Abstract
Objective:
Although the rabbit elastase-induced aneurysm model is currently used widely for endovascular research and device testing, procedural causes leading to animal morbidity and mortality have not yet been clearly described. We conducted a retrospective study to analyse factors contributing to neurological deficits in rabbits that underwent the elastase-induced aneurysm creation procedure at our research center from 2002 to 2005 in order to improve the technique and reduce procedure-related morbidity and mortality.
Methods:
A total sample of 38 animals that underwent the procedure under the same conditions was analysed in two groups: animals that presented neurological deficits (ND, n=15) and animals that were neurological deficit free (NDF, n=23). Data were collected by reviewing the animal records and radiographic images from the procedures. Statistical analyses using the Mann–Whitney test, unpaired t-test with Welch correction and Fisher's exact tests were performed to compare the two groups based on variables associated with endothelial injury and activation of the coagulation cascade.
Results:
The variables of animal weight (signifying state of health of the animal), total procedure time, total balloon occlusion time and clot formation were found to be significantly and/or very significantly correlated to ND presentation.
Discussion:
Successful creation of the rabbit elastase-induced aneurysm model depends on careful control over several technical details. Important variables governing outcome have been identified here. A specific, improved endovascular arrangement that facilitates maneuvering of the devices and reduces the risk of air emboli is presented.
Keywords: Saccular cerebral aneurysm, experimental aneurysm, elastase-induced aneurysm, neurological deficit, rabbit model
INTRODUCTION
The endovascular elastase-induced aneurysm model in rabbits has been described in literature as easy, reliable, reproducible, and as possessing features that are considered desirable for an intracranial aneurysm model1,2. Moreover, different authors have stated that these aneurysms are histologically similar to naturally occurring aneurysms3-5. All the features noted above make this model suitable for hemodynamic and pathophysiologic investigations, therapeutic strategies and evaluation of endovascular devices for interventional neuroradiologic practice, among others.
The most commonly cited problems with the elastase-induced aneurysm model in rabbits are related to the reliability of the technique or the aneurysm outcome. However, only a few studies report on issues directly related to rabbit morbidity and mortality6-8. The attrition rate for the aneurysm creation procedure has not been reported clearly in the available literature. Massoud et al.6 noted that there is a ‘high incidence of procedure-related mortality’ associated with this species (rabbit) such as anesthesia-related deaths and post-operative complications within 2 days of surgery in as many as 20% of rabbits, even when the procedure has been performed by experienced hands. Others report mortality rates from 25 (Ref. 7) to 54%8 brought about by anatomical variations. In our practice with the elastase-induced aneurysm model during 2002–2005, the mortality varied from 20 to 7.4%. The high mortality associated with this procedure was due to neurological deficit (ND), defined as the clinical expression of a structural or functional, transient or permanent abnormality that can be ascribed to a particular region of the nervous system9. Endo et al.10 have previously graded neurological deficits in rabbits based on observed symptoms such as paresis of the legs or abnormal gait (circling movement or difficulty walking): (1) grade 1: no neurological deficit; (2) grade 2: minimum or suspicious neurological deficit; (3) grade 3: mild neurological deficit without abnormal movement; (4) grade 4: severe neurological deficit with abnormal movement.
Symptoms of neurological deficit observed in the animals in our laboratory include nystagmus, dyspnea, head tilt, level of consciousness varying from clouded to comatose, loss of balance and in severe cases, rolling and pedaling. Mild cases were able to stand, drink and largely were able to recover after 24–48 hours. Although the existence of such deficits is well known, their causes have not been closely examined yet. We conducted a retrospective statistical investigation to analyse the causes of neurological impairment in rabbits that underwent this procedure to improve the technique and reduce related mortality.
METHODS
Animal model
All procedures were performed according to protocols approved by the Animal Care and Use Committee of our institution. The general technique for aneurysm creation was the same in all cases. Pre-anesthesia was induced by injection of 0.01 mg/kg glycopyrrolate (American Regent Inc., Shirley, NY, USA). Anesthesia was then induced by ketamine 35 mg/kg and xylazine 5 mg/kg and maintained by inhalation of 1.0–1.5% isoflurane. After induction of anesthesia, the right common carotid artery (RCCA) was exposed and a 5 Fr introducer sheath (Cordis Endovascular, Miami, FL, USA) was placed retrogradely in the RCCA towards its origin. A non-detachable silicone balloon (Endeavor, Target Therapeutics, Fremont, CA, USA) filled with contrast and a microcatheter (Prowler 14, Cordis Endovascular, Miami, FL, USA) were advanced side by side through the sheath. Some animals received an extra intravenous dose of heparin (100 units/kg), depending on the researcher performing the procedure on that date. The balloon was inflated to occlude the origin of the RCCA. A solution of porcine pancreatic elastase (Sigma-Aldrich Co., St Louis, MO, USA) and contrast was perfused via the microcatheter into the dead space between the balloon and the sheath. After incubating for a period of time between 5 and 20 minutes, the elastase was drained and digital subtraction angiography (DSA) was performed. The process was repeated until widening of the RCCA root was evident, then the devices were removed and the RCCA was ligated below the entry point. The stump of the RCCA was allowed to remodel and mature into an aneurysm for a term of 3 weeks.
Statistical analysis
Endovascular aneurysm creation by elastase incubation has been conducted at our research center with an overall attrition rate of 10.2%. To investigate the major factors contributing to the attrition from ND, we selected a cohort of 38 animals. This cohort was separated into two groups: animals with neurological deficits (ND, n=15) and neurological deficit free (NDF, n=23) animals. The NDF group thus falls under grade 1 as per the grading scale mentioned above, while the ND group falls under grades 2 through 4. Data for each animal were collected from its chart and from examination of all the angiographic images acquired during the aneurysm induction procedure. The following variables were analysed for possible correlation with ND: animal weight, total endovascular procedure time, total balloon occlusion time, number of balloon occlusions, balloon position indicated by total or partial occlusion of the subclavian artery, and clot formation assessed by visualization of clots in the DSA images.
The collected database was organized in a spreadsheet and the Mann–Whitney test, unpaired t-test with Welch correction, and Fisher's exact tests were performed to compare the two groups using the InStat (GraphPad Software Inc., San Diego, CA, USA) statistical software.
RESULTS
Fifty-two percent of the animals in the selected cohort survived the procedure without ND symptoms while 8% of the animals died without manifesting any ND symptoms. Neurological injury symptoms were noticed in the remaining 40% of the cohort (Table 1). Of this ND group, 11% recovered and 29% died after seizures during the post-operative period or were euthanized.
Table 1.
Neurological deficits and morbidity/mortality in the selected cohort of rabbits after elastase-induced aneurysm creation (2002–2005)
| Total sample (n=38, 100%) | Animals free of neurological deficits (NDF) (n=23, 60%) | Animals with neurological deficits (ND) (n=15, 40%) | ||
|---|---|---|---|---|
| Outcome | Survived | Died within 24 hours | Died/killed | Recovered |
| Animals | 52% | 8% | 29% | 11% |
The weight of the rabbit at time of surgery was significantly correlated with ND (p=0.019) with heavier animals being more prone to present complications. The average weight of the rabbits in the ND group was 3.7 kg with a standard deviation (SD) of 0.62 kg, nearly 20% more than the average weight of those in the NDF group (3.2 ± 0.73 kg).
The time elapsed between acquisition of the first diagnostic angiogram and acquisition of the last one to confirm effectiveness of the treatment was recorded as the total endovascular procedure time. The mean total endovascular procedure time for the ND group was 32.2 ± 8.06 minutes compared to 25.7 ± 5.66 minutes for the NDF group. The length of the endovascular procedure was highly correlated (p=0.006) to ND.
The number of times the occlusion balloon was inflated and deflated during the procedure (balloon occlusion occurrences) was also considered, but this variable did not show a significant correlation with the ND outcome. However, the total occlusion time of the parent vessel (brachiocephalic trunk) did correlate very significantly (p=0.0096).
Flow arrest in the subclavian artery due to its total occlusion by the balloon occurred in 67% of the procedures. Partial occlusion of the subclavian artery occurred in 23% of the cases and in the remainder of cases (10%), flow through the left common carotid artery (LCCA) was also compromised (Figure 1). In eight of the selected 38 cases, we had no documentation about the precise balloon position, so they were excluded from the balloon positioning analysis and the subsequent thrombus formation analysis. Correlation between balloon position, in terms of the number of arteries occluded simultaneously, and ND, did not reach significance in this cohort.
Figure 1.
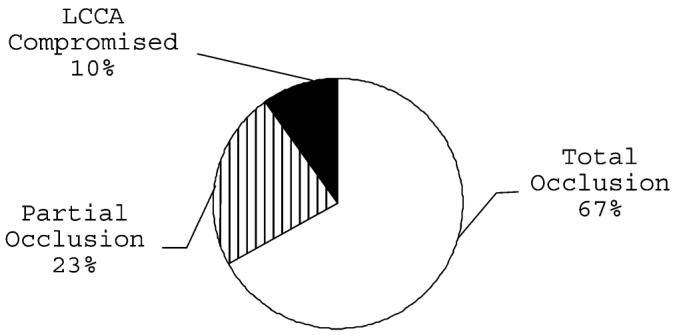
Distribution of balloon positions in relation to subclavian artery occlusion (n=30)
The significance of intra-procedural thrombus formation to neurological injury was assessed using Fisher's exact test. This analysis was conducted for only 30 animals of the selected cohort because requisite data were not available for the other eight animals. Clot formation along the parent vessel walls (Figure 2) was identified on available digital subtraction angiograms acquired during the procedure. Clot formation was evident in six of the 30 animals used for this analysis. Four of these animals belonged to the ND group while the other two belonged to the NDF group. Table 2 shows the distribution of cases used to calculate the significance of thrombus formation to ND. The presence of clot formation was significantly (p=0.047) correlated to ND.
Figure 2.
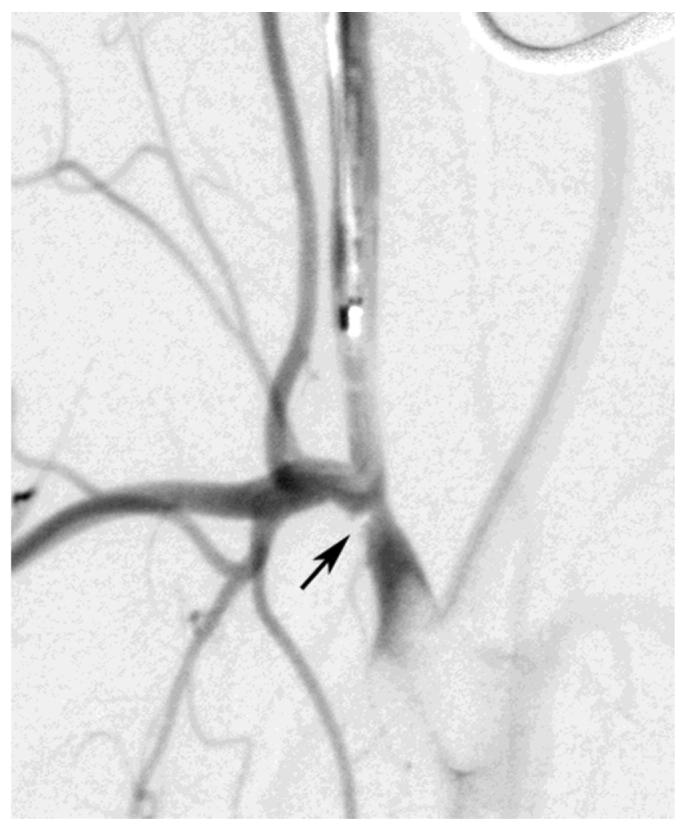
Clot (arrow) formed along the parent vessel
Table 2.
Number of cases in which clot formation was visualized on digitally subtracted angiograms acquired during the procedure (n=30)
| Outcome | Clot formation (positive) |
Clot formation (negative) |
|---|---|---|
| Neurological deficit (n=10) | 4 | 6 |
| Neurological deficit free (n=20) | 2 | 18 |
| Total sample (n=30) | 20% | 80% |
DISCUSSION
During 2002 and 2003, neurological dysfunction was the primary cause of morbidity and mortality in rabbits which underwent the endovascular elastase-induced aneurysm creation procedure at our laboratory. During 2002, the mortality rate was 20% and many modifications to the procedure were implemented to reduce the complication rate as discussed in detail below. Consequently, the mortality rate dropped to only 7.4% during 2005 as a result of changes such as switching the animal vendor, use of younger hence lighter animals, shortening of the endovascular procedure time, reduction in elastase incubation time and reduction in balloon occlusion time.
The weight is directly related to age, thus it is not surprising that the animal weight correlated significantly with ND and in our case to the overall health of the animal. As per our experience, older (i.e. heavier) animals (adults older than 4 months, weighing 3.5 kg or more) were more prone to post-operative complications including respiratory problems during or right after surgery, neurological deficits or a slow recovery period with subsequent days of sickness if survived. In some cases, Pasteurella multocida was documented through nasal culture from survivors.
We initially used New Zealand rabbits that weighed between 3.7 and 5.4 kg resulting in good recovery in only 30% of the animals (Figure 3a). In addition, rabbits confined in cages become easily overweight due to ad libitum feeding and poor exercise. These circumstances increase the susceptibility of these animals to complications during the procedure. We subsequently restricted our practice to young adult rabbits (2.7–3.2 kg), resulting in drastically improved outcomes (Figure 3b). Nonetheless, to elucidate the relationship between age, weight and overall health of rabbits in a research facility will require a separate investigation.
Figure 3.
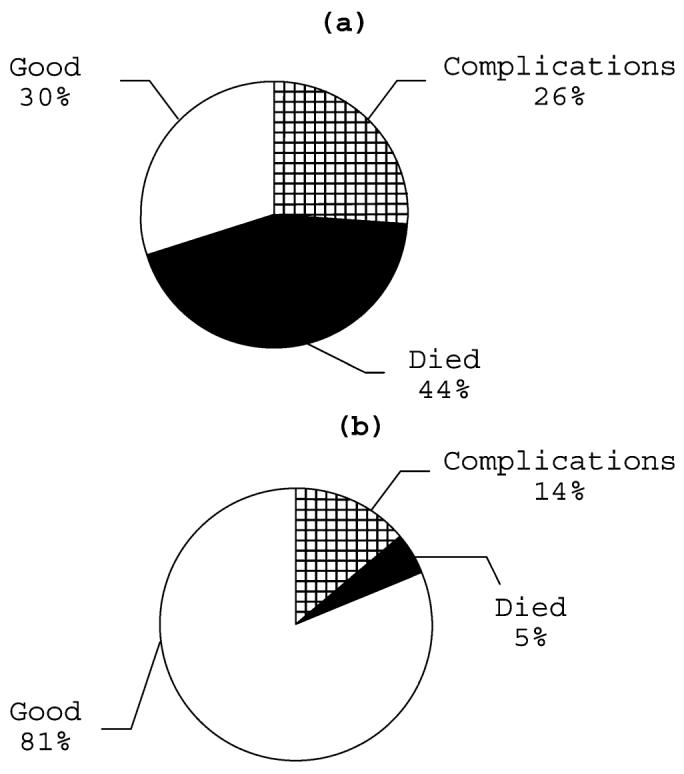
Post-surgery outcome in New Zealand rabbits. (a) Animal weight range 3.7–5.1 kg (n=27); (b) animal weight range 2.5–3.1 kg (n=22)
Our results show that longer procedure times are likely to result in poor neurological outcomes because of the cumulative effect of the added manipulations and the increased probability of procedural complications. A careful analysis of the DSA images revealed that the following events were the main contributors to delays and lengthy endovascular procedures:
difficulty in positioning the balloon and achieving sufficient seal to avoid elastase leakage during incubation;
spasm of the brachiocephalic trunk requiring angioplasty;
long elastase incubation times;
poor results with the initial elastase incubation necessitating additional treatments.
Extensive maneuvering of the catheters inside the vessels and frequent manipulation of the blood flow can trigger activation of the coagulation cascade and clot formation, which was found to be significantly correlated (p=0.034) to the total endovascular procedure time for these animals. However, when the length of the endovascular part of the procedure was limited to less than 25 minutes11, no complications were observed.
MacDonald et al.12 demonstrated that temporary arterial occlusion with a balloon catheter in pigs induces endothelial injury similar to that of microvascular clipping, but more widespread because of the large surface area of direct contact. They compared the injury the vessel incurred over different time periods using either technique via a grading scale to assess injury. Balloon occlusion for 5 and 10 minutes showed minimal to moderate injury while occlusion for 30 minutes showed marked injury. Regardless of occlusion technique, the injury grade increased with increasing occlusion time. In our study, 70% of the ND group experienced a total occlusion time of more than 20 minutes while in 70% of the NDF group, the occlusion time was less than 20 minutes. Nevertheless, it has been previously reported13 that increased vascular wall thrombogenicity together with substantial blood flow reduction is crucial for occlusive thrombus formation in a very short period of time. Small mural thrombi composed of aggregated platelets were produced after balloon injury and flow reduction. Further injury and substantial reduction in flow to less than 25% promoted occlusive thrombus formation within 71–160 seconds in rabbit femoral arteries.
During the aneurysm creation procedure, the inflated balloon is pulled backwards and repositioned until a seal is achieved. Chafing the inflated balloon against the artery wall may also contribute to increased injury size and severity12. Endothelial damage may lead to the exposure of the subendothelium and activation of the coagulation cascade. When the balloon is deflated and flow restored, swept emboli may reach the brain via the right vertebral artery (RVA). The possibility of such deleterious events increases when considering that the infusion of heparin as an antithrombotic agent was not a consistent practice during the procedures included in this cohort.
We evaluated total balloon injury as a factor in inducing ND in two different ways. First, we evaluated balloon injury by assuming that it was a cumulative sum of individual injuries inflicted each time the balloon was inflated regardless of the occlusion time (referred herein as number of occurrences). Second, we calculated the total occlusion time of the balloon regardless of the number of occurrences. The number of occurrences was not found to be a statistically significant contributing factor to ND, although it was nearly significant (p=0.06). The total occlusion time, however, showed a highly significant (p=0.0096) correlation to ND. Endothelial damage due to long balloon occlusion times and persistent manipulation can contribute to a failure in creating aneurysms, shorter periods of aneurysm patency and a high likelihood of ND. Concentration of elastase and its time of incubation in the RCCA appear to have no effect on the size of the aneurysm14, although high doses could be fatal15. Occlusion times no longer than 15 minutes divided into two consecutive incubation sessions, the first of which is 5 minutes in duration followed closely by a 10 minute incubation (after angiographic evaluation of the results of the first occlusion), have demonstrated excellent results11.
Balloon positioning alters aneurysm morphology. Balloons positioned and inflated with most of their volume in the brachiocephalic trunk give rise to broad-base aneurysms, whereas balloons inflated with most of their volume in the root of the RCCA tend to generate circumscribed aneurysm necks, and larger aneurysms are obtained by increasing the distance between the balloon and sheath16. Moreover, the precise location of the balloon within the vascular zone of interest (Figure 1) bears on subsequent ND presentation since the number of arteries supplying blood to the brain is temporarily decreased and the probability of thrombus formation is increased.
Balloon positioning was evaluated from the digitally subtracted angiograms of 30 cases for which documentation was present and categorized as follows:
Ppartial occlusion of the subclavian artery when at least one-third of the balloon was located in the RCCA or its origin (n=7);
total occlusion of the subclavian artery and thus total occlusion of the right vertebral artery (n=20);
partial occlusion of the LCCA when the balloon was located low in the brachiocephalic trunk, occluding the subclavian artery, with the distal part of the balloon reaching the origin of the LCCA (n=3).
The three cases of the third group above represent the worst case scenario because three arteries (RCCA, RVA and LCCA) that supply blood to the brain are occluded simultaneously. This situation is usually brought about by long balloons and anatomical variations in the rabbit vasculature. An LCCA that emerges from the distal portion of the brachiocephalic trunk rather than the aortic arch can be reached and covered by the balloon. In our population sample, one of the three cases in the third group above presented with ND and the final angiogram showed occlusion of the LCCA.
As expected, clot formation correlated significantly with ND (p=0.047).
Clots were seen attached to the arterial wall or obstructing collateral branches (Figure 2). This condition can be aggravated by insufficient antithrombotic agent on board. However, there was no statistically significant correlation between rabbits that received intravenous injection of heparin and clot formation when compared with rabbits that did not receive the extra dose of i.v. heparin. It should be noted that heparin is not a potent antithrombotic agent and its activity against platelet deposition and thrombus formation has been assessed as moderate17.
While we were able to identify and document the presence of clot formation, there were not enough reliable data to analyse cerebral arterial air embolism. Cerebral arterial air embolism is a known risk factor for neurological deficits and it may occur during this procedure even at the hands of an experienced interventionalist. Bendszus et al. demonstrated that after diagnostic and interventional cerebral angiography in humans, embolic events caused by blood clots or small air bubbles are more frequent than the neurological complication rate18. Cerebral arterial air emboli may also occur primarily at the time of injection of angiographic contrast, possibly by the formation of cavitation bubbles under pressure, as previously shown with data collected by transcranial Doppler19.
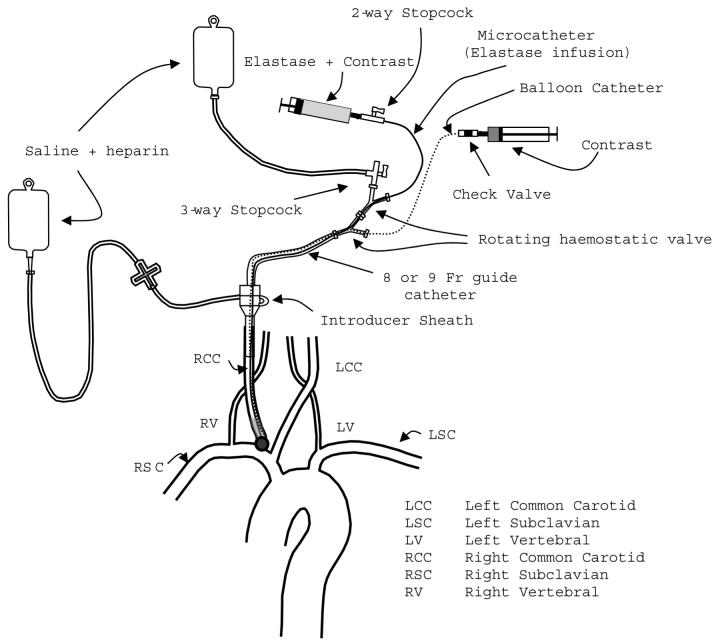
Nevertheless, since air embolism is a known risk factor, we have developed an experimental protocol to minimize such risk. Figure 4 shows schematically our recommended set-up for preventing the accidental introduction of air into the circulation and to facilitate smooth and secure maneuvering of the balloon and microcatheter. Using an 8 or 9 Fr guide catheter, the port of entry for the balloon and microcatheter into the introducer sheath is relatively protected against the accidental entry of air during the manipulation and replacement of these devices. In addition, the two rotating hemostatic valves, the stopcocks, and the check valve help to create a closed environment and reduce the risk of air infiltration. However, administration of fluids must be carefully controlled to prevent pulmonary edema.
Figure 4.
Schematic diagram of the modified endovascular arrangement
CONCLUSION
Factors related to neurological deficits in rabbits after elastase-induced aneurysm creation were analysed retrospectively. Rabbit weight (representing the animal's state of health), total procedure time, total balloon occlusion time and clot formation were analysed and found to be correlated to neurological deficits at a significant and/or highly significant level. Endothelial cell damage caused by balloon inflation, blood flow reduction due to arterial spasm and balloon obstruction, and an insufficient concentration level of an antithrombotic agent, may be the principal reasons for activation of the coagulation cascade and clot formation. The inadvertent introduction of air into the animal can be controlled and minimized by reducing access of air to the endovascular ports through a closed set-up like the one presented in Figure 4.
ACKNOWLEDGEMENTS
Most of the aneurysm induction procedures analysed were conducted for in vivo device tests sponsored by the following companies (alphabetical order): Boston Scientific/Target, Cordis Neurovascular, Micrus Inc. This work was also supported in part by the National Institutes of Health under grant no. R01 NS045753-01A1 to BBL.
REFERENCES
- 1.Kallmes DF, Helm GA, Hudson SB, et al. Histologic evaluation of platinum coil embolization in an aneurysm model in rabbits. Radiology. 1999;213:217–222. doi: 10.1148/radiology.213.1.r99oc16217. [DOI] [PubMed] [Google Scholar]
- 2.Cloft HJ, Altes TA, Marz WF, et al. Endovascular creation of an in vivo bifurcation aneurysm in rabbits. Radiology. 1999;213:223–228. doi: 10.1148/radiology.213.1.r99oc15223. [DOI] [PubMed] [Google Scholar]
- 3.Miskolczi L, Guterman LR, Flaherty JD, et al. Saccular aneurysm induction by elastase digestion of the arterial wall: A new animal model. Neurosurgery. 1998;43:595–600. doi: 10.1097/00006123-199809000-00110. [DOI] [PubMed] [Google Scholar]
- 4.Abruzzo T, Shengelaia GG, Dawson RC, et al. Histologic and morphologic comparison of experimental aneurysms with human intracranial aneurysms. AJNR Am J Neuroradiol. 1998;19:1309–1314. [PMC free article] [PubMed] [Google Scholar]
- 5.Altes TA, Cloft HJ, Short JG, et al. Creation of saccular aneurysms in the rabbit: A model suitable for testing endovascular devices. AJR Am J Roentgenol. 2000;174:349–354. doi: 10.2214/ajr.174.2.1740349. [DOI] [PubMed] [Google Scholar]
- 6.Massoud TF, Guglielmi G, Ji C, et al. Experimental saccular aneurysms. I. Review of surgically-constructed models and their laboratory applications. Neuroradiology. 1994;36:537–546. doi: 10.1007/BF00593517. [DOI] [PubMed] [Google Scholar]
- 7.Möller-Hartmann W, Krings T, Stein KP, et al. Aberrant origin of the superior thyroid artery and tracheoesophageal branch from the common carotid artery: A source of failure in elastase-induced aneurysms in rabbits. AJR Am J Roentgenol. 2003;181:739–741. doi: 10.2214/ajr.181.3.1810739. [DOI] [PubMed] [Google Scholar]
- 8.Thiex R, Hans FJ, Krings T, et al. Haemorrhagic tracheal necrosis as a lethal complication of an aneurysm model in rabbits via endoluminal incubation with elastase. Acta Neurochir (Wien) 2004;146:285–289. doi: 10.1007/s00701-003-0198-8. [DOI] [PubMed] [Google Scholar]
- 9.De Toffol B, Hommet C, Corcia P, et al. Indications for emergency EEG. Focal neurologic deficits and partial epileptic seizures in adults. Neurophysiol Clin. 1997;27:383–389. doi: 10.1016/s0987-7053(97)88804-1. [DOI] [PubMed] [Google Scholar]
- 10.Endo S, Branson PJ, Alksne JF. Experimental model of symptomatic vasospasm in rabbits. Stroke. 1988;19:1420–1425. doi: 10.1161/01.str.19.11.1420. [DOI] [PubMed] [Google Scholar]
- 11.Miskolczi L, Cesar L, Gounis M, et al. Elastase-induced saccular aneurysms in rabbits: Instructions “for the rest of us”; Presented at the 42nd Annual Scientific Meeting of the American Society of Neuroradiology; Seattle, WA, USA. 2004. [Google Scholar]
- 12.MacDonald JD, Gyorke A, Jacobs JM, et al. Acute phase vascular endothelial injury: A comparison of temporary arterial occlusion using an endovascular occlusive balloon catheter versus a temporary aneurysm clip in a pig model. Neurosurgery. 1994;34:876–881. doi: 10.1227/00006123-199405000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita A, Furukoji E, Marutsuka K, et al. Increased vascular wall thrombogenicity combined with reduced blood flow promotes occlusive thrombus formation in rabbit femoral artery. Arterioscler Thromb Vasc Biol. 2004;24:2420–2424. doi: 10.1161/01.ATV.0000147767.61336.de. [DOI] [PubMed] [Google Scholar]
- 14.Kallmes DF, Fujiwara NH, Berr SS, et al. Elastase-induced saccular aneurysms in rabbits: A dose-escalation study. AJNR Am J Neuroradiol. 2002;23:295–298. [PMC free article] [PubMed] [Google Scholar]
- 15.Krings T, Möller-Hartmann W, Hans FJ, et al. A refined method for creating saccular aneurysms in the rabbit. Neuroradiology. 2003;45:423–429. doi: 10.1007/s00234-003-0976-2. [DOI] [PubMed] [Google Scholar]
- 16.Thiex R, Moller-Hartmann W, Hans FJ, et al. Are the configuration and neck morphology of experimental aneurysms predictable? A technical approach. Neuroradiology. 2004;46:571–576. doi: 10.1007/s00234-004-1218-y. [DOI] [PubMed] [Google Scholar]
- 17.Zaman AG, Osende JI, Chesebro JH, et al. In vivo dynamic real-time monitoring and quantification of platelet-thrombus formation: Use of a local isotope detector. Arterioscler Thromb Vasc Biol. 2000;20:860–865. doi: 10.1161/01.atv.20.3.860. [DOI] [PubMed] [Google Scholar]
- 18.Bendszus M, Koltzenburg M, Burger R, et al. Silent embolism in diagnostic angiography and neurointerventional procedures. Lancet. 1999;354:1594–1597. doi: 10.1016/S0140-6736(99)07083-X. [DOI] [PubMed] [Google Scholar]
- 19.Markus H, Loh A, Israel D, et al. Microscopic air embolism during cerebral angiography and strategies for its avoidance. Lancet. 1993;341:784–787. doi: 10.1016/0140-6736(93)90561-t. [DOI] [PubMed] [Google Scholar]