Abstract
One potential physiological target for new antischistosomals is the parasite’s system for excretion of wastes and xenobiotics. P-glycoprotein (Pgp), a member of the ATP-binding cassette superfamily of proteins, is an ATP-dependent efflux pump involved in transport of toxins and xenobiotics from cells. In vertebrates, increased expression of Pgp is associated with multidrug resistance in tumor cells. Pgp may also play a role in drug resistance in helminths. In this report, we examine the relationship between praziquantel (PZQ), the current drug of choice against schistosomiasis, and Pgp expression in Schistosoma mansoni. We show that levels of RNA for SMDR2, a Pgp homolog from S. mansoni, increase transiently in adult male worms following exposure to sublethal concentrations (100 – 500 nM) of PZQ. A corresponding, though delayed, increase in anti-Pgp immunoreactive protein expression occurs in adult males following exposure to PZQ. The level of anti-Pgp immunoreactivity in particular regions of adult worms also increases in response to PZQ. Adult worms from an Egyptian S. mansoni isolate with reduced sensitivity to PZQ express increased levels of SMDR2 RNA and anti-Pgp-immunoreactive protein, perhaps indicating a role for multidrug resistance proteins in development or maintenance of PZQ resistance.
Keywords: Schistosoma mansoni, P-glycoprotein, multidrug resistance, praziquantel, ABC transporter
INTRODUCTION
Trematode flatworms of the genus Schistosoma are the causative agents of schistosomiasis, a major tropical parasitic disease. Adult schistosomes reside in the blood vessels of the host, where, among other functions, they must take up nutrients and dispose of toxic metabolites.
The current drug of choice against schistosomiasis is praziquantel (PZQ), a pyrazinoisoquinoline anthelmintic that is active against all schistosome species, shows minimal side effects, and is also effective against other trematode and cestode infections [1–4]. Indeed, because of these advantages over other chemotherapeutics, PZQ has in recent years become effectively the only antischistosomal commercially available [5,6], making the prospect of emerging resistance to PZQ particularly troubling [7].
The export of biomolecules, including metabolite disposal, is commonly performed by members of the ATP-binding-cassette (ABC) superfamily of proteins. One of the members of this class, P-glycoprotein (Pgp), is an ATP-dependent efflux pump that in vertebrates serves as one of a set of major membrane transporters for toxic and xenobiotic compounds. Pgp is the product of the multidrug-resistance 1 (MDR1, ABCB1) gene, which is amplified and overexpressed in certain mammalian tumor cells that show broad drug resistance [8–11]. Pgp expression levels and allele frequencies are also altered in anthelmintic-resistant populations of nematodes [13–18], and the potential roles of Pgp in parasite drug resistance and as a possible site for pharmacological modulation in helminths have recently been reviewed [19–21].
Investigation of schistosome and platyhelminth Pgps and other drug transporters has been limited. Several years ago, two S. mansoni cDNAs coding for ABC proteins were cloned and sequenced [22]. One of these cDNAs (SMDR2) encodes a Pgp-like protein, with 12 transmembrane regions and two ATP-binding domains predicted. A partial ABC transporter sequence from the liver fluke, Fasciola hepatica has also been reported [23]. Sato et al [24, 25] have used fluorescent substrates of Pgp and multidrug resistance-like proteins (MRPs) to visualize the excretory system of S. mansoni. They also used compounds known to inhibit these transporter activities in mammals to perturb excretory function in schistosomes. PZQ has dramatic effects on excretion of resorufin, a likely Pgp substrate [26]. Thus, schistosome excretory activity, which is probably mediated by a Pgp-like molecule such as SMDR2, can be altered by PZQ, as well as other pharmacological and environmental agents. Furthermore, PZQ has been shown [27] to be an inhibitor of Pgp-mediated [3H]-taxol transport in a human intestinal epithelial cell line, with a Kiapp = 20 μM (the Kiapp value represents the extracellular concentration of test compound bringing about 50% inhibition of active transport; unlike an IC50 value, it is independent of the affinity and concentration of the reference substrate).
Here, we examine the relationship between PZQ and schistosome Pgp in more detail, by examining the expression patterns of SMDR2 RNA and anti-Pgp immunoreactive protein in response to PZQ. We find that exposure of adult schistosomes to a sub-lethal concentration of PZQ results in transiently increased expression of the SMDR2 transcript as well as a ~140 kDa band that is immunolabeled by a monoclonal antibody against conserved epitopes of Pgp. In addition, we show that adults from EE2, an Egyptian S. mansoni isolate with reduced susceptibility to PZQ [28], expresses significantly higher levels of SMDR2 RNA and anti-Pgp immunoreactive protein than adults from control, PZQ-sensitive strains.
MATERIALS AND METHODS
Reagents
Praziquantel (Sigma) was dissolved in dimethyl sulfoxide for stock solutions, which were subsequently diluted to an appropriate concentration in culture media. The mouse monoclonal antibody against Pgp [29] was from Abcam (C219). The anti-rabbit tubulin antibody was from Santa Cruz Biotechnology (H-235).
Isolation and treatment of adult schistosomes
Female Swiss Webster mice infected with S. mansoni (NMRI strain) were obtained from the NIAID Schistosomiasis Resource Center at the Biomedical Research Institute in Rockville, MD. Adult S. mansoni were collected by perfusion, as described [30], and maintained in RPMI (Invitrogen) plus 10% FBS (Sigma) at 37°C and 5% CO2. Following an overnight incubation, worms were exposed to PZQ for variable periods and at different concentrations. In some experiments, the mixed worm population was separated into male and female groups, and then exposed to PZQ for various time points. Following incubation, adults were either used for RNA and protein extraction immediately, or quick-frozen in liquid nitrogen and stored at −80°C until use. Both the EE2 and CD1 worms were obtained from the Theodor Bilharz Research Institute, Giza, Egypt. EE2 worms were originally isolated from Egyptian patients not cured following three successive doses of PZQ. These worms were also shown to exhibit an approximately threefold reduction in PZQ susceptibility when tested in murine infections [31] as well as reduced susceptibility to PZQ in vitro [28]. Subsequently, following maintenance of the EE2 isolate over several years and through multiple laboratory life cycles without exposure to PZQ, EE2 worms continued to exhibit approximately three-fold reduced susceptibility to PZQ compared to PZQ-susceptible worms [32]. The PZQ-susceptible CD1 isolate has been maintained at the Theodor Bilharz Research Institute, Giza, Egypt for more than two decades, and has not knowingly been in contact with PZQ. These adult worms were recovered by perfusion, placed in RNAlater (Ambion), and stored first at room temperature and then at −20°C until use.
RNA extractions and RT-PCR
RNA was extracted from adult schistosomes using either RNAqueous-4-PCR (Ambion) or the PARIS miRVANA kit (Ambion) using the manufacturer’s instructions. All RNA preparations were treated with either DNAase 1 or Turbo-DNAase (Ambion) and DNAase Inactivation Reagent (Ambion) prior to reverse transcription. Equal amounts of total RNA from each sample were primed with 500 ng random hexamers (Promega) and were reverse-transcribed using SuperScript II reverse transcriptase (Invitrogen) at 42°for 1 h. Sequences of interest were amplified from the cDNA using GoTaq Green Master Mix (Promega), which contains 200 μM dNTPs and 1.5 mM MgCl2 in the final reaction, with primers added to 10 μM. Alternatively, one-step RT-PCR (Reverse-it 1-step RT-PCR kit, ABgene) was used to amplify the sequences. Primers used for amplification of SMDR2 were: SMDR2 RT1 (5′-GGAGTCGCTGCAGGACGTTTAACT-3′; SMDR RT2 (5′-AGTGCACCAGGTCGATTGTCAGAT-3′); SMDR2-1 (5′-CCTATGGTGATAATAGTCGGA-3′); and SMDR2-2 (5′-CGATGTGCAATTATAATAGAATG-3′). Primers used for amplification of 18S ribosomal RNA were: 18S-1 (5′-TTAACGAGGACCAATTGGAGGG-3′; and 18S-2 (5′-CCCCGTCTGTCCCTCTTAACCA-3′). Following a 1 min hot start at 94°C, PCR conditions were as follows: 94°C for 30 sec, 50°C for 30 sec, 72°C for 1 min, with the appropriate number of cycles determined empirically. Band intensities were quantified using Quantity One software (BioRad).
Real-time RT-PCR
Real-time PCR using SYBR Green was performed using the Brilliant SYBR Green QPCR Reagent kit (Statagene) on an Opticon 2 Real-Time PCR Detection System (BioRad). S. mansoni glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference sequence in these experiments. Primers used for amplification of SMDR2 were: SmMDR2-Sy1 (5′-TGCTCTAGTCGGTTCTAGTGGTTCTG-3′) and SmMDR2-Sy2 (5′-GCATTAGCTTTGATGGCAGCTTCG-3′). Primers used for amplification of GAPDH cDNA were SmGAPDH-1 (5′-AATTATGGCGAGATGGCCGT-3′) and SmGAPDH-2 (5′-TTTGGCAGCACCAGTGGAA-3′). Following optimization experiments, PCR cycles were set as follows: 95°C 3 min, 95°C 30 s, 56.0°C 1 min, 45 cycles performed total, with a melting curve from 52°C–95°C included.
Real time PCR using Taqman probes was performed with either iQSupermix iSCript Reverse transcriptase for One step RT-PCR (Biorad), or the TaqMan One-Step RT-PCR kit (Applied Biosystems). S. mansoni 18S ribosomal RNA gene and GAPDH were used as reference sequences in these experiments. Primers used for amplification of SMDR2 were SMDR2-TM-F1 (5′-TCTGACAATCGACCTGGTG-3′) and SMDR2-TM-R1 (5′-CCAAGGAAGCAATGACTAAAAC-3′), and the probe was: 5′FAM-ACTGCATTTCTGTCCACAGATGCTTC-3′BHQ1. Primers used for amplification of GAPDH were: GAPDH-TM-F1 (5′-GCACTCATTTACGGCTACAC-3′) and GAPDH-TM-R1 (5′-GCTGGAATGACTTTTCCCAC-3′), with 5′HEX- ACCATCCTCAAAATTATGGCGAGATGGC-3′BHQ1 as the probe. Primers used for amplification of 18S RNA were: Sm-18S-TM-1 (5′-AGGAATTGACGGAAGGGCAC-3′) and Sm-18S-TM-2 (5′-ACCACCCACCGAATCAAGAAAG-3′), with 5′HEX-AGCCTGCGGTTTAATTCGACTCAACACGG-3′BHQ1 as the probe. PCR cycles were as follows: 50°C 10 min, 95°C 5 min., 95°C 13 min, 56°C 30min, with 45 cycles performed total. Experiments were performed on either an Opticon 2 Real-Time PCR Detection System (BioRad) or an Applied Biosystems 7500 Fast system. The templates for those experiments using CD1 and EE2 were amplified, adapter-ligated cDNA pools made from CD1 and EE2 total RNA. These cDNA samples were made using primers and protocols of Matz Method B [33]. Quantitative analysis of expression levels using this approach have been validated using “virtual northern blots”, and accurately reflect differential RNA expression patterns [34]. An equal amount of RNA from the two isolates was reverse-transcribed and amplified by PCR using Superscript II (Invitrogen) and the adapter-primers described in [33], and amplified by PCR. These cDNA pools were diluted 1:50 in water prior to use as templates for real time PCR experiments.
Analysis of real time RT-PCR data was carried out using the 2−ΔΔCt method, with appropriate calibrators and corrections for amplification efficiency, as described [35]. A one sample t-test was used to determine statistically significant differences from a value of 1 (P ≤ 0.05), which would indicate no change in expression. Data are presented as mean ± SEM.
Protein extraction and analysis
Protein was extracted using the PARIS miRVANA kit (Ambion) using the manufacturer’s instructions. Additionally, a protease inhibitor mix (Sigma) was added to the cell disruption buffer. Protein concentrations were determined using the Bio-Rad Protein assay (Bio-rad). Equal amounts of protein were loaded onto NuPage 4–12 % Bis-Tris gradient gels (Invitrogen), transferred to nitrocellulose at 30 V for 1 h, and reversibly stained using Memcode Reversible Protein Stain kit (Pierce). The membrane was blocked in 5% dry milk dissolved in phosphate-buffered saline containing 0.1% Tween 20 (PBST) at 4°C overnight. Blots were incubated with primary antibody for 2 h. at room temperature in 5% dry milk-PBST. Anti-mouse-Pgp (Abcam) was used at a 1:1000 dilution and anti-rabbit tubulin at a 1:200 dilution. Following three washes in PBS, blots were incubated in either peroxidase-conjugated goat anti- mouse IgG (1:10,000, Jackson ImmunoResearch) or goat anti-rabbit IgG (1:10,000, Jackson ImmunoResearch). Blots were then washed three times in PBS, incubated in Supersignal West Pico Chemiluminescent Substrate (Pierce) for 5 min., and exposed to CL-Xposure film X-ray film (Pierce). Band intensities were quantified using either Quantity One (BioRad) or Scion Image (Scioncorp) software.
Immunostaining
Adult S. mansoni (NMRI strain) were exposed for 18 hours in culture to either PZQ (100 nM) or control RPMI prior to fixation in 4% (w/v) paraformaldehyde in PBS for 1 hr. Worms were washed 6 times in 0.1 M PBS, pH 7.4, containing 0.1% (w/v) Triton X-100, 1% (w/v) BSA, and 0.1% NaN3 [36]. Schistosomes were incubated in the anti-mouse Pgp monoclonal antibody at a 1:40 dilution for 96 hr. at 4°C. Following an overnight wash, worms were treated with goat anti-mouse IgG-Rhodamine (Jackson ImmunoResearch; 1:200) for 48 hrs., washed, and mounted with PBS/glycerol. Controls consisted of omission of primary antisera.
RESULTS
Levels of SMDR2 RNA increase transiently in adult males in response to sub-lethal concentrations of PZQ
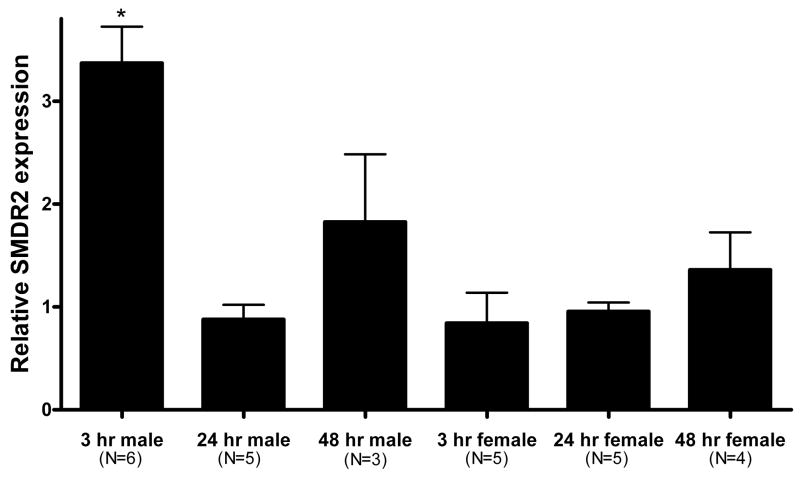
Adult S. mansoni in culture were exposed to a sublethal concentration of PZQ (100 nM) for 3h. Total RNA was extracted from these parasites, as well as controls, and reverse-transcribed into cDNA using random hexamers as primers. Initially, we found that a mixed-sex population of worms treated with PZQ showed increased levels of SMDR2 RNA as determined by RT-PCR. Further experiments in which male and female worms were separated prior to PZQ exposure showed that the increase in SMDR2 RNA levels is occurring only in males (Fig. 1). Real-time RT-PCR analysis shows a significant 3.37 ± 0.35-fold increase in SMDR2 expression in males following 3 h PZQ exposure. However, by 24 h, SMDR2 mRNA expression levels have returned to baseline levels (relative SMDR2 expression = 0.88 ± 0.14). We also typically see 2–5-fold higher SMDR2 RNA levels in control females (ie, incubated in RPMI medium without PZQ) compared to males, consistent with earlier observations by others [22]. For example, relative SMDR2 RNA levels are 3.0 ± 0.43-fold higher in 3h control female worms than in 3h control males (n = 4). There is also some indication of an increase in relative SMDR2 mRNA expression following a 48-h PZQ exposure, although the change is not significant (1.83 ± 0.66). Interestingly, a mixed-sex population of adults appears to show higher relative levels of SMDR2 RNA following 6 days in 100 nM PZQ (2.81-fold). However, as the elimination half-life of PZQ and its metabolites is on the order of hours [34], the relevance of any such long-term effects in actual treatment regimens is not clear.
Figure 1. Exposure of adult males to PZQ results in increased SMDR2 RNA expression.
Real time RT-PCR on S. mansoni adult males and females treated with 100 nM PZQ in vitro for 3 hours, 24 hours, or 48 hours using primers to SMDR2 and GAPDH. The 2−ΔΔCt method [32] was used to determine the relative expression ratio between SMDR2 and GAPDH mRNAs. Asterisk indicates a statistically significant difference from a value of 1, which would indicate no change (one sample t-test, P = 0.001).
Adult males exposed to different concentrations of PZQ show increased SMDR2 mRNA expression at 3 h, as measured by quantitative RT-PCR. Thus, at 300 nM PZQ, SMDR2 mRNA expression increases 2.17-fold, while 500 nM PZQ results in a 5.6-fold increase. As with 100 nM PZQ, the increase is transient. After exposure to 300 nM PZQ for 24 h, the ratio of SMDR2 mRNA expression compared to controls is 0.6. The level of expression following 24 h exposure to 500 nM PZQ could not be measured accurately due to excessive worm lethality. We have not yet determined a minimal PZQ concentration that can produce the transient increase in SMDR2 mRNA expression.
Levels of an S. mansoni anti-Pgp immunoreactive protein increase in males in response to PZQ
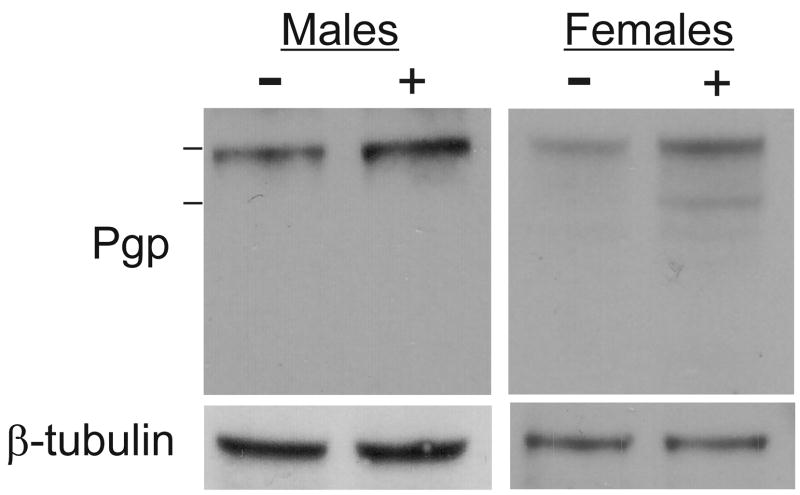
We have used western blots to measure relative levels of schistosome SMDR2 protein (or a cross-reacting Pgp-like protein species). For these experiments, we used a monoclonal antibody against mammalian Pgp [29]. This antibody targets two Pgp epitopes that are largely conserved in the SMDR2 sequence: VQAALD at the N-terminus (VQGALD in SMDR2); and VQEALD at the C-terminus (VQKALD in SMDR2). The anti-Pgp antibody detects a single, cross-reacting ~140 kDa band in protein extracts from adult S. mansoni (Figs. 2, 5), and detects SMDR2 recombinant protein (~140 kDa) expressed in mammalian cells (Kasinathan and Greenberg, unpublished results). Exposure of adult male schistosomes to 100 nM PZQ for 24 hours in culture results in an increase in relative levels of the Pgp-immunoreactive band compared to control adult male worms (Fig. 2). Average Pgp band intensity (relative to β-tubulin) increases 1.92 ± 0.57-fold (n = 5; P = 0.024, paired t-test). We sometimes see evidence for an increase in band intensity in females as well, though the change is not significant. Tubulin levels did not increase in response to PZQ. Interestingly, additional, anti-Pgp-immunoreactive bands of slightly lower molecular weight are sometimes apparent in these experiments following PZQ exposure, in both males and females (Fig. 2). An increase is also apparent in male worms exposed to PZQ for 48 h, though to a lesser extent, and no detectable changes in expression levels of anti-Pgp immunoreactivity are observed in S. mansoni exposed to PZQ for either 15 min or 3 h (data not shown). Interestingly, the higher level of SMDR2 RNA found in females does not appear to be reflected at the protein level, as there is no significant difference in relative band intensity (compared to β-tubulin) between control males and females.
Figure 2. Exposure of adult male schistosomes to PZQ results in increased levels of Pgp-immunoreactive protein.
Proteins were extracted from S. mansoni adult males and females following treatment of worms in vitro with 100 nM PZQ (+) or parallel controls (−) for 24 h. Proteins were electrophoresed and immunoblotted as described in Materials and Methods. Bars on left are molecular weight markers, from top, 160 kDa and 110 kDa. Primary antibodies were against conserved Pgp epitopes (Pgp) or against β-tubulin.
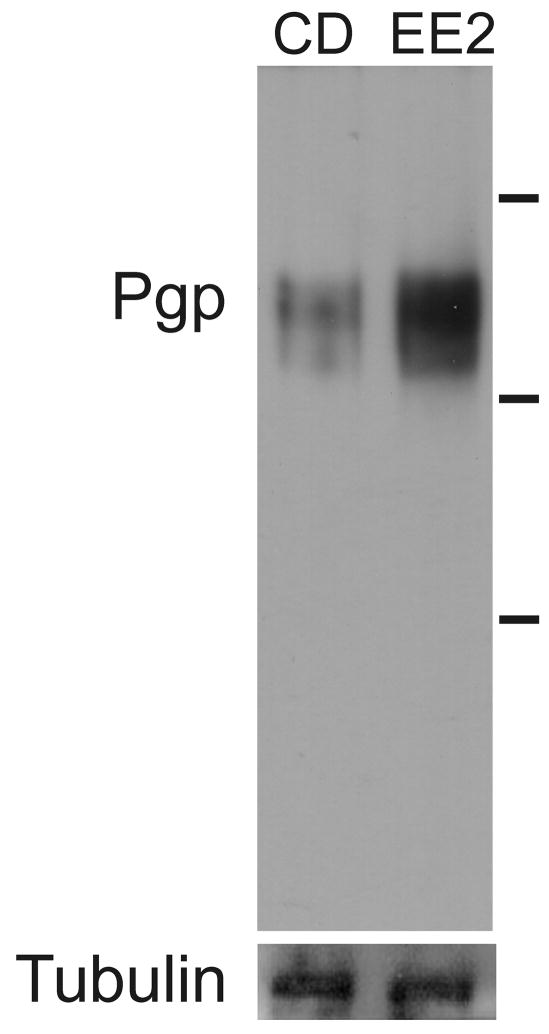
Figure 5. Expression of a Pgp-immunoreactive protein expression is higher in a S. mansoni isolate with reduced susceptibility to PZQ.
Proteins were extracted from S. mansoni CD1 and EE2 adults. Proteins were electrophoresed and immunoblotted as described in Materials and Methods. Primary antibodies were against conserved Pgp epitopes (Pgp) or against β-tubulin (Tubulin). Lines at right of image designate size markers. Top line = 188 kDa; middle line = 97 kDa; bottom line = 52 kDa.
Immunohistochemical labeling was used to localize changes in schistosome anti-Pgp immunoreactivity in whole mounts of adult schistosomes. Adult S. mansoni males exposed to 100 nM PZQ for 18 h showed prominent anti-Pgp immunoreactivity within the gut (Fig. 3A) and this labeling was also present surrounding the gut (Fig. 3D). This immunoreactivity was not detected in untreated control worms (Fig. 3B), nor in samples not incubated with the primary antibody (Fig. 3C).
Figure 3. PZQ exposure increases anti-Pgp immunoreactivity in adult worms.
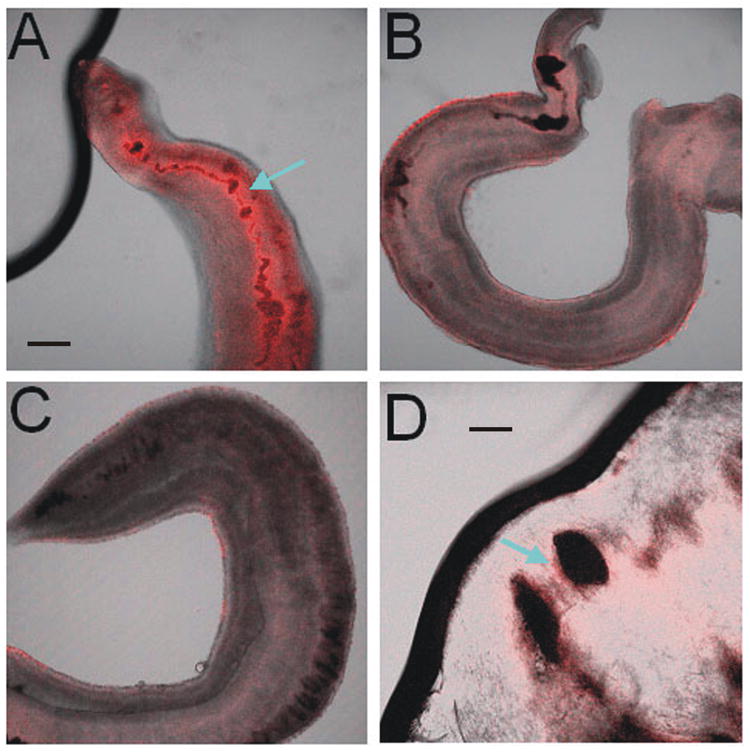
Immunostaining using the monoclonal antibody to conserved Pgp epitopes shows positive Pgp-like immunoreactivity in the gut of worms exposed to 100 nM PZQ for 18 h (A, arrow), which is not present in control schistosomes (B), or in worms incubated with secondary antibody alone (C). Black material in the gut in (B) is likely digested hemoglobin. Immunoreactivity also appears in the epithelial layer surrounding the gut (D, arrow). The thick black line (A) is an artifact, and the faint surface staining in control worms (B) appears inconsistently, and is likely also artifactual. A–C, scale bar = 60 μm, D, scale bar = 20 μm.
SMDR2 RNA and an anti-Pgp-immunoreactive protein are expressed at higher levels in a schistosome isolate with reduced PZQ susceptibility
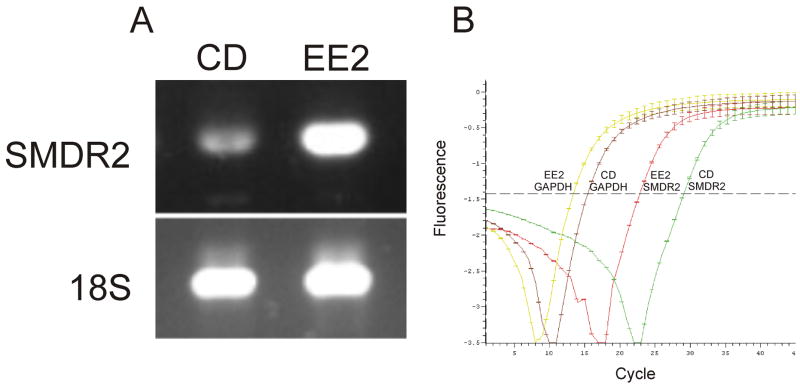
We examined the expression levels of S. mansoni SMDR2 RNA and anti-Pgp immunoreactive protein in adult EE2 and CD1 schistosomes. EE2 is an Egyptian isolate with reduced susceptibility to PZQ, while CD1 is a PZQ-sensitive isolate that has never been exposed to PZQ [38]. Semiquantitative RT-PCR indicates that SMDR2 RNA is expressed at higher levels in the EE2 worms (Fig. 4a). In contrast, 18S ribosomal RNA levels in EE2 and CD1 worms are relatively constant. Based on quantitation of band intensity and normalization to 18S band intensity, SMDR2 RNA is expressed at a 2.68 ± 0.75 fold higher level in EE2 worms compared with CD1 worms (P = 0.013, two-tailed t-test, n=5). SMDR2 RNA is also expressed at higher levels in EE2 worms than in adult worms from the NMRI strain of S. mansoni. Quantitation of band intensity, with normalization to 18S band intensity, indicates SMDR2 RNA is expressed at a 2.92-fold higher level in EE2 adults than in NMRI worms.
Figure 4. SMDR2 RNA transcript is expressed at higher levels in an isolate of S. mansoni with reduced PZQ susceptibility.
(A) RT-PCR amplification (35 cycles) of SMDR2 or 18S ribosomal RNA from adult parasites of the PZQ-sensitive CD1 isolate (CD), or the EE2 isolate, which has reduced sensitivity to PZQ. (B) Real-time RT-PCR amplification curves for SMDR2 and GAPDH cDNA pools from CD1 and EE2 isolates. Source of template and identity of amplified sequence are denoted on the plot.
We also tested relative SMDR2 RNA levels using real time quantitative PCR (Fig. 4b). In this case, highly representative, amplified cDNA samples [33] made from EE2 and CD1 schistosomes were used as templates. These types of cDNA samples have been verified by others to be accurate reflections of differential gene expression [34]. From these experiments, we determined that the normalized expression ratio of SMDR2 RNA, using GAPDH as the reference sequence, is 12.4 in the EE2 isolate compared to the CD1 isolate.
Anti-Pgp immunoreactive protein is also expressed at higher levels in the EE2 isolate (Fig. 5). In contrast, levels of β-tubulin are equivalent in EE2 and CD1 worms. This increased level of putative Pgp expression has been observed in four separate blots. Schistosome anti-Pgp immunoreactivity is also higher in EE2 adults compared to adult worms from the NMRI strain of S. mansoni (data not shown).
DISCUSSION
Multidrug resistance was originally characterized in mammalian tumor cells that had been selected for resistance to a single drug, but which also showed an unpredictable cross-resistance against several structurally unrelated compounds. A major mediator of this effect is Pgp, an ATP-dependent transporter that functions to remove toxic and xenobiotic compounds from cells. Alterations of Pgp expression levels and allele frequencies have also been implicated in drug resistance in various parasites, including helminths [17, 18]. We have demonstrated in this report that treatment of schistosomes with sublethal PZQ concentrations in vitro results in an apparently transient increase in SMDR2 RNA and anti-Pgp immunoreactive protein in adult male parasites. Increases in SMDR2 protein levels are not coincident with the increase in SMDR2 mRNA, indicating a delay between transcription of SMDR2 mRNA and translation and processing of SMDR2 protein. Our results also point to higher levels of Pgp being associated with reduced susceptibility to PZQ in S. mansoni.
It remains to be determined whether this increased Pgp level is involved in the mechanism by which PZQ resistance can develop, or is, instead, simply a correlate of this process. However, the apparent upregulation of SMDR2 RNA and Pgp protein in adult parasites following exposure to PZQ, as well as the recent observation that PZQ is an inhibitor of mammalian Pgp [27], indicate that PZQ may be interacting in important ways with schistosome Pgp transporters. If PZQ is also an inhibitor, or possibly a substrate, of schistosome Pgp, increased Pgp levels might serve as a mechanism by which the parasite can escape the effects of standard or reduced doses of PZQ. If PZQ does inhibit schistosome Pgp, the worm may respond by increasing Pgp levels to compensate for the deleterious effects of this reduced excretory activity. If, instead, or in addition, PZQ is a substrate for Pgp, then upregulated expression of Pgp (and perhaps other drug transporters) may help clear the drug from cells. Interestingly, we find that PZQ treatment often results in the appearance of a second, slightly lower MW anti-Pgp immunoreactive protein on western blots. This band may represent a second cross-reacting Pgp-like protein, or possibly a differentially modified (eg, glycosylated) form of SMDR2, and may be involved in mediating parasite defenses against PZQ.
PZQ is the drug of choice against schistosomiasis and has more recently become effectively the sole treatment available for the disease. However, approximately three decades following its introduction, a detailed mode of action for PZQ remains elusive, and reports of emerging PZQ resistance are a cause of particular concern [7, 39, 40]. One strategy for overcoming drug resistance might be to potentiate the effectiveness of current drugs by including additional agents targeted against different, but perhaps interacting, sites of action, or against cellular components, such as Pgp, that regulate rates of drug efflux. Indeed, PZQ has been shown to affect excretion of Pgp substrates in schistosomes [26], perhaps a functional correlate of the effects we see on Pgp expression. Although investigation of the effects of higher, more clinically relevant PZQ concentrations (eg, ≥500 nM) on Pgp expression would have been interesting, those concentrations result in significant worm lethality over the extended periods examined in these experiments. In this study, we were particularly interested in examining the effects on adult worms of exposure to sublethal concentrations of PZQ in order to begin the dissection of the physiological responses of schistosomes to PZQ, and also the possible mechanisms by which PZQ resistance might develop. Further experiments examining parasite responses within the host should be particularly informative in this regard. Additional experiments on other worm stages that are less susceptible to PZQ, as well as a more detailed examination of SMDR2 levels in various schistosome isolates with lower sensitivity to PZQ may help define the role these transporters might play in reduced drug susceptibility. It will also be interesting to determine effects of PZQ on other schistosome drug and xenobiotic transporters (eg, multidrug resistance-like proteins) and interacting proteins, and whether other Pgp substrates and inhibitors potentiate or reduce PZQ antischistosomal activity.
In this study, we have investigated the effects of PZQ on S. mansoni SMDR2 expression. However, the functional and pharmacological properties of SMDR2 and other schistosome drug transporters remain to be determined. Direct functional assays of schistosome drug transporters, along with genetic manipulation, may provide more complete information about the interplay of PZQ and these proteins, and may also provide clues for development of novel anthelmintics.
Acknowledgments
SMM, RSK, WM, and RMG were supported by NIH grants R01 AI40522 and R01 AI 73660. RMG was also supported by the Neal Cornell Research Fund at the Marine Biological Laboratory. RMG and SMM were also supported in part by NIH/NSF Woods Hole Center for Oceans and Human Health grant WHOI-A100354/A100360. Support was also received from the NIH Biocurrents Research Center at MBL (P41 RR001395). We thank Fred Lewis and the NIAID Schistosome Resource Center for supplying the schistosome life cycle and Vicenta Salvador-Recatala for helpful comments. We thank Sanaa Botros for supplying EE2 and CD1 schistosomes.
List of abbreviations
- PZQ
Praziquantel
- Pgp
P-glycoprotein
- ABC
ATP-binding-cassette
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greenberg RM. Praziquantel: Mechanism of action. In: Maule A, Marks NJ, editors. Parasitic Flatworms: Molecular Biology, Biochemistry, Immunology and Physiology. Oxfordshire, UK: CAB International; 2006. pp. 269–281. [Google Scholar]
- 2.Greenberg RM. Are Ca2+ channels targets of praziquantel action? Int J Parasitol. 2005;35:1–9. doi: 10.1016/j.ijpara.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Cioli D, Pica-Mattoccia L. Praziquantel. Parasitol Res. 2003;90(Supp 1):S3–S9. doi: 10.1007/s00436-002-0751-z. [DOI] [PubMed] [Google Scholar]
- 4.Andrews P. Praziquantel: mechanisms of anti-schistosomal activity. Pharmacol & Ther. 1985;29:129–156. doi: 10.1016/0163-7258(85)90020-8. [DOI] [PubMed] [Google Scholar]
- 5.Fenwick A, Savioli L, Engels D, et al. Drugs for the control of parasitic diseases: current status and development in schistosomiasis. Trends Parasitol. 2003;19:509–515. doi: 10.1016/j.pt.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Hagan P, Appleton CC, Coles GC, et al. Schistosomiasis control: keep taking the tablets. Trends Parasitol. 2004;20:92–97. doi: 10.1016/j.pt.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Day TA, Botros S. Drug resistance in schistosomes. In: Maule A, Marks NJ, editors. Parasitic Flatworms: Molecular Biology, Biochemistry, Immunology and Physiology. Oxfordshire, UK: CAB International; 2006. pp. 256–268. [Google Scholar]
- 8.Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22:7468–7485. doi: 10.1038/sj.onc.1206948. [DOI] [PubMed] [Google Scholar]
- 9.Leonard GD, Fojo T, Bates SE. The role of ABC transporters in clinical practice. The Oncologist. 2003;8:411–424. doi: 10.1634/theoncologist.8-5-411. [DOI] [PubMed] [Google Scholar]
- 10.Lin JH, Yamazaki M. Clinical relevance of P-glycoprotein in drug therapy. Drug Metab Rev. 2003;35:417–454. doi: 10.1081/dmr-120026871. [DOI] [PubMed] [Google Scholar]
- 11.Paulsen IT. Multidrug efflux pumps and resistance: regulation and evolution. Current Opinion Microbiol. 2003;6:446–451. doi: 10.1016/j.mib.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Blackhall WJ, Liu HY, Xu M, et al. Selection at a P-glycoprotein gene in ivermectin- and moxidectin-selected strains of Haemonchus contortus. Mol Biochem Parasitol. 1998;95:193–201. doi: 10.1016/s0166-6851(98)00087-5. [DOI] [PubMed] [Google Scholar]
- 13.Xu M, Molento M, Blackhall W, et al. Ivermectin resistance in nematodes may be caused by alteration of P-glycoprotein homolog. Mol Biochem Parasitol. 1998;91:327–335. doi: 10.1016/s0166-6851(97)00215-6. [DOI] [PubMed] [Google Scholar]
- 14.Sangster NC, Bannan SC, Weiss AS, et al. Haemonchus contortus: sequence heterogeneity of internucliotide binding domains from P-glycoproteins and an association with avermectin/milbemycin resistance. Exp Parasitol. 1999;91:250–257. doi: 10.1006/expr.1998.4373. [DOI] [PubMed] [Google Scholar]
- 15.Smith JM, Prichard RK. Localization of p-glycoprotein mRNA in the tissues of Haemonchus contortus adult worms and its relative abundance in drug-selected and susceptible strains. J Parasitol. 2002;88:612–620. doi: 10.1645/0022-3395(2002)088[0612:LOPGMI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Prichard RK, Roulet A. ABC transporters and beta-tubulin in macrocyclic lactone resistance: prospects for marker development. Parasitol. 2007;134:1123–1132. doi: 10.1017/S0031182007000091. [DOI] [PubMed] [Google Scholar]
- 17.Blackhall WJ, Prichard RK, Beech RN. P-glycoprotein selection in strains of Haemonchus contortus resistant to benzimidazoles. Veterinary Parasitology. 2008;152:101–107. doi: 10.1016/j.vetpar.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Bourguinat C, Ardelli BF, Pion SD, Kamgno J, Gardon J, Duke BO, Boussinesq M, Prichard RK. P-glycoprotein-like protein, a possible genetic marker for ivermectin resistance selection in Onchocerca volvulus. Molec Biochem Parasitol. 2008;158:101–111. doi: 10.1016/j.molbiopara.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 19.Kerboeuf D, Blackhall W, Kaminsky R, von Samson-Himmelstjerna G. P-glycoprotein in helminths: function and perspectives for anthelmintic treatment and reversal of resistance. Int J Antimicrob Agents. 2003;22:332–346. doi: 10.1016/s0924-8579(03)00221-8. [DOI] [PubMed] [Google Scholar]
- 20.Jones PM, George AM. Multidrug resistance in parasites: ABC transporters, P-glycoproteins and molecular modelling. Int J Parasitol. 2005;35:555–566. doi: 10.1016/j.ijpara.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Lespine A, Alvinerie M, Vercruysse J, Prichard RK, Geldhof P. ABC transporter modulation: a strategy to enhance the activity of macrocyclic lactone anthelmintics. Trends in Parasitology. 2008;24:293–298. doi: 10.1016/j.pt.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Bosch IB, Wang Z-X, Tao L-F, Shoemaker CB. Two Schistosoma mansoni cDNAs encoding ATP-binding cassette (ABC) family proteins. Molec Biochem Parasitol. 1994;65:351–356. doi: 10.1016/0166-6851(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 23.Reed MB, Panaccio M, Strugnell RA, Spithill TW. Developmental expression of a Fasciola hepatica sequence homologous to ABC transporters. Int J Parasitol. 1998;28:1375–1381. doi: 10.1016/s0020-7519(98)00102-7. [DOI] [PubMed] [Google Scholar]
- 24.Sato H, Kusel JR, Thornhill J. Functional visualization of the excretory system of adult Schistosoma mansoni by the fluorescent marker resorufin. Parasitology. 2002;125:527–535. doi: 10.1017/s0031182002002536. [DOI] [PubMed] [Google Scholar]
- 25.Sato H, Kusel JR, Thornhill J. Excretion of fluorescent substrates of mammalian multidrug resistance-associated protein (MRP) in the Schistosoma mansoni excretory system. Parasitology. 2004;128:43–52. doi: 10.1017/s0031182003004177. [DOI] [PubMed] [Google Scholar]
- 26.Oliviera FA, Kusel JR, Ribeiro F, Coelho PMZ. Responses of the surface membrane and excretory system of Schistosoma mansoni to damage and to treatment with praziquantel and other biomolecules. Parasitology. 2006;132:321–330. doi: 10.1017/S0031182005009169. [DOI] [PubMed] [Google Scholar]
- 27.Hayeshi R, Masimirembwa C, Mukanganyama S, Ungell ALB. The potential inhibitory effect of antiparasitic drugs and natural products on P-glycoprotein mediated efflux. Eur J Pharmaceut Sci. 2006;29:70–81. doi: 10.1016/j.ejps.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Ismail M, Botros S, Metwally A, et al. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am J Trop Med Hyg. 1999;60:932–935. doi: 10.4269/ajtmh.1999.60.932. [DOI] [PubMed] [Google Scholar]
- 29.Georges E, Bradley G, Gariepy J, Ling V. Detection of P-glycoprotein isoforms by gene-specific monoclonal antibodies. Proc Natl Acad Sci USA. 1990;87:152–156. doi: 10.1073/pnas.87.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis FA. Schistosomiasis. In: Coligan JE, Kruisbeek AM, Margulies DH, et al., editors. Current Protocols in Immunology. New York: John Wiley & Sons, Inc; 1998. pp. 19.1.1–19.1.28. [Google Scholar]
- 31.Ismail M, Metwally A, Farghaly A, et al. Characterization of isolates of Schistosoma mansoni from Egyptian villagers that tolerate high doses of praziquantel. Am J Trop Med Hyg. 1996;55:214–218. doi: 10.4269/ajtmh.1996.55.214. [DOI] [PubMed] [Google Scholar]
- 32.Cioli D, Botros S, Wheatcroft-Francklow K, et al. Determination of ED50 values for praziquantel in praziquantel-resistant and -susceptible Schistosoma mansoni isolates. Int J Parasitol. 2004;34L:979–987. doi: 10.1016/j.ijpara.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Matz MV. Amplification of representative cDNA samples from microscopic amounts of invertebrate tissue to search for new genes. Methods in Molecular Biology. 2002;183:3–18. doi: 10.1385/1-59259-280-5:003. [DOI] [PubMed] [Google Scholar]
- 34.Franz O, Bruchhaus I, Roeder T. Verification of differential gene transcription using virtual northern blotting. Nucleic Acids Research. 1999;27:e3. doi: 10.1093/nar/27.11.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative Ct method. Nature Protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 36.Mair GR, Maule AG, Day TA, Halton DW. A confocal microscopical study of the musculature of adult Schistosoma mansoni. Parasitology. 2000;121:163–170. doi: 10.1017/s0031182099006174. [DOI] [PubMed] [Google Scholar]
- 37.Leopold G, Ungethüm W, Groll E, et al. Clinical pharmacology in normal volunteers of praziquantel, a new drug against schistosomes and cestodes. An example of a complex study covering both tolerance and pharmacokinetics. Eur J Clin Pharmacol. 1978;14:281–291. doi: 10.1007/BF00560463. [DOI] [PubMed] [Google Scholar]
- 38.William S, Sabra A, Ramzy F, et al. Stability and reproductive fitness of Schistosoma mansoni isolates with decreased sensitivity to praziquantel. Int J Parasitol. 2001;31:1093–1100. doi: 10.1016/s0020-7519(01)00215-6. [DOI] [PubMed] [Google Scholar]
- 39.Cioli D. Praziquantel: is there real resistance and are there alternatives? Curr Opin Infect Dis. 2000;13:659–663. doi: 10.1097/00001432-200012000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Doenhoff MJ, Kusel JR, Coles GC, Cioli D. Resistance of Schistosoma mansoni to praziquantel: is there a problem? Trans Royal Soc Trop Med Hyg. 2002;96:465–469. doi: 10.1016/s0035-9203(02)90405-0. [DOI] [PubMed] [Google Scholar]