Abstract
The enzyme sterol 24-c-methyltranferase (SMT) is required for the biosynthesis of ergosterol, the major membrane sterol in Leishmania parasites. SMT and ergosterol are not found in mammals, so this protein may be an attractive target for anti-leishmanial vaccines and drugs. We have previously demonstrated that SMT from L. infantum, which causes visceral leishmaniasis, is a protective antigen against this parasite. Because this protein is highly conserved among Leishmania species, we evaluated the potential of SMT to cross-protect against a different form of leishmaniasis. Here, we show that immunization with L. infantum SMT, formulated with monophosphoryl lipid A in stable emulsion (MPL-SE), protects mice from cutaneous leishmaniasis caused by L. major. In BALB/c mice the vaccine preparation induced antigen-specific multifunctional CD4+ T cells capable of producing IFN-γ, IL-2, and/or TNF-α upon antigen re-exposure, and MPL-SE was indispensable to direct immune responses to SMT towards Th1. Mice immunized with the SMT/MPL-SE vaccine developed significantly smaller lesions following ear challenge with L. major. These results suggest that SMT is a promising vaccine antigen for multiple forms of leishmaniasis.
Keywords: sterol 24-c-methyltranferase, leishmaniasis, vaccine
Introduction
Leishmaniasis represents a spectrum of diseases caused by protozoan parasites of the genus Leishmania. According to the WHO Health Report 2004, the disease is endemic in 88 countries, putting 350 million people at risk and resulting in 12 million cases. Each year, it is estimated there are 2 million new cases of leishmaniasis and 59,000 deaths. Leishmaniasis can be classified into three general types of disease, cutaneous leishmaniasis (CL), mucosal leishmaniasis (ML) and visceral leishmaniasis (VL) based on the clinical manifestations of the disease. These three forms of leishmaniasis are caused by different parasite species, including Leishmania major which causes CL, and Leishmania donovani and Leishmania infantum which cause VL. Because many countries are endemic for multiple Leishmania species, ideal leishmaniasis control strategies such as vaccination and chemotherapy would not be limited to controlling a single parasite species. There has been an on-going effort toward characterizing the protective efficacy of individual Leishmania antigens, including thiol-specific antioxidant (TSA), stress inducible protein-1 (LmSTI1), kinetoplastid membrane protein-11 (KMP11), hydrophilic acylated surface protein B (HASPB), A2, and cysteine proteinase B (CPB) using animal models of leishmaniasis [1–9]. However, few antigens have demonstrated protective efficacy against multiple species.
In a previous study we demonstrated that L. infantum sterol 24-c-methyltransferase (SMT) is a protective vaccine antigen against experimental VL [10]. SMT is a requisite enzyme in the ergosterol biosynthetic pathway of trypanosomatid parasites, fungi and higher plants, but not in mammals, making this protein a potential target of anti-leishmanial vaccines and drugs. Since amphotericin B binds ergosterol but not cholesterol, it is a potent and specific leishmanicidal and fungicidal agent [11]. SMT is present in other Leishmania species, and its amino acid sequence is highly conserved in many of these species [10]. However, whether this antigen is protective against multiple Leishmania species has not been previously analyzed. In this report we show that immunization with L. infantum SMT plus MPL-SE protects mice from CL caused by L. major. Potential mechanisms of vaccine-induced protection were also investigated and are consistent with a role for multi-functional CD4+ T cells in this process.
2. Materials and Methods
2.1. Animals and parasites
Female BALB/c and BALB/c nu/nu mice as well as LVG golden Syrian hamsters were purchased from Charles River Laboratories (Wilmington, MA), and maintained in specific-pathogen-free conditions. Mice that were six to eight-weeks-old at the beginning of each experiment were used. The L. major Friedlin strain was kindly provided by Dr. David Sacks (The National Institute of Health, Bethesda, MD), and maintained as promastigotes as previously described [12]. L. infantum promastigotes were cultured as previously described [10]. Amastigotes of L. major were harvested from footpads of BALB/c nu/nu mice (6 to 8 weeks old, Charles River Laboratories) eight weeks after infection. Amastigotes of L. infantum were harvested from livers of infected hamsters [13]. Both L. major and L. infantum amastigotes were purified by passing through 8 μm followed by 5 μm pore size membranes (Millipore, Billerica, MA), as previously described [14].
2.2. Antigens
Recombinant SMT has been made in our previous studies [10]. L. major soluble leishmanial antigen (SLA) was made by sonicating stationary phase promastigotes. After centrifuging the homogenate at 10,000 g, the supernatant was collected and filtered through 0.22 μm pore size membrane. All the antigens used in this study had endotoxin levels less than 100 EU/mg, as determined by a Limulus amebocyte lysate test (Cambrex Corporation, East Rutherford, NJ)
2.3. Western blotting
Samples for immunoblotting were prepared by suspending promastigotes or amastigotes in SDS sample buffer followed by boiling for 5 min. Samples containing 5 × 105 parasites were separated by SDS-PAGE and blotted on a polyvinylidene difluoride membrane (Invitrogen, Carlsbad, CA). Sera from rabbits immunized six times with 250 μg of either recombinant SMT or KMP11 formulated in Freund’s incomplete adjuvant were used as the primary antibody. Pre-immunization serum from one of those rabbits was used at the same dilution as a control. The membrane was then probed with HRP-conjugated F(ab′)2-fragment donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Development was performed using Chemiluminescent Super Sensitive HRP Substrate Kit (BioFX Laboratories, Owings Mills, MD).
2.4. Immunization of mice
BALB/c mice were injected with 10 μg of SMT plus 20 μg of MPL-SE (GlaxoSmithKline Biologicals, Rixensant, Belgium) in a volume of 0.1 ml. The mice were immunized three times at three-week intervals subcutaneously at the base of the tail. As a control, mice were given either saline alone, MPL-SE alone, or SMT alone three times at three-week intervals.
2.5. Antibody ELISA
The timing of serum collections from mice is described in the Results section. Recombinant protein (200 ng/well) or SLA (1 μg/well) were diluted in ELISA coating buffer, and 96-well plates were coated with the diluted antigen followed by blocking with phosphate-buffered saline containing 0.05% Tween-20 and 1% bovine serum albumin. To detect antibodies, mouse serum samples were diluted to 1:100 and applied to the plates in five-fold serial dilutions. The plates were incubated with HRP-conjugated goat anti-mouse IgG1 or IgG2a (Southern Biotech, Birmingham, AL) and developed with tetramethylbenzidine peroxidase substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD), and the enzyme-substrate reaction was stopped by adding 1N H2SO4. The plates were read at 450 nm by a microplate reader.
2.6. Cytokine assay using spleen cells
Mouse splenocytes (2 × 105 cells/well) in complete RPMI medium (RPMI supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml of penicillin and 100 μg/ml of streptomycin) were plated in a 96-well plate and stimulated with con A (3 μg/ml), SLA (10 μg/ml), recombinant protein (10 μg/ml), or medium alone. Culture supernatants were collected after 72 hr cultivation, and the IFN-γ, IL-4 and IL-10 levels were determined by sandwich ELISA (eBioscience, San Diego, CA).
2.7. Intracellular staining and Flow cytometry
One million splenocytes in 100 μl of complete RPMI were plated per well in a round bottom 96-well plate and stimulated with PMA/ionomycin, 10 μg/ml of SMT, or medium alone. Co-stimulation antibodies anti-CD28 (eBioscience) and anti-CD49d (eBioscience) were added for a final concentration of 1 μg/ml. After 2 hours incubation at 37°C, brefeldin A (GolgiPlug: BD Biosciences, San Jose, CA) was added to the wells, and the incubation resumed for an additional 12 hrs at 37°C. Cells were treated with anti-CD16/32 (eBioscience) 1:50, stained with AlexaFluor 700-anti-CD3 (eBioscience), PerCP-anti-CD4 (BD Biosciences), and PE-anti-CD8 (BD Biosciences), and fixed using the Cytofix/Cytoperm kit (BD Biosciences). Cells were again blocked with anti-CD16/32 and then stained intracellularly with FITC-anti-TNF-α (eBioscience), Pacific Blue-anti-IL-2 (eBioscience) and PE-Cy7-anti-IFN-γ (BD Biosciences). Cells were analyzed with a LSRII FACS machine (BD Biosciences) and DIVA software.
2.8. Infection of mice with L. major
For evaluating the efficacy of the vaccines, a BALB/c mouse – ear infection model was used. Three weeks after the last immunization, mice injected with saline, SMT alone, MPL-SE alone or SMT/MPL-SE were infected with 2,000 L. major metacyclic promastigotes given intradermally into both the right and left ears. Purification of metacyclic promastigotes were performed by negative selection with peanut agglutinin as previously described [15]. Lesion sizes were measured weekly for eight weeks using vernier calipers. These mice were sacrificed at eight weeks of infection, ears collected for quantifying parasites by limiting dilution, as previously described [16], and sera and spleens collected for immunological analyses.
3. Results
3.1. Expression of SMT by L. major amastigotes
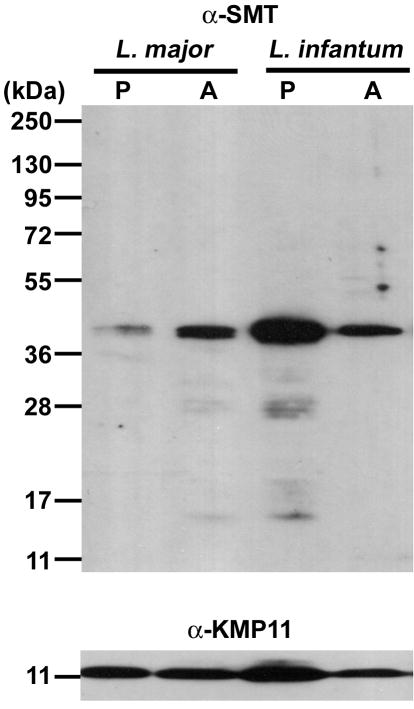
A vaccine antigen suitable for use against L. major infection is expected to be expressed by the parasite and to be recognized by the immune system during infection. Amastigotes, but not promastigotes, are the replicating developmental stage of Leishmania parasites in mammalian hosts. We previously demonstrated the expression of SMT protein by L. major promastigotes, the developmental form in the sandfly vectors, using a polyclonal antibody to recombinant L. infantum SMT [10]. Western blot analysis with this same antibody revealed that the protein is also expressed by L. major amastigotes (Fig. 1). The same number of promastigotes and amastigotes (5 × 105 cells) were loaded on a gel, and expression levels of KMP11 were relatively constant among both developmental stages of L. major and L. infantum. By contrast to KMP11, SMT expression levels seemed affected by the developmental stage, with L. major amastigotes accumulating more SMT than promastigotes; the opposite was observed in L. infantum stages. A further indication that SMT is expressed by L. major amastigotes is that BALB/c mice produced anti-SMT antibodies in response to L. major infection (data not shown).
Figure 1.
Expression of SMT by L. major. Proteins from 5 × 105 L. major or L. infantum promastigotes (P) or amastigotes (A) were separated in the indicated lanes by SDS-PAGE and transferred to membranes. Membranes were probed with rabbit anti-SMT (top) or anti-KMP11 polyclonal antibody (bottom), and developed by chemiluminescence. None of these bands were detected when the membrane was probed with pre-immunization rabbit serum.
3.2. Immunization with SMT/MPL-SE induces antigen-specific, multi-functional Th1 cells
BALB/c mice immunized with SMT, either alone or formulated with MPL-SE, were examined for titers of anti-SMT IgG1 and IgG2a, and for production of IFN-γ, IL-4 and IL-10 by splenocytes upon antigen recall (Fig. 2). Injection of saline or MPL-SE alone did not induce any significant antigen-specific responses (either antibodies or cytokines). Mice receiving SMT, either by itself and adjuvanted with MPL-SE, showed antigen-specific responses with mixed Th1/Th2 characteristics. However, the balance of Th1/Th2 and the quality of Th1 responses were distinct for these two groups. Immunization with SMT alone resulted in induction of only an IgG1 response to the antigen, and the IgG1 titers were at least 125 times higher than IgG2a titers (Fig. 2A). In contrast, antigen-specific IgG2 was readily detected when SMT was co-administrated with MPL-SE; comparable levels of IgG1 and IgGa2 were produced. IFN-γ, IL-4 and IL-10 production by spleen cells upon SMT recall were detected in both SMT alone and SMT/MPL-SE groups (Fig. 2B). The IFN-γ level was significantly higher in the SMT/MPL-SE group than the SMT alone group. In contrast, no significant differences were found in IL-4 or IL-10 levels between these two groups.
Figure 2.

Antigen-specific responses induced by immunization with SMT/MPL-SE in BALB/c mice. Mice were inoculated three times with saline, MPL-SE alone, SMT alone, or SMT/MPL-SE, as described in Materials and Methods. (A) Anti-SMT IgG1 and IgG2a responses were evaluated by ELISA one week after the last immunization. (B) IFN-γ, IL-4 and IL-10 production after ex vivo antigen stimulation of splenocytes from those immunized mice was determined by sandwich ELISA. *P<0.05, ns: not significant by unpaired t test.
FACS analyses also revealed that antigen-specific CD4+ T-cells having Th1 characteristics were generated following immunization with SMT/MPL-SE. CD4+ T-cells producing TNF-α, IL-2 or IFN-γ in response to SMT recall were found in splenocytes from mice immunized with SMT/MPL-SE, and the numbers of these cells were higher in this group than those immunized with SMT alone (Fig. 3A). In contrast, no antigen-specific CD8+ T-cells producing these same cytokines were detected over background, even in the SMT/MPL-SE group. Further analysis of the expression patterns of TNF-α, IL-2 and IFN-γ in CD4+ T cells at a single cell level revealed that the SMT/MPL-SE vaccine induced multi-functional CD4+ cells with the capacity to produce more than one cytokine (Fig. 3B). Upon antigen recall, CD4+ T-cells producing all the three cytokines and those producing IL-2 and TNF-α were significantly elevated in the SMT/MPL-SE group, compared to SMT alone or other groups examined. The triple-positive CD4+ T-cells accounted for 55% of IFN-γ producing CD4+ T-cells in the SMT/MPL-SE group, but only 19% in the SMT alone group.
Figure 3.

Induction of multi-functional, antigen-specific CD4+ T-cells by SMT/MPL-SE immunization in BALB/c mice. (A) Spleen cells from mice immunized three time with saline, MPL-SE alone, SMT alone, or SMT/MPL-SE were stimulated in vitro with medium alone (none) or SMT. (A) The total frequency of CD4+ or CD8+ T-cells producing IFN-γ, IL-2 or TNF-α were analyzed by flow cytometry. (B) The CD4+ T-cells were further analyzed at the single-cell level for abundance of each of the seven possible expression pattern combinations of these three cytokines.
3.3. Immunization with SMT/MPL-SE protects mice against L. major infection
We examined the protective efficacy of the SMT/MPL-SE vaccine against L. major infection. Unimmunized control mice or mice immunized with SMT/MPL-SE were challenged with L. major promastigotes by intradermal injection into the ear. The control mice developed lesions in the ear, and the disease progressed over time (Fig. 4A). A significant reduction in lesion size was found in mice vaccinated with SMT/MPL-SE compared with the control mice during the eight weeks of infection (Fig. 4A). The smaller lesions found in the immunized mice were associated with a significantly lower number of parasites at the lesion site: a geometric mean of 75 compared to 1.9 × 107 parasites in the control group (Fig. 4B). In separate experiments the SMT/MPL-SE vaccine was compared against MPL-SE alone and SMT using the same model. Neither MPL-SE alone nor SMT alone produced evidence of protecting mice against L. major challenge infection. In contrast, again, the SMT/MPL-SE vaccine induced significant protection against L. major infection over the eight weeks of infection (Fig. 4C)
Figure 4.
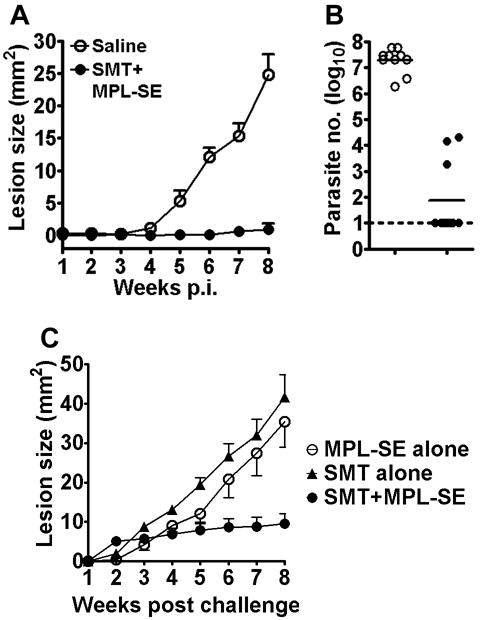
Protection against L. major infection by immunization with SMT/MPL-SE. Mice inoculated subcutaneously with saline (○) or SMT/MPL-SE (●) were challenged with L. major intradermally in the both right and left ears. (A) Ear lesion sizes were measured weekly for eight weeks. Mean and SEM of five mice (10 ears) in each group are shown. (B) The number of parasites in each ear was measured eight weeks after infection by limiting dilution. Horizontal bars represent the mean values of five mice (10 ears) in individual groups. The dotted line indicates the assay detection limit. The Mann-Whitney test was used to compare vaccinated groups with the saline group. (C) In separate experiments the SMT/MPL-SE vaccine was compared with either MPL-SE alone or SMT alone for protection against L. major infection. Data shown are representative of at least two independent experiments with similar results.
3.4. Correlation of Th1/Th2 balance with protection against L. major
To examine the nature of protection against L. major induced by SMT/MPL-SE vaccination, antibody and cytokine responses were analyzed at the time parasite burden was determined. Strong vaccine antigen-specific antibody and cytokine responses were found in the SMT/MPL-SE immunized mice after infection; however, since there were no significant differences between the pre- and post-challenge immune responses, the magnitude and types of SMT-specific responses were due to immunization and were not affected by infection (data not shown). In contrast, protected (SMT/MPL-SE) and non-protected mice (saline) differed in their post-infection responses to SLA. When T-cell responses to SLA were analyzed ex vivo using splenocytes from infected mice, the vaccine-protected mice produced significantly less IL-4 than the unvaccinated mice (Fig. 5A). IFN-γ production was moderately higher in the vaccinated mice (not statistically different), and the ratio of IFN-γ/IL-4 in response to SLA clearly shifted towards Th1 in the vaccinated and protected group of mice. The IgG1-dominant antibody response to SLA observed in the unvaccinated mice after L. major infection was strongly suppressed in mice protected by SMT/MPL-SE vaccination (Fig. 5B), reflecting this shift to a Th1 response with vaccination.
Figure 5.
Correlation of post-challenge immune responses to parasite lysate with protection against L. major following SMT/MPL-SE vaccination. Eight weeks after challenge with L. major, BALB/c mice pre-treated with either saline or SMT/MPL-SE were examined for their immune responses to crude parasite antigen (SLA). (A) IFN-γ and IL-4 production by spleen cells upon antigen recall (saline: open bars, SMT/MPL-SE: closed bars). (B) IgG1 and IgG2a responses to SLA by ELISA (OD values are shown). Mean and SEM of five mice are shown. ns: not significant, *P<0.05 by unpaired t-test.
4. Discussion
Since the distribution of different Leishmania species may overlap geographically, a vaccine effective against multiple species would be a powerful tool in the effort to reduce leishmaniasis. One purpose of this study was to examine whether SMT from L. infantum is cross-protective against L. major, which causes a form of disease distinct from the VL caused by L. infantum. Several Leishmania antigens, including parasite lysate [17, 18], and defined antigens, such as cysteine proteinase A/B [7, 19], FML/nucleoside hydrolase [6, 20, 21], and Leish-111f [13, 22], have shown protective efficacy against multiple Leishmania species. We showed here that L. infantum SMT also protected mice against heterologous challenge. The efficacy of a vaccine antigen derived from the sequence of one species to act against heterologous species may depend on antigen conservation among species. The high amino acid sequence conservation of L. infantum and L. major SMT (96.6% identity in amino acid sequence) [10] together with obvious expression in L. major amastigotes undoubtedly had positive impacts on the ability of L. infantum SMT to protect mice against L. major infection.
Another purpose of this study was to characterize the mechanism(s) of protection. SMT/MPL-SE induced mixed Th1/Th2 responses, but those responses were more pronounced and Th1-biased than those of SMT alone, and individual CD4+ T cells gained the capacity to produce multiple cytokines (IFN-γ, TNF-α and/or IL-2) than as a result of SMT alone vaccination. Functionally, the Th1 cells induced by antigen plus adjuvant vaccination respond rapidly to Leishmania infection with the production of IFN-γ and TNF-α, cytokines pivotal for the control of CL [23, 24], that activate macrophage-mediated killing of the parasites [25, 26]. Furthermore, there is a correlation between the induction of multi-functional Th1 cells, which are individually capable of producing more than one Th1 cytokine, and protection against L. major infection [27]. This appears due to a synergistic effect of these cytokines, especially IFN-γ and TNF-α, in the killing of parasites [28]. Separate experiments have shown that IFN-γ alone is not sufficient to control CL [29, 30]. This may be reflected in our observation that even though SMT alone could induce IFN-γ production, it was not sufficient for protection against L. major infection. Alternatively, the magnitude of the response may not have reached the necessary threshold of a protective response. These results suggest that magnitude and/or quality/multi-functionality are important factors for vaccine-induced Th1 response necessary for protection against CL.
We also showed here that the post-challenge responses in the SMT/MPL-SE immunized and protected mice were associated with an improved Th1/Th2 balance to parasites, as represented by higher IFN-γ/IL-4 ratios and decreased IgG1 responses to SLA. These findings confirm that levels of IL-4, as a Th2 cytokine, have an impact on the fate of the disease during L. major infection in BALB/c mice [29, 31]. Because increased amounts of anti-Leishmania IgG appear to contribute to disease exacerbation in L. major-infected mice [32, 33], the vaccine-mediated suppression of anti-L. major antibody production observed in this study might have a great impact on our ability to control the disease. Although further studies are needed to clarify if the improved post-infection response to SLA is a cause or a result of protection, the prophylactic effects of the SMT/MPL-SE vaccine may be attributable to induction of multi-functional Th1 responses, which upon infection with L. major, contribute to balancing Th1/Th2 for effective immune control of infection.
In summary, we have demonstrated that vaccination with SMT is protective against two diverse species of Leishmania causing distinct disease forms. The absence of a homologous protein in the mammalian proteome further suggests SMT as a safe and effective vaccine for the control of leishmaniasis.
Acknowledgments
This work was supported by the National Institutes of Health grant AI25038 and a grant from the Bill and Melinda Gates Foundation. We are grateful to Alex Picone, Raodoh Mohamath and Garrett Poshusta for technical assistances.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Basu R, Bhaumik S, Basu JM, Naskar K, De T, Roy S. Kinetoplastid membrane protein-11 DNA vaccination induces complete protection against both pentavalent antimonial-sensitive and -resistant strains of Leishmania donovani that correlates with inducible nitric oxide synthase activity and IL-4 generation: evidence for mixed Th1- and Th2-like responses in visceral leishmaniasis. J Immunol. 2005 Jun 1;174(11):7160–71. doi: 10.4049/jimmunol.174.11.7160. [DOI] [PubMed] [Google Scholar]
- 2.Stager S, Smith DF, Kaye PM. Immunization with a recombinant stage-regulated surface protein from Leishmania donovani induces protection against visceral leishmaniasis. J Immunol. 2000 Dec 15;165(12):7064–71. doi: 10.4049/jimmunol.165.12.7064. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh A, Zhang WW, Matlashewski G. Immunization with A2 protein results in a mixed Th1/Th2 and a humoral response which protects mice against Leishmania donovani infections. Vaccine. 2001 Oct 12;20(1–2):59–66. doi: 10.1016/s0264-410x(01)00322-x. [DOI] [PubMed] [Google Scholar]
- 4.Wilson ME, Young BM, Andersen KP, Weinstock JV, Metwali A, Ali KM, et al. A recombinant Leishmania chagasi antigen that stimulates cellular immune responses in infected mice. Infection and immunity. 1995 May;63(5):2062–9. doi: 10.1128/iai.63.5.2062-2069.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tewary P, Jain M, Sahani MH, Saxena S, Madhubala R. A heterologous prime-boost vaccination regimen using ORFF DNA and recombinant ORFF protein confers protective immunity against experimental visceral leishmaniasis. J Infect Dis. 2005 Jun 15;191(12):2130–7. doi: 10.1086/430348. [DOI] [PubMed] [Google Scholar]
- 6.Aguilar-Be I, da Silva Zardo R, Paraguai de Souza E, Borja-Cabrera GP, Rosado-Vallado M, Mut-Martin M, et al. Cross-protective efficacy of a prophylactic Leishmania donovani DNA vaccine against visceral and cutaneous murine leishmaniasis. Infection and immunity. 2005 Feb;73(2):812–9. doi: 10.1128/IAI.73.2.812-819.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rafati S, Zahedifard F, Nazgouee F. Prime-boost vaccination using cysteine proteinases type I and II of Leishmania infantum confers protective immunity in murine visceral leishmaniasis. Vaccine. 2006 Mar 15;24(12):2169–75. doi: 10.1016/j.vaccine.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Webb JR, Campos-Neto A, Ovendale PJ, Martin TI, Stromberg EJ, Badaro R, et al. Human and murine immune responses to a novel Leishmania major recombinant protein encoded by members of a multicopy gene family. Infection and immunity. 1998 Jul;66(7):3279–89. doi: 10.1128/iai.66.7.3279-3289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campos-Neto A, Porrozzi R, Greeson K, Coler RN, Webb JR, Seiky YA, et al. Protection against cutaneous leishmaniasis induced by recombinant antigens in murine and nonhuman primate models of the human disease. Infection and immunity. 2001 Jun;69(6):4103–8. doi: 10.1128/IAI.69.6.4103-4108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goto Y, Bogatzki LY, Bertholet S, Coler RN, Reed SG. Protective immunization against visceral leishmaniasis using Leishmania sterol 24-c-methyltransferase formulated in adjuvant. Vaccine. 2007 Oct 16;25(42):7450–8. doi: 10.1016/j.vaccine.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pourshafie M, Morand S, Virion A, Rakotomanga M, Dupuy C, Loiseau PM. Cloning of S-adenosyl-L-methionine:C-24-Delta-sterol-methyltransferase (ERG6) from Leishmania donovani and characterization of mRNAs in wild-type and amphotericin B-Resistant promastigotes. Antimicrobial agents and chemotherapy. 2004 Jul;48(7):2409–14. doi: 10.1128/AAC.48.7.2409-2414.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belkaid Y, Von Stebut E, Mendez S, Lira R, Caler E, Bertholet S, et al. CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. J Immunol. 2002 Apr 15;168(8):3992–4000. doi: 10.4049/jimmunol.168.8.3992. [DOI] [PubMed] [Google Scholar]
- 13.Coler RN, Goto Y, Bogatzki L, Raman V, Reed SG. Leish-111f, a recombinant polyprotein vaccine that protects against visceral Leishmaniasis by elicitation of CD(4+) T cells. Infection and immunity. 2007 Sep;75(9):4648–54. doi: 10.1128/IAI.00394-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goto Y, Sanjoba C, Asada M, Saeki K, Onodera T, Matsumoto Y. Adhesion of MRP8/14 to amastigotes in skin lesions of Leishmania major-infected mice. Experimental parasitology. 2008 May;119(1):80–6. doi: 10.1016/j.exppara.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Belkaid Y, Mendez S, Lira R, Kadambi N, Milon G, Sacks D. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J Immunol. 2000 Jul 15;165(2):969–77. doi: 10.4049/jimmunol.165.2.969. [DOI] [PubMed] [Google Scholar]
- 16.Anderson CF, Mendez S, Sacks DL. Nonhealing infection despite Th1 polarization produced by a strain of Leishmania major in C57BL/6 mice. J Immunol. 2005 Mar 1;174(5):2934–41. doi: 10.4049/jimmunol.174.5.2934. [DOI] [PubMed] [Google Scholar]
- 17.Tonui WK, Mejia JS, Hochberg L, Mbow ML, Ryan JR, Chan AS, et al. Immunization with Leishmania major exogenous antigens protects susceptible BALB/c mice against challenge infection with L. major. Infection and immunity. 2004 Oct;72(10):5654–61. doi: 10.1128/IAI.72.10.5654-5661.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tonui WK, Titus RG. Cross-protection against Leishmania donovani but not L. Braziliensis caused by vaccination with L. Major soluble promastigote exogenous antigens in BALB/c mice. The American journal of tropical medicine and hygiene. 2007 Mar;76(3):579–84. [PubMed] [Google Scholar]
- 19.Rafati S, Kariminia A, Seyde-Eslami S, Narimani M, Taheri T, Lebbatard M. Recombinant cysteine proteinases-based vaccines against Leishmania major in BALB/c mice: the partial protection relies on interferon gamma producing CD8(+) T lymphocyte activation. Vaccine. 2002 Jun 7;20(19–20):2439–47. doi: 10.1016/s0264-410x(02)00189-5. [DOI] [PubMed] [Google Scholar]
- 20.Al-Wabel MA, Tonui WK, Cui L, Martin SK, Titus RG. Protection of susceptible BALB/c mice from challenge with Leishmania major by nucleoside hydrolase, a soluble exo-antigen of Leishmania. The American journal of tropical medicine and hygiene. 2007 Dec;77(6):1060–5. [PubMed] [Google Scholar]
- 21.Gamboa-Leon R, Paraguai de Souza E, Borja-Cabrera GP, Santos FN, Myashiro LM, Pinheiro RO, et al. Immunotherapy against visceral leishmaniasis with the nucleoside hydrolase-DNA vaccine of Leishmania donovani. Vaccine. 2006 May 29;24(22):4863–73. doi: 10.1016/j.vaccine.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Coler RN, Skeiky YA, Bernards K, Greeson K, Carter D, Cornellison CD, et al. Immunization with a polyprotein vaccine consisting of the T-Cell antigens thiol-specific antioxidant, Leishmania major stress-inducible protein 1, and Leishmania elongation initiation factor protects against leishmaniasis. Infection and immunity. 2002 Aug;70(8):4215–25. doi: 10.1128/IAI.70.8.4215-4225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Titus RG, Sherry B, Cerami A. Tumor necrosis factor plays a protective role in experimental murine cutaneous leishmaniasis. J Exp Med. 1989 Dec 1;170(6):2097–104. doi: 10.1084/jem.170.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott P. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol. 1991 Nov 1;147(9):3149–55. [PubMed] [Google Scholar]
- 25.Titus RG, Kelso A, Louis JA. Intracellular destruction of Leishmania tropica by macrophages activated with macrophage activating factor/interferon. Clin Exp Immunol. 1984 Jan;55(1):157–65. [PMC free article] [PubMed] [Google Scholar]
- 26.Liew FY, Parkinson C, Millott S, Severn A, Carrier M. Tumour necrosis factor (TNF alpha) in leishmaniasis. I. TNF alpha mediates host protection against cutaneous leishmaniasis. Immunology. 1990 Apr;69(4):570–3. [PMC free article] [PubMed] [Google Scholar]
- 27.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007 Jul;13(7):843–50. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 28.Liew FY, Li Y, Millott S. Tumor necrosis factor-alpha synergizes with IFN-gamma in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990 Dec 15;145(12):4306–10. [PubMed] [Google Scholar]
- 29.Sadick MD, Heinzel FP, Holaday BJ, Pu RT, Dawkins RS, Locksley RM. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. The Journal of experimental medicine. 1990 Jan 1;171(1):115–27. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tobin JF, Reiner SL, Hatam F, Zheng S, Leptak CL, Wirth DF, et al. Transfected Leishmania expressing biologically active IFN-gamma. J Immunol. 1993 Jun 1;150(11):5059–69. [PubMed] [Google Scholar]
- 31.Himmelrich H, Launois P, Maillard I, Biedermann T, Tacchini-Cottier F, Locksley RM, et al. In BALB/c mice, IL-4 production during the initial phase of infection with Leishmania major is necessary and sufficient to instruct Th2 cell development resulting in progressive disease. J Immunol. 2000 May 1;164(9):4819–25. doi: 10.4049/jimmunol.164.9.4819. [DOI] [PubMed] [Google Scholar]
- 32.Miles SA, Conrad SM, Alves RG, Jeronimo SM, Mosser DM. A role for IgG immune complexes during infection with the intracellular pathogen Leishmania. The Journal of experimental medicine. 2005 Mar 7;201(5):747–54. doi: 10.1084/jem.20041470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kane MM, Mosser DM. The role of IL-10 in promoting disease progression in leishmaniasis. J Immunol. 2001 Jan 15;166(2):1141–7. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]