Abstract
Background
The biologic mechanisms linking socioeconomic position and psychosocial factors to cardiovascular disease (CVD) are not well understood. Immune response to persistent pathogens may be one of these mechanisms.
Methods
We analyzed cross-sectional data from the Multi-Ethnic Study of Atherosclerosis (N=999) composed of adults age 45–84. Log-binomial regression and ordinal logistic regression models were used to examine associations of socioeconomic factors and psychosocial factors with pathogen burden and immune response among those infected. Pathogen burden was assessed based on seroprevalence of Helicobacter pylori, cytomegalovirus, herpes simplex virus-1, and Chlamydia pneumoniae and antibody levels were used to characterize high immune response to all four pathogens.
Results
Low education was a strong and significant independent predictor of higher pathogen burden after adjustment for covariates (adjusted odds ratio (OR) 95% confidence interval (CI) 1.37, 1.19–1.57). Among subjects seropositive for all four pathogens, low education and a higher level of chronic psychosocial stress showed a positive association with higher antibody response, although associations were no longer significant in models with all covariates included (OR = 1.64, 95%CI 0.82–3.31 for lowest vs. highest educational category and OR= 1.29, 95%CI 0.96–1.73 for a one level increase in chronic stress).
Conclusion
Pathogen burden and heightened immune response may represent a biological pathway by which low socioeconomic position and chronic stress are related to increased rates of cardiovascular disease.
Keywords: Infection, inflammation, epidemiology, cardiovascular diseases
INTRODUCTION
Strong inverse associations of cardiovascular diseases with socioeconomic and psychosocial factors have been observed (Everson-Rose and Lewis, 2005; Kaplan and Lynch, 1999). Many persist after adjustment for health behaviors, suggesting that other processes may be involved. A ubiquitous factor potentially linking socioeconomic and psychosocial factors with cardiovascular disease is infection.
Several pathogens have been linked to cardiovascular disease in animal models and human epidemiological studies (Epstein, 2002; Zhu et al., 2000). The implicated infectious agents include cytomegalovirus (CMV), herpes simplex virus-1 (HSV-1), Helicobacter pylori (H. pylori) and Chlamydia pneumoniae (C. pneumoniae), but relationships have not always been consistent (Epstein, 2002; Zhu et al., 2000). Animal models have shown that persistent pathogens may cause direct or secondary pathological damage to cardiovascular tissue by acting as pro-inflammatory stimuli resulting in endothelial tissue damage in the vasculature (Muhlestein et al., 1998; Takaoka et al., 2008).
Although conclusions regarding the association between individual pathogens and cardiovascular disease in human populations have been conflicting, recent studies have more consistently identified associations between multiple persistent pathogens and cardiovascular disease processes (Georges et al., 2003; Zhu et al., 2001). Although some studies have investigated racial/ethnic and socioeconomic differences in infections with individual pathogens (McQuillan et al., 2004; Staras et al., 2006; Xu et al., 2002), few studies have examined cumulative pathogen burden. A recent study showed that increased pathogen burden, including HSV-1, CMV, Hepatitis B virus, and H. pylori, was significantly associated with lower income and education in a US nationally representative study (Zajacova et al., In press). An earlier study from the UK reported that employment grade was related to pathogen burden (sum of seropositive status to CMV, HSV-1 and C. pneumoniae.) (Steptoe et al., 2007). Examining the role of psychosocial stress is also important, given the observed associations of chronic stressors with susceptibility to novel infections and reactivation of persistent pathogens such as CMV and HSV (Cohen et al., 1998; Glaser et al., 1985; Stowe et al., 2001). To our knowledge, there have been no studies that have directly examined the relationship of psychosocial stressors/distress to pathogen burden or immune response to multiple pathogens implicated in cardiovascular disease.
Using data from the Multiethnic Study of Atherosclerosis (MESA), we examined whether adverse socioeconomic conditions and psychosocial stressors/distress (depression, chronic stress, and cynical distrust) are related to seropositivity to four pathogens implicated in cardiovascular diseases and to higher antibody responses among those seropositive. We hypothesized that lower socioeconomic position would be associated with a higher prevalence of pathogens since socioeconomic position may influence exposure and susceptibility. In addition, we hypothesized that socioeconomic stressors may lead to pathogen reactivation and therefore a higher immune response among adults who are seropositive to persistent pathogens. Since these pathogens are generally acquired at an early age, we hypothesized that current levels of psychosocial stressors/distress (depression, chronic stress, and cynical distrust) would only be weakly associated with seropositivity status. However, because psychosocial stress/distress may cause reactivation of latent infections, we hypothesized that these psychosocial measures would be associated with higher immune response among adults who are seropositive to all four persistent pathogens.
MATERIALS AND METHODS
Study Population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a longitudinal, multi-site study aimed at identifying risk factors for atherosclerosis in individuals free of clinical cardiovascular disease and aged 45–84 years at baseline (Bild et al., 2002). Cross-sectional data from the baseline examination were used for these analyses. Of 6814 MESA participants, 1000 were randomly selected for testing for several CVD related infections (CMV, HSV, and H. pylori). In addition, C. pneumoniae was assayed in the entire cohort. Of the 1000, 999 had complete information on all four pathogens and were included in these analyses. The study was reviewed by the Institutional Review Board at each participating site. All subjects provided written informed consent.
Measures
Information on sociodemographic, psychosocial, and behavioral factors was collected via questionnaire at baseline. Annual income was categorized as <US$25,000, $25,000–$50,000, and >$50,000. Education was trichotomized as High School or Less; Some College, Associate’s Degree, or Technical School; and Bachelor’s or Graduate Degree.
Psychosocial measures included depression, chronic burden of stress, and cynical distrust, shown to influence immune function and/or induce subclinical reactivation of herpesviruses (Cohen and Herbert, 1996; Gerra et al., 2003; Kiecolt-Glaser et al., 2002; Miller et al., 2005). Some of these psychosocial measures have also been implicated in susceptibility to H.pylori infection (Levenstein, 2000) and linked to C. pneumoniae (Appels et al., 2000). Depression was measured using the Centers for Epidemiological Studies of Depression Scale (CES-D); the score was dichotomomized as ≥16 versus < 16, a cutoff value often used to screen for depression (Kohout et al., 1993). Chronic burden of stress was assessed by means of four items that measured ongoing problems in several domains, including: health problems of someone close to them, ongoing job difficulties, ongoing financial problems, and personal relationship difficulties (Diez Roux et al., 2006). Participants were first asked if they had experienced ongoing difficulties in each domain. Those who responded yes were then asked to rate their ongoing difficulties as not very stressful, moderately stressful, or very stressful. A chronic burden of stress scale was then created by summing the number of domains for which participants reported moderately or severely stressful problems. Scores ranged from 0 to 4 with higher scores indicating greater chronic stress. This scale was developed for the Health Women’s Study (Bromberger and Matthews, 1996) and was shown to be predictive of inflammatory markers in prior work (Ranjit et al., 2007). The chronic stress variable was treated as a continuous variable when the relationship to the outcome appeared linear, and categorical otherwise.
Cynical distrust was measured by the 8-item validated Cynical Distrust Scale, derived from the Cook-Medley Hostility Scale (Greenglass, 1996; Greenglass and Julkunen, 1989). This subset has previously been used in studies investigating the relationship between inflammation and adverse health outcomes (Christensen et al., 2004; Ranjit et al., 2007) and has been shown to be related to the progression of atherosclerosis (Julkunen et al., 1994). The scale score was obtained by summing item scores; higher scores indicate a higher level of cynical distrust (Ranjit et al., 2007). The distribution of the scale scores in the study population was first examined and then categorized by quartiles to identify potential threshold effects. Based on the similarity of the effect estimates across the two highest quartiles (5 or higher on the scale score) a cut point was made and the scale score dichotomized as low (scoring 0–4) and high cynical distrust scores (scoring 5 or higher).
Demographic covariates included age, gender, race/ethnicity, and number of children living in participants’ households. Race/ethnicity was self-designated as Black non-Hispanic, Hispanic, Chinese, and white non-Hispanic. Covariates representing health behaviors and those related to susceptibility to infection were also examined, as follows: Tobacco and alcohol use were categorized as current, never or former; presence of ongoing health problems was defined as no health problems or not very stressful ongoing health problems versus moderately or severely stressful ongoing health problems; diabetes was defined as fasting glucose level of >125 mg/dL [>6.99 mmol/L] or hypoglycemic medication use; and impaired glucose tolerance as fasting glucose level of 100–125 mg/dL [5.60–6.99 mmol/L ]). As weight and height have been associated with higher pathogen burden in previous studies (Ekesbo et al., 2000; Fernandez-Real et al., 2006), we also explored BMI (body mass index), waist to hip ratio, and height as covariates.
Laboratory Tests
Serum IgG antibodies to CMV, HSV, and H. pylori, were detected using commercially available kits, employing an indirect enzyme immunoassay (DiaMedex Corp. Miami, FL). The sensitivity and specificity of the tests ranged from 94–100% (DiaMedex Corp. Miami, FL). IgG antibodies to C. pneumoniae, were detected using a microimmunoflourescent antibody assay (Focus Technologies, Cypress, CA).
Each infection was examined using continuous or dichotomously defined antibody levels. Individuals were classified as negative for CMV if values <8.0 ELISA Units (EU)/mL. Values higher than 10.0 were classified as positive, and those between 8.0 and 9.9 were classified as equivocal. Cutoff values for HSV-1 seropositivity were ≥ 20.0 EU/mL, with equivocal values at 16.0–19.9 and values <16.0 as negative. For H.pylori values of < 0.90 were interpreted as negative, 0.90–1.09 as equivocal, and ≥ 1.10 as positive. All equivocal values (CMV n=2, HSV n=1, H. pylori n=13, C. pneumoniae n=0) were added to the positive category. Given the very small number of equivocal values, assigning them to the negative category had no impact on results.
Statistical Analysis
Using the categorical (presence/absence of infection) and continuous antibody levels, we constructed two composite measures: 1) overall pathogen burden and 2) cumulative immune response among participants seropositive to all four pathogens.
Pathogen burden was defined as the number of pathogens for which a subject tested seropositive for IgG antibodies. Based on prior work (Rothenbacher et al., 2003), pathogen burden was categorized as low (seropositive for 0–2 pathogens) and high (seropositive for 3–4 pathogens). Prevalence ratios of a high pathogen burden by levels of socioeconomic position and psychosocial factors were estimated using log-binomial regression (Spiegelman and Hertzmark, 2005) in three sequential models. As in many epidemiologic analyses, the model building approach was driven by our research questions and hypotheses (Martin, 2008; Tripepi et al., 2008). We first estimated age and gender adjusted prevalence ratios of high pathogen burden for socioeconomic position, race/ethnicity, and each psychosocial factor modeled separately. This model allowed us to examine the age- and gender-adjusted associations of each predictor with the outcomes. A second model included race/ethnicity, socioeconomic measures and psychosocial factors in the same model (in addition to age and gender) in order to examine the independent effect of each predictor of interest. A final model added number of children in household, smoking, alcohol use, diabetes/impaired glucose tolerance, BMI, waist to hip ratio, height, and ongoing health problems. This model allowed us to estimate associations after adjustment for possible confounders or mediators of the socioeconomic or psychosocial effects.
The cumulative immune response outcome was investigated among subjects seropositive to all four infections. Continuous antibody levels to CMV, HSV, H.pylori, and C. pneumoniae were dichotomized at the median based on the distribution of each of these infections in the study population (coded as 1 for at or above the median, 0 below), and summed across all four pathogens for a total score ranging from zero (low antibody levels to all four pathogens) to four (high antibody levels to all four pathogens). The highest two categories (3 and 4) were combined since only 11 subjects had high antibody levels to all four pathogens resulting in a final antibody level outcome score of zero to three. This approach was a practical way to summarize antibody levels across the four pathogens in a simple and intuitive manner, consistent with the approach used in prior work (Aiello et al., 2006; Aiello et al., 2008). Proportional odds models (a.k.a ordinal logistic regressions) were used to estimate odds ratios of cumulative immune response associated with socioeconomic position, race/ethnicity, and psychosocial stress/distress among persons seropositive to all four infections. The model building approach was analogous to that described for pathogen burden above. All analyses were conducted using SAS software Version 9.1 (SAS Institute, Inc., Cary, NC).
RESULTS
The proportion of seropositive subjects for each infection out of the total sample (N=999) was 76.8% for CMV, 84.9% for HSV, 71.0% for C. pneumoniae and 46.2% for H. pylori. The percentage of participants with 0, 1, 2, 3, and 4 infections were 2.2, 11.9, 21.5, 33.6, and 30.7%, respectively. Among the 30.7% (N=307) of participants with all four infections, 9.1% were in the lowest cumulative immune response category (cumulative score 0 as described in Methods), 32.6% were in the second category (score 1), 35.2% in the third category (score 2) and 23.1% in the highest category (score 3–4).
Table 1 shows the distribution of demographic and psychosocial characteristics (means and proportions) for participants within infection seropositivity categories. The mean age of the 999 participants was 59 years, 43% were male, 46% were white, 23% Hispanic, 21% Black, and 10% Chinese, 36.9% had a Bachelor’s degree or more, and 44.5% had an annual income greater than or equal to $50,000 (Table 1). Fourteen percent had depressive symptoms scores ≥ 16 and 48.8% had scores in lowest chronic burden category, and the mean cynical distrust score was 21.5.
Table 1.
Characteristics of participants included in the analyses by categories of pathogen burden and cumulative immune response in the Multi-ethnic Study of Atherosclerosis (July 2000 —July 2002)
| Characteristics* | Number of Pathogens | Cumulative Immune Response (Participants seropositive for all 4 pathogens) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 0–2 | 3–4 | DF | Value | p-value | Total | Low | High | DF | Value | p-value | |
| N§ | 999 | 356 | 643 | 307 | 236 | 71 | ||||||
| Age at baseline (years) | 59 ± 9.7 | 59 ± 9.5 | 59 ± 10.0 | 997 | −0.60 | 0.55 | 60 ± 9.5 | 59 ± 9.8 | 62 ± 8.3 | 305 | −1.99 | 0.05 |
| Male | 42.9 | 44.4 | 42.1 | 1 | 0.47 | 0.49 | 44.0 | 45.3 | 39.4 | 1 | 0.77 | 0.38 |
| Race/Ethnicity | 3 | 194.15 | <0.001 | 3 | 7.20 | 0.07 | ||||||
| White | 45.9 | 75.0 | 29.9 | 19.2 | 20.8 | 14.1 | ||||||
| Hispanic | 23.1 | 10.1 | 30.3 | 35.2 | 34.3 | 38.0 | ||||||
| Black | 21.0 | 10.1 | 27.1 | 30.3 | 27.5 | 39.4 | ||||||
| Chinese | 9.9 | 4.8 | 12.8 | 15.3 | 17.4 | 8.5 | ||||||
| Education | NA | 9.56 | <0.001 | NA | 2.49 | 0.01 | ||||||
| High School or Less | 34.4 | 18.8 | 43.1 | 52.0 | 47.2 | 67.6 | ||||||
| Some College, Associate’s Degree, or Technical School | 28.7 | 25.6 | 30.4 | 27.8 | 31.1 | 16.9 | ||||||
| Bachelor’s or Graduate Degree | 36.9 | 55.6 | 26.5 | 20.3 | 21.7 | 15.5 | ||||||
| Income (Annual) | NA | 7.35 | <0.001 | NA | 1.79 | 0.07 | ||||||
| <$25,000 | 27.1 | 15.6 | 33.6 | 42.1 | 38.7 | 53.7 | ||||||
| $25,000–50,000 | 28.4 | 25.5 | 30.0 | 26.3 | 28.3 | 19.4 | ||||||
| >$50,000 | 44.5 | 58.9 | 36.3 | 31.6 | 33.0 | 26.9 | ||||||
| Depressive Symptoms‡ | 14.0 | 11.5 | 15.4 | 1 | 2.89 | 0.09 | 17.3 | 15.7 | 22.5 | 1 | 1.76 | 0.19 |
| Chronic Burden of Stress‡ | 3 | 12.76 | 0.005 | NA | −3.04 | 0.002 | ||||||
| Level 0 | 48.8 | 41.6 | 52.8 | 56.0 | 60.8 | 40.0 | ||||||
| Level 1 | 30.1 | 33.7 | 28.0 | 26.8 | 24.6 | 34.3 | ||||||
| Level 2 | 13.9 | 17.3 | 12.0 | 11.3 | 9.9 | 15.7 | ||||||
| Level 3–4 | 7.3 | 7.4 | 7.2 | 6.0 | 4.7 | 10.0 | ||||||
| Cynical Distrust‡ | 21.5 | 11.5 | 27.4 | 1 | 31.67 | <0.001 | 31.1 | 30.7 | 32.8 | 1 | 0.10 | 0.75 |
| Number of Children in Household | NA | −1.88 | 0.06 | NA | 1.55 | 0.12 | ||||||
| None | 77.3 | 80.4 | 75.6 | 72.4 | 69.7 | 81.2 | ||||||
| 1 | 12.2 | 11.4 | 12.7 | 12.8 | 14.5 | 7.2 | ||||||
| ≥2 | 10.5 | 8.2 | 11.7 | 14.8 | 15.8 | 11.6 | ||||||
| Current smokers | 15.5 | 12.6 | 17.0 | 1 | 3.34 | 0.07 | 16.67 | 15.3 | 21.1 | 1 | 1.32 | 0.25 |
| Current drinkers | 58.5 | 70.9 | 51.6 | 1 | 35.11 | <0.001 | 46.41 | 48.1 | 40.8 | 1 | 1.15 | 0.28 |
| Height (cm) | 167 ± 9.9 | 169 ± 10.0 | 165 ± 9.6 | 997 | 5.29 | <0.001 | 165 ± 9.5 | 165 ± 9.7 | 164 ± 9.0 | 305 | 0.96 | 0.34 |
| BMI||, kg/m2 | 28 ± 5.6 | 28 ± 5.2 | 29 ± 5.8 | 997 | −2.30 | 0.02 | 29 ± 5.7 | 29 ± 5.5 | 29 ± 6.1 | 305 | −0.64 | 0.52 |
| Diabetes | 2 | 12.66 | 0.002 | 2 | 4.06 | 0.13 | ||||||
| Negative | 65.5 | 72.4 | 61.7 | 58.0 | 60.2 | 50.7 | ||||||
| Impaired Fasting Glucose | 21.9 | 18.9 | 23.6 | 24.8 | 22.0 | 33.8 | ||||||
| Positive | 12.5 | 8.7 | 14.6 | 17.3 | 17.8 | 15.5 | ||||||
| Ongoing Health Problems (self-rated) | 14.7 | 12.9 | 15.8 | 1 | 1.46 | 0.23 | 16.4 | 16.7 | 15.5 | 1 | 0.05 | 0.82 |
Note: Values in table are mean ± SD for continuous characteristics and percents for categorical characteristics. NA=Not applicable.
T-tests were used to compare continuous characteristics by pathogen burden and immune response categories, chi-squared tests for categorical characteristics, and trend tests for ordinal characteristics. The chronic stress variable was assessed using a chi-square test rather than a trend test for pathogen burden since the relationship did not follow a linear trend.
Cumulative immune response was measured among subjects seropositive for all four pathogens. A high cumulative immune response was defined as having above median antibody response to at least 3 of the 4 pathogens versus having high immune response to only 0 or 2 out of the four pathogens.
Depressive symptoms were defined as CES-D ≥ 16 versus < 16, chronic stress is presented categorically with level 0 representing no chronic stress, and high cynical distrust was defined as a score of ≥ 5 versus < 5 based on the median value in the study population.
Within the 0–2 category for number of pathogens: 14 participants were missing values on number of children in household, 2 on alcohol use, and 1 diabetes. Within the 3–4 category for number of pathogens: 19 were missing data on number of children in household, 3 on alcohol use, 2 on smoking, diabetes status, and ongoing health problems. Within the low cumulative immune response category: 8 participants were missing values on number of children in household, 1 on smoking status and alcohol use. Within the high cumulative immune response category: 2 were missing data on number of children in household and 2 for ongoing health problems.
BMI=Body Mass Index
NA: A trend test for ordinal characteristics doesn’t have degree of freedom.
Participants with a high pathogen burden (3–4 infections) were more likely to be non-white (Chi Square =194.15, df=3, p <0.001), and to be in the lower educational (z-test =9.56, p <0.001) and income categories (z-test =7.35, p <0.001) than those with a low pathogen burden (0–2 infections) (Table 1). Those with a higher pathogen burden had a slightly higher level of depressive symptoms (Chi Square =2.89, df=1, p = 0.09), and were significantly more likely to report a higher level of cynical distrust (Chi Square =31.67, df=1, p <0.001) compared to those with a low pathogen burden. A greater proportion of subjects with a high pathogen burden reported lower levels of chronic stress compared to those with a low pathogen burden but differences across other categories were inconsistent (Chi Square =12.76, df=3, p = 0.005). Subjects with a higher pathogen burden were also more likely to be current smokers (Chi Square =3.34, df=1, p = 0.07), less likely to be current drinkers (Chi Square =35.11, df=1, p <0.001), more likely to be diabetic (Chi Square =12.66, df=2, p = 0.002) and had shorter height (t-test=5.29, df=997, p <0.001) and higher BMI (t-test=−2.30, df=997, p = 0.02) compared to those with a low pathogen burden (Table 1). In addition, participants with a higher pathogen burden were more likely to have two or more children compared to those with a low pathogen burden, but this difference was not statistically significant (z-test= −1.88, p = 0.06).
Table 1 also shows characteristics of subjects who were seropositive to all four pathogens (N=307, 31%) and differences in characteristics by immune response categories. Those with a higher cumulative immune response were older (t-test=−1.99, df=305, p = 0.05), less likely to be white non-Hispanic (Chi Square =7.20, df=3, p = 0.07), and had lower income (z-test= 1.79, p = 0.07), although these differences were not statistically significant. Participants with a higher cumulative immune response were also more likely to be in the lower educational categories than those with a lower immune response (z-test = 2.49, p = 0.01). Participants with a higher cumulative immune response reported significantly higher levels of chronic stress (z-test = −3.04, p= 0.002).
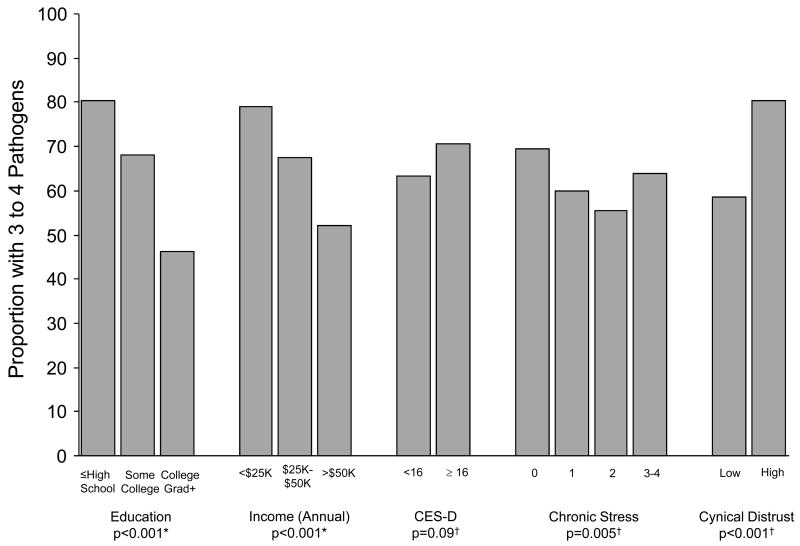
Figure 1 shows the proportion of subjects seropositive to 3–4 pathogens by education, income, CES-D, chronic stress levels, and cynical distrust. A greater proportion of participants with lower education and lower income were positive for 3–4 pathogens compared to those with higher education (z-test =9.56, p <0.001) and income (z-test =7.35, p <0.001). Subjects with higher levels of cynical distrust were more likely to be positive for 3–4 pathogens than those with lower cynical distrust (Chi Square =34.06, df=1, p <0.001). There were also differences by chronic stress level whereby those in the lowest and highest categories of chronic stress had a higher proportion of subject positive for 3–4 pathogens versus the two middle categories of chronic stress (Chi Square=12.76, df=3, p =0.005). There were no statistically significant differences in the proportion of subjects seropositive for 3–4 pathogens, by CESD categories (Chi Square=2.89, df=1, p =0.09).
Figure 1. Percent of persons with 3–4 pathogens by socioeconomic and psychosocial factors in a subset (n=999) of the Multi-ethnic Study of Atherosclerosis (July 2000 — July 2002).
Depressive symptoms were defined as CES-D ≥ 16 versus < 16, chronic stress is presented categorically with level 0 representing no chronic stress, and high cynical distrust was defined as a score of ≥ 5 versus < 5 based on the median value in the study population. *P-values were computed using trend tests. † P-values were computed using Chi-squared tests.
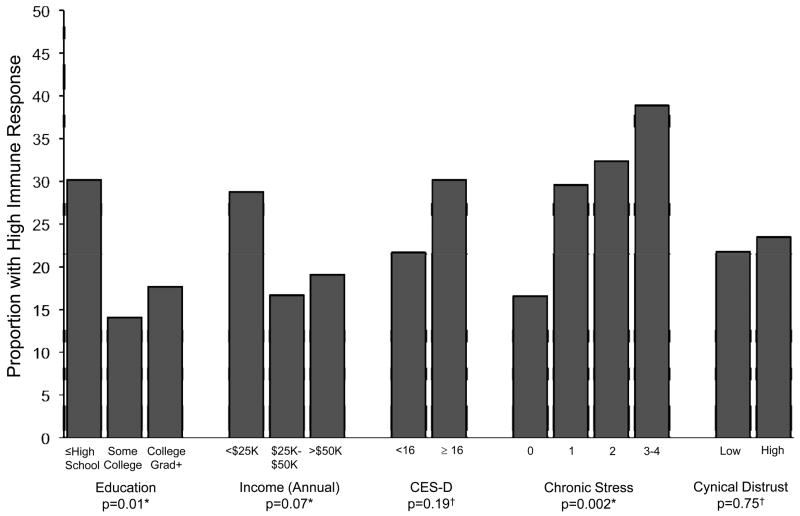
Figure 2 shows the proportion of subjects with a high cumulative immune response by education, income, CES-D, chronic stress levels, and cynical distrust, among the 307 participants seropositive for all infections. Participants with lower education and income were more likely to have a higher cumulative immune response than those with higher education (z-test =2.49, p=0.01) and income (z-test =1.79, p= 0.07). Greater levels of chronic stress were associated with a greater probability of having a higher immune response (z-test =−3.04, p= 0.002). Neither depressive symptoms nor cynical distrust were significantly associated with a higher cumulative immune response (Chi-square=1.76, df=1, p= 0.19 and Chi Square=0.10, df=1, p= 0.75, respectively).
Figure 2. Percent of persons with high IgG antibody response among participants with all four pathogens (N=307) by socioeconomic and psychosocial factors in a subset of the Multi-ethnic Study of Atherosclerosis (July 2000 — July 2002).
Cumulative immune response was measured among subjects seropositive for all four pathogens. A high cumulative immune response was defined as having above median antibody response to at least 3 of the 4 pathogens versus having high immune response to only 0 or 2 out of the four pathogens. Depressive symptoms were defined as CES-D ≥ 16 versus < 16, chronic stress is presented categorically with level 0 representing no chronic stress, and high cynical distrust was defined as a score of ≥ 5 versus < 5 based on the median value in the study population. *P-values were computed using trend tests. † P-values were computed using Chi-squared tests.
Table 2 shows adjusted associations of socioeconomic and psychosocial factors with pathogen burden. In age and gender adjusted models, lower education, lower income, and higher cynical distrust were associated with a higher prevalence of infection with multiple pathogens. Depression was associated with a higher prevalence of infection with multiple pathogens but the association was only marginally statistically significant (Table 2, Model 1). Surprisingly, subjects with moderate chronic stress (levels 1 and 2) had a significantly lower prevalence of pathogens compared to those with the lowest levels of chronic (level 0) in the age and gender adjusted model (Table 2, Model 1).
Table 2.
Prevalence ratios of having a high pathogen burden by socioeconomic and psychosocial characteristics among the random subset of participants in the Multi-ethnic Study of Atherosclerosis. (July 2000—July 2002)*
| Models 1† |
Model 2‡ (N=891)
|
Model 3§ (N=865)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | PR | 95% CI | p-value | PR† | 95% CI | p-value | PR† | 95% CI | p-value | |
| Education | 997 | |||||||||
| High School or Less | 1.75 | (1.55, 1.98) | <0.001 | 1.43 | (1.24, 1.63) | <.0001 | 1.37 | (1.19, 1.57) | <.0001 | |
| Some College, Associate’s Degree, or Technical School | 1.48 | (1.29, 1.69) | <0.001 | 1.26 | (1.10, 1.45) | 0.001 | 1.23 | (1.07, 1.41) | 0.005 | |
| Bachelor’s or Graduate Degree | 1.00 | -- | -- | 1.00 | -- | -- | 1.00 | -- | -- | |
| Income (Annual) | 972 | |||||||||
| <$25,000 | 1.54 | (1.38, 1.73) | <0.001 | 1.06 | (0.94, 1.20) | 0.35 | 1.02 | (0.90, 1.16) | 0.72 | |
| $25,000–$50,000 | 1.30 | (1.15, 1.47) | <0.001 | 1.05 | (0.93, 1.19) | 0.41 | 1.03 | (0.91, 1.16) | 0.65 | |
| >$50,000 | 1.00 | -- | -- | 1.00 | -- | -- | 1.00 | -- | -- | |
| Psychosocial Stress# | ||||||||||
| Depressive Symptoms | 998 | 1.12 | (0.99, 1.26) | 0.07 | 1.01 | (0.89, 1.14) | 0.90 | 1.01 | (0.89, 1.15) | 0.88 |
| Chronic Stress | 988 | |||||||||
| Level 0 | 1.00 | -- | -- | 1.00 | -- | -- | 1.00 | -- | -- | |
| Level 1 | 0.86 | (0.77, 0.96) | 0.01 | 0.94 | (0.85, 1.05) | 0.29 | 0.95 | (0.85, 1.06) | 0.33 | |
| Level 2 | 0.79 | (0.67, 0.93) | 0.005 | 0.85 | (0.73, 1.00) | 0.05 | 0.84 | (0.72, 0.99) | 0.04 | |
| Level 3–4 | 0.92 | (0.76, 1.10) | 0.37 | 0.94 | (0.78, 1.14) | 0.52 | 0.93 | (0.76, 1.15) | 0.52 | |
| Cynical distrust | 919 | 1.38 | (1.26, 1.52) | <0.001 | 1.03 | (0.94, 1.13) | 0.56 | 1.04 | (0.95, 1.14) | 0.39 |
Outcome is dichotomized pathogen burden (3–4) vs. (0–2).
Adjusted for gender and age.
Adjusted for income, education, psychosocial stress, race/ethnicity, gender and age.
Adjusted for income, education race/ethnicity, psychosocial stress, number of children in household, BMI, waist to hip ratio, diabetes, height, smoking, alcohol use, ongoing health problems, gender and age.
PR = Prevalence Ratio and CI=Confidence Interval
Depressive symptoms were defined as CES-D ≥ 16 versus < 16, chronic stress was examined categorically with level 0 representing no chronic stress, and cynical distrust was defined as a score of ≥ 5 versus <5 based on the study population median value.
Only lower education remained significantly associated with higher probability of high pathogen burden when all covariates were simultaneously adjusted (PR=1.37, CI 1.19–1.57 for lowest vs. highest category and PR=1.23, CI 1.07–1.41 for middle category vs. highest category) (Table 2, Model 3). Level 2 chronic stress remained associated with lower probability of higher pathogen burden after full adjustment, but the association was only marginally statistically significant (Table 2, Model 3).
Table 3 shows adjusted associations of socioeconomic and psychosocial factors with odds of a higher cumulative immune response among subjects seropositive to all four pathogens. In age and gender adjusted proportional odds models, the lowest education level and a unit increase in the level of chronic stress were each associated with greater cumulative immune response (Table 3, Model 1). Depression, cynical distrust, and low income were also associated with odds of a higher cumulative immune response but associations were not statistically significant at the p < 0.05 level. The direction of the association of low education and high chronic stress with greater odds of a cumulative immune response was unchanged in fully adjusted models (Table 3, Model 3) although the confidence intervals were wide and the associations were no longer statistically significant.
Table 3.
Odds ratios of having a high immune response among individuals in the Multi-ethnic Study of Atherosclerosis (July 2000—July 2002) infected with all four pathogens by socioeconomic and psychosocial factors*
| Models 1† |
Model 2‡ (N=266)
|
Model 3§ (N=259)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | OR|| | 95% CI | p-value | OR|| | 95% CI | p-value | OR|| | 95% CI | p-value | |
| Education | 306 | |||||||||
| High School or Less | 2.13 | (1.23, 3.69) | 0.01 | 1.54 | (0.79, 2.99) | 0.20 | 1.64 | (0.82, 3.31) | 0.16 | |
| Some College, Associate’s Degree, or Technical School | 1.22 | (0.67, 2.22) | 0.52 | 0.91 | (0.47, 1.78) | 0.79 | 0.91 | (0.46, 1.80) | 0.79 | |
| Bachelor’s or Graduate Degree | 1.00 | -- | -- | 1.00 | -- | -- | 1.00 | -- | -- | |
| Income (Annual) | 297 | |||||||||
| <$25,000 | 1.60 | (0.96, 2.65) | 0.07 | 1.23 | (0.66, 2.29) | 0.51 | 1.15 | (0.59, 2.23) | 0.68 | |
| $25,000–$50,000 | 1.09 | (0.63, 1.87) | 0.76 | 1.06 | (0.58, 1.95) | 0.85 | 0.99 | (0.53, 1.86) | 0.99 | |
| >$50,000 | 1.00 | -- | -- | 1.00 | -- | -- | 1.00 | -- | -- | |
| Psychosocial Stress# | ||||||||||
| Depressive Symptoms | 306 | 1.25 | (0.73, 2.17) | 0.42 | 0.83 | (0.43, 1.62) | 0.59 | 0.79 | (0.39, 1.60) | 0.51 |
| Chronic Stress | 302 | 1.40 | (1.10, 1.77) | 0.01 | 1.25 | (0.95, 1.64) | 0.11 | 1.29 | (0.96, 1.73) | 0.09 |
| Cynical Distrust | 273 | 1.15 | (0.72, 1.84) | 0.57 | 0.87 | (0.51, 1.48) | 0.61 | 0.89 | (0.51, 1.54) | 0.67 |
The outcome for these models is the ranked antibody response for all four infections among seropositive subjects.
Adjusted for gender and age.
Adjusted for income, education, race/ethnicity, psychosocial stress, gender and age.
Adjusted for income, education race/ethnicity, psychosocial stress, number of children in household, BMI (body mass index), waist to hip ratio, diabetes, height, smoking, alcohol use, ongoing health problems, gender and age.
Odds Ratio (OR) represents the probability of having a higher ordinal antibody response and CI=Confidence Interval.
Depressive symptoms were defined as CES-D ≥ 16 versus < 16, chronic stress score was treated as a continuous variable and therefore represents the effect of a one level increase in the chronic stress score, and cynical distrust was defined as a score of ≥ 5 versus <5 based on the study population median value.
DISCUSSION
Low education was associated with a higher probability of being seropositive for multiple pathogens implicated in cardiovascular disease. Moreover, among individuals seropositive to all four pathogens, those who reported lower education or higher levels of chronic stress were more likely to have a higher cumulative antibody response. These findings suggest that social and psychosocial factors may influence pathogen burden and immune response to pathogens implicated in cardiovascular disease. Strengths of this study include: a large well characterized population-based multi-ethnic sample; availability of serostatus and immune response to several important pathogens implicated in cardiovascular disease; data from a range of social and psychological variables; and subjects free of cardiovascular disease. Our results therefore suggest that social and psychosocial determinants may influence pathogen burden and immune response even among generally healthy community dwelling adults.
To our knowledge, only two studies have examined the relationship between socioeconomic position and pathogen burden (Steptoe et al., 2007; Zajacova et al., In press). Using data from the National Health and Nutrition Examination Survey, Zajacova et al. showed significant education, income, and race/ethnic disparities in the burden of infection (as measured by seropositivity to H. pylori, cytomegalovirus, herpes simplex virus, and hepatitis B) in the US population ages 17–90 (Zajacova et al., In press). In a study of 451 civil servants in the UK, subjects with lower grades of employment were shown to have a higher pathogen burden (CMV, HSV-1, and C. pneumoniae) (Steptoe et al., 2007). Our results, based on a more race/ethnically diverse population-based sample, are consistent with these earlier findings and add to this research by including both H. pylori and C.pneumoniae in our pathogen burden variable. Unlike these earlier studies, we also assessed associations with psychosocial factors and examined immune response among seropositive individuals.
We found more robust associations with education than with income. Although it is difficult to tease out “independent effects” of income and education in studies like ours, educational attainment may be a better marker of socioeconomic position over the lifecourse than current annual income in our relatively older sample (mean age of 59 years). Continuous measures of education (years) and income (household annual) were positively but only moderately correlated (r= .48, p<0.001).
Low socioeconomic position may influence pathogen burden in two ways. First, it may increase the frequency of exposure and transmission of these pathogens through crowding and poor living conditions in childhood. Indeed, socioeconomic gradients in pathogen burden are likely to emerge at young ages since most of the pathogens examined in this study are generally acquired in childhood/young adulthood (Lin et al., 2004; Malaty et al., 2002; McQuillan et al., 2004; Schillinger et al., 2004). Second, low socioeconomic position may be associated with greater susceptibility to infection through poorer nutrition, higher levels of stress, and biological risk factors (e.g. obesity and type 2 diabetes), which may enhance immunological susceptibility to these pathogens (Ekesbo et al., 2000; Fernandez-Real et al., 2006). Thus, those who are of low socioeconomic position may be more likely to come in contact with these pathogens and once exposed may be more immunologically susceptible to infection. In addition, individuals of low socioeconomic position may be continuously exposed to a larger number of pathogens, a higher infectious dose, and more severe primary infection or re-infection.
Although our data did not allow us to determine which of these mechanisms is most important, we did find that associations with low education persisted after adjustment for a range of covariates previously linked to pathogen exposure, such as number of children in the household, diabetes, smoking, and chronic health conditions. It is possible that some of these demographic and behavioral covariates are mediators of the relationship between socioeconomic position or psychosocial factors and pathogen burden. For example, several studies have shown that exposure to children is a risk factor for acquisition of CMV, HSV, C. pneumoniae and H. pylori (Jackson et al., 1996; Malaty et al., 2002). Diabetes and smoking (which are more common in lower socioeconomic groups) may enhance susceptibility to infection and also lead to increased reactivation of persistent pathogens through their effects on immune response (Albrecht et al., 2004; Ekesbo et al., 2000; Fernandez-Real et al., 2006). In full models, adjustment for these potential mediators may have reduced our ability to detect significant associations. Limited sample size together with the cross-sectional nature of our analyses made it difficult to differentiate confounders from mediators in these analyses. The mediating role of these factors should be explored with more appropriate designs and larger sample sizes.
Consistent with our hypothesis we found no evidence of strong positive associations between psychosocial factors (chronic stress, depression, or cynical distrust) and pathogen burden. Surprisingly, there appeared to be a u-shaped relationship between chronic stress and pathogen burden with the lowest proportion of subjects seropositive for 3–4 pathogens found among those reporting moderate levels of chronic stress (levels 1 and 2). However, this pattern was weakened and associations were no longer statistically significant (or were only marginally statistically significant) after adjustment for race/ethnicity, education and income. Further analyses (not shown) revealed that this unexpected pattern was largely the result of confounding by education. Nevertheless studies with larger sample sizes and better measures of chronic stress are necessary to better investigate the relation between chronic stress and pathogen burden. The correlations among the psychosocial variables were 0.30 or less, indicating moderate to week correlations (Hinkle et al., 1988). Chronic stress and depressive symptoms showed the highest correlation (r=0.30, p < 0.001) suggesting a moderate but significant correlation between these variables. There was no significant correlation between cynical distrust and depression (r= −0.01 p=0.85), suggesting that these two variables tap into different psychological domains. There was also a very low correlation between the chronic burden of stress scale and ongoing health issues (r=0.17, p=0.02) indicating no or a negligible correlation. Thus, most of our psychosocial variables appear to be relatively independent of each other.
Our results also suggest that low education and chronic stress, are related to a heightened antibody response among subjects who were seropositive to all four pathogens. Although associations were not statistically significant in final models (most likely due to limited sample size) point estimates were largely unchanged after adjustment, suggesting that these patterns are not confounded by the other factors we measured. This implies that socioeconomic position and stress may not only alter exposure patterns and susceptibility through behavior or environmental factors but may also directly impact immunological stress-related reactivation of persistent pathogens among those that are infected. Higher antibody response to persistent pathogens, such as HSV and CMV, is one of the most consistent indicators of altered cell mediated immunity related to psychosocial stress in experimental studies (Herbert and Cohen, 1993). Once infected, persons of lower socioeconomic position and those with higher levels of chronic stress may be more likely to experience chronic reactivation of persistent pathogen burden. Some studies have shown that higher IgG antibody response is related to poorer cardiovascular outcomes, suggesting that antibody response may be an important mediator of socioeconomic or psychosocial differences in cardiovascular risk (Adam et al., 1987; Nieto et al., 1996; Paldanius et al., 2006). The combination of earlier pathogen exposure, increased susceptibility to a wider array of pathogens and greater likelihood of stress-related reactivation may be important factors contributing to socioeconomic disparities in cardiovascular disease.
Recent studies suggest that total pathogen burden rather than the effects of single pathogens may play a significant role in cardiovascular outcomes (Georges et al., 2003; Zhu et al., 2001). The mechanisms by which infection with multiple pathogens may contribute to cardiovascular disease include effects of infection on induction of a systemic pro-inflammatory environment (Epstein, 2002). Studies have shown that socioeconomic position, depressive symptoms, and chronic stress, are related to heightened levels of circulating inflammatory markers associated with cardiovascular disease, including C-reactive protein (CRP) and Interleukin 6 (IL-6) (Appels et al., 2000; Glaser and Kiecolt-Glaser, 2005; Kiecolt-Glaser et al., 2003; McDade et al., 2006; Ranjit et al., 2007). CRP has been shown to be correlated with pathogen burden, suggesting that persistent pathogen burden may function as a biological inducer of inflammation (Zhu et al., 2000).
Our study has a number of limitations. MESA was not designed to be a representative sample of the general US population (Bild et al., 2002). Therefore the prevalence estimates may not be generalizable. However, MESA prevalence estimates were in fact only slightly lower or quite comparable to those observed in national samples. For example, CMV and H.pylori in the age range of subjects 59–69 were 83% and 51% respectively in the US National Health and Nutrition Survey III (NHANES) compared to 77% and 48%, respectively, among subjects in the same age range in MESA. The prevalence of HSV in subjects aged 59–69 was identical (83%) in NHANES and MESA. Population based prevalence estimates of C. pneumoniae are not available in NHANES, but the Cardiovascular Health Study (CHS), another large population based sample of adults 65 and over, found that 83% of healthy controls (no cardiovascular disease) ages 65+ were seropositive for C. pneumoniae (Siscovick et al., 2000) compared to 79% in MESA subjects in the same age group. Regardless of whether prevalence estimates are generalizable, there is no reason to believe that associations of social and psychosocial factors with infections (our main research question) would not be generalizable.
MESA also excluded persons with clinical cardiovascular disease. The examination of the associations in a sample free of cardiovascular disease is advantageous because it avoids possible bias from reverse causation. It is well known that clinical CVD is strongly patterned by SES. If persons with clinical disease are more likely to become infected or reactivate infections, the examination of associations in a sample with clinical disease could result in a biased estimate of the causal association between social factors and infection.
In addition, the use of cross-sectional data prevented us from distinguishing between current or past infection. Most of the pathogens that we investigated in our study, however, are acquired during the first or second decade of life and remain in a persistent without clinically apparent infection in most healthy individuals. Another limitation is that our analyses of antibody response are limited to persons seropositive to four pathogens. Because low education is associated with pathogen burden, the immune response sample is weighted towards persons of lower socioeconomic position. Thus limited variability in socioeconomic position in this subset together with small sample size may have limited our ability to detect associations with immune response. We also assessed whether socioeconomic and psychosocial factors were related to high immune response among subjects seropositive for only two pathogens (CMV and HSV-1) and found similar trends. The measurement of psychosocial factors is always a challenge. Although the chronic stress measure we used has been linked to biological markers in the MESA sample (Ranjit et al., 2007), it undoubtedly has important measurement error and could have severely limited our ability to detect associations. Future studies should include larger sample sizes and validated and more detailed measures of chronic stress.
In conclusion, socioeconomic and psychosocial gradients in pathogen burden and immune response exist among generally healthy adults. Higher pathogen burden and heightened antibody response to multiple pathogens implicated in cardiovascular disease may increase risk of infection-related inflammation and cardiovascular damage. Our results suggest that pathogen burden and heightened immune response to co-infection may be biological pathways by which the effects of low socioeconomic position and chronic stress ultimately lead to disparities in cardiovascular morbidity and mortality. Studies examining intervention targets for social and biological pathways related to persistent infection are warranted.
Acknowledgments
We acknowledge Amber K. Price, Vicki J Lawrence and Amanda Simanek for help with organizing initial data analyses.
Funding Sources. These analyses were supported in part by R01 HL076831 from the National Heart Lung and Blood Institute to A.D.R. The Multi-ethnic Study of Atherosclerosis is supported by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute. A.E. Aiello was supported by a grant from the Center for Integrative Approaches to Health Disparities 1P60MD00249-01.
Footnotes
Disclosures. None of the authors have conflicts of interest associated with this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam E, Melnick JL, Probtsfield JL, Petrie BL, Burek J, Bailey KR, McCollum CH, DeBakey ME. High levels of cytomegalovirus antibody in patients requiring vascular surgery for atherosclerosis. Lancet. 1987;2:291–293. doi: 10.1016/s0140-6736(87)90888-9. [DOI] [PubMed] [Google Scholar]
- Aiello AE, Haan M, Blythe L, Moore K, Gonzalez JM, Jagust W. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc. 2006;54:1046–1054. doi: 10.1111/j.1532-5415.2006.00796.x. [DOI] [PubMed] [Google Scholar]
- Aiello AE, Haan MN, Pierce CM, Simanek AM, Liang J. Persistent infection, inflammation, and functional impairment in older Latinos. J Gerontol A Biol Sci Med Sci. 2008;63:610–618. doi: 10.1093/gerona/63.6.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht T, Deng CZ, Abdel-Rahman SZ, Fons M, Cinciripini P, El-Zein RA. Differential mutagen sensitivity of peripheral blood lymphocytes from smokers and nonsmokers: effect of human cytomegalovirus infection. Environmental and molecular mutagenesis. 2004;43:169–178. doi: 10.1002/em.20012. [DOI] [PubMed] [Google Scholar]
- Appels A, Bar FW, Bar J, Bruggeman C, de Baets M. Inflammation, depressive symptomtology, and coronary artery disease. Psychosom Med. 2000;62:601–605. doi: 10.1097/00006842-200009000-00001. [DOI] [PubMed] [Google Scholar]
- Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- Bromberger JT, Matthews KA. A longitudinal study of the effects of pessimism, trait anxiety, and life stress on depressive symptoms in middle-aged women. Psychology and aging. 1996;11:207–213. doi: 10.1037//0882-7974.11.2.207. [DOI] [PubMed] [Google Scholar]
- Christensen U, Lund R, Damsgaard MT, Holstein BE, Ditlevsen S, Diderichsen F, Due P, Iversen L, Lynch J. Cynical hostility, socioeconomic position, health behaviors, and symptom load: a cross-sectional analysis in a Danish population-based study. Psychosom Med. 2004;66:572–577. doi: 10.1097/01.psy.0000126206.35683.d1. [DOI] [PubMed] [Google Scholar]
- Cohen S, Frank E, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr Types of stressors that increase susceptibility to the common cold in healthy adults. Health Psychol. 1998;17:214–223. doi: 10.1037//0278-6133.17.3.214. [DOI] [PubMed] [Google Scholar]
- Cohen S, Herbert TB. Health psychology: psychological factors and physical disease from the perspective of human psychoneuroimmunology. Annu Rev Psychol. 1996;47:113–142. doi: 10.1146/annurev.psych.47.1.113. [DOI] [PubMed] [Google Scholar]
- Diez Roux AV, Ranjit N, Powell L, Jackson S, Lewis TT, Shea S, Wu C. Psychosocial factors and coronary calcium in adults without clinical cardiovascular disease. Ann Intern Med. 2006;144:822–831. doi: 10.7326/0003-4819-144-11-200606060-00008. [DOI] [PubMed] [Google Scholar]
- Ekesbo R, Nilsson PM, Lindholm LH, Persson K, Wadstrom T. Combined seropositivity for H. pylori and C. pneumoniae is associated with age, obesity and social factors. J Cardiovasc Risk. 2000;7:191–195. doi: 10.1177/204748730000700305. [DOI] [PubMed] [Google Scholar]
- Epstein SE. The multiple mechanisms by which infection may contribute to atherosclerosis development and course. Circ Res. 2002;90:2–4. [PubMed] [Google Scholar]
- Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- Fernandez-Real JM, Lopez-Bermejo A, Vendrell J, Ferri MJ, Recasens M, Ricart W. Burden of infection and insulin resistance in healthy middle-aged men. Diabetes Care. 2006;29:1058–1064. doi: 10.2337/diacare.2951058. [DOI] [PubMed] [Google Scholar]
- Georges JL, Rupprecht HJ, Blankenberg S, Poirier O, Bickel C, Hafner G, Nicaud V, Meyer J, Cambien F, Tiret L. Impact of pathogen burden in patients with coronary artery disease in relation to systemic inflammation and variation in genes encoding cytokines. Am J Cardiol. 2003;92:515–521. doi: 10.1016/s0002-9149(03)00717-3. [DOI] [PubMed] [Google Scholar]
- Gerra G, Monti D, Panerai AE, Sacerdote P, Anderlini R, Avanzini P, Zaimovic A, Brambilla F, Franceschi C. Long-term immune-endocrine effects of bereavement: relationships with anxiety levels and mood. Psychiatry Res. 2003;121:145–158. doi: 10.1016/s0165-1781(03)00255-5. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK, Speicher CE, Holliday JE. Stress, loneliness, and changes in herpesvirus latency. J Behav Med. 1985;8:249–260. doi: 10.1007/BF00870312. [DOI] [PubMed] [Google Scholar]
- Greenglass ER. Anger, hostility and CHD: Implications for coronary heart disease. In: Brebner JMT, Greenglass E, Laungani P, O’Roark AM, editors. Stress and Emotion: Anxiety, Anger and Curiosity. Taylor & Francis; London: 1996. pp. 205–207. [Google Scholar]
- Greenglass ER, Julkunen J. Construct validity and sex differences in Cook-Medley hostility. Personality and Individual Differences. 1989;19:209–218. [Google Scholar]
- Herbert TB, Cohen S. Stress and immunity in humans: a meta-analytic review. Psychosom Med. 1993;55:364–379. doi: 10.1097/00006842-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Hinkle DE, Wiersma W, Jurs SG. Applied Statistics for the Behavioral Sciences. Houghton Mifflin Co; Boston: 1988. [Google Scholar]
- Jackson LA, Stewart LK, Solomon SL, Boase J, Alexander ER, Heath JL, McQuillan GK, Coleman PJ, Stewart JA, Shapiro CN. Risk of infection with hepatitis A, B or C, cytomegalovirus, varicella or measles among child care providers. Pediatr Infect Dis J. 1996;15:584–589. doi: 10.1097/00006454-199607000-00005. [DOI] [PubMed] [Google Scholar]
- Julkunen J, Salonen R, Kaplan GA, Chesney MA, Salonen JT. Hostility and the progression of carotid atherosclerosis. Psychosom Med. 1994;56:519–525. doi: 10.1097/00006842-199411000-00007. [DOI] [PubMed] [Google Scholar]
- Kaplan GA, Lynch JW. Socioeconomic considerations in the primordial prevention of cardiovascular disease. Prev Med. 1999;29:S30–35. doi: 10.1006/pmed.1999.0540. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: new perspectives from psychoneuroimmunology. Annu Rev Psychol. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J Aging Health. 1993;5:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- Levenstein S. The very model of a modern etiology: a biopsychosocial view of peptic ulcer. Psychosom Med. 2000;62:176–185. doi: 10.1097/00006842-200003000-00003. [DOI] [PubMed] [Google Scholar]
- Lin TM, Kuo CC, Chen WJ, Lin FJ, Eng HL. Seroprevalence of Chlamydia pneumoniae infection in Taiwan. J Infect. 2004;48:91–95. doi: 10.1016/s0163-4453(03)00129-4. [DOI] [PubMed] [Google Scholar]
- Malaty HM, El-Kasabany A, Graham DY, Miller CC, Reddy SG, Srinivasan SR, Yamaoka Y, Berenson GS. Age at acquisition of Helicobacter pylori infection: a follow-up study from infancy to adulthood. Lancet. 2002;359:931–935. doi: 10.1016/S0140-6736(02)08025-X. [DOI] [PubMed] [Google Scholar]
- Martin W. Linking causal concepts, study design, analysis and inference in support of one epidemiology for population health. Prev Vet Med. 2008;86:270–288. doi: 10.1016/j.prevetmed.2008.02.013. [DOI] [PubMed] [Google Scholar]
- McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health, aging, and social relations study. Psychosom Med. 2006;68:376–381. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- McQuillan GM, Kruszon-Moran D, Kottiri BJ, Curtin LR, Lucas JW, Kington RS. Racial and ethnic differences in the seroprevalence of 6 infectious diseases in the United States: data from NHANES III, 1988–1994. Am J Public Health. 2004;94:1952–1958. doi: 10.2105/ajph.94.11.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Freedland KE, Duntley S, Carney RM. Relation of depressive symptoms to C-reactive protein and pathogen burden (cytomegalovirus, herpes simplex virus, Epstein-Barr virus) in patients with earlier acute coronary syndromes. Am J Cardiol. 2005;95:317–321. doi: 10.1016/j.amjcard.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Muhlestein JB, Anderson JL, Hammond EH, Zhao L, Trehan S, Schwobe EP, Carlquist JF. Infection with Chlamydia pneumoniae accelerates the development of atherosclerosis and treatment with azithromycin prevents it in a rabbit model. Circulation. 1998;97:633–636. doi: 10.1161/01.cir.97.7.633. [DOI] [PubMed] [Google Scholar]
- Nieto FJ, Adam E, Sorlie P, Farzadegan H, Melnick JL, Comstock GW, Szklo M. Cohort study of cytomegalovirus infection as a risk factor for carotid intimal-medial thickening, a measure of subclinical atherosclerosis. Circulation. 1996;94:922–927. doi: 10.1161/01.cir.94.5.922. [DOI] [PubMed] [Google Scholar]
- Paldanius M, Leinonen M, Virkkunen H, Tenkanen L, Savykoski T, Manttari M, Saikku P. Chlamydia pneumoniae antibody levels before coronary events in the Helsinki Heart Study as measured by different methods. Diagnostic microbiology and infectious disease. 2006;56:233–239. doi: 10.1016/j.diagmicrobio.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Diez-Roux AV, Shea S, Cushman M, Seeman T, Jackson SA, Ni H. Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Arch Intern Med. 2007;167:174–181. doi: 10.1001/archinte.167.2.174. [DOI] [PubMed] [Google Scholar]
- Rothenbacher D, Brenner H, Hoffmeister A, Mertens T, Persson K, Koenig W. Relationship between infectious burden, systemic inflammatory response, and risk of stable coronary artery disease: role of confounding and reference group. Atherosclerosis. 2003;170:339–345. doi: 10.1016/s0021-9150(03)00300-9. [DOI] [PubMed] [Google Scholar]
- Schillinger JA, Xu F, Sternberg MR, Armstrong GL, Lee FK, Nahmias AJ, McQuillan GM, Louis ME, Markowitz LE. National seroprevalence and trends in herpes simplex virus type 1 in the United States, 1976–1994. Sex Transm Dis. 2004;31:753–760. doi: 10.1097/01.olq.0000145852.43262.c3. [DOI] [PubMed] [Google Scholar]
- Siscovick DS, Schwartz SM, Corey L, Grayston JT, Ashley R, Wang SP, Psaty BM, Tracy RP, Kuller LH, Kronmal RA. Chlamydia pneumoniae, herpes simplex virus type 1, and cytomegalovirus and incident myocardial infarction and coronary heart disease death in older adults : the Cardiovascular Health Study. Circulation. 2000;102:2335–2340. doi: 10.1161/01.cir.102.19.2335. [DOI] [PubMed] [Google Scholar]
- Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Shamaei-Tousi A, Gylfe A, Henderson B, Bergstrom S, Marmot M. Socioeconomic status, pathogen burden and cardiovascular disease risk. Heart (British Cardiac Society) 2007;93:1567–1570. doi: 10.1136/hrt.2006.113993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe RP, Mehta SK, Ferrando AA, Feeback DL, Pierson DL. Immune responses and latent herpesvirus reactivation in spaceflight. Aviat Space Environ Med. 2001;72:884–891. [PubMed] [Google Scholar]
- Takaoka N, Campbell LA, Lee A, Rosenfeld ME, Kuo CC. Chlamydia pneumoniae infection increases adherence of mouse macrophages to mouse endothelial cells in vitro and to aortas ex vivo. Infect Immun. 2008;76:510–514. doi: 10.1128/IAI.01267-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripepi G, Jager KJ, Dekker FW, Zoccali C. Testing for causality and prognosis: etiological and prognostic models. Kidney Int. 2008 doi: 10.1038/ki.2008.416. [DOI] [PubMed] [Google Scholar]
- Xu F, Schillinger JA, Sternberg MR, Johnson RE, Lee FK, Nahmias AJ, Markowitz LE. Seroprevalence and coinfection with herpes simplex virus type 1 and type 2 in the United States, 1988–1994. J Infect Dis. 2002;185:1019–1024. doi: 10.1086/340041. [DOI] [PubMed] [Google Scholar]
- Zajacova A, Dowd JB, Aiello AE. Socioeconomic and race/ethnic patterns in persistent infection burden among U.S. adults. J Gerontol A Biol Sci Med Sci. doi: 10.1093/gerona/gln012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Nieto FJ, Horne BD, Anderson JL, Muhlestein JB, Epstein SE. Prospective study of pathogen burden and risk of myocardial infarction or death. Circulation. 2001;103:45–51. doi: 10.1161/01.cir.103.1.45. [DOI] [PubMed] [Google Scholar]
- Zhu J, Quyyumi AA, Norman JE, Csako G, Waclawiw MA, Shearer GM, Epstein SE. Effects of total pathogen burden on coronary artery disease risk and C-reactive protein levels. Am J Cardiol. 2000;85:140–146. doi: 10.1016/s0002-9149(99)00653-0. [DOI] [PubMed] [Google Scholar]