Abstract
Curcumin (diferuloylmethane), a yellow pigment in turmeric, has been shown to inhibit the activation of NF-κB, a transcription factor closely linked to chemoresistance in multiple myeloma (MM) cells. Whether curcumin can overcome chemoresistance and enhance the activity of thalidomide and bortezomib, used to treat patients with MM, was investigated in vitro and in xenograft model in nude mice. Our results show that curcumin inhibited the proliferation of human MM cells regardless of their sensitivity to dexamethasone, doxorubicin, or melphalan. Curcumin also potentiated the apoptotic effects of thalidomide and bortezomib by downregulating the constitutive activation of NF-κB and Akt; and this correlated with the suppression of NF-κB-regulated gene products, including cyclin D1, Bcl-xL, Bcl-2, TRAF1, cIAP-1, XIAP, survivin, and VEGF. Furthermore, in a nude mice model, we found that curcumin potentiated the antitumor effects of bortezomib (P < 0.001, vehicle vs. bortezomib plus curcumin; P < 0.001, bortezomib vs. bortezomib plus curcumin), and this correlated with suppression of Ki-67 (P < 0.001 vs. control), CD31 (P < 0.001 vs. vehicle), and VEGF (P < 0.001 vs. vehicle) expression. Collectively, our results suggest that curcumin overcomes chemoresistance and sensitizes MM cells to thalidomide and bortezomib by downregulating NF-κB and NF-κB-regulated gene products.
Keywords: Curcumin, multiple myeloma, NF-κB, chemotherapy
Introduction
In the United States, 50,000 patients are affected by multiple myeloma (MM), 16,000 patients are newly diagnosed with MM, and 11,000 MM patients die of the disease each year (1). MM is a late-stage B-cell malignancy characterized by the infiltration of malignant plasma cells in bone marrow, and is associated with high monoclonal (M) protein in the blood. MM can occur de novo or it can evolve from benign monoclonal gammapathy of undetermined significance (MGUS) that involves in low levels of bone marrow plasmacytosis and M protein. Approximately 1% of patients with MGUS develop MM each year. The current standard treatments for MM are high-dose chemotherapy and stem cell transplantation. Although a wide range of new drugs, including bortezomib (Velcade), thalidomide (Thalomid), and lenalidomide (Revlimid), a thalidomide analogue, have been recently approved, MM remains an incurable disease (2). The majority of MM patients eventually experience a relapse, their disease becomes chemoresistant, and they die of the disease.
Why MM patients develop resistance to chemotherapy is not well understood, but numerous lines of evidence suggest that the transcription factor NF-κB may plays a major role in pathogenesis of MM. First, NF-κB has been shown to be constitutively active in MM cell lines(3) and in CD138+ MM cells obtained from patients (4). Second, the NF-κB-regulated cyclin D1 gene is frequently dysregulated in MM patients (5). Third, IL-6, which is also regulated by NF-κB, can suppress apoptosis in MM cells (6). Fourth, the NF-κB-regulated antiapoptotic proteins, C-reactive protein (CRP), and cell adhesion molecules have been linked to chemoresistance in MM cells (7–9). Fifth, two recent reports used a whole genome-based screen approach and showed that cells from MM patients have alterations in numerous genes that control the constitutive activation of NF-κB (10, 11). The mechanisms involved are the inactivation of TRAF2, TRAF3, CYLD, cIAP-1, and cIAP-2 and the activation of NF-κ B1, NF-κ B2, CD40, LTBR, TACI, and NIK. Sixth, NF-κB signaling in stromal cells can lead to production of IL-6, BAFF, or APRIL, which can activate NF-κB and cause proliferation of MM cells (12). Based on these lines of evidence, agents that can inhibit NF-κB activation and are pharmacologically safe have the potential to overcome chemoresistance and potentiate the antitumor effects of existing chemotherapeutic agents, such as thalidomide and bortezomib.
Because of its ability to suppress NF-κB activation (13), we hypothesize that curcumin (diferuloylmethane), a pharmacologically safe agent, can reverse chemoresistance and potentiate effects of existing chemotherapy. First, curcumin can suppress NF-κB activation and inhibit the proliferation of MM cells (3, 4). Additional support for this hypothesis include the following: curcumin can downregulate cyclin D1 expression (14); curcumin has been shown to suppress the expression of IL-6 (15); curcumin can inhibit cell survival, proliferation, invasion, and angiogenic gene products (16); curcumin can downregulate the activation of STAT3 (4, 15), a transcription factor that has also been linked with chemoresistance in MM cells (17); curcumin binds directly to P-glycoprotein, inhibits drug transport (18). In addition, a clinical trial of curcumin showed that it is safe even when consumed at 12 g/day (19). Furthermore, the administration of curcumin at a dose of 2 g/day for 4 weeks to asymptomatic MM patients downregulated NF-κB activation in peripheral blood lymphocytes (20).
We tested our hypothesis by examining whether curcumin overcomes the resistance of MM cells to chemotherapy and potentiates the antitumor effects of thalidomide and bortezomib. Our results show that curcumin can overcome the chemoresistance of MM cells to conventional chemotherapeutic agents and potentiate the effects of bortezomib and thalidomide by inhibiting the NF-κB activation pathway, which leads to the downregulation of NF-κB-regulated antiapoptotic gene products.
Materials and Methods
Materials
Curcumin (77.5% curcumin, 18.27% demethoxycurcumin, 4.21% bisdemethoxycurcumin; also called C3 complex) was kindly supplied by Sabinsa Corp. (Piscataway, NJ). A 25-mM solution was prepared in dimethyl sulfoxide, stored as small aliquots at −20°C, and diluted as needed in cell culture medium. Penicillin, streptomycin, and RPMI 1640 medium were obtained from Invitrogen (Grand Island, NY). Fetal bovine serum (FBS) was obtained from Atlanta Biologicals (Lawrenceville, GA). Bortezomib (PS-341) was obtained from Millennium (Cambridge, MA), and thalidomide was obtained from Tocris Cookson (St. Louis, MO). Dexamethasone, melphalan, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), and anti-β-actin were obtained from Sigma-Aldrich Chemicals (St. Louis, MO). Antibodies against PARP, cIAP-1, Bcl-2, Bcl-xL, and TRAF1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-XIAP and survivin antibodies were obtained from BD Biosciences (San Jose, CA). Anti-VEGF and Ki-67 antibodies were obtained from NeoMakers (Fremont, CA). Polyclonal antibody against p65 was obtained from Abcam, Inc. (Cambridge, MA). γ32P-ATP was obtained from ICN Pharmaceuticals (Costa Mesa, CA). Phospho-specific anti-Akt (Ser 473) and anti-Akt antibodies were obtained from Cell Signaling (Beverly, MA).
Cell lines
Human MM cell lines U266, RPMI-8226, RPMI-8226-Dox-6 (a doxorubicin-resistant clone), RPMI-8226-LR-5 (a melphalan-resistant clone), MM.1 (also called MM.1S) and MM.1R (a dexamethasone-resistant variant of MM.1) cell lines were described previously (21). The U266, RPMI-8226, MM.1, and MM.1R cells as well as the Dox-6 and LR-5 variants were cultured in RPMI 1640 medium supplemented with 10% FBS and 1 × antibiotic/antimycotic.
Proliferation assay
The antiproliferative effects of curcumin on drug-sensitive and drug-resistant cells were determined by the MTT uptake method as described previously (21).
Activation of NF-κB in MM cells and tumor samples
To assess NF-κB activation, we isolated nuclei from MM cell lines and tumor samples and carried out electrophoretic mobility shift assays (EMSAs) essentially as described previously (22). In brief, nuclear extracts prepared from MM cancer cells (1 × 106/mL) and tumor samples were incubated with 32P-end-labeled 45-mer double-stranded NF-κB oligonucleotide from the HIV long terminal repeat (5′-TTGTTACAAGGGACTTTC CGCTG GGGACTTTC CAGGGA GGCGT GG-3′; boldface indicates NF-κB binding sites) for 15 minutes at 37°C. The resulting DNA-protein complex was separated from free oligonucleotides on 6.6% native polyacrylamide gels. To verify the equal loading of nuclear proteins, the binding of Oct-1 to DNA was determined by incubating 15 μg of nuclear extracts with 16 fmol of 32P-end-labeled with the octamer-binding protein (Oct-1) consensus oligonucleotide 5′-TGTCGAATGCAAATCACTAGAA-3′ (boldface indicates Oct-1 binding site) for 30 min at 37°C, and then analysed using 5% native polyacrylamide gel. The dried gels were visualized, and radioactive bands were quantitated with a PhosphorImager (Molecular Dynamics, Sunnyvale, CA) using ImageQuant software.
Western blot analysis
To determine the expression levels of the cIAP-1, XIAP, survivin, Bcl-2, Bcl-xL, TRAF1, VEGF, and PARP proteins in the curcumin-treated cells, we subjected the whole-cell extracts to Western blot analysis as described previously (23). In brief, the whole-cell extracts were prepared by lysing curcumin-treated cells with lysis buffer (20 mM Tris [pH 7.4], 250 mM NaCl, 2 mM EDTA [pH 8.0], 0.1% Triton X-100, 0.01 mg/mL aprotinin, 0.005 mg/mL leupeptin, 0.4 mM PMSF, and 4 mM sodium orthovanadate) and loaded. After electrophoresis, the proteins were electrotransferred to nitrocellulose membranes, blocked with 5% nonfat milk, and probed with various antibodies overnight at 4°C. The blots were then washed, exposed to horseradish peroxidase-conjugated secondary antibodies for 1 hour, and finally examined using an enhanced chemiluminescence (ECL) reagent (Amersham, Piscataway, NJ).
Apoptosis assay
To determine whether curcumin potentiates the apoptotic effects of bortezomib and thalidomide in MM cells, we used a Live/Dead assay kit (Molecular Probes, Eugene, OR), which determines intracellular esterase activity and plasma membrane integrity. This assay uses calcein, a polyanionic, green fluorescent dye that is retained within live cells, and a red fluorescent ethidium bromide homodimer dye that can enter cells through damaged membranes and bind to nucleic acids but is excluded by the intact plasma membranes of live cells (23). In brief, cells (1×106 well) were incubated in 12 well plates, pretreated with curcumin for 4 hours, and treated with either thalidomide or bortezomib for 24 hours. Cells were then stained with the assay reagents for 30 minutes at ambient temperature. Cell viability was determined under a fluorescence microscope by counting live (green) and dead (red) cells. This experiment was repeated twice, and the statistical analysis was performed. The values were initially subjected to one-way ANOVA, which revealed significant differences between groups, and then compared among groups using an unpaired Student’s t-test (P < 0.05 considered to be statistically significant), which revealed significant differences between the two sample means.
Annexin V assay
One of the early indicators of apoptosis is the rapid translocation and accumulation of the membrane phospholipid phosphatidylserine from the cell’s cytoplasmic interface to the extracellular surface. This loss of membrane asymmetry can be detected by using the binding properties of annexin V. This assay was performed as described previously (23).
Animals
Male athymic nu/nu mice (4 weeks old) were obtained from the breeding colony of the Department of Experimental Radiation Oncology at The University of Texas M. D. Anderson Cancer Center. The animals were housed (5 mice/cage) in the standard mice plexiglass cages in a room maintained at constant temperature and humidity under 12-hour light and dark cycle and fed with regular autoclaved mouse chow with water ad libitum. None of the mice exhibited any lesions and all were tested pathogen-free. Before initiating the experiment, we acclimatized all mice to a pulverized diet for 3 days. Our experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee at The University of Texas M. D. Anderson Cancer Center.
Subcutaneous implantation of U266 cells
U266 cells were injected subcutaneously into the mice as described previously (24). In brief, U266 cells were harvested from subconfluent cultures, washed once in serum-free medium, and resuspended in PBS. Only suspensions consisting of single cells, with >90% viability, were used for the injections. Mice were anesthetized with a ketamine-xylazine solution, and U266 cells [2 × 106 cells/100 μL PBS:Matrigel (1:1)] were injected subcutaneously into the left flank of the mice using a 25-gauge needle and a calibrated push button-controlled dispensing device (Hamilton Syringe Co., Reno, NV). To prevent leakage, a cotton swab was held cautiously for 1 minute over the site of injection.
Experimental protocol
After 1 week of implantation, tumor diameters were measured using Vernier calipers. The mice were then randomized into treatment groups (n = 5) based on the tumor volume. Group I (control) was treated with corn oil (100 μL; orally; daily) and saline (100 μL; orally; once a week); group II was treated with curcumin alone (1 g/kg; orally; daily); group III was treated with bortezomib alone (0.25 mg/kg; 100 μL; once in a week; i.p.); and group IV was treated with a combination of curcumin (1 g/kg; orally; daily) and bortezomib (0.25 mg/kg; 100 μL; once in a week). Treatment was continued for up to 20 days from the date of randomization (Day 0). The tumor volume was measured at 5-day intervals. The mice were killed 25 days after randomization. The tumors were carefully excised and measured to calculate tumor volume. The tumor volume was derived using the formula V = 2/3 πr3, where r is the mean of the 3 dimensions (length, width, and depth). The tumor volumes were initially subjected to one-way ANOVA and then later compared among groups using an unpaired Student’s t-test, with P < 0.05 considered to be significant.
Preparation of nuclear extract from tumor samples
MM tumor tissues (75–100 mg/mouse) from control and treated mice were minced and incubated on ice for 30 minutes in 0.5 mL of ice-cold buffer A (10 mM HEPES [pH 7.9], 1.5 mM KCl, 10 mM MgCl2, 0.5 mM DTT, 0.1% Igepal CA-630, and 0.5 mM PMSF). The minced tissue was homogenized using a Dounce homogenizer and centrifuged at 16,000 × g at 4°C for 10 minutes. The resulting nuclear pellet was suspended in 0.2 mL of buffer B (20 mM HEPES [pH 7.9], 25% glycerol, 1.5 mM MgCl2, 420 mM NaCl, 0.5 mM DTT, 0.2 mM EDTA, 0.5 mM PMSF, and 2 μg/mL leupeptin) and incubated on ice for 2 hours with intermittent mixing. The suspension was then centrifuged at 16,000 × g at 4°C for 30 minutes. The supernatant (nuclear extract) was collected and stored at −70°C until use (25).
Immunolocalization of NF-κB p65 and VEGF in tumor samples
The nuclear localization of p65 and the expression of VEGF was examined using an immunohistochemical method described previously (25). In brief, MM tumor samples were embedded in paraffin and fixed with paraformaldehyde. After being washed in PBS, the slides were blocked with protein block solution (DakoCytomation, Carpinteria, CA) for 20 minutes and then incubated overnight with rabbit polyclonal anti-human p65 and mouse monoclonal anti-human VEGF antibodies (1:400 and 1:100, respectively). Slides were washed and then incubated with biotinylated link universal antiserum followed by horseradish peroxidase-streptavidin conjugate (LSAB+kit). The slides were rinsed, and color was developed using 3,3′-diaminobenzidine hydrochloride as a chromogen. Finally, sections were rinsed in distilled water, counterstained with Mayer’s hematoxylin, and mounted with DPX mounting medium for evaluation. Pictures were captured with a Photometrics CoolSnap CF color camera (Nikon, Lewisville, TX) using MetaMorph software (v. 4.6.5; Universal Imaging, Downingtown, PA).
Ki-67 immunohistochemistry
Frozen sections (5 μm) were stained with anti-Ki-67 antibody as described previously (25). Results were expressed as percent of Ki-67+ cells ± SE per x40 magnification. A total of ten x40 fields were examined and counted from 3 tumors of each treatment group. The values were initially subjected to one-way ANOVA and then later compared among groups using an unpaired Student’s t-test, with P < 0.05 considered to be significant.
Microvessel density
Frozen sections (5 μm) were stained with rat anti-mouse CD31 monoclonal antibody (Pharmingen, San Diego, CA) as described previously (25). Areas of greatest vessel density were then examined under higher magnification (×100) and counted. Any distinct area of positive staining for CD31 was counted as a single vessel. Results were expressed as the mean number of vessels ± SE per high-power field (HPF, or ×100). A total of 20 HPFs were examined and counted from 3 tumors of each treatment group. The values were initially subjected to one-way ANOVA and then later compared among groups using an unpaired Student’s t-test, with P < 0.05 considered to be significant.
Results
The goals of this study were, first, to determine whether curcumin (Figure 1A) can sensitizes chemoresistant multiple myeloma MM cells that have developed resistance to conventional chemotherapeutic agents; second, to determine whether curcumin can potentiates the antitumor effects of thalidomide and bortezomib, two chemotherapeutic agents used extensively to treat MM patients; third, to determine whether curcumin potentiates the effects of these chemotherapeutic agents in vivo; fourth, to determine the mechanism by which curcumin sensitizes MM cells to these drugs. We used MM cell lines that are sensitive and resistant to dexamethasone, doxorubicin, and melphalan in our studies.
Figure 1. Curcumin suppresses the proliferation of drug-resistant MM cells.
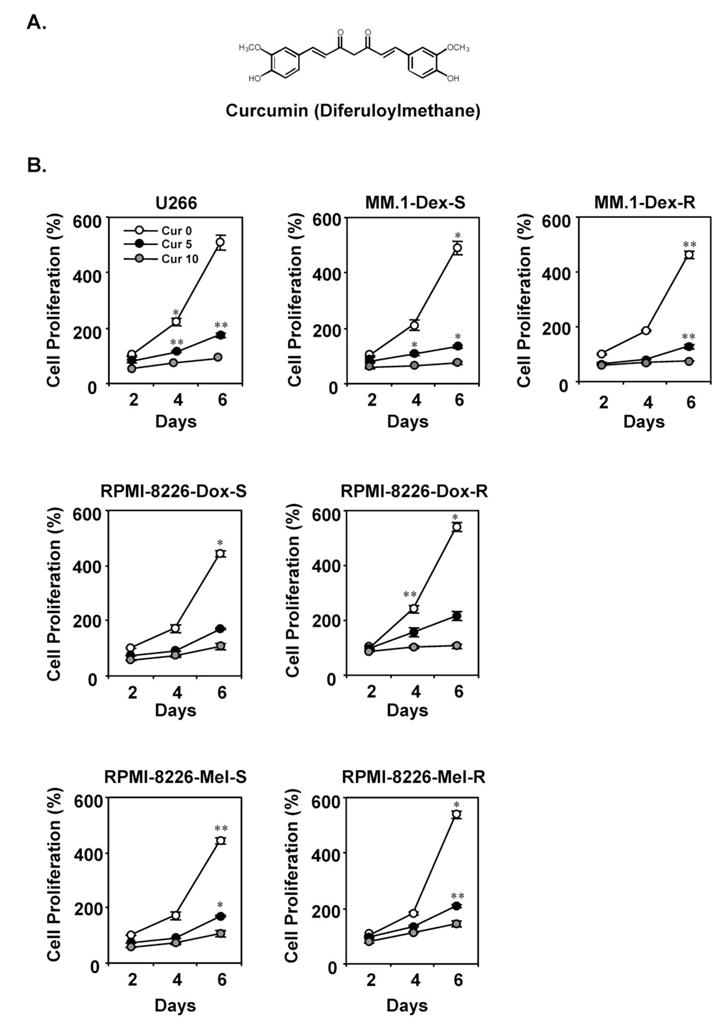
(A) Structure of curcumin. (B) U266 cells (5 × 103 cells) treated with various concentrations of curcumin, dexamethasone-sensitive (top-middle panel) and dexamethasone-resistant (top-right panel) MM.1 cells (5 × 103 cells), doxorubicin-sensitive (middle-left panel) and doxorubicin-resistant (middle-right panel) RPMI-8266 cells (5 × 103 cells), and melphalan-sensitive (bottom-right panel) and melphalan-resistant (bottom-right panel) RPMI-8266 cells were plated in triplicate, treated with 5 and 10 μM curcumin, and then subjected to MTT assay on days 2, 4, or 6 to analyze MM cell proliferation. Data are means ± SD for three experiments; **, P < 0.01; *, P < 0.05, vs. control.
Curcumin suppresses the proliferation of drug-sensitive and drug-resistant MM cells
To determine whether curcumin suppresses the proliferation of drug-sensitive and drug-resistant human MM cells, we tested the cell proliferation using an MTT assay. Curcumin suppressed the proliferation of all MM cell types tested, including U266 cells, MM.1R cells (resistant to dexamethasone), RPMI-8226-Dox-6 cells (resistant to doxorubicin), and RPMI-8226-LR-5 cells (resistant to melphalan) in a dose-dependent and time-dependent manner (Figure 1B). Whether a cell line is a sensitive or resistant to a conventional chemotherapeutic agent, no difference in the sensitivity to curcumin was observed.
Curcumin potentiates the apoptotic effects of bortezomib and thalidomide against MM cells
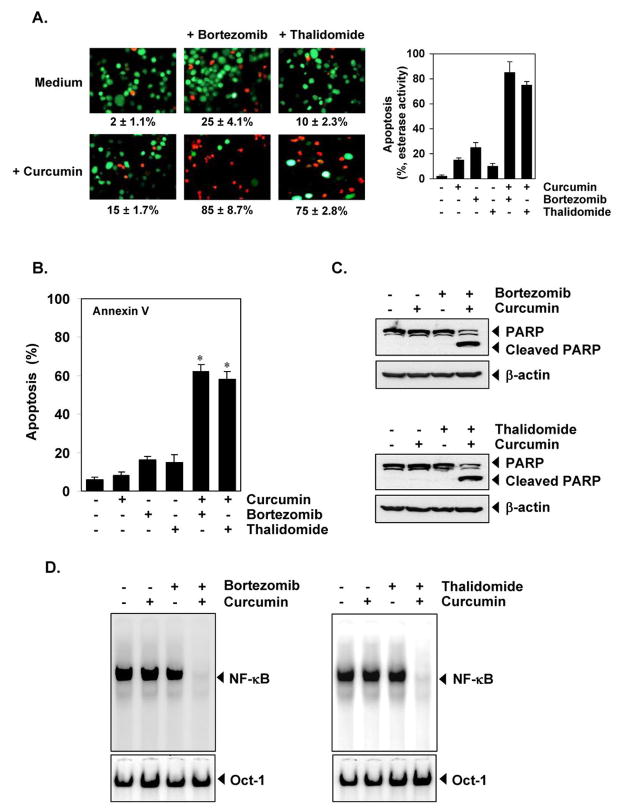
Bortezomib, a proteasome inhibitor, and thalidomide, a TNF inhibitor, have been approved for the conventional treatment of MM patients. To determine whether curcumin potentiates the apoptotic effect of these drugs, we treated U266 cells with curcumin combined with either bortezomib or thalidomide, and then examined these cells with a Live/Dead assay. Curcumin potentiated the apoptotic effect of bortezomib from 25% to 85% (Figure 2A, middle panel) and of thalidomide from 10% to 75% (Figure 2A, right panel).
Figure 2. Curcumin potentiates the apoptotic effect of bortezomib and of thalidomide.
(A) (left) U266 cells (1 × 106/mL) were treated with 25 μM curcumin, 20 nM bortezomib, or 10 μg/mL thalidomide alone or a combination of these two agents with curcumin for 24 hours at 37°C. Cells were stained with a Live/Dead assay reagent for 30 minutes and then analyzed under a fluorescence microscope. Percentage of apoptosis is indicated in the inset. (right) a graphic representation of the data with U266 cells. (B) U266 cells (1 × 106/mL) were treated with 25 μM curcumin, 20 nM bortezomib, or 10 μg/mL thalidomide alone or a combination of these agents with curcumin for 12 hours at 37°C. Cells were incubated with anti-annexin V antibody conjugated with fluorescein isothiocyanate and then analyzed with a flow cytometery to detect early apoptotic effects. Data are means ± SD for three experiments; *, P < 0.001, vs. control as well as single agent. (C) U266 cells (1 × 106/mL) were treated with 25 μM curcumin, 20 nM bortezomib, or 10 μg/mL thalidomide alone or a combination of these agents with curcumin for 12 hours at 37°C. Whole-cell extracts were prepared, separated on SDS-PAGE, and subjected to Western blot analysis using antibody against PARP. The same blots were stripped and reprobed with β-actin antibody to show equal protein loading. The results shown represent 3 independent experiments. (D) U266 cells (1 × 106/mL) were treated with 5 μM curcumin or 10 nM bortezomib alone or a curcumin+bortezomib combination for 12 hours at 37°C and then tested for NF-κB activation by EMSA (left panel). U266 cells (1 × 106/mL) were treated with 5 μM curcumin or 10 μg/mL thalidomide alone or a curcumin+thalidomide combination for 12 hours at 37°C and then tested for NF-κB activation by EMSA (right panel). Oct-1 EMSA served as a loading control. The results shown represent 3 independent experiments.
To further confirm the potentiating effect of curcumin on bortezomib and thalidomide, we used the annexin V method, which detects an early stage of apoptosis. Again, curcumin enhanced the apoptotic effects of bortezomib from 16% to 60% and of thalidomide from 18% to 57% (Figure 2B).
When we examined the PARP cleavage, which indicates caspase-3 activation, a well-known characteristic of apoptosis, we found that curcumin potentiated the effect of bortezomib (Figure 2C, top) and of thalidomide (Figure 2C, bottom). The potentiating effect of curcumin was more pronounced on thalidomide than on bortezomib. This may be because bortezomib induces apoptosis by activating caspase-9, whereas thalidomide induces apoptosis by activating caspase-8 (26).
Curcumin potentiates the inhibitory effect of bortezomib and thalidomide on constitutive NF-κB activation in MM cells
Our results indicate that curcumin can potentiate the apoptotic effects of bortezomib and thalidomide. How the effects were potentiated, was investigated. The constitutive activation of NF-κB is associated with growth and survival of cancer cells, including MM cells (27, 28). In addition, studies have shown that bortezomib and thalidomide have been shown to suppress NF-κB activation (29, 30). To determine whether the apoptotic effects of the curcumin alone and of the combination of curcumin with these drugs, correlates with down-regulation of NF-κB activation was examined. To investigate this, we incubated U266 cells with suboptimal concentrations of curcumin, bortezomib, and thalidomide alone or a combination with curcumin and these drugs and then examined NF-κB activation by EMSA. Curcumin potentiated the inhibitory effect of bortezomib and of thalidomide on NF-κB activation in MM cells (Figure 2D).
Curcumin suppresses Akt activation in MM cells
The phosphatidylinositol 3-kinase (PI3K)/Akt pathway is one of the signaling pathways whose dysregulation has been linked with chemoresistance in MM cells (31). We investigated whether curcumin suppressed the activation of Akt in MM cells. We found that Akt was constitutively active in U266 cells and that curcumin suppressed the levels of phosphorylated Akt in a time-dependent manner (Figure 3A), indicating that these reduced levels of activated Akt may contribute towards increasing the apoptosis in MM cells.
Figure 3. Curcumin suppresses the expression of antiapoptotic proteins in MM cells.
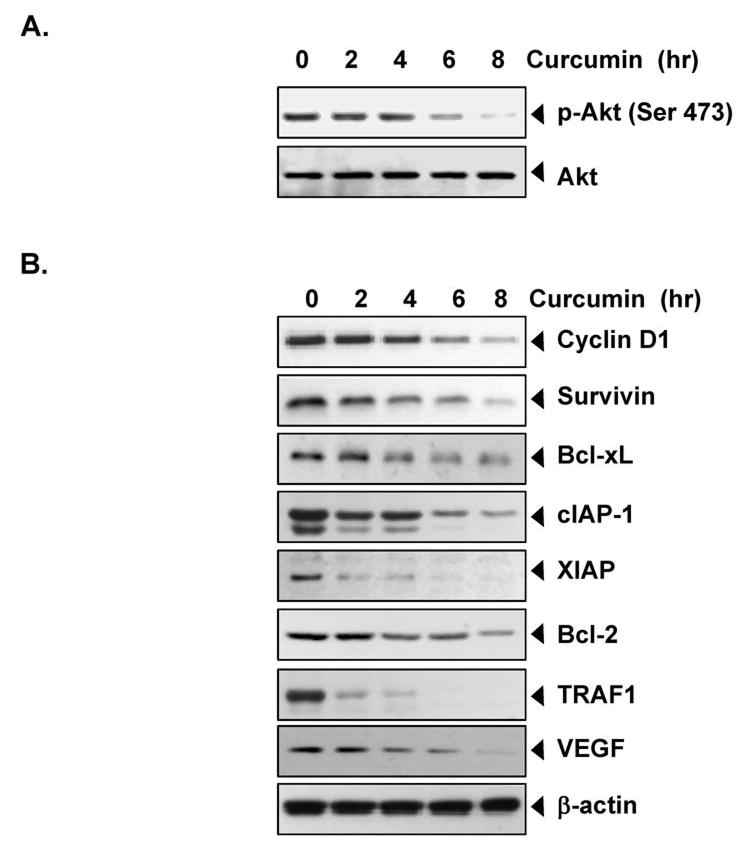
(A) Curcumin suppresses Akt activation in U266 cells. U266 cells (2 × 106/mL) were treated with 50 μM curcumin for the indicated times. Whole-cell extracts were prepared, separated on SDS-PAGE, and subjected to Western blot analysis using the indicated proteins. The same blots were stripped and reprobed with Akt antibody to show equal protein loading. (B) Curcumin inhibits the expression of antiapoptotic gene products. U266 cells (2 × 106/mL) were treated with 50 μM curcumin for the indicated times. Whole-cell extracts were prepared, separated on SDS-PAGE, and subjected to Western blot analysis using the indicated proteins. The same blots were stripped and reprobed with Akt and β-actin antibody to show equal protein loading.
Curcumin inhibits the expression of NF-κB-regulated gene products in MM cells
Numerous proteins, including cyclin D1, survivin, Bcl-xL, cIAP-1, XIAP, Bcl-2, TRAF1, and VEGF, have been linked with chemoresistance; all regulated by NF-κB. To determine whether curcumin mediates its effects by downregulating the expression of NF-κB-regulated proteins, we examined the effect of curcumin on the expression of NF-κB-regulated gene products implicated in cell proliferation (cyclin D1), antiapoptosis (cIAP-1, XIAP, survivin, Bcl-2, Bcl-xL, and TRAF1), and angiogenesis (VEGF). Our results showed that curcumin downregulated the constitutive expression of cyclin D1, cIAP-1, XIAP, survivin, Bcl-2, Bcl-xL, and TRAF1 and VEGF in U266 cells (Figure 3B). The effect of curcumin on apoptosis showed the consistent to our previous report (3).
Curcumin potentiates the antitumor effects of bortezomib in human MM xenograft in nude mice
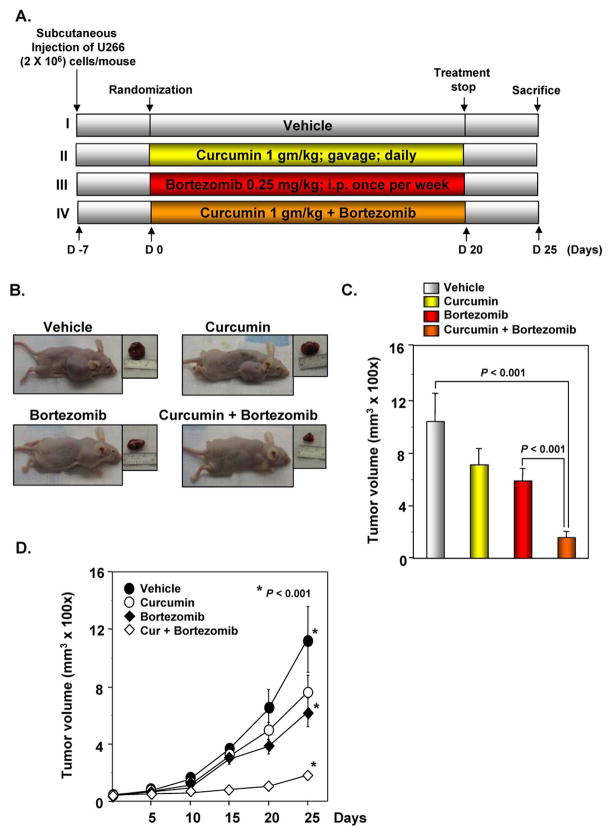
To determine whether curcumin enhances the antitumor effects of bortezomib against MM, we developed a human MM xenograft in nude mice using U266 cells. A week after implantation, the animals were randomized into 4 treatment groups based on tumor volume. Treatment was started 1 week after tumor cell implantation and was continued up to 20 days, in accordance with the experimental protocol (Figure 4A). The tumor diameters were measured at 5-day intervals. Animals were killed 32 days after tumor cell injection and 25 days after the treatment start date (Figure 4A), and the tumors were excised and the tumor diameters were measured. We found that the tumor volume increased rapidly in the control group compared with the other treatment groups (Figure 4B). Both curcumin alone and bortezomib alone significantly decreased the tumor volume; the tumor volume in the curcumin+bortezomib group was significantly lower than that in the bortezomib alone group and in the control group at Day 25 after treatment (P < 0.001, vehicle vs. curcumin+bortezomib; P < 0.001, bortezomib vs. curcumin+bortezomib) (Figure 4C). When examined for tumor volume on different days, we found that curcumin+bortezomib combination was much more effective in reducing the tumor volume compared to either agent alone (P < 0.001 vs. vehicle) (Figure 4D).
Figure 4. Curcumin potentiates the antitumor effects of bortezomib in myeloma tumor growth in nude mice induced by U266 cells.
(A) Schematic representation of the experimental protocol as described in “Materials and Methods.” U266 cells (2×106/mice) were injected subcutaneously into the left flank of the mice. The animals were randomized after 1 week of tumor cell injection into four groups based on tumor volume. Group I was treated with corn oil (100 μL; orally; daily) and saline (100 μL; orally; once a week), group II was treated with curcumin alone (1 g/kg daily; orally), group III was treated with bortezomib alone (0.25 mg/kg; orally; once a week), and group IV with a combination of curcumin (1 g/kg orally; daily) and bortezomib (0.25 mg/kg; orally; once a week) (n = 6). (B) The tumor diameters were measured at 5-day intervals with Vernier calipers, and the tumor volumes were calculated using the formula V = 2/3 πr3 (n = 5). (C, left panel) Necropsy photographs of mice bearing xenotransplanted MM. (C, right panel) Tumor volumes (mean ± SE) calculated using the formula V = 2/3 πr3 (n = 6) after tumor diameters were measured on the last day of the experiment at autopsy using Vernier calipers. (D) The tumor volume of mice at different time intervals.
Curcumin inhibits NF-κB activation in human MM xenograft in nude mice
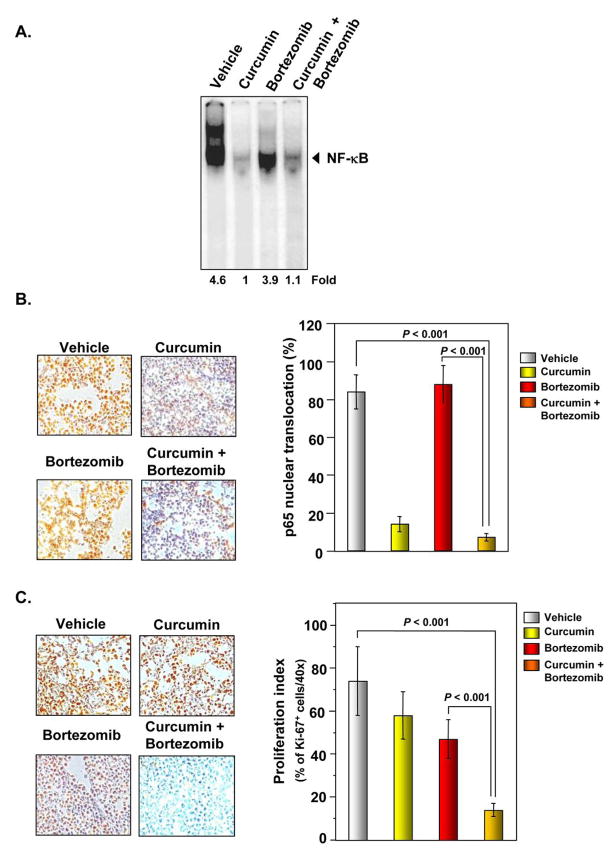
We evaluated whether the effects of curcumin on MM tumor growth was associated with the inhibition of NF-κB activation. The DNA binding assay for NF-κB in nuclear extracts from tumor samples showed that curcumin alone significantly suppressed NF-κB activation. The effect of bortezomib alone was less pronounced than curcumin in inhibiting NF-κB activation (Figure 5A). Interestingly, the curcumin+bortezomib combination was not more effective than curcumin alone (P < 0.001 vs. vehicle).
Figure 5. Curcumin enhances the inhibitory effect of bortezomib on the activation of NF-κB and expression of the cell proliferation marker Ki-67 in MM tumor samples.
(A) EMSA analysis revealed that curcumin inhibited NF-κB activation in nuclear extracts from animal tissue. (B, left panel) Immunohistochemical analysis of nuclear p65 in tumor tissues showed that curcumin alone and in combination with bortezomib inhibited NF-κB activation. (B, right panel) Quantification of NF-κB expression in MM tumor samples. Percentages indicate p65 nuclear positive cells. Samples from 3 animals in each treatment group were analyzed, and representative data are shown. (C, left panel) Immunohistochemical analysis of Ki-67+ cells in MM indicated that curcumin alone and in combination with bortezomib suppressed cell proliferation. Samples from 3 animals in each treatment group were analyzed, and representative data are shown. (C, right panel) Quantification of Ki-67 proliferation index as described in “Materials and Methods.” Values are represented as mean ± SE of triplicate.
We also examined whether the EMSA results were consistent with the immunohistochemical analyses. We found that the nuclear translocation of p65 was significantly inhibited by curcumin alone but not by bortezomib alone, which confirmed the EMSA results (Figure 5B, left and right panel).
Curcumin downregulates the expression of the cell proliferation marker Ki-67 in human MM xenograft in nude mice
To determine whether curcumin decreases MM tumor growth by inhibiting proliferation, we examined the expression of Ki-67+ cells in MM tumors from mice. Our results showed that curcumin alone and bortezomib alone significantly decreased the expression of Ki-67 in tumor tissue (Figure 5C, left and right panel). The curcumin+bortezomib combination was more effective in reducing Ki-67 expression than either agent alone (P < 0.001 vs. vehicle).
Curcumin inhibits angiogenesis and downregulates the expression of VEGF in human MM xenograft in nude mice
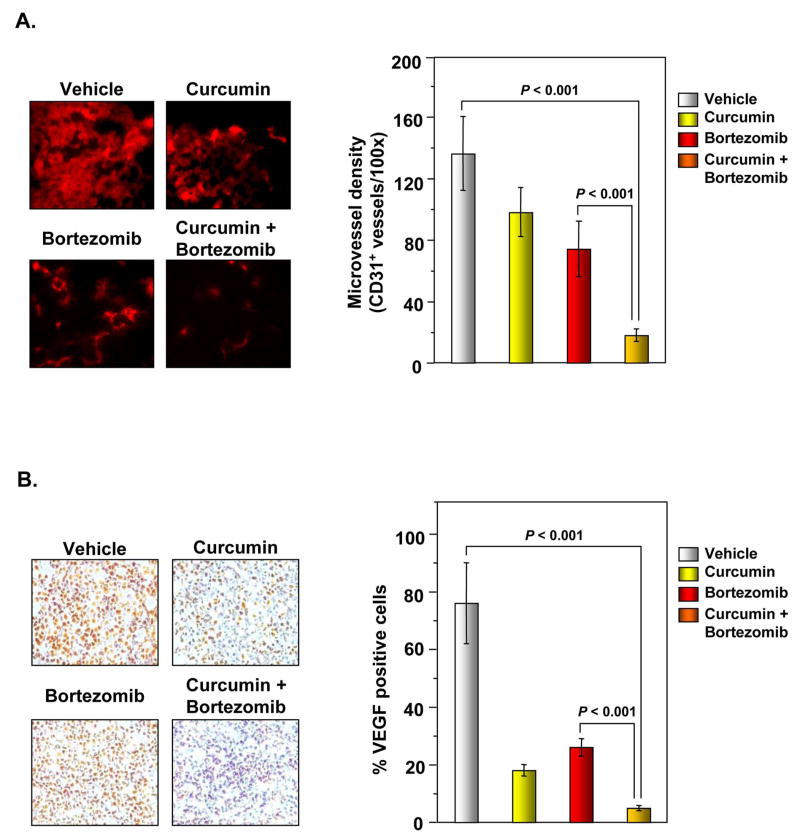
To determine whether curcumin decreases MM tumor growth by inhibiting angiogenesis, we examined the expression of the microvessel density marker CD31+ in MM tumors from nude mice. Our results showed that curcumin alone and bortezomib alone significantly reduced microvessel density. The curcumin+bortezomib combination was more effective in reducing microvessel density than either agent alone (Figure 6A, left and right panel) (P < 0.001 vs. vehicle).
Figure 6. Curcumin enhances effects of bortezomib to inhibit angiogenesis in MM tumor samples.
(A, left panel) Immunohistochemical analysis of the microvessel density marker CD31 in MM tumor samples. Samples from 3 animals in each treatment group were analyzed, and representative data are shown. (A, right panel) Quantification of CD31+ microvessel density as described in “Materials and Methods.” Values are represented as mean ± SE of triplicate. (B, left panel) Immunohistochemical analysis of VEGF in MM tumor samples revealed that curcumin alone and in combination with bortezomib suppresses angiogenesis. Samples from 3 animals in each treatment group were analyzed, and representative data are shown. (B, right panel) Quantification of VEGF as described in “Materials and Methods.” Values are represented as mean ± SE of triplicate.
Because VEGF plays an important role in angiogenesis, we also examined its expression in MM tumors. We found that both curcumin alone and bortezomib alone effectively suppressed VEGF expression and that the curcumin+bortezomib combination had the highest inhibitory effect on VEGF expression in MM tumors (Figure 6B, left and right panel).
Discussion
Despite the fact that several treatments for MM are currently available, MM remains an incurable disease, with a median survival time of 3–5 years (32–34). The disease relapses in the majority of MM patients, regardless of the treatment regimen or their initial response to a given treatment. Recently, 3 new agents (bortezomib, thalidomide, and lenalidomide) were approved for the treatment of MM patients. Bortezomib (Velcade) was approved for the treatment of MM patients who have received at least one prior therapy; thalidomide (Thalomid) in combination with dexamethasone was approved for the treatment of newly diagnosed MM patients; and the thalidomide analogue lenalidomide (Revlimid) in combination with dexamethasone was recently approved for the treatment of MM patients who have received one prior therapy (35). All these three drugs mediate their antimyeloma activities through modulation of intracellular signaling pathways within tumor cells and microenvironments (36, 37). Preclinical studies have shown that these drugs induce apoptosis in myeloma cells resistant to melphalan, doxorubicin, and dexamethasone and potentiate the antimyeloma activity of conventional therapies (38, 39). However, these drugs exhibit several side effects, such as fatigue, anemia, and peripheral neuropathy. Furthermore, studies have shown that patients eventually develop resistance to these drugs. Thus, new treatment approaches that are more effective than conventional therapies against MM are required to improve the outcome and extend the survival of MM patients.
Because the transcription factor NF-κB has been closely linked with chemoresistance, cell survival, and proliferation, we investigated whether curcumin suppresses the proliferation of MM cells that have developed resistance to chemotherapeutic agents. We found that curcumin suppressed human MM cell proliferation regardless of the cells’ sensitivity or resistance to melphalan, doxorubicin, or dexamethasone. Similar to our results these drug-resistant cells have been shown to be sensitive to bortezomib and thalidomide (38, 40). We also found that curcumin potentiated the antitumor effects of bortezomib and thalidomide. What is the mechanism of resistance of MM to melphalan, doxorubicin, or dexamethasone? First, cells that resistant to chemotherapeutic agents have been shown to express increased activation of NF-κB and suppression of this NF-κB can sensitize the cells to the drug (41). Second, multiple myeloma cells and mantel cell lymphoma are known to express constitutive active NF-κB that is resistant to bortezomib (42, 43). Our results indicate that the mechanism of potentiation is the suppression of NF-κB activation. Why NF-κB is constitutively active in MM cells, is not fully understood. Recently, two studies found mutations on the genes encoding positive and negative regulators of NF-κB signaling in many MM cell lines and primary tumor cells; these mutations are thought to mediate the constitutive activation of NF-κB in MM cells (10, 11). It is likely that the constitutive activation of NF-κB in MM cells leads to chemoresistance. For example, CRP, whose expression is regulated by NF-κB, has been shown to enhance the proliferation of myeloma cells and protect myeloma cells from chemotherapy-induced apoptosis both in vitro and in vivo (8). CRP has also been shown to bind to Fcgamma receptors and activate Akt, ERK, and NF-κB pathways. CRP also enhanced the secretion of IL-6 and synergized with IL-6 to protect myeloma cells from chemotherapy-induced apoptosis. In our study, we showed that curcumin downregulates Akt activation, another possible mechanism which curcumin sensitizes tumor cells to chemotherapeutic agents. Previously, we showed that curcumin also downregulates the expression of IL-6 (4), a major growth factor for MM cells. We also found that curcumin downregulated various proliferative and antiapoptotic proteins, including cyclin D1, cIAP-1, XIAP, survivin, Bcl-2, Bcl-xL, and TRAF1. As mentioned above, cyclin D1, Bcl-2, Bcl-xL and survivin have been linked with chemoresistance in MM cells (44–46). Thus, in addition to the suppression of NF-κB activation, these mechanisms may help explain the effects of curcumin.
Like curcumin, bortezomib inhibits NF-κB activation but through different mechanism. The pathway by which bortezomib prevents degradation of IκB, differs from that of curcumin. Bortezomib inhibits the proteasome, resulting in the accumulation of IκBα, whereas curcumin prevents IκB phosphorylation, thus blocking its subsequent ubiquitination and degradation through suppression of upstream kinase IKK. Thus, these different mechanisms of NF-κB suppression provide the rationale for combining these agents to effectively inhibit NF-κB activation. Furthermore, other mechanisms bortezomib and curcumin may support their combination to maximize their cytotoxicity against MM cells. For instance, curcumin is a potent blocker of STAT3 activation (15), a transcription factor that has been linked with chemoresistance (47). Moreover, bortezomib has been shown to overcome chemoresistance in MM cells both in the laboratory (41, 48) and in the clinic (49). Indeed, bortezomib has been shown to exhibit considerable clinical efficacy against MM. Whether this activity is due to its anti-NF-κB activity is not clear (35); as its ability to suppress NF-κB in patients has not been demonstrated.
We next determined whether curcumin potentiates the effects of bortezomib on human MM xenografts in nude mice. Our results showed that curcumin alone and bortezomib alone inhibited the MM xenograft in nude mice. We found that the curcumin+bortezomib combination had a higher antimyeloma effect than either agent alone. When investigated the mechanism, we found that curcumin downregulated the expression of NF-κB, the cell proliferation marker Ki-67, and the microvessel density marker CD31, all which have been linked with chemoresistance. Since curcumin alone inhibited most of NF-κB activity whit partial anti-tumor effect, the downregulation of NF-κB by curcumin alone may not be sufficient to explain the effect of this agent in vivo. Moreover, when combined with bortezomib in vivo there is slight increase in NF-κB DNA binding activity even though this combination leads to the greatest effect on myeloma growth in vivo. Thus, it is possible that in vivo effect of curcumin are not entirely mediated though its ability to suppress NF-κB. Curcumin has been shown to modulate multiple targets (50) that may be involved in its in vivo antitumor action.
MM is usually diagnosed in elderly patients who are unable to tolerate highly toxic drugs, so curcumin, a pharmacologically safe compound, is a viable therapeutic agent for these MM patients. A recent phase I study of curcumin demonstrated that this agent can be administered safely at oral doses of up to 12 g/day (19). There was no dose-limiting toxicity; dosing was limited by the number of pills that patients could or would swallow daily. In addition, a recent phase II clinical trial with curcumin in pancreatic cancer patients showed that tumor progression was suppressed by 73% in some patients (51). Clinical trials with asymptomatic MM patients have shown that curcumin downregulates NF-κB activation in peripheral blood mononuclear cells even at dose of 2 g/day (20). Thus, curcumin can be used, first, to enhance the effects of current chemotherapeutic agents and, second, to overcome chemoresistance in MM to conventional therapy. Fatigue and peripheral neuropathy are two symptoms commonly observed in MM patients. Because these symptoms are mediated through the expression of proinflammatory cytokines, curcumin may be used to alleviate these symptoms. In fact, curcumin has been shown to reverse these symptoms (52–54), which further supports its use in MM patients.
In conclusion, the chemoresistance remains a major challenge in the treatment of patients with MM as well as other cancers. MM patients who have relapsed after conventional-dose chemotherapy or stem cell transplantation are typically treated with high-dose corticosteroids, thalidomide, or bortezomib. However, a large number of these patients do not respond to treatment with these agents. Moreover, prolonged exposure leads to the development of resistance and toxicity, nd progression-free and overall survival times for MM patients are short. The ability of curcumin to suppress NF-κB activation, downregulate the expression of cyclin D1 and Bcl-xL, inhibit cell proliferation, potentiate the effects of bortezomib and thalidomide, and overcome chemoresistance provides a sound basis for conducting clinical trials with curcumin, alone or in combination with other agents, to enhance treatment efficacy, reduce toxicity, and overcome chemoresistance of relapsed or refractory MM.
Acknowledgments
Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was supported by a grant from the Clayton Foundation for Research (B.B.A.), a core grant from the National Institutes of Health (CA-16 672), National Institutes of Health (NIH CA-124787-01A2), and grant from Center for Targeted Therapy of M.D. Anderson Cancer Center. We thank Lionel Santibañez for carefully editing this manuscript.
Abbreviations
- cIAP-1
cellular inhibitor of apoptosis-1
- CRP
C-reactive protein
- EMSA
electrophoretic mobility shift assay
- ERK
extracellular signal-regulated kinase
- IKK
IκB kinase
- IL-6
interleukin-6
- MM
multiple myeloma
- NF-κB
nuclear factor-kappaB
- PARP
poly (ADP-ribose) polymerase
- PI3K
phosphatidylinositol 3-kinase
- TRAF1
TNF receptor associated factor 1
- VEGF
vascular endothelial growth factor
- XIAP
X-linked IAP
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Ries LG, Alves MC, Berzin F. Asymmetric activation of temporalis, masseter, and sternocleidomastoid muscles in temporomandibular disorder patients. Cranio. 2008;26:59–64. doi: 10.1179/crn.2008.008. [DOI] [PubMed] [Google Scholar]
- 2.Hideshima T, Anderson KC. Preclinical studies of novel targeted therapies. Hematol Oncol Clin North Am. 2007;21:1071–91. viii–ix. doi: 10.1016/j.hoc.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharti AC, Donato N, Singh S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–62. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 4.Bharti AC, Shishodia S, Reuben JM, et al. Nuclear factor-kappaB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood. 2004;103:3175–84. doi: 10.1182/blood-2003-06-2151. [DOI] [PubMed] [Google Scholar]
- 5.Bergsagel PL, Kuehl WM, Zhan F, Sawyer J, Barlogie B, Shaughnessy J., Jr Cyclin D dysregulation: an early and unifying pathogenic event in multiple myeloma. Blood. 2005;106:296–303. doi: 10.1182/blood-2005-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lichtenstein A, Tu Y, Fady C, Vescio R, Berenson J. Interleukin-6 inhibits apoptosis of malignant plasma cells. Cell Immunol. 1995;162:248–55. doi: 10.1006/cimm.1995.1076. [DOI] [PubMed] [Google Scholar]
- 7.Schwarze MM, Hawley RG. Prevention of myeloma cell apoptosis by ectopic bcl-2 expression or interleukin 6-mediated up-regulation of bcl-xL. Cancer Res. 1995;55:2262–5. [PubMed] [Google Scholar]
- 8.Yang J, Wezeman M, Zhang X, et al. Human C-reactive protein binds activating Fcgamma receptors and protects myeloma tumor cells from apoptosis. Cancer Cell. 2007;12:252–65. doi: 10.1016/j.ccr.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Landowski TH, Gleason-Guzman MC, Dalton WS. Selection for drug resistance results in resistance to Fas-mediated apoptosis. Blood. 1997;89:1854–61. [PubMed] [Google Scholar]
- 10.Keats JJ, Fonseca R, Chesi M, et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–44. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annunziata CM, Davis RE, Demchenko Y, et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–30. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreaux J, Legouffe E, Jourdan E, et al. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103:3148–57. doi: 10.1182/blood-2003-06-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–5000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 14.Mukhopadhyay A, Bueso-Ramos C, Chatterjee D, Pantazis P, Aggarwal BB. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene. 2001;20:7597–609. doi: 10.1038/sj.onc.1204997. [DOI] [PubMed] [Google Scholar]
- 15.Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–71. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 17.Catlett-Falcone R, Landowski TH, Oshiro MM, et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–15. doi: 10.1016/s1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 18.Limtrakul P, Chearwae W, Shukla S, Phisalphong C, Ambudkar SV. Modulation of function of three ABC drug transporters, P-glycoprotein (ABCB1), mitoxantrone resistance protein (ABCG2) and multidrug resistance protein 1 (ABCC1) by tetrahydrocurcumin, a major metabolite of curcumin. Mol Cell Biochem. 2007;296:85–95. doi: 10.1007/s11010-006-9302-8. [DOI] [PubMed] [Google Scholar]
- 19.Lao CD, Ruffin MTt, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vadhan-Raj S, Weber D, Giralt S, et al. Curcumin downregulates NF-kappaB and related gene in patients with multiple myeloma: Results in phase 1/2 study. American Society of Hematology. 2007 [Google Scholar]
- 21.Bhardwaj A, Sethi G, Vadhan-Raj S, et al. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 22.Chaturvedi MM, Mukhopadhyay A, Aggarwal BB. Assay for redox-sensitive transcription factor. Methods Enzymol. 2000;319:585–602. doi: 10.1016/s0076-6879(00)19055-x. [DOI] [PubMed] [Google Scholar]
- 23.Sung B, Pandey MK, Ahn KS, et al. Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyltransferase, suppresses expression of nuclear factor-kappaB-regulated gene products involved in cell survival, proliferation, invasion, and inflammation through inhibition of the inhibitory subunit of nuclear factor-kappaBalpha kinase, leading to potentiation of apoptosis. Blood. 2008;111:4880–91. doi: 10.1182/blood-2007-10-117994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhutani M, Pathak AK, Nair AS, et al. Capsaicin is a novel blocker of constitutive and interleukin-6-inducible STAT3 activation. Clin Cancer Res. 2007;13:3024–32. doi: 10.1158/1078-0432.CCR-06-2575. [DOI] [PubMed] [Google Scholar]
- 25.Kunnumakkara AB, Diagaradjane P, Guha S, et al. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin Cancer Res. 2008;14:2128–36. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 26.Mitsiades N, Mitsiades CS, Poulaki V, et al. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood. 2002;99:4525–30. doi: 10.1182/blood.v99.12.4525. [DOI] [PubMed] [Google Scholar]
- 27.Ni H, Ergin M, Huang Q, et al. Analysis of expression of nuclear factor kappa B (NF-kappa B) in multiple myeloma: downregulation of NF-kappa B induces apoptosis. Br J Haematol. 2001;115:279–86. doi: 10.1046/j.1365-2141.2001.03102.x. [DOI] [PubMed] [Google Scholar]
- 28.Hideshima T, Chauhan D, Richardson P, et al. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277:16639–47. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 29.Palombella VJ, Conner EM, Fuseler JW, et al. Role of the proteasome and NF-kappaB in streptococcal cell wall-induced polyarthritis. Proc Natl Acad Sci U S A. 1998;95:15671–6. doi: 10.1073/pnas.95.26.15671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majumdar S, Lamothe B, Aggarwal BB. Thalidomide suppresses NF-kappa B activation induced by TNF and H2O2, but not that activated by ceramide, lipopolysaccharides, or phorbol ester. J Immunol. 2002;168:2644–51. doi: 10.4049/jimmunol.168.6.2644. [DOI] [PubMed] [Google Scholar]
- 31.Kim D, Dan HC, Park S, et al. AKT/PKB signaling mechanisms in cancer and chemoresistance. Front Biosci. 2005;10:975–87. doi: 10.2741/1592. [DOI] [PubMed] [Google Scholar]
- 32.Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–20. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 33.Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 34.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–83. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 35.Richardson PG, Mitsiades C, Schlossman R, Munshi N, Anderson K. New drugs for myeloma. Oncologist. 2007;12:664–89. doi: 10.1634/theoncologist.12-6-664. [DOI] [PubMed] [Google Scholar]
- 36.Rajkumar SV, Richardson PG, Hideshima T, Anderson KC. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005;23:630–9. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 37.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4:314–22. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 38.Hideshima T, Chauhan D, Shima Y, et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943–50. [PubMed] [Google Scholar]
- 39.Mitsiades N, Mitsiades CS, Richardson PG, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101:2377–80. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 40.Chauhan D, Li G, Podar K, et al. Targeting mitochondria to overcome conventional and bortezomib/proteasome inhibitor PS-341 resistance in multiple myeloma (MM) cells. Blood. 2004;104:2458–66. doi: 10.1182/blood-2004-02-0547. [DOI] [PubMed] [Google Scholar]
- 41.Ma MH, Yang HH, Parker K, et al. The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin Cancer Res. 2003;9:1136–44. [PubMed] [Google Scholar]
- 42.Markovina S, Callander NS, O’Connor SL, et al. Bortezomib-resistant nuclear factor–kappaB activity in multiple myeloma cells. Mol Cancer Res. 2008;6:1356–64. doi: 10.1158/1541-7786.MCR-08-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang DT, Young KH, Kahl BS, Markovina S, Miyamoto S. Prevalence of bortezomib-resistant constitutive NF-kappaB activity in mantle cell lymphoma. Mol Cancer. 2008;7:40. doi: 10.1186/1476-4598-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kornmann M, Danenberg KD, Arber N, Beger HG, Danenberg PV, Korc M. Inhibition of cyclin D1 expression in human pancreatic cancer cells is associated with increased chemosensitivity and decreased expression of multiple chemoresistance genes. Cancer Res. 1999;59:3505–11. [PubMed] [Google Scholar]
- 45.Reed JC, Kitada S, Takayama S, Miyashita T. Regulation of chemoresistance by the bcl-2 oncoprotein in non-Hodgkin’s lymphoma and lymphocytic leukemia cell lines. Ann Oncol. 1994;5 (Suppl 1):61–5. doi: 10.1093/annonc/5.suppl_1.s61. [DOI] [PubMed] [Google Scholar]
- 46.Tran J, Master Z, Yu JL, Rak J, Dumont DJ, Kerbel RS. A role for survivin in chemoresistance of endothelial cells mediated by VEGF. Proc Natl Acad Sci U S A. 2002;99:4349–54. doi: 10.1073/pnas.072586399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boehm AL, Sen M, Seethala R, et al. Combined targeting of epidermal growth factor receptor, signal transducer and activator of transcription-3, and Bcl-X(L) enhances antitumor effects in squamous cell carcinoma of the head and neck. Mol Pharmacol. 2008;73:1632–42. doi: 10.1124/mol.107.044636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–6. [PubMed] [Google Scholar]
- 49.Berenson JR, Yang HH, Sadler K, et al. Phase I/II trial assessing bortezomib and melphalan combination therapy for the treatment of patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2006;24:937–44. doi: 10.1200/JCO.2005.03.2383. [DOI] [PubMed] [Google Scholar]
- 50.Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trend Pharmacol Sci. 2008 doi: 10.1016/j.tips.2008.11.002. (in press) [DOI] [PubMed] [Google Scholar]
- 51.Dhillon N, Aggarwal BB, Newman RA, et al. Phase II Trial of Curcumin in Patients with Advanced Pancreatic Cancer. Clin Cancer Res. 2008;14:4491–9. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 52.Sharma RA, Euden SA, Platton SL, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–54. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 53.Maes M, Mihaylova I, Bosmans E. Not in the mind of neurasthenic lazybones but in the cell nucleus: patients with chronic fatigue syndrome have increased production of nuclear factor kappa beta. Neuro Endocrinol Lett. 2007;28:456–62. [PubMed] [Google Scholar]
- 54.Davis JM, Murphy EA, Carmichael MD, et al. Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2168–73. doi: 10.1152/ajpregu.00858.2006. [DOI] [PubMed] [Google Scholar]