple Hfq molecules. Of note, the pattern of binding of invE RNA to Hfq in low-salt buffer was remarkably similar to that seen in low temperature conditions [11]. That indicates that the distribution of RNA-Hfq interaction strength upon the ionic circumstance exists in a similar range, which is defined by the thermodynamic distribution of Hfq binding between 30°C and 37°C. To date, specific molecular sensors of low osmotic conditions or mild temperature change have not been identified. Our results suggest that low osmotic conditions evoke a decrease in intracellular ionic strength, resulting in a similar effect on the strength of the RNA-Hfq interaction as that of decreased temperature. This raises the interesting possibility that post-transcriptional regulation itself represents a sensing system for changes in temperature and osmotic pressure.
The lack of active translation of invE mRNA could result in its destabilization [24]. In fact, one of the mechanisms of post-transcriptional regulation is the regulation of mRNA stability [35]. The degradosome is a well-characterized mRNA degradation system that consists of RNaseE, as well as Hfq (46). We examined the role of RNaseE in TTSS synthesis using a deletion mutant (Δrne701–892) of the C-terminal region of RnaseE and E. coli rne-3071ts strain N3431 [36] carrying expression plasmids for virF, invE and TTSS genes (pJK1143 and pJK1142, respectively) [4]. TTSS synthesis was unaffected in either of the two strains (data not shown), which indicates that an as-yet unidentified degradation pathway involving Hfq likely plays a role in the degradation of invE mRNA.
Similar to other bacterial species, hfq mutants of S. sonnei and S. flexneri exhibited decreased virulence in vivo. If the up-regulation of virulence gene expression due to hfq deletion leads to efficient antigen presentation for the host immune-system, then the hfq deletion is a potentially viable candidate for the development of a more effective Shigella vaccine, one that goes beyond the serotype-specific effects seen in current vaccine development [37]. In fact, a Shigella hfq mutant is currently under evaluation for use as a vaccine in the guinea pig model [38]. Shigella can survive in a range of environmental conditions, such as low osmotic pressure and low temperature, where strict repression of virulence gene expression is required. The development of a bi-functional sensing system for osmolarity and temperature represents an important adaptation for survival by this organism.
Conclusion
Changes in TTSS synthesis in response to osmotic pressure in Shigella involve in part the transcriptional regulation of the master regulator virF. In the current study, we demonstrated that post-transcriptional regulation of InvE expression is also involved in TTSS synthesis. This mechanism of post-transcriptional regulation of InvE synthesis was abolished in mutants that lacked hfq. The stability of invE mRNA was increased in the absence of Hfq, a major RNA chaperone in gram-negative bacteria. We propose that the synthesis of TTSS and pathogenesis of Shigella in varying temperature and osmolarity environments is dependent on the post-transcriptional regulation of InvE.
Methods
Media, reagents and bacterial culture conditions
Luria-Bertani (LB) medium (LB Lenox, Difco Laboratory, Detroit MI) and YENB medium (0.75% Difco Yeast extract, 0.8% Difco Nutrient broth) [12] were used for the low osmotic media. YENB medium containing 150 mM NaCl (Wako Chemical, Tokyo Japan) was used as the physiological osmotic medium. YENB medium containing 155 mM KCl (Wako) or 260 mM sorbitol (Sigma Co., St. Louis MO) was used as a control for osmotic pressure. The osmotic pressure of each type of medium was measured by the decreasing freezing point method [39] in a clinical inspection facility (SRL Co., Tokyo Japan). The concentrations of antibiotics were as follows: ampicillin (Wako), 50 μg/ml; chloramphenicol (Wako), 12.5 μg/ml; rifampicin (R3501 Sigma), 200 μg/ml. Concentrations are also specified in the Figure legends for each experiment. For all experiments, the indicated strains were inoculated into 2 ml of LB medium and grown overnight at 30°C with shaking (150 rpm) in a water-bath. The cultures were diluted 100-fold in 5 ml of fresh YENB medium with or without salt. The samples were incubated at 37°C with shaking at 150 rpm, and monitored for turbidity at 600 nm (A600) by spectroscopy (Spectronic™ 20+, Shimadzu Co., Kyoto Japan). Cells were harvested when they reached an A600 of 0.8. Aliquots of the culture were used for measuring β-galactosidase activity (50 μl), as previously described [40], or subjected to 10% SDS-PAGE and Western blot analysis (10 μl) [41]. The control experiments, indicated by black bars in Figure 1C (NC, negative control), were conducted by ΔcpxR strain MS2830 (Graph 1), or strain MS506 cured of virulence plasmid (Graphs 2 and 3) carrying the indicated reporter plasmid. All controls were grown in YENB plus 150 mM NaCl. The percentages indicated in the text were calculated after data was normalized to the negative control. Data represents the means and standard deviation of at least two independent experiments. IpaB and InvE proteins were detected using an anti-IpaB monoclonal antibody and an anti-InvE polyclonal antibody [13], respectively. For the detection of CpxR and H-NS, 5 μl of whole cell culture were separated by 15–20% tricine gradient gel electrophoresis (Wako), and then analysed by Western blot using an anti-CpxR [28] and anti-H-NS antibody, respectively, as previously described [42,43].
Construction of mutant strains
The bacterial strains and plasmids used in this study are listed in Table 2. Strain MS506 is a tetracycline-sensitive derivative of an avirulent strain, HW506, that was isolated by fusaric acid selection, as described previously [13]. For the construction of a partial deletion mutant of rne, we used a PCR-based gene disruption technique and wild-type S. sonnei strain MS390. A kanamycin resistant gene cassette in the plasmid pKD13 was amplified with the following primers: rne701us, 5'-GATGATAAACGTCAGGCGCAACAAGAAGCGAAGGCGCTGAATGTTGAAGAGTGAGGCTGGAGCTGCTTCG-3'; and rne701ds, 5'-GCATTTACCGATATGCAGGGATTGTCGCTCTTCCAGCTCAACAAATAATTTCCGGGGATCCGTCGAC-3'. The amplified fragment was inserted into the bacterial chromosome, as described previously [44].
Table 2.
Bacterial strains and plasmids used in this study
| Bacterial strains and plasmids | Genotypes | (references) |
|---|---|---|
| E. coli | ||
| N3431 | rne-3071ts, lacZ43, LAM-, relA1, spoT1 (CGSC#6975) | [36] |
| S. sonnei | ||
| HW383 | S. sonnei wild-type strain, (Tcr) | [7] |
| HW506 | S. sonnei HW383 without pSS120 plasmid (Tcr, non invasive) | [7] |
| MS506 | HW506 (Tcs) | This study |
| MS390 | HW383 (Tcs) | [13] |
| MS1632 | MS390ΔinvE | [11] |
| MS2830 | MS390ΔcpxR (cpxR: chromosomal activator of virF gene) | [13] |
| MS4831 | MS390Δhfq | [11] |
| MS4841 | MS390Δhns (non invasive) | [11] |
| MS5400 | MS390Δrne701–892::aphA | This study |
| MS5512 | MS390ΔpinvE::paraBAD | [11] |
| S. flexneri | ||
| 2457T | S. flexneri 2a wild-type strain, | [49] |
| 2457O | 2457T carrying mutation in virF gene (non-invasive) | [50] |
| MF4835 | 2457TΔhfq::aphA | [11] |
| Plasmids | ||
| pBAD-invE | PCR-amplified invE gene was cloned into pBAD24 (Apr) | [11] |
| pHW848 | virF-lacZ translational fusion plasmid (Cmr) | [8] |
| pJK1142 | invE and ipa-mxi-spa (TTSS) genes encoding plasmid (Kmr) | [4] |
| pJK1143 | virF-encoding plasmid (Cmr) | [4] |
| pJM4320 | invE-lacZYA transcriptional fusion in pTH18cs5(Cmr) | [13] |
| pJM4321 | invE-lacZYA translational fusion in pTH18cs5(Cmr) | [13] |
| pTrc99A | IPTG inducible expression plasmid(Apr) | [51] |
| pTrc-hfq | PCR-amplified hfq gene was cloned into pTrc99A(Apr) | [11] |
Measurement of intracellular K+ ion concentration
Intracellular K+ ion concentration was measured by potassium-electrode, as described previously [17]. An avirulent S. sonnei strain, MS506, was grown to an A600 of 0.8 in 45 ml of YENB medium or YENB medium plus 150 mM NaCl at 37°C, and then the culture was chilled on ice for 15 min. The culture was divided into triplicate tubes (15 ml Falcon tubes, #430766, Corning Inc., Corning NY), and then bacterial cells were collected by centrifugation at 5000 × g for 15 min at 4°C. An aliquot of each culture was diluted and plated on LB agar for measuring colony counts. The bacterial cells were washed twice at 4°C with 5 ml of hypotonic buffer (20 mM Na-Phosphate pH7.0 for the YENB cultures) or isotonic buffer (20 mM Na-Phosphate pH7.0, 150 mM NaCl for the YENB plus 150 mM NaCl cultures). Cells were suspended in 2 ml of hypotonic buffer and then sonicated using a SONIFIER-250D (Branson Ultrasonic Co., Danbury CT) until microscopic examination confirmed that all the cells were completely disrupted. The samples were cleared by centrifugation at 12000 × g for 30 min at 4°C, and the K+ ion concentration of the supernatants was measured by potassium electrode [17] at SRL Co. (Tokyo Japan).
RNA preparation and detection
Two ml of whole cell culture were quickly mixed with 150 μl of 5% (v/v) water-saturated phenol in ethanol to prevent RNA degradation [45]. virF and invE mRNAs were purified and analysed using a Titan™ one tube RT-PCR kit (Roche, Indianapolis IN) and Perfect Real-time™ (Takara Bio Co., Shiga Japan), as described previously [11]. For the detection of virF mRNA by real-time PCR, virFc-314F (5'-GGAGACGTTTATTTGTATATTTCGCTCTA-3', 120 nM) and virFc-398R (5'-GACGGTTAGCTCAGGCAATGAT-3', 120 nM) primers and the fluorescent probe virFc-345T (5'-FAM-AAAGCAATTTGCCCTTCATCGAT-TAMRA-3', 32 nM) were designed by ABI primer design software (Applied Biosystems Inc., Foster CA) and synthesized by ABI Japan (Tokyo). Real-time PCR analysis was performed using an ABI PRISM 2000 Thermal Cycler, as described previously [11]. RNA preparation and real-time PCR analysis were repeated at least 3 times with similar results.
Gel-shift assay
The labelled RNA probe (20 fmoles), corresponding to 140 nucleotides of the invE gene (starting from the transcription start site at +1) [11], and purified Hfq protein (0, 1, 2, 4, 8, or 16 nM Hfq hexamer) were mixed in a volume of 10 μl in one of two RNA binding buffers (40 mM NH4Cl, 10 mM Tris-HCl pH7.5, 5 mM magnesium acetate, 0.1 mM dithiothreitol; or 100 mM NH4Cl, 10 mM Tris-HCl pH7.5, 5 mM magnesium acetate, 0.1 mM dithiothreitol) at 37°C for 10 min. Gel-shift analysis was performed at 37°C as described previously [11].
Surface Plasmon Resonance (Biacore Analysis)
Surface plasmon resonance was performed with Biacore 2000 optical sensor device using the same 140 nucleotide invE RNA probe for the gel-shift assay as described previously [11]. The probe was immobilized onto a sensor tip SA (GE Healthcare Co., Piscataway NJ), causing a change of nearly 150 resonance units. Purified Hfq protein was diluted to a final concentration of 0, 1, 2, 4 or 8 nM (Hfq hexamer) in one of two RNA binding buffers, as described for gel-shift assays, and then injected for 180 seconds through two flow cells (flow cell 1, blank; flow cell 2, invE RNA) at a flow rate of 20 ml/min at 37°C. Non-specific proteins were dissociated from the chip by washing (for 700 seconds). Bound Hfq protein was subsequently removed with 2 M NaCl. The response value of the reference cell (flow cell 1, blank) was subtracted from the response value of flow cell 2 (invE RNA) to correct for nonspecific binding, and the results are expressed as difference units (D.U.). The right panels of Figure 5A and 5B are reprinted from our previous issue [11] with the permission of the American Society for Biochemistry and Molecular Biology (Copyright © 2008), which were performed with the identical materials to the left panels in the same experimental period.
Invasion assay
Pre-cultures in LB media were inoculated into 5 ml of YENB medium and then incubated for 2 hrs at 37°C with shaking. For strains carrying expression plasmids, IPTG was added to a final concentration of 0.1 mM 40 min after inoculation, and then the cultures were allowed to incubate for an additional 80 min at 37°C. Bacterial invasion into HeLa cells using the gentamicin protection assay was performed as previously described [11].
Animal experiments
Three groups (6 total) of male Hartley guinea pigs (2 weeks old, SLC Co., Hamamatu Japan) were infected with S. sonnei and S. flexneri strains for the Sereny test, an experimental animal model of conjunctivitis [46]. Fresh LB cultures of the indicated strains were harvested at an A600 of 0.8 and then collected by centrifugation. Bacterial cells (5 × 108) in 10 μl of LB medium were deposited into the conjunctival sac of each eye of 2 animals for two consecutive days. Four day later, the symptoms of each animal were recorded by digital photography. Sera were obtained two weeks after infection, and the levels of antibodies against soluble effector molecules of TTSS were measured by ELISA using peroxidase-conjugated anti-guinea pig IgG as the secondary antibody (A5545 Sigma). The source of effector molecules was a culture supernatant of strain MS390 grown at 37°C in LB medium containing 10 μg/ml Congo Red (C6767 Sigma), with which the effector molecules of TTSS are known to be secreted [47]. The culture supernatant (200 μl) was plated onto polystyrene microtiter plates (Costar #3369, Corning) and the plates were incubated at 4°C for 18 hours (hrs). Serial dilutions (25, 100, 400, 1600-fold in phosphate-buffered saline) of guinea pig sera were added to the plate and allowed to react for 2 hrs at 37°C, after which the secondary antibody (5000-fold dilution) was added for 1 hr at room temperature. Absorbance at 620 nm was measured using a Multiskan Ascent microplate reader (Thermo Labsystem, Helsinki Finland) after the addition of 1-Step™ ABTS (2,2'-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt) (#37615 Pierce, Rockford IL), as described by the manufacture. All animal experiments were conducted in compliance with the Animal Welfare Act, and adhered to the principles stated in the Guide for Care and Use of Laboratory Animals [48] after approval as #209002-2 by a board of experimental animals at the National Institute of Infectious Diseases (NIDD), Japan.
Abbreviations
The abbreviations used are: TTSS: Type three secretion system; LB: Luria-Bertani medium; IPTG: isopropyl-1-thio-β-D-galactoside; RT: reverse transcriptase; FAM: 6-carboxyfluorescein; TAMRA: 6-carboxytetramethylrhodamine.
Authors' contributions
JM carried out the experiments other than invasion analysis. TMI carried out the invasion analysis. AI and HW conceived the study, helped in the biological interpretation, and drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Jiro Mitobe, Email: jmitobe@nih.go.jp.
Tomoko Morita-Ishihara, Email: ishihara@nih.go.jp.
Akira Ishihama, Email: aishiham@hosei.ac.jp.
Haruo Watanabe, Email: haruwata@nih.go.jp.
Acknowledgements
This research was supported by a grant-in-aid for Exploratory Research 19657043 from the Ministry of Education, Science and Technology (KAKENHI), Ministry of Health, Labor and Welfare (H19·kokusai-igaku) of the Japanese Government. An E. coli strain N3431 was kindly provided from the Coli Genetic Stock Center (Yale University, CT). We thank Shu-ichi Nakayama for providing anti-CpxR antibody, Nobuo Koizumi and Ken Shimuta for assistance of the animal experiments.
References
- Porter ME, Dorman CJ. A role for H-NS in the thermo-osmotic regulation of virulence gene expression in Shigella flexneri. J Bacteriol. 1994;176(13):4187–4191. doi: 10.1128/jb.176.13.4187-4191.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli AT, Sansonetti PJ. Identification of a chromosomal gene controlling temperature-regulated expression of Shigella virulence. Proc Natl Acad Sci USA. 1988;85(8):2820–2824. doi: 10.1073/pnas.85.8.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli AT, Blackmon B, Curtiss R 3rd. Temperature-dependent expression of virulence genes in Shigella species. Infect Immun. 1984;43(1):195–201. doi: 10.1128/iai.43.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J, Ito K, Nakamura A, Watanabe H. Cloning of regions required for contact hemolysis and entry into LLC-MK2 cells from Shigella sonnei form I plasmid: virF is a positive regulator gene for these phenotypes. Infect Immun. 1989;57(5):1391–1398. doi: 10.1128/iai.57.5.1391-1398.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe T, Yoshikawa M, Mizuno T, Sasakawa C. Transcriptional control of the invasion regulatory gene virB of Shigella flexneri: activation by virF and repression by H-NS. J Bacteriol. 1993;175(19):6142–6149. doi: 10.1128/jb.175.19.6142-6149.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler B, Sasakawa C, Tobe T, Makino S, Komatsu K, Yoshikawa M. A dual transcriptional activation system for the 230 kb plasmid genes coding for virulence-associated antigens of Shigella flexneri. Mol Microbiol. 1989;3(5):627–635. doi: 10.1111/j.1365-2958.1989.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Arakawa E, Ito K, Kato J, Nakamura A. Genetic analysis of an invasion region by use of a Tn3-lac transposon and identification of a second positive regulator gene, invE, for cell invasion of Shigella sonnei: significant homology of invE with ParB of plasmid P1. J Bacteriol. 1990;172(2):619–629. doi: 10.1128/jb.172.2.619-629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S, Watanabe H. Involvement of cpxA, a sensor of a two-component regulatory system, in the pH-dependent regulation of expression of Shigella sonnei virF gene. J Bacteriol. 1995;177(17):5062–5069. doi: 10.1128/jb.177.17.5062-5069.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniya T, Mitobe J, Nakayama S, Mingshan Q, Okuda K, Watanabe H. Determination of the InvE binding site required for expression of IpaB of the Shigella sonnei virulence plasmid: involvement of a ParB boxA-like sequence. J Bacteriol. 2003;185(17):5158–5165. doi: 10.1128/JB.185.17.5158-5165.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beloin C, Dorman CJ. An extended role for the nucleoid structuring protein H-NS in the virulence gene regulatory cascade of Shigella flexneri. Mol Microbiol. 2003;47(3):825–838. doi: 10.1046/j.1365-2958.2003.03347.x. [DOI] [PubMed] [Google Scholar]
- Mitobe J, Morita-Ishihara T, Ishihama A, Watanabe H. Involvement of RNA-binding protein Hfq in the post-transcriptional regulation of invE gene expression in Shigella sonnei. J Biol Chem. 2008;283(9):5738–5747. doi: 10.1074/jbc.M710108200. [DOI] [PubMed] [Google Scholar]
- Sharma RC, Schimke RT. Preparation of electrocompetent E. coli using salt-free growth medium. Biotechniques. 1996;20(1):42–44. doi: 10.2144/96201bm08. [DOI] [PubMed] [Google Scholar]
- Mitobe J, Arakawa E, Watanabe H. A sensor of the two-component system CpxA affects expression of the type III secretion system through posttranscriptional processing of InvE. J Bacteriol. 2005;187(1):107–113. doi: 10.1128/JB.187.1.107-113.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux A, Beloin C, Ghigo JM. Combined inactivation and expression strategy to study gene function under physiological conditions: application to identification of new Escherichia coli adhesins. J Bacteriol. 2005;187(3):1001–1013. doi: 10.1128/JB.187.3.1001-1013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajitani M, Ishihama A. Identification and sequence determination of the host factor gene for bacteriophage Q beta. Nucleic Acids Res. 1991;19(5):1063–1066. doi: 10.1093/nar/19.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajitani M, Kato A, Wada A, Inokuchi Y, Ishihama A. Regulation of the Escherichia coli hfq gene encoding the host factor for phage Q beta. J Bacteriol. 1994;176(2):531–534. doi: 10.1128/jb.176.2.531-534.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleyer M, Schmid R, Bakker EP. Transient, specific and extremely rapid release of osmolytes from growing cells of Escherichia coli K-12 exposed to hypoosmotic shock. Arch Microbiol. 1993;160(6):424–431. doi: 10.1007/BF00245302. [DOI] [PubMed] [Google Scholar]
- Harold FM, Maloney PC. In: in Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. 2. Neidhardt FC, Ingraham JL, Magasanik B, Low KB, Schaechter M, Umbarger HE, editor. American Society for Microbiology, Washington, D. C; 1987. p. 293. [Google Scholar]
- Afonyushkin T, Vecerek B, Moll I, Blasi U, Kaberdin VR. Both RNase E and RNase III control the stability of sodB mRNA upon translational inhibition by the small regulatory RNA RyhB. Nucleic Acids Res. 2005;33(5):1678–1689. doi: 10.1093/nar/gki313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNealy TL, Forsbach-Birk V, Shi C, Marre R. The Hfq homolog in Legionella pneumophila demonstrates regulation by LetA and RpoS and interacts with the global regulator CsrA. J Bacteriol. 2005;187(4):1527–1532. doi: 10.1128/JB.187.4.1527-1532.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson GT, Roop RM Jr. The Brucella abortus host factor I (HF-I) protein contributes to stress resistance during stationary phase and is a major determinant of virulence in mice. Mol Microbiol. 1999;34(4):690–700. doi: 10.1046/j.1365-2958.1999.01629.x. [DOI] [PubMed] [Google Scholar]
- Sonnleitner E, Hagens S, Rosenau F, Wilhelm S, Habel A, Jager KE, Blasi U. Reduced virulence of a hfq mutant of Pseudomonas aeruginosa O1. Microb Pathog. 2003;35(5):217–228. doi: 10.1016/S0882-4010(03)00149-9. [DOI] [PubMed] [Google Scholar]
- Ding Y, Davis BM, Waldor MK. Hfq is essential for Vibrio cholerae virulence and downregulates sigma expression. Mol Microbiol. 2004;53(1):345–354. doi: 10.1111/j.1365-2958.2004.04142.x. [DOI] [PubMed] [Google Scholar]
- Storz G, Opdyke JA, Zhang A. Controlling mRNA stability and translation with small, noncoding RNAs. Curr Opin Microbiol. 2004;7(2):140–144. doi: 10.1016/j.mib.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Sittka A, Pfeiffer V, Tedin K, Vogel J. The RNA chaperone Hfq is essential for the virulence of Salmonella typhimurium. Mol Microbiol. 2007;63(1):193–217. doi: 10.1111/j.1365-2958.2006.05489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman CJ, Bhriain NN, Higgins CF. DNA supercoiling and environmental regulation of virulence gene expression in Shigella flexneri. Nature. 1990;344(6268):789–792. doi: 10.1038/344789a0. [DOI] [PubMed] [Google Scholar]
- Falconi M, Colonna B, Prosseda G, Micheli G, Gualerzi CO. Thermoregulation of Shigella and Escherichia coli EIEC pathogenicity. A temperature-dependent structural transition of DNA modulates accessibility of virF promoter to transcriptional repressor H-NS. Embo J. 1998;17(23):7033–7043. doi: 10.1093/emboj/17.23.7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S, Watanabe H. Identification of cpxR as a positive regulator essential for expression of the Shigella sonnei virF gene. J Bacteriol. 1998;180(14):3522–3528. doi: 10.1128/jb.180.14.3522-3528.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse E, Gottesman S. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc Natl Acad Sci USA. 2002;99(7):4620–4625. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ER, Payne SM. RyhB, an iron-responsive small RNA molecule, regulates Shigella dysenteriae virulence. Infect Immun. 2007;75(7):3470–3477. doi: 10.1128/IAI.00112-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Kashiwagi K, Shigemasa A, Taniguchi S, Yamamoto K, Makinoshima H, Ishihama A, Igarashi K. A unifying model for the role of polyamines in bacterial cell growth, the polyamine modulon. J Biol Chem. 2004;279(44):46008–46013. doi: 10.1074/jbc.M404393200. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Maris C, Allain FH, Narberhaus F. Molecular basis for temperature sensing by an RNA thermometer. Embo J. 2006;25(11):2487–2497. doi: 10.1038/sj.emboj.7601128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narberhaus F, Waldminghaus T, Chowdhury S. RNA thermometers. FEMS Microbiol Rev. 2006;30(1):3–16. doi: 10.1111/j.1574-6976.2005.004.x. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Ragaz C, Kreuger E, Narberhaus F. Temperature-controlled structural alterations of an RNA thermometer. J Biol Chem. 2003;278(48):47915–47921. doi: 10.1074/jbc.M306874200. [DOI] [PubMed] [Google Scholar]
- Masse E, Escorcia FE, Gottesman S. Coupled degradation of a small regulatory RNA and its mRNA targets in Escherichia coli. Genes Dev. 2003;17(19):2374–2383. doi: 10.1101/gad.1127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apirion D. Isolation, genetic mapping and some characterization of a mutation in Escherichia coli that affects the processing of ribonuleic acid. Genetics. 1978;90(4):659–671. doi: 10.1093/genetics/90.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, Adak GK, Levine MM. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999;77(8):651–666. [PMC free article] [PubMed] [Google Scholar]
- Hartman AB, Powell CJ, Schultz CL, Oaks EV, Eckels KH. Small-animal model to measure efficacy and immunogenicity of Shigella vaccine strains. Infect Immun. 1991;59(11):4075–4083. doi: 10.1128/iai.59.11.4075-4083.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter RW, Clynne MA, Brown DL. Freezing point depression of aqueous sodium chloride solutions. Economic Geology. 1978;73(2):284–285. doi: 10.2113/gsecongeo.73.2.284. [DOI] [Google Scholar]
- Miller JH. A short course in bacterial genetics. 3. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, New York; 1992. [Google Scholar]
- Sambrook J, Russel DW. Molecular Cloning, a laboratory manual. 3. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, New York; 2002. [Google Scholar]
- Azam TA, Ishihama A. Twelve species of the nucleoid-associated protein from Escherichia coli. Sequence recognition specificity and DNA binding affinity. J Biol Chem. 1999;274(46):33105–33113. doi: 10.1074/jbc.274.46.33105. [DOI] [PubMed] [Google Scholar]
- Jishage M, Ishihama A. A stationary phase protein in Escherichia coli with binding activity to the major sigma subunit of RNA polymerase. Proc Natl Acad Sci USA. 1998;95(9):4953–4958. doi: 10.1073/pnas.95.9.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97(12):6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagwat AA, Phadke RP, Wheeler D, Kalantre S, Gudipati M, Bhagwat M. Computational methods and evaluation of RNA stabilization reagents for genome-wide expression studies. J Microbiol Methods. 2003;55(2):399–409. doi: 10.1016/S0167-7012(03)00175-1. [DOI] [PubMed] [Google Scholar]
- Sereny B. Experimental Shigella conjunctivitis. Acta Microbiol Acad Sci Hung. 1957;4(4):367–376. [PubMed] [Google Scholar]
- Bahrani FK, Sansonetti PJ, Parsot C. Secretion of Ipa proteins by Shigella flexneri: inducer molecules and kinetics of activation. Infect Immun. 1997;65(10):4005–4010. doi: 10.1128/iai.65.10.4005-4010.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guide for the care and use of laboratory animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- Wei J, Goldberg MB, Burland V, Venkatesan MM, Deng W, Fournier G, Mayhew GF, Plunkett G 3rd, Rose DJ, Darling A. Complete genome sequence and comparative genomics of Shigella flexneri serotype 2a strain 2457T. Infect Immun. 2003;71(5):2775–2786. doi: 10.1128/IAI.71.5.2775-2786.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JA, Venkatesan MM, Baron LS, Buysse JM. Spontaneous insertion of an IS1-like element into the virF gene is responsible for avirulence in opaque colonial variants of Shigella flexneri 2a. Infect Immun. 1992;60(1):175–182. doi: 10.1128/iai.60.1.175-182.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann E, Ochs B, Abel KJ. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988;69(2):301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]