Abstract
The objective of this review is to examine the role that HIF-1 plays in the initiation of angiogenesis and in radiotherapy response. Although these two phenomena may at first seem unrelated, there are parallelisms to be drawn associated with the importance of reactive oxygen species in controlling the transcriptional activity of HIF-1, independently of its main driving force, hypoxia. Knowledge of the mechanisms underlying the control of HIF-1 leads to rationale for its inhibition in a range of clinical scenarios.
Keywords: Hypoxia, HIF-1, angiogenesis, radiotherapy response
Introduction
The advent of fluorescent reporter genes in the mid 1990’s has permitted unprecedented ability to monitor a variety of cellular functions, both in vitro and in vivo. Use has been widespread in vivo, permitting direct evaluation of a large range of behaviours in vivo, including metastasis, alternative gene splicing1, gene silencing2 and promoter activity3. Our group has examined interactions between tumor cells and surrounding normal tissue microvasculature in the initiation of angiogenesis, using the skin-fold window chamber model implanted into nude mice4. These initial studies demonstrated that interactions were occurring between tumor cells and the host vasculature within 24h of tumor transplant and angiogenesis initiation occurred at a very early stage, when only a few hundred cells were present. Thus, the next question was whether angiogenesis was being initiated by hypoxia.
We engineered tumor cells to express hypoxia regulated reporter genes to pursue this question. Hypoxia responsive reporter genes were engineered to increase expression under control of the hypoxia-responsive transactivator, HIF-1 (Hypoxia-inducible factor -1). HIF-1 is generally thought of as the master regulator of the hypoxia response and is known to regulate dozens of genes involved in regulation of metabolism, angiogenesis and metastasis5. When active in promoting gene expression, it is a heterodimer, consisting of HIF1α and HIF-1β subunits that enter the nucleus and bind to specific promoter regions of responsive genes. Both subunits are constitutively expressed, but the HIF-1α subunit is rapidly degraded via prolyl hydroxylation, recognition by the VHL complex and is targeted for proteosome degradation under normoxic conditions. The degradation process is so efficient that the transactivator complex is normally silent in normoxia. The HIF-1 reporter cell lines were used to evaluate the role of HIF-1 in angiogenesis and in tumor responses to radiotherapy.
Hypoxia and Angiogenesis Initiation
In initial studies we utilized green fluorescent protein (GFP) under control of a constitutive promoter, to evaluate tumor-host microvascular interactions prior to and after initiation of angiogenesis. There were profoundly obvious interactions occurring between tumor cells and host vasculature, well before the initiation of angiogenesis. Increases in vasodilation and vascular tortuosity occurred within 24–48h after tumor cell transplant. Concomitantly, tumor cells tended to proliferate and migrate toward pre-existing microvasculature4 (Figure 1). Angiogenesis initiation occurred at a very early stage, when cell numbers in the growing tumor were less than 200 cells. These observations led to the question of whether hypoxia was responsible for angiogenesis initiation.
Figure 1.
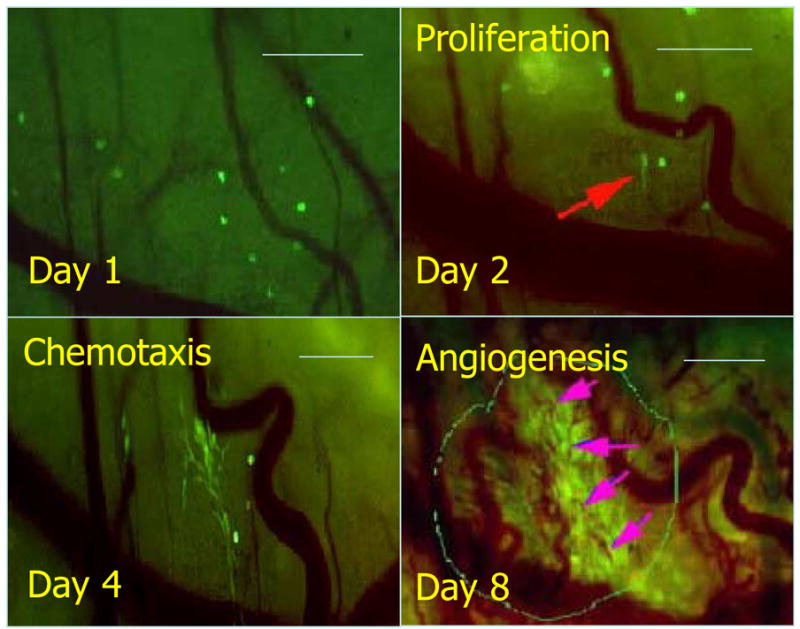
Serial observation of tumor cell – vascular interactions during early tumor growth. Vasodilation and increased vascular tortuosity is observed within 24h following transplant of only a few tumor cells. Red arrow: an elongated 4T1GFP tumor cell (Day 2). Vascular cooption is observed as tumor cells proliferate and migrate toward existing host vasculature (Day 4). Angiogenesis is first observed when total tumor size is less than 200 μm diameter. Arrows: tumor (localized in the marked circle)-associated new microvessels (Day 8). Bar, 200 μm. Figure reconfigured from Li et al.4, with permission from the publisher.
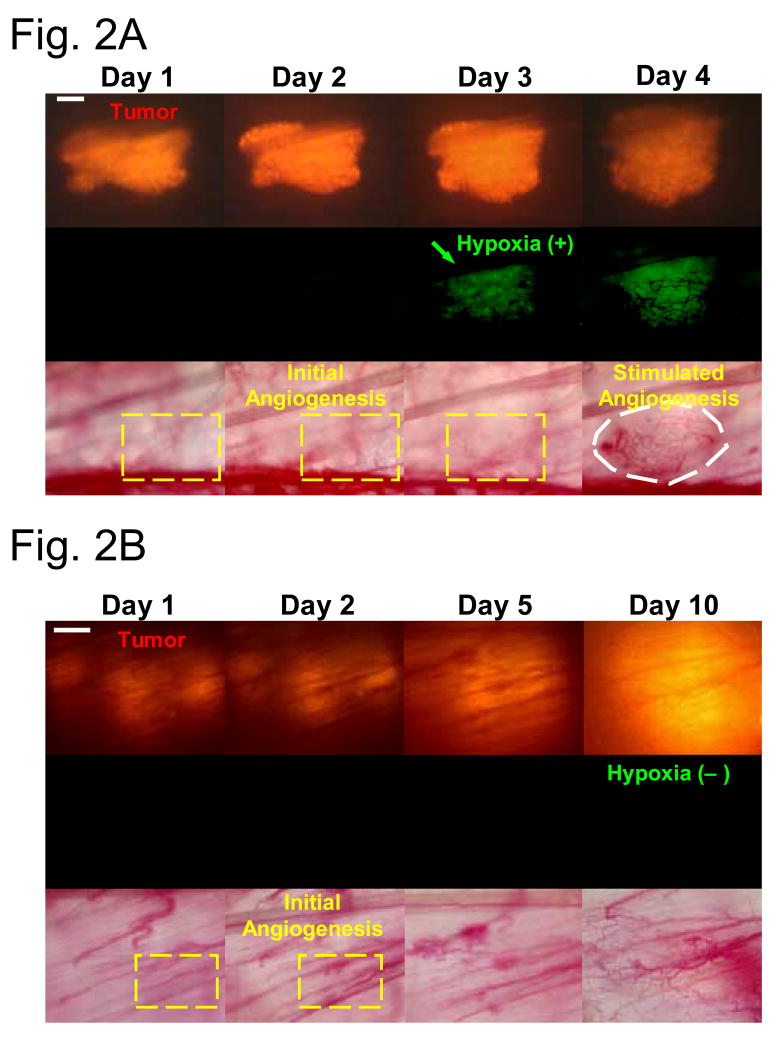
Using doubly transfected cell lines that contained red fluorescence protein (RFP) under control of a constitutive promoter and GFP under control of HIF-1, we found that the initiation of angiogenesis occurred prior to detection of HIF-1 activity in two different tumor lines (HCT116 and 4T1) 6. To accomplish this goal, we monitored CMV-RFP and HIF-1-GFP expression and vascular density on a daily basis, following tumor cell transplantation into window chambers. In initial studies we found that HIF-1-GFP expression typically followed initial detection of angiogenesis, by 1–2 days. To further verify that angiogenesis was initiated prior to hypoxia, we administered tirapazamine, a hypoxia selective cytotoxin, to the mice in order to selectively kill hypoxic cells. In these experiments, we hypothesized that the initiation of angiogenesis should be prolonged, if HIF-1 expression were important for angiogenesis initiation. Tirapazamine significantly prolonged the time until detection of HIF-1-GFP, but it had no effect on the time of angiogenesis initiation (Figure 2). Importantly, angiogenesis appeared to accelerate after HIF-1 expression was detected. Interestingly, angiogenesis initiation did not seem to result in later downregulation of HIF-1. Since one would presume that oxygenation would be improved once angiogenesis was initiated, this observation was opposite of what we expected.
Figure 2.
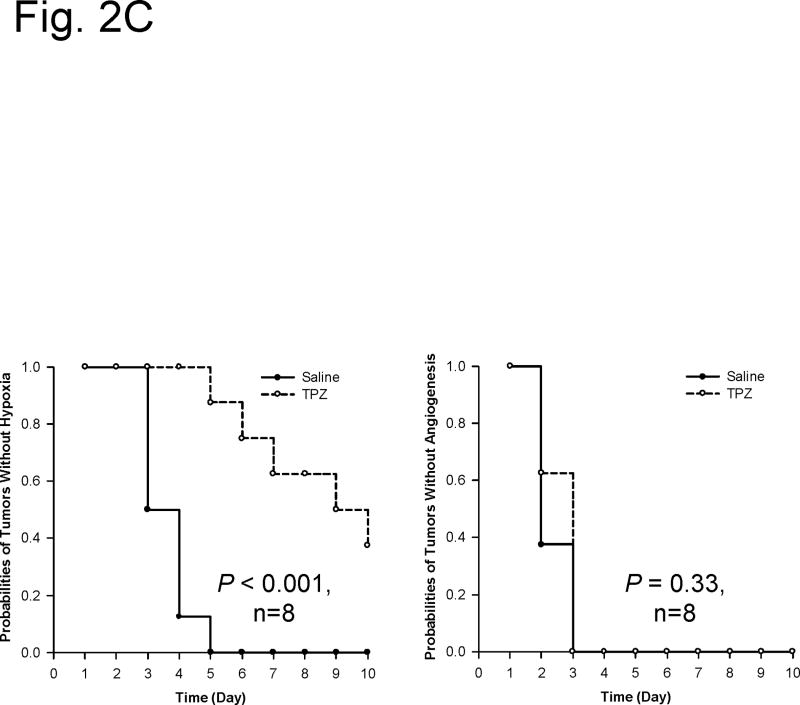
Selectively killing hypoxic cells by tirapazamine does not delay incipient tumor angiogenesis. (A) Representative window chamber images of a saline-treated HCT116 tumor (transduced with the double fluorescent reporter CMV-RFP/HIF-1-GFP) revealing incipient angiogenesis (Day 2) before hypoxic response (Day 3, GFP fluorescence). HIF-1 expression is followed by more robust angiogenesis (Day 4; white dashed circle). Bar, 300 μm. (B) Representative window chamber images of a tirapazamine-treated HCT116 tumor revealing incipient angiogenesis (Day 2) and its development into a vascular plexus (Day 10; white field ) in the absence of hypoxic response (no GFP fluorescence). Bar, 300 μm. (C) Kaplan Meier analysis of time required for the initial hypoxic response and angiogenesis in the above tirapazamine versus saline-treated HCT116 window chamber tumors. Tirapazamine treatment delayed the initial hypoxic response when compared with saline treatment (median time: 9.5 days in the tirapazamine-treated group versus 3.5 days in the saline-treated group; log-rank test, P < 0.001). In contrast, there was no difference in the probabilities of times required for the onset of incipient angiogenesis in tirapazamine versus saline-treated HCT116 window chamber tumors (log-rank test, P = 0.33). Figure modified from Cao et al.6, with permission of the publisher.
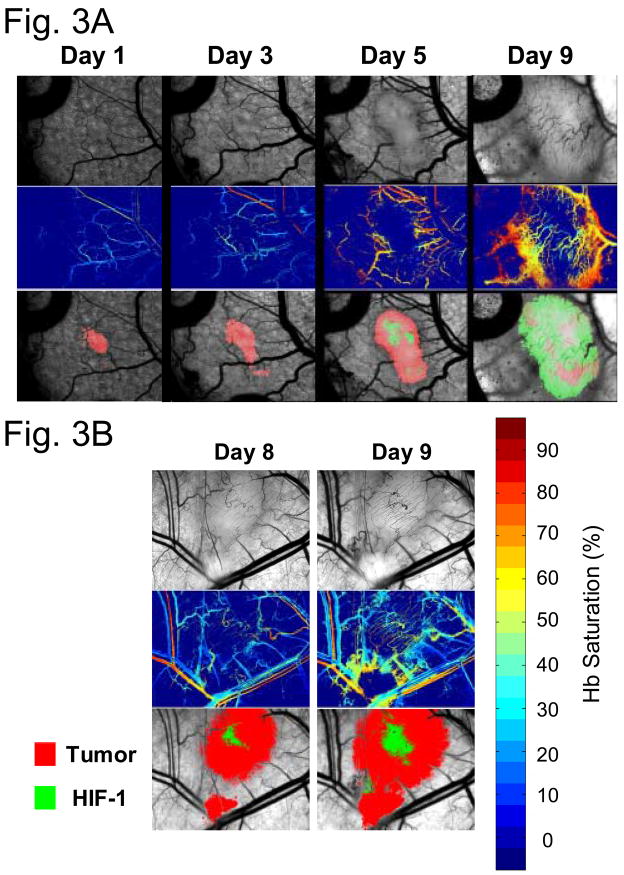
In more recent studies, we have also used optical measurement of hemoglobin saturation to examine changes in vascular oxygen content during early tumor growth 7. These studies have strikingly shown that the first vascular reaction to tumor cells is arteriolar vasodilation with concomitant increase in hemoglobin saturation. Increased venular pO2 follows, particularly in microvessels surrounding the growing tumor mass (Figure 3). The response is strikingly similar to an inflammatory reaction. HIF-1-GFP expression occurred as the oxygenation state of the supplying microvessels increased.
Figure 3.
Serial observation of hemoglobin saturation during early tumor growth. (A) An increase in arterial pO2 is the first reaction to the presence of tumor cells (a). followed by increases in venular pO2 (b). During the continued process of angiogenesis, HIF-1 expression level increases, suggesting that improvements in pO2 within the growing tumor mass are not alleviating HIF-1 activity (c). (B) Comparison of HIF-1 reporter gene expression, relative to location of tumor microvessels. In this example case, HIF-1 reporter gene shows strong expression around tumor microvessels. The average hemoglobin saturation is between 30–50%, suggesting that the HIF-1 expression is not the result of hypoxia. Alternatively, instabilities in oxygenation, caused by fluctuations in red cell flux within vessels of this caliber may contribute to oxidative stress and stabilization of HIF-1 (See Figure 4 for additional detail).
As will be discussed below, we believe that the initiation of HIF-1-GFP expression is driven by oxidative stress, in this case caused by instabilities in oxygenation, leading to hypoxia reoxygenation injury and generation of free radical species that increase HIF-1 levels in tumor cells. This result suggests that administration of therapies that inhibit oxidative stress could prove very useful as a means to inhibit tumor angiogenesis during early tumor growth, perhaps in the adjuvant setting.
HIF-1 response following radiotherapy
We initiated studies, using the CMV-RFP/HIF-1-GFP tumor lines to examine the kinetics of reoxygenation following radiotherapy. Since radiation treatment typically causes reoxygenation, we hypothesized that HIF-1-GFP expression would decrease after radiotherapy and then gradually return, as the tumors re-established a hypoxic state. Again, much to our surprise, we found that HIF-1 protein levels actually increased after radiation therapy, at a time when the tumors were reoxygenating8. Two mechanisms were identified for this effect that depended initially on the presence of hypoxia in the tumor prior to radiotherapy. Protein-mRNA complexes, referred to as stress granules, were found to increase in number during hypoxic stress. These complexes served to protect HIF-1 mediated mRNAs from being translated to protein during hypoxia. Upon reoxygenation, the complexes disaggregated, leading to a burst of HIF-1 regulated proteins. The second mechanism underlying this effect was the formation of excessive free radical species in the hours to days after radiation treatment. Treatment of animals with a ubiquitous SOD mimetic, that catalytically inactivates many free radical species, including superoxide anion, hydroxyl radical and nitric oxide, effectively blocked the upregulation of HIF-1 after radiation treatment and resulted in vascular damage (Figure 4) and prolongation of growth delay after radiotherapy9. There has been speculation that HIF-1 can be stabilized by nitric oxide in aerobic conditions, based on work by Moncada’s laboratory10. Hess speculated that a particular cysteine residue in the oxygen dependent degradation domain of HIF-1 may be a target for nitrosylation by nitric oxide and further hypothesized that this would stabilize HIF-111. Alternatively, there is recent evidence from Joachim Fandrey’s laboratory that nitric oxide can increase prolyl hydroxlyase activity under hypoxic conditions, thus providing a negative regulating effect on HIF-1 activity under hypoxia12. Based on very recent work by Li et al., we now know that S-nitrosylation of that the cysteine residue by nitric oxide plays a major role in HIF-1 stabilization after radiotherapy13. Additionally, the process of hypoxia-reoxygenation injury may be less important than activation of macrophages in the tumor mass following radiotherapy, which appears to be the main source for nitric oxide in this setting. It is not known whether the negative regulatory effect of NO on HIF-1 in hypoxia is important in this context.
Figure 4.
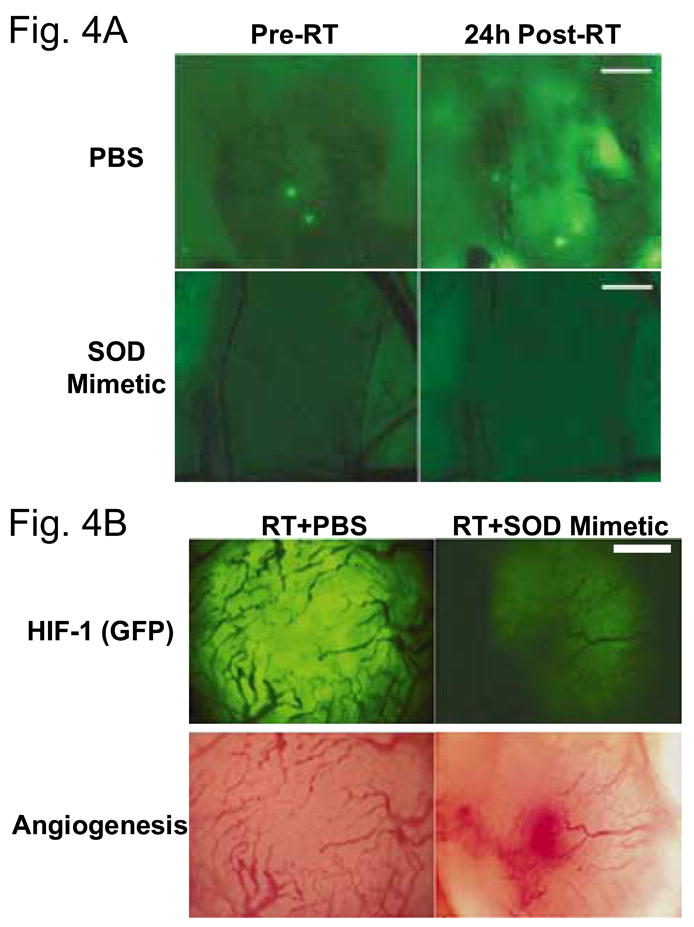
Observation of free radicals in skin fold window chamber model, prior to and after radiotherapy. (A) Fluore scence intensity of ROS sensitive dye is detectable prior to radiotherapy, but the level of fluorescence increases 24h after radiotherapy. This suggests some baseline level of oxidative stress that increases after radiotherapy. Administration of an SOD mimetic inhibits the increase in free radical production after radiotherapy (B) Administration of SOD mimetic inhibits increase in HIF-1-GFP expression and causes significant vascular damage, as observed at 48h after treatment. Modified from Moeller et al.8, with permission from the publisher. Bar, 300 μm.
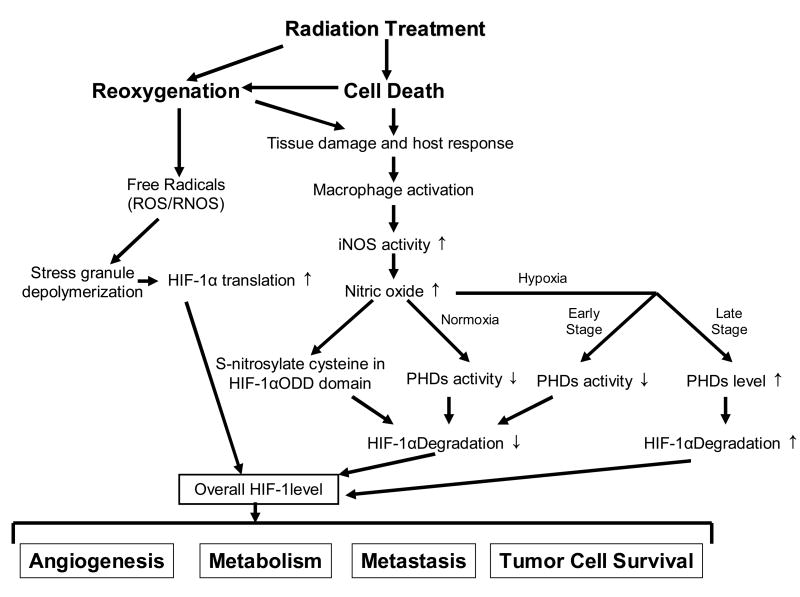
The consequences of HIF-1 activation following radiation therapy are complex, involving alterations in both tumor and endothelial cell behavior and survival14. On the one hand, an increase in HIF-1 leads to upregulation of VEGF and other HIF-1 regulated pro-angiogenic factors. VEGF protects tumor microvasculature from cytotoxic effects of radiation15. On the other hand, upregulation of HIF-1 promotes apoptosis and decreases clonogenic survival of P53(+) tumor cells, thereby sensitizing them to being killed more efficiently by radiotherapy. Alternatively, upregulation of HIF-1 after radiotherapy has no effect on the radiosensitivity of P53(−) cells. Additional effects of the tumor microenvironment, relating to the combination of hypoxia and nutrient depletion can contribute to slight radioresistance in the tumor cells that can survive this type of environment. The issue of sequencing between radiotherapy and initiation of HIF-1 inhibition is yet to be clarified. In P53(+) cells, it is clear that HIF-1 inhibition after radiotherapy treatment is preferred. A summary of the overreaching effects of microenvironmental factors on HIF-1 expression is depicted in Figure 5.
Figure 5.
Summary of effects of radiation therapy on HIF-1. HIF-1: hypoxia inducible factor-1; ROS: reactive oxygen species; RNOS: reactive nitric oxygen species (RNOS); iNOS: inducible nitric oxide synthase (iNOS); ODD: oxygen-dependent degradation; PHD: prolyl hydroxylase. Not depicted in the diagram are other sources of HIF-1 control, such as hypoxia and oncogene/suppressor gene regulation.
Future Directions
Several papers have been published, suggesting potential benefits for HIF-1 inhibition in combination with radiotherapy and chemotherapy16–19. There are a number of unanswered questions, however. First, it will be important to determine the dose modifying factor for HIF-1 inhibition, when combined with curative doses of radiotherapy, particularly with a fractionated regimen. Second, radiotherapy is rarely administered by itself in modern radiotherapy practice. It is usually combined with either traditional chemotherapeutic agents, or with targeted therapeutics such as EGFR inhibitors or anti-angiogenic agents. It will be important to determine how HIF-1 expression is influenced by combination therapies that mirror modern radiotherapy practice. As with any form of therapy, it will also be important to determine whether HIF-1 inhibition has any deleterious effects on normal tissue responses to radiotherapy and/or chemotherapy. In this regard, current results suggest that HIF-1 inhibition by SOD mimetics after radiotherapy actually protects against normal tissue, such as lung, from radiation damage20, 21. This effect is largely attributed to the prevention of inflammatory reactions in the post radiation period.
Acknowledgments
Work supported by grants from the NIH/NCI CA40355, NBIB EB001882, NIH/NCI CA81512, the Duke SPORE for breast cancer, the Hughes Foundation for Medical Research, the Duke Medical Scientist Training Program and the Susan G. Komen Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- 1.Oltean S, Sorg BS, Albrecht T, et al. Alternative inclusion of fibroblast growth factor receptor 2 exon IIIc in Dunning prostate tumors reveals unexpected epithelial mesenchymal plasticity. Proc Natl Acad Sci U S A. 2006;103:14116–14121. doi: 10.1073/pnas.0603090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin JQ, Gao J, Shao R, et al. siRNA agents inhibit oncogene expression and attenuate human tumor cell growth. J Exp Ther Oncol. 2003;3:194–204. doi: 10.1046/j.1359-4117.2003.01092.x. [DOI] [PubMed] [Google Scholar]
- 3.Dewhirst MW, Shan S, Cao Y, et al. Intravital fluorescence facilitates measurement of multiple physiologic functions and gene expression in tumors of live animals. Dis Markers. 2002;18:293–311. doi: 10.1155/2002/820102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li CY, Shan S, Huang Q, et al. Initial stages of tumor cell-induced angiogenesis: evaluation via skin window chambers in rodent models. J Natl Cancer Inst. 2000;92:143–147. doi: 10.1093/jnci/92.2.143. [DOI] [PubMed] [Google Scholar]
- 5.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 6.Cao Y, Li CY, Moeller BJ, et al. Observation of incipient tumor angiogenesis that is independent of hypoxia and hypoxia inducible factor-1 activation. Cancer Res. 2005;65:5498–5505. doi: 10.1158/0008-5472.CAN-04-4553. [DOI] [PubMed] [Google Scholar]
- 7.Sorg BS, Moeller BJ, Donovan O, et al. Hyperspectral imaging of hemoglobin saturation in tumor microvasculature and tumor hypoxia development. J Biomed Opt. 2005;10:44004. doi: 10.1117/1.2003369. [DOI] [PubMed] [Google Scholar]
- 8.Moeller BJ, Cao Y, Li CY, et al. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 9.Moeller BJ, Batinic-Haberle I, Spasojevic I, et al. A manganese porphyrin superoxide dismutase mimetic enhances tumor radioresponsiveness. Int J Radiat Oncol Biol Phys. 2005;63:545–552. doi: 10.1016/j.ijrobp.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Quintero M, Brennan PA, Thomas GJ, et al. Nitric oxide is a factor in the stabilization of hypoxia-inducible factor-1alpha in cancer: role of free radical formation. Cancer Res. 2006;66:770–774. doi: 10.1158/0008-5472.CAN-05-0333. [DOI] [PubMed] [Google Scholar]
- 11.Hess DT, Matsumoto A, Kim SO, et al. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 12.Berchner-Pfannschmidt U, Yamac H, Trinidad B, et al. Nitric oxide modulates oxygen sensing by hypoxia-inducible factor 1-dependent induction of prolyl hydroxylase 2. J Biol Chem. 2007;282:1788–1796. doi: 10.1074/jbc.M607065200. [DOI] [PubMed] [Google Scholar]
- 13.Li F, Sonveaux P, Rabbani ZN, et al. Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moeller BJ, Dreher MR, Rabbani ZN, et al. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell. 2005;8:99–110. doi: 10.1016/j.ccr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–3378. [PubMed] [Google Scholar]
- 16.Cao Y, Linden P, Shima D, et al. In vivo angiogenic activity and hypoxia induction of heterodimers of placenta growth factor/vascular endothelial growth factor. J Clin Invest. 1996;98:2507–2511. doi: 10.1172/JCI119069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pore N, Gupta AK, Cerniglia GJ, et al. Nelfinavir down-regulates hypoxia-inducible factor 1alpha and VEGF expression and increases tumor oxygenation: implications for radiotherapy. Cancer Res. 2006;66:9252–9259. doi: 10.1158/0008-5472.CAN-06-1239. [DOI] [PubMed] [Google Scholar]
- 18.Sasabe E, Zhou X, Li D, et al. The involvement of hypoxia-inducible factor-1alpha in the susceptibility to gamma-rays and chemotherapeutic drugs of oral squamous cell carcinoma cells. Int J Cancer. 2007;120:268–277. doi: 10.1002/ijc.22294. [DOI] [PubMed] [Google Scholar]
- 19.Williams KJ, Telfer BA, Xenaki D, et al. Enhanced response to radiotherapy in tumours deficient in the function of hypoxia-inducible factor-1. Radiother Oncol. 2005;75:89–98. doi: 10.1016/j.radonc.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, et al. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med. 2002;33:857–863. doi: 10.1016/s0891-5849(02)00980-2. [DOI] [PubMed] [Google Scholar]
- 21.Rabbani ZN, Batinic-Haberle I, Anscher MS, et al. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int J Radiat Oncol Biol Phys. 2007;67:573–580. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]