Abstract
Emerging evidence suggests that acute psychological stress modulates inflammatory competence; however, not all findings are consistent. Gender is one factor that may impact magnitude of response. To explore this possibility, we examined the effects of acute mental stress on lipopolysaccharide-induced production of pro-inflammatory cytokines interleukin (IL)- 1 β, IL-6, and tumor necrosis factor (TNF)- α among a relatively healthy sample of midlife men (n=28) and women (n=34). Blood samples for the assessment of cytokine production were drawn before, immediately after and 30 minutes following subjects’ performance of an evaluative speech task. Relative to baseline evaluations, the speech stressor elicited a significant increase in stimulated production of all 3 pro-inflammatory cytokines, as measured 30 minutes following the end of the task. There were no gender differences in the magnitude of this effect. However, men showed a significant decrease in cytokine production from before to immediately following the stressor, whereas women showed no change across this period. Menopausal status partially accounted for these gender differences, with postmenopausal women displaying greater increases in IL-6 and TNF-α production from baseline-to-post task when compared to men. These data provide further evidence that acute psychological stress primes the immune system to mount larger inflammatory responses and initial support for gender differences in the patterning of stress-related cytokine activity. In addition, this study presents novel evidence that post-menopausal women may be particularly susceptible to stress-related inflammatory responses. The possibility that this contributes to the increased risk of inflammatory disease observed among older women warrants investigation.
Introduction
Consistent evidence shows that brief psychological stress modulates components of the immune system. Early studies focused on stress-induced changes in cellular immune parameters (for review, see Segerstrom and Miller, 2004); however, more recent interest has shifted to the effects of acute laboratory stress on immune processes involved in the inflammatory response. In this regard, evidence shows reliable, stress-related increases in circulating levels of inflammatory mediators, including interleukin (IL)-6, IL-1β, and C-reactive protein (Steptoe et al., 2007). These circulating signaling proteins are assumed to reflect systemic inflammation, with higher levels predicting increased risk for inflammatory disease (Libby and Ridker, 1999; Ridker et al., 2000a; Ridker et al., 2000b). However, the interpretation of circulating levels of these proteins is complicated by the multiple cell types that produce them, including adipocytes and endothelial cells (Mohamed-Ali et al., 1997; Papanicolaou et al., 1998), raising the possibility that they may not reflect a primary inflammatory response. In order to more directly assess inflammatory competence, recent studies have begun to examine the effects of acute stress on the functional ability of immune cells to produce pro-inflammatory mediators when stimulated in vitro.
The inflammatory response begins when monocytes/macrophages are activated by pathogens or tissue damage to release pro-inflammatory cytokines (e.g. IL-6, IL-1β, TNF-α), which, in turn, recruit leukocytes to the area of injury, up-regulate the expression of cellular adhesion molecules on the endothelium, and promote the systemic release of acute phase proteins (e.g. C-reactive protein) (Maier and Watkins, 1998). The magnitude of the pro-inflammatory cytokine response to immune activation is critical; insufficient response may leave the organism vulnerable to infection, whereas excessive response can increase risk for inflammatory diseases (Nathan, 2002; Pavlov and Tracey, 2004). Convergent evidence from the animal and in vitro literatures shows that the autonomic nervous system plays a key role in regulating the magnitude of this response. Signaling by the sympathetic nervous system (SNS) can up- and down-regulate production of pro-inflammatory cytokines by activated monocytes/macrophages and thus modulate inflammatory potential (Elenkov et al., 2000; Hasko and Szabo, 1998; van der Poll et al., 1994; van der Poll and Lowry, 1997). The parasympathetic nervous system also plays a role in the down-regulation of the inflammatory response (Pavlov and Tracey, 2005; Tracey, 2002). Although there are wide and stable individual differences in the magnitude of autonomic responses to acute laboratory stress, in general, the ratio of sympathetic to parasympathetic activation increases, which may prime immune cells to mount larger pro-inflammatory responses to inflammatory stimuli.
To date, findings from experimental studies that examine whether acute stress alters stimulated cytokine production have been inconsistent. In a recent review of the literature, Steptoe and colleagues (2007) identified 8 studies that examined endotoxin-stimulated inflammatory responses to acute laboratory stress in healthy adults (Steptoe et al., 2007). Of the studies that measured stress-induced changes in IL-6 production, 4 showed an increase (Gaab et al., 2005; Goebel et al., 2000; Peters et al., 1999; Rohleder et al., 2003), 2 showed a decrease (Jacobs et al., 2001; Rohleder et al., 2001), and 2 showed no effect (Miller et al., 2005; Suarez et al., 2006). Similarly variable results were observed on examination of stimulated production of TNF-α. As noted by Steptoe et al (2007), reasons for these inconsistencies are unclear due to small samples sizes, variation in sample characteristics, timing of measures, and the types of challenges employed. One characteristic of participants that may contribute to differences across studies is gender. A fairly robust literature exists on gender differences in cardiovascular responses to acute psychological stress, with men exhibiting greater increases in indices of sympathetic activation than women (Allen et al., 1993; Matthews and Stoney, 1988). To the extent that autonomic activation modulates inflammatory competence, one might expect gender differences in stimulated cytokine production following an acute stressor. To date, only one study has directly examined this possibility, comparing endotoxin-stimulated production of IL-6 and TNF-α in response to the Trier Social Stress Test among 18 women and 27 men (Rohleder et al., 2001). Results show a pre- to 60 minute post-task decrease in stimulated production of IL-6 among males, but not females. These findings are inconsistent with predictions based on the autonomic regulation of inflammatory competence; however, it is possible that measures taken closer to the end of the challenge would reveal a different pattern of findings. To explore this possibility, the present study examines the capacity of immune cells to produce IL-6, IL-1β, and TNF-α following in vitro stimulation with the bacterial product LPS, as measured before, immediately after, and 30 minutes following a speech task among males and females. Based on evidence that males show larger autonomic responses to acute psychological stress than females, it was hypothesized that men would show greater stress-induced increases in the production of these pro-inflammatory cytokines than women. In secondary analyses, the possibility that menopausal status moderates the magnitude of inflammatory responses in women was also explored. To date, no studies have examined this possibility despite evidence that (1) immune cells have receptors for estrogen and progesterone (Turgeon et al., 2006), (2) in vitro treatment of immune cells with estrogen and progesterone modulates pro-inflammatory cytokine production (Rogers & Eastell, 2001; Turgeon et al., 2006; Zhang et al., 2001), and (3) risk for inflammatory disease increases following menopause when levels of these hormones are reduced (Moxley et al., 2002; Turgeon et al., 2006).
Methods
Participants
Study participants were 28 men and 34 women (92% Caucasian) between the ages of 40 and 59 enrolled in the Vaccination Immunity Project (VIP), a longitudinal study investigating psychological, behavioral, and physiological predictors of antibody response to hepatitis B vaccination. Participants were recruited via mass mail solicitation in Western Pennsylvania (principally Allegheny County). Eligible participants were non-smokers, reported being in good general health (including no history or symptoms of myocardial infarction, asthma, cancer treatment in the past year, psychotic illness, or other systemic diseases known to affect the immune system), and free from medications known to affect the nervous, endocrine, or immune systems in the past 3 months (not including oral contraceptives). Women who were pregnant or lactating were also ineligible. No women in the sub-study endorsed taking hormone replacement therapy. Appointments were scheduled so that all participants were free of signs and symptoms of infection for 2 weeks prior to the acute laboratory session. Participants included in this sub-study were a random subset of participants in the parent project on whom we drew blood samples for the measurement of endotoxin-stimulated pro-inflammatory cytokine production. Informed consent was acquired in compliance with guidelines of the University of Pittsburgh Institutional Review Board.
Laboratory Stress Testing
Data used in the present analyses were collected at a single laboratory session that lasted approximately 2 hours and started between 7:00 AM and 9:00 AM. Prior to the session, subjects abstained from food, beverages (except water), and caffeine for 12 hours, strenuous physical activity and non-prescription medications for 24 hours, and alcohol consumption for 48 hours. On arrival, subjects completed a battery of paper and pencil questionnaires, including assessment of current symptoms of acute illness, medical history, and menopausal status. After completion of questionnaires, subjects’ height and weight was recorded for determination of body mass index (BMI; kg/m2) and they were escorted to a testing chamber. Here, an intravenous catheter was inserted into the antecubital fossa of the non-dominant arm for the collection of blood samples, and an occluding cuff was placed on the other arm for automated measurement of heart rate (HR) and systolic blood pressure (SBP) and diastolic BP (DBP) (Critikon Dinamap 8100 Vital Signs Monitor, Tampa, FL). Following instrumentation, subjects rested for a 30-minute adaptation period. During the last 6 minutes of this period, BP and HR were recorded 4 times (every 90 seconds) and a 20ml blood sample was drawn. Subjects were then asked to perform a simulated public speaking task, consisting of 2 minutes of preparation for a speech defending themselves against an alleged transgression, followed by 3 minutes of videotaped speech delivery. HR and BP were measured every 90 seconds during speech preparation and delivery, and a second 20ml blood sample was collected immediately after task performance. Following the task, subjects rested for a 30-minute recovery period. HR and BP were recorded 4 times (every 90 seconds) during the last 6 minutes of this period and a final 20ml blood sample was drawn at the end of this period.
Stimulated Cytokine Production
Whole blood was collected in heparin-treated vacutainer tubes and stimulated with lipopolysaccharide (LPS, serotype 026:B6, Sigma) at a final concentration of 2.5 ug/ml without antibiotics in polypropylene tubes under sterile conditions (stimulated sample). Control samples, containing whole blood without LPS, were set up in parallel to measure spontaneous levels of cytokine production (unstimulated sample). The samples were incubated at 37°C with 5% CO2 humidified atmosphere for 24 hours. Following incubation, the tubes were centrifuged at 1000g for 10 minutes. Supernatants were collected and frozen at −80°C until the study was complete.
At the end of the study, LPS-stimulated and unstimulated supernatants were assayed in one batch using a multiplex analysis system, as previously described (Marsland et al., 2007b). Briefly, multiplex bead kits, based on the principle of solid phase sandwich immunoassays, were employed. All reagents, working standards, and samples were prepared as per the manufacturer’s specifications and were run in duplicates. Samples were read within 24 hours using Bio-Plex reader (Luminex 100™). Stimulated levels of IL-6, IL-1β, and TNF-α were determined using the Bio-Plex Manager Software (Bio-rad Corporation, Hercules, CA), interpolating from the standard curve (Logistic-5PL curve fit). Pooled plasma controls were included on all plates. Inter- and intra- assay coefficients of variability were less than or equal to 10%. Stimulated cytokine production was quantified by subtracting unstimulated from stimulated cytokine levels.
Menopausal Status
Menopausal status was defined on the basis of reported menstrual history over the prior 12 months, with premenopausal being regular, normal cycles; perimenopausal being irregular cycles and other related symptoms; and postmenopausal being no cycles.
Statistical Analyses
All analyses were performed using SPSS for windows (version 14.0). Stimulated pro-inflammatory cytokines were natural log transformed prior to analyses to better approximate normal distributions. Comparisons of demographic characteristics and baseline cytokine concentrations between male and female participants were carried out using independent t tests or χ2 statistics. To evaluate the effects of the speech task on cardiovascular and immune parameters, repeated-measures analyses of covariance (ANCOVAs) were conducted on each dependent variable. For these analyses, HR and BP data were reduced by calculating mean values for baseline, task, and recovery periods. These values, and the cytokine production data, were then subjected to 2 (Gender women, men) × 3 (Period baseline, task, recovery) repeated-measures ANCOVAs. In light of significant correlations between baseline cytokine production and magnitude of stress-related changes in cytokine production (r’s= −.30–−.49, p<.05), baseline cytokine production was included as a covariate in immune analyses. Greenhouse-Geisser corrections for repeated measures were calculated where appropriate. Significant effects were followed by Bonferroni post-hoc comparisons. Sex differences were further explored by stratifying women by menopausal status (premenopausal and postmenopausal). All α’s for statistical analyses were set to .05 and two-tailed tests of significance were used.
Results
Preliminary Analyses
Mean values of IL-6, IL-1β, and TNF-α with and without stimulation at each study time point (baseline, task, recovery) are presented in Table 1. As expected, whole blood stimulation with LPS induced a marked increase in the levels of IL-1β, IL-6, and TNF-α and baseline stimulated levels of the 3 pro-inflammatory cytokines were significantly correlated with one another (r’s= .67–.79). Demographic characteristics of the study sample are displayed in Table 2. The only significant gender difference was in resting BP, with men showing higher DBP than women [t (1,60) = −2.46, p<.02]. There were no significant associations between baseline levels of cytokine production and any of the demographic characteristics.
Table 1.
Mean (standard deviation) stimulated and unstimulated cytokine levels across the whole sample (n=62).
| Baseline | Task | Recovery | ||||
|---|---|---|---|---|---|---|
| Stimulated | Unstimulated | Stimulated | Unstimulated | Stimulated | Unstimulated | |
| IL-6 (pg/ml) | 65,616 (37,054) | 1,008 (1,957) | 67,937 (43,447) | 1,278 (2,363) | 85,843 (55,077) | 1,582 (3,743) |
| IL-1β (pg/ml) | 6,072 (3,154) | 209 (267) | 6,398 (3,527) | 197 (250) | 8,230 (3,810) | 308 (393) |
| TNF-α (pg/ml) | 7,651 (8,881) | 77 (267) | 7,651 (8,950) | 69 (192) | 12,838 (13,907) | 449 (1,788) |
Table 2.
Mean (standard deviation) baseline characteristics among men and women (further stratified by menopausal status)
| Men (n=28) | Females (n=34) | Premenopausal (n=10) | Postmenopausal (n=15) | |
|---|---|---|---|---|
| Age (years) | 49.5 (4.7)± | 51.2 (5.6) | 44.8 (2.6) ± | 55.9 (2.8) |
| Race (% Caucasian) | 93% Caucasian, 7% Other | 91% Caucasian, 9% Other | 70% Caucasian, 30% other± | 100% Caucasian |
| BMI (kg/m2) | 27.3 (3.5) | 26.0 (4.0) | 25.1 (4.3) | 27.7 (3.8) |
| Heart rate (beats/min) | 58.7 (7.4) | 61.2 (6.6) | 63.6 (8.0) | 59.6 (4.1) |
| Systolic blood pressure (mmHg) | 115.5 (14.1) | 111.4 (12.2) | 114.7 (12.2) | 111.8 (13.6) |
| Diastolic blood pressure (mmHg) | 75.2 (7.8)*± | 69.8 (9.3) | 74.4 (10.7) | 68.0 (6.2) |
| IL-6 production (pg/ml)a | 62,518 (24,010) | 66,509 (45,602) | 48,923 (22,779) | 79,650 (61,802) |
| IL-1β production (pg/ml)a | 6,718 (3,027) | 5,367 (3,180) | 4,965 (2,761) | 5,485 (3,890) |
| TNF-α production (pg/ml)a | 7,682 (6,780) | 7,563 (10,367) | 5,122 (6,137) | 10,711 (14,282) |
corrected for unstimulated values;
p<.05, when compared to women;
p<.05, when compared to postmenopausal women
Effect of the speech task on HR, BP, and stimulated production of pro-inflammatory cytokines
Table 3 displays means and standard error of the means (SEM) for the cardiovascular and inflammatory measures as measured during baseline, task and recovery periods. Task-related changes in HR and BP were evaluated first, as an index of the physiologic effect of the experimental stressor. As expected, these analyses revealed a significant Period main effect of HR (F(1.7, 106.1)=110.85, p<.001), SBP (F(1.4, 86.2)=133.11, p<.001), and DBP (F(1.6, 97.1)= 133.66, p<.001). In each instance, task values were significantly higher than baseline measures, with values returning to baseline levels by 30 minutes post-task. We also observed a significant task-related increase in the numbers of white blood cells (WBCs) in peripheral circulation (F(2, 122)=16.13, p<.001), with task values being significantly higher than those measured at baseline and remaining elevated throughout the recovery period. Because concomitant increases in circulating WBCs could account for within and between subject differences in stimulated cytokine production, cytokine levels were calculated on a per 1 × 106 WBC basis to control for cell number. These adjusted cytokine production levels were used in all subsequent analyses. With respect to inflammatory measures, the ANCOVAs revealed a significant Period main effect on analysis of stimulated production of IL-6 (F(2, 116)=11.45, p<.001), TNF-α (F(1.8, 108.2)=23.76, p<.001), and IL-1β(F(2, 120)=27.00, p<.001). Here, post hoc comparisons among means showed no significant change from baseline to task measures, but a significant increase in stimulated concentrations of all 3 pro-inflammatory cytokines from baseline to 30 minutes post-task. There were no stress-related changes in the levels of unstimulated cytokine production (data not shown). The magnitudes of cardiovascular and inflammatory responses to the stressor were unrelated.
Table 3.
Mean (SEM) levels of stimulated cytokines and cardiovascular parameters during baseline, task, and recovery
| Whole sample (n=62) | Men (n=28) | Women (n=34) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Task | Recovery | Baseline | Task | Recovery | Baseline | Task | Recovery | |
| Heart Rate (beats/min) | 60 (.90) | 68 (1.2) | 60 (.99) | 59 (1.3) | 65 (1.7) | 58 (1.4) | 61 (1.2) | 70 (1.5) | 61 (1.3) |
| Systolic BP (mmHg) | 113 (1.7) | 132 (2.2) | 116 (1.8) | 115 (2.5) | 135 (3.2) | 119 (2.6) | 111 (2.2) | 130 (2.9) | 113 (2.4) |
| Diastolic BP (mmHg) | 72 (1.1) | 82 (1.3) | 73 (1.0) | 75 (1.6) | 85 (1.9) | 76 (1.5) | 70 (1.5) | 79 (1.7) | 70 (1.4) |
| IL-6 production (pg/ml)a | 64,706 (4,725) | 66,804 (5,512) | 84,435 (7,026) | 62,518 (4,537) | 60,854 (6,600) | 76,555 (5,951) | 66,509 (7,821) | 71,703 (8,453) | 90,461 (11,519) |
| IL-1β production (pg/ml)a | 5,977 (401) | 6,303 (448) | 8,071 (480) | 6,718 (572) | 6,514 (639) | 8,892 (677) | 5,367 (545) | 6,129 (631) | 7,395 (660) |
| TNF-α production (pg/ml)a | 7,617 (1,125) | 7,622 (1,113) | 12,671 (1,612) | 7,682 (1,281) | 7,189 (1,319) | 14,864 (2,224) | 7,563 (1,778) | 7,978 (1,774) | 10,864 (2,281) |
corrected for ustimulated levels
Gender differences in cardiovascular and stimulated cytokine responses to acute stress
Next, we investigated whether gender moderated the magnitude of cardiovascular and inflammatory responses to acute stress (see Table 3). These analyses revealed a significant Gender × Period interaction on analysis of stimulated production of IL-6 (F(2, 114) = 3.35, p=.04) and TNF-α (F(2, 118) = 6.25, p=.004), and to a lesser degree IL-1β (F(2, 118) = 2.73, p=.07). As displayed in Figure 1, women showed little change in stimulated IL-6 production from baseline to task, but a significant increase in IL-6 production 30 minutes following the task. In contrast, men showed baseline-to-task decreases in IL-6 production followed by a significant increase in production, as measured during recovery. A similar gender pattern was also seen on examination of stimulated TNF-α production. Post-hoc analyses revealed that sex differences in cytokine production during the task period accounted for the Gender × Period interaction of IL-6 and TNF-α (p’s<.05). Contrary to expectations, there were no significant Gender × Period interactions on analysis of cardiovascular responses to the speech task.
Figure 1.
Natural log transformed IL-6 production at baseline, task, and recovery among males (n=28) and females (n=34). Vertical lines indicate standard errors of the means. All values are adjusted for baseline levels.
Role of menopausal status
To examine the possibility that menopause influences the magnitude of inflammatory responses, female participants were stratified by menopausal status. Of the 34 women in the sample, 9 indicated that they were perimenopausal. Given difficulties in the accurate delineation of perimenopause (Burger et al., 2007), we excluded these 9 women from the analyses and focused on a comparison of pre-and post-menopausal women with men. Of note, preliminary analyses showed that pre- and postmenopausal women did not differ from perimenopausal women on demographic characteristics or baseline stimulated production of cytokines (p’s>.05).
Table 2 displays demographic and baseline inflammatory measures for men (n=28), premenopausal women (n= 10), and postmenopausal women (n= 15). As expected, the postmenopausal group was significantly older than the premenopausal women and the men. Premenopausal women were also more racially diverse than postmenopausal women, with 30% of premenopausal group being non-Caucasian compared to the postmenopausal group which was comprised of solely Caucasian women. Men continued to show higher resting DBP than pre- and postmenopausal women; otherwise, there were no significant group differences.
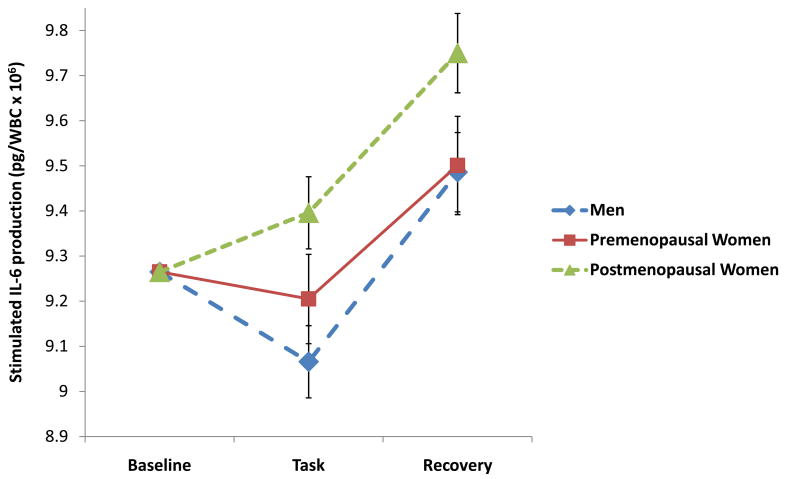
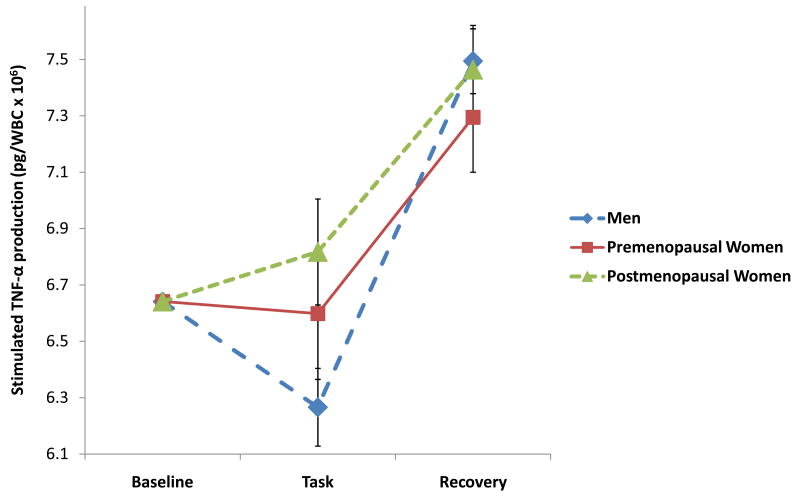
Stimulated production of pro-inflammatory cytokine data were subjected to 3 (Group premenopausal women, postmenopausal women, and men) × 3 (Period baseline, task, recovery) repeated measures ANCOVAs to evaluate whether the groups differed in responses to the experimental stressor. These analyses revealed significant Group × Period interactions on analysis of IL-6 (F(4, 94) = 2.75, p=.03) and TNF-α production (F(4, 98) = 2.73, p=.04), but not IL-1β (F(4, 98) = .62, p=.65). As shown in Figure 2A, the pattern of stimulated production of IL-6 was similar across the 3 groups; however, post hoc comparisons reveal that postmenopausal women showed significantly higher levels of stimulated IL-6 production when compared to men during both the task and recovery periods (p’s<.02). Similarly, post hoc comparisons (see Figure 2B) indicated that postmenopausal women showed greater levels of TNF-α production than men, but only during the task period (p<.05). Taken together, these findings suggest that observed gender differences in stress-induced cytokine production are largely attributable to differences between men and postmenopausal women. To explore whether age contributed to the observed differences in cytokine reactivity between men, pre-menopausal, and post-menopausal women, we conducted additional correlational analyses. We found no significant correlations between age and stress-related changes in cytokine production for any of the cytokines (r’s= .05–.08), suggesting that age does not account for the differences observed in this sample.
Figure 2.
Figure 2A. Natural log transformed IL-6 production at baseline, task, and recovery among men (n=28) and pre- (n=10) and postmenopausal women (n=15). All values are adjusted for baseline levels.
Figure 2B. Natural log transformed TNF-α production at baseline, task, and recovery among men (n=28) and pre- (n=10) and postmenopausal women (n=15). All values are adjusted for baseline levels.
Discussion
The present study provides further evidence that brief laboratory stress primes peripheral immune cells to produce higher levels of pro-inflammatory cytokines in response to endotoxin, here among a community sample of relatively healthy midlife men and women. Consistent with a growing literature suggesting that acute stress is associated with activation of innate inflammatory pathways (Steptoe et al., 2007), we found that a public speaking task resulted in significant increases in cellular production of IL-6, TNF-α, and to a lesser extent, IL-1β, as measured 30 minutes after task completion. Although there were no gender differences in the magnitude of the increase in cytokine production from baseline to 30 minutes post-task, we did observe differences in stimulated production of IL-6, TNF-α, and IL-1β from baseline to immediately post-task, with men showing a significant decrease and women no change. Thus, there were notable gender differences in the pattern of cytokine production responses to acute stress, with women showing no change from baseline to post-task, but a significant increase during the 30 minute recovery period and men showing a more biphasic response, with a significant decrease from baseline to post-task and an increase during recovery. Interestingly, this pattern of gender differences is inconsistent with the only other study that we know of that has examined gender differences in cytokine production following acute stress. In this study, Rohleder (2001) showed a significant decrease in stimulated production of IL-6 from before to 60 minutes after the Trier Social Stress Test for males, but no change for females. One possible explanation for the inconsistent findings across these studies is differences in the timing of measures, with stress-related increases in cytokine production having returned to baseline levels by 60 minutes post-task. In this regard, evidence suggests that many immune responses to acute stress return to baseline levels precipitously following the end of the stressor (Segerstrom and Miller, 2004). Thus, further research is warranted to better delineate the role gender plays in modulating inflammatory response trajectories following acute challenge.
The speech stressor used in the current study was associated with significant increases in HR and BP, yet we did not observe gender differences in the magnitude of these effects. This raises questions regarding the mechanism of gender differences in cytokine production in response to the task. Convincing animal and in vitro evidence suggests that the autonomic nervous system can up- and down-regulate production of pro-inflammatory cytokines (Nance and Sanders, 2007). For example, experimental evidence shows that stress-induced increases in catecholamines upregulate the NF-kB signaling pathways responsible for pro-inflammatory cytokine production (Bierhaus et al., 2003). Conversely, LPS-stimulated cells treated with catecholamines or β-agonists show decreased pro-inflammatory cytokine production (Izeboud et al., 1999; van der Poll et al., 1994). Recent evidence also suggests that the parasympathetic division of the ANS can regulate inflammatory competence in real time via the vagus nerve (Pavlov and Tracey, 2005). Indeed, cross-sectional evidence shows that heart rate variability, an index of sympathovagal balance, covaries inversely with stimulated pro-inflammatory cytokine production (Marsland et al., 2007a; Sloan et al., 2007), and that this relationship appears to be moderated by gender (O’Connor et al., 2007). This raises the possibility that gender differences in sympathovagal activation in response to acute stress may contribute to differences in the inflammatory response.
Our findings suggest that menopausal status partially accounts for gender differences in stress-induced cytokine production, with post-menopausal women showing greater increases in cytokine production from baseline to immediately following the stressor than pre-menopausal women or men. Though preliminary, given small sample sizes, this is the first study to document menopausal differences in stress-induced cytokine reactivity. Hormonal differences between pre and postmenopausal women may help explain these findings. Plasma concentrations of estradiol are lower in postmenopausal women (< 15 pg/ml) than premenopausal women (50–250 pg/ml) or men (50 pg/ml) (Walsh and Shiff, 2001; Winters, 2001). Estradiol acts to inhibit pro-inflammatory cytokine gene expression, NF-kB binding, and production of pro-inflammatory cytokines (Deshpande et al., 1997; Liu et al., 2005; Ray et al., 1997). Thus, it is possible that the increased inflammatory reactivity we observed among post-menopausal women is related to lower circulating levels of reproductive hormones. Further research on the effects of stress on cytokine production in immune cells treated in vitro with reproductive hormones is warranted. Moreover, future studies are needed to explore whether this increased inflammatory propensity contributes to the heightened susceptibility to inflammatory disease that is observed among older women.
The present findings should be interpreted in context of a number of study limitations. First, this study did not include a control condition that would allow us to rule out alternative explanations for stress-related changes in cytokine production, such as prolonged IV catheterization and repeated blood draws. Levels of circulating pro-inflammatory mediators are largely unaffected by these factors (Steptoe et al., 2001; von Kanel et al., 2006); however, it remains unclear whether this is the case for the cellular production of cytokines (Steptoe et al., 2007). Second, information regarding menstrual phase was not collected in our sample. Given that reproductive hormones, including progesterone and estrogen, regulate inflammatory activity, future work should consider menstrual phase in interpreting gender and menopausal status effects. In this regard, Rohleder and colleagues (2003) reported a modest increase in IL-6 production following a laboratory stressor among women taking oral contraceptives whereas levels among women in their luteal phase decreased. In the current study, only one premenopausal woman reported taking oral contraceptives; however, the reported findings remained unchanged when this person was dropped from the analyses. Finally, it should be noted that demonstrating gender differences in the magnitude of responses to a speech task offers only limited evidence for gender differences in stress-induced cytokine reactivity. To conclude that there are generalized gender differences will require that the current findings be replicated and reproduced with diverse behavioral stressors.
The current study employed a whole blood assay designed to permit the quantification of cytokines produced by the full array of immune cells acting in concert within their normal milieu and is thought to simulate the in vivo environment (De Groote et al., 1992). In contrast, others have measured cytokine production by activated monocytes or mononuclear cells (e.g., Suarez et al., 2003, 2004), which provides greater specificity, but a less accurate picture of the total immune response. Evidence suggests that these differing methods can influence results. For example, in samples taken from the same healthy individuals, the stimulation of isolated mononuclear cells resulted in higher levels of IL-1 than the stimulation of whole blood cultures that contained identical concentrations of mononuclear cells, suggesting a downregulation of the cytokine response by elements in whole blood (DeGroote et al., 1992). Given the goals of the current study, we selected to use a whole blood assay that offers a closer approximation of in vivo immune processes. Future work may benefit from the isolation of cells to permit an examination of the specific immune cells contributing to the observed inflammatory response (e.g. intracellular cytokine staining for flow cytometry).
It is well documented that women are more susceptible than men to a number of inflammatory conditions (e.g., autoimmune diseases) (Gleicher and Barad, 2007; Lockshin, 2001). However, the mechanisms of these differences are poorly elucidated. Although gender differences in cytokine production may contribute to this differential risk, it is important to note that the clinical significance of individual differences in pro-inflammatory cytokine production remain unclear. That said, initial evidence shows a positive association of stimulated cytokine production with health risk. For example, higher levels of LPS-stimulated inflammatory activity have been observed in patients with rheumatoid arthritis relative to healthy controls (Scuderi et al., 2003) and among post myocardial infarction patients who go on to experience heart failure when compared with those who do not (Satoh et al., 2006). Thus, further prospective investigation of gender differences in stress induced cytokine reactivity is justified to determine whether individual response patterns contribute to susceptibility to inflammatory disease.
Acknowledgments
This study was supported by a grant from the National Institute of Nursing Research (NR008237; ALM) and a National Institute of Health fellowship (HL007560; AAP). The expert technical assistance of Adele Marrangoni at the University of Pittsburgh Cancer Institute Luminex Core Facility is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen MT, Stoney CM, Owens JF, Matthews KA. Hemodynamic adjustments to laboratory stress: the influence of gender and personality. Psychosom Med. 1993;55:505–517. doi: 10.1097/00006842-199311000-00006. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger H, Woods NF, Dennerstein L, Alexander JL, Kotz K, Richardson G. Nomenclature and endocrinology of menopause and perimenopause. Expert Rev Neurother. 2007;7:S35–43. doi: 10.1586/14737175.7.11s.S35. [DOI] [PubMed] [Google Scholar]
- De Groote D, Zangerle PF, Gevaert Y, Fassotte MF, Beguin Y, Noizat-Pirenne F, Pirenne J, Gathy R, Lopez M, Dehart I, Igot D, Baudrihaye M, Delacroix D, Franchimont P. Direct stimulation of cytokines (IL-1β, TNF-α, IL-6, IL-2, IFN-γ and GM-CSF) in whole blood. Cytokine. 1992;4:239–248. doi: 10.1016/1043-4666(92)90062-v. [DOI] [PubMed] [Google Scholar]
- Deshpande R, Khalili H, Pergolizzi RG, Michael SD, Chang MD. Estradiol down-regulates LPS-induced cytokine production and NFkB activation in murine macrophages. Am J Reprod Immunol. 1997;38:46–54. doi: 10.1111/j.1600-0897.1997.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- Gaab J, Rohleder N, Heitz V, Engert V, Schad T, Schurmeyer TH, Ehlert U. Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrinology. 2005;30:188–198. doi: 10.1016/j.psyneuen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Gleicher N, Barad DH. Gender as risk factor for autoimmune diseases. J Autoimmun. 2007;28:1–6. doi: 10.1016/j.jaut.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Goebel MU, Mills PJ, Irwin MR, Ziegler MG. Interleukin-6 and tumor necrosis factor-alpha production after acute psychological stress, exercise, and infused isoproterenol: differential effects and pathways. Psychosom Med. 2000;62:591–598. doi: 10.1097/00006842-200007000-00019. [DOI] [PubMed] [Google Scholar]
- Hasko G, Szabo C. Regulation of cytokine and chemokine production by transmitters and co-transmitters of the autonomic nervous system. Biochem Pharmacol. 1998;56:1079–1087. doi: 10.1016/s0006-2952(98)00153-1. [DOI] [PubMed] [Google Scholar]
- Izeboud CA, Monshouwer M, van Miert AS, Witkamp RF. The beta-adrenoceptor agonist clenbuterol is a potent inhibitor of the LPS-induced production of TNF-alpha and IL-6 in vitro and in vivo. Inflamm Res. 1999;48:497–502. doi: 10.1007/s000110050493. [DOI] [PubMed] [Google Scholar]
- Jacobs R, Pawlak CR, Mikeska E, Meyer-Olson D, Martin M, Heijnen CJ, Schedlowski M, Schmidt RE. Systemic lupus erythematosus and rheumatoid arthritis patients differ from healthy controls in their cytokine pattern after stress exposure. Rheumatology (Oxford) 2001;40:868–875. doi: 10.1093/rheumatology/40.8.868. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM. Novel inflammatory markers of coronary risk: theory versus practice. Circulation. 1999;100:1148–1150. doi: 10.1161/01.cir.100.11.1148. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu K, Bodenner DL. Estrogen receptor inhibits interleukin-6 gene expression by disruption of nuclear factor kappaB transactivation. Cytokine. 2005;31:251–257. doi: 10.1016/j.cyto.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Lockshin MD. Invited review: sex ratio and rheumatic disease. J Appl Physiol. 2001;91:2366–2373. doi: 10.1152/jappl.2001.91.5.2366. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Prather AA, Jennings JR, Neumann SA, Manuck SB. Stimulated production of pro-inflammatory cytokines covaries inversely with heart rate variability. Psychosom Med. 2007a;69:709–716. doi: 10.1097/PSY.0b013e3181576118. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Sathanoori R, Muldoon MF, Manuck SB. Stimulated production of interleukin-8 covaries with psychosocial risk factors for inflammatory disease among middle-aged community volunteers. Brain Behav Immun. 2007b;21:218–228. doi: 10.1016/j.bbi.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Stoney CM. Influences of sex and age on cardiovascular responses during stress. Psychosom Med. 1988;50:46–56. doi: 10.1097/00006842-198801000-00006. [DOI] [PubMed] [Google Scholar]
- Miller GE, Rohleder N, Stetler C, Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosom Med. 2005;67:679–687. doi: 10.1097/01.psy.0000174172.82428.ce. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- Moxley G, Posthuma D, Carlson P, Estrada E, Han J, Benson LL, Neale MC. Sexual dimorphism in innate immunity. Arthritis Rheum. 2002;46:250–258. doi: 10.1002/1529-0131(200201)46:1<250::AID-ART10064>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Motivala SJ, Valladares EM, Olmstead R, Irwin MR. Sex differences in monocyte expression of IL-6: role of autonomic mechanisms. Am J Physiol Regul Integr Comp Physiol. 2007;293:R145–151. doi: 10.1152/ajpregu.00752.2006. [DOI] [PubMed] [Google Scholar]
- Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. Neural regulators of innate immune responses and inflammation. Cell Mol Life Sci. 2004;61:2322–2331. doi: 10.1007/s00018-004-4102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005;19:493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Peters ML, Godaert GL, Ballieux RE, Brosschot JF, Sweep FC, Swinkels LM, van Vliet M, Heijnen CJ. Immune responses to experimental stress: effects of mental effort and uncontrollability. Psychosom Med. 1999;61:513–524. doi: 10.1097/00006842-199907000-00016. [DOI] [PubMed] [Google Scholar]
- Ray P, Ghosh SK, Zhang DH, Ray A. Repression of interleukin-6 gene expression by 17 beta-estradiol: inhibition of the DNA-binding activity of the transcription factors NF-IL6 and NF-kappa B by the estrogen receptor. FEBS Lett. 1997;409:79–85. doi: 10.1016/s0014-5793(97)00487-0. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000a;101:2149–2153. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000b;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- Rogers A, Eastell R. The effects of 17beta-estradiol on production of cytokines in cultures of peripheral blood. Bone. 2001;29:30–34. doi: 10.1016/s8756-3282(01)00468-9. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Schommer NC, Hellhammer DH, Engel R, Kirschbaum C. Sex differences in glucocorticoid sensitivity of pro-inflammatory cytokine production after psychosocial stress. Psychosom Med. 2001;63:966–972. doi: 10.1097/00006842-200111000-00016. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Wolf JM, Piel M, Kirschbaum C. Impact of oral contraceptive use on glucocorticoid sensitivity of pro-inflammatory cytokine production after psychosocial stress. Psychoneuroendocrinology. 2003;28:261–273. doi: 10.1016/s0306-4530(02)00019-7. [DOI] [PubMed] [Google Scholar]
- Satoh M, Shimoda Y, Maesawa C, Akatsu T, Ishikawa Y, Minami Y, Hiramori K, Nakamura M. Activated toll-like receptor 4 in monocytes is associated with heart failure after acute myocardial infarction. Int J Cardiol. 2006;109:226–234. doi: 10.1016/j.ijcard.2005.06.023. [DOI] [PubMed] [Google Scholar]
- Saurez EC, Krishnan RR, Lewis JG. The relation of the severity of depressive symptoms to monocyte-associated proinflammatory cytokines and chemokines in apparently healthy men. Psychsom Med. 2003;65:362–368. doi: 10.1097/01.psy.0000035719.79068.2b. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Lewis JG, Krishnan RR, Young KH. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology. 2004;29:1119–1128. doi: 10.1016/j.psyneuen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Scuderi F, Convertino R, Molino N, Provenzano C, Marino M, Zoli A, Bartoccioni E. Effect of pro-inflammatory/anti-inflammatory agents on cytokine secretion by peripheral blood mononuclear cells in rheumatoid arthritis and systemic lupus erythematosus. Autoimmunity. 2003;36:71–77. doi: 10.1080/0891693031000079275. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan RP, McCreath H, Tracey KJ, Sidney S, Liu K, Seeman T. RR interval variability is inversely related to inflammatory markers: the CARDIA study. Mol Med. 2007;13:178–184. doi: 10.2119/2006-00112.Sloan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Willemsen G, Owen N, Flower L, Mohamed-Ali V. Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clin Sci (Lond) 2001;101:185–192. [PubMed] [Google Scholar]
- Suarez EC, Boyle SH, Lewis JG, Hall RP, Young KH. Increases in stimulated secretion of pro-inflammatory cytokines by blood monocytes following arousal of negative affect: the role of insulin resistance as moderator. Brain Behav Immun. 2006;20:331–338. doi: 10.1016/j.bbi.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, Wise PM. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: Insights from basic science and clinical studies. Endocr Rev. 2006;27:575–605. doi: 10.1210/er.2005-0020. [DOI] [PubMed] [Google Scholar]
- van der Poll T, Jansen J, Endert E, Sauerwein HP, van Deventer SJ. Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect Immun. 1994;62:2046–2050. doi: 10.1128/iai.62.5.2046-2050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Poll T, Lowry SF. Epinephrine inhibits endotoxin-induced IL-1 beta production: roles of tumor necrosis factor-alpha and IL-10. Am J Physiol. 1997;273:R1885–1890. doi: 10.1152/ajpregu.1997.273.6.R1885. [DOI] [PubMed] [Google Scholar]
- von Kanel R, Kudielka BM, Preckel D, Hanebuth D, Fischer JE. Delayed response and lack of habituation in plasma interleukin-6 to acute mental stress in men. Brain Behav Immun. 2006;20:40–48. doi: 10.1016/j.bbi.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Walsh B, Shiff I. Menopause. In: Becker, editor. Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins; Philadelphia, PA: 2001. pp. 982–991. [Google Scholar]
- Winters SJ. Evaluation of Testicular Function. In: Becker, editor. Principles and Practice of Endocrinology and Metabolism. Lippincott, Williams & Wilkins; Philadelphia, PA: 2001. pp. 1115–1125. [Google Scholar]
- Zhang X, Wang L, Zhang H, Guo D, Qiao Z, Qiao J. Estrogen inhibits lipopolysaccharide-induced tumor necrosis factor-alpha release from murine macrophages. Methods Find Exp Clin Pharmacol. 2001;23:169–173. doi: 10.1358/mf.2001.23.4.634640. [DOI] [PubMed] [Google Scholar]