Abstract
JCV is a human polyomavirus of the Polyomaviridae family, which also includes BK virus and simian vacuolating virus 40 (SV40). JC virus (JCV) was first isolated in 1971 from the brain of a patient with Progressive Multifocal Leukoencephalopathy (PML). Like other polyomaviruses, JCV has a restricted host range. The virus infects the majority of the human population with seroconversion occurring during adolescence. JCV has a limited and specific tissue tropism infecting the kidney and oligodendrocytes and astrocytes in the central nervous system (CNS). Initial JCV infection is generally asymptomatic in immunocompetent hosts, and it establishes a persistent infection in the kidney and possibly bone marrow. In immunocompromised individuals JCV can cause a lytic infection in the CNS and lead to development of the fatal, demyelinating disease PML. The name polyoma is derived from the Greek terms: poly, meaning many, and oma, meaning tumors, owing to the capacity of this group of viruses to cause tumors. JCV inoculation of small animal models and non-human primates, which are not permissive to a productive JCV infection, leads to tumor formation. Given the ubiquitous nature of the virus and its strong association with cancer in animal models, it is hypothesized that JCV plays a role in human cancers. However, the role for JCV in human cancers and tumor formation is not clear. Some researchers have reported an association of JCV with human cancers including brain tumors, colorectal cancers, and cancers of the gastrointestinal tract, while other groups report no correlation. Here, we review the role of JCV in cancers in animal models and present the findings on JCV in human cancers.
Keywords: JC virus, polyomavirus, cancer, DNA tumor virus, PML
1. Introduction
JC virus (JCV) is a human polyomavirus of the Polyomaviridae family and is the causative agent of Progressive Multifocal Leukoencephalopathy (PML). The name polyoma is derived from the Greek terms: poly, meaning many, and oma, meaning tumors, and refers to the capacity of these viruses to cause tumors. The relationship between Polyomaviruses and cancer is a topic that has intrigued researchers in the field for the past 50 years. The debate about polyomaviruses in human cancer arose from the findings that polyomaviruses can transform cells and are oncogenic in non-permissive hosts. Furthermore, attempts to develop a model system to study PML led to the observation that JCV is oncogenic in experimental animal models. Given the oncogenic potential of JCV and that the majority of the human population is seropositive for JCV, the role of polyomaviruses in human cancer development is an obvious question. Many elegant studies have been performed to investigate the association of JCV and human cancers yet a causative role for JCV in cancer has not been established. In this review we examine the evidence of JCV in cancer in both animals and humans and try to demystify the ongoing debate.
2. JC Virus
2.1. JCV History and Disease
JCV is a human polyomavirus of the Polyomaviridae family, which also includes BK virus and simian vacuolating virus 40 (SV40). JC virus (JCV) was first isolated in 1971 from the brain of a patient with Progressive Multifocal Leukoencephalopathy (PML) [1]. This virus, termed Mad-1, is the JCV prototype strain and has been used in most in vitro and in vivo studies of JCV. Most individuals become infected with JC virus during childhood or adolescence, and by adulthood approximately 50–80% of individuals are seropositive [2,3]. Although the exact mechanism of JCV transmission is unknown, it is thought to be spread via a fecal-oral route as JCV can be detected in untreated urban sewage [4,5]. Initial JCV infection is thought to occur in the tonsils [6,7,8], after which the virus spreads to infect the epithelium of the kidney, [9,10] where it establishes a life-long, persistent infection [11]. JCV infection in immunocompetent hosts is usually subclinical and localized in the kidney. It is not clear whether JCV reactivation causes viral spread to the CNS or if a latent infection in the CNS becomes locally reactivated. However, JCV is permissive in B-lymphocytes of the bone marrow and peripheral blood [12,13,14,15,16,17], suggesting that viral spread after primary replication may occur via a hematogenous route. In the CNS, JCV infects glial cells including astrocytes and myelin-producing cells, known as oligodendrocytes [18,19]. In cases of immunosuppression, JCV can become reactivated leading to enhanced viral replication and a lytic infection of the CNS [20,15,14,21] causing cytolytic destruction of oligodendroglia, resulting in the fatal disease PML [22,23]. The exact mechanism of oligodendroglia cell death in PML is unknown (reviewed in [23]). PML is characterized by multiple foci of demyelination of cerebral white matter. Disease diagnosis is made by identification of white matter lesions through magnetic resolution imaging [24] and PCR analysis of cerebrospinal fluid for JCV DNA [25]. Patients with PML develop symptoms including ataxia, hemiplegia, paralysis, vision loss, speech impairment, and loss of cognitive function [22,26]. The prognosis for PML is dismal as it proves fatal within 6–12 months of the onset of symptoms [27,28,29,30,31].
PML has been reported in individuals with immunosuppression as a result of human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS) [32,33], organ transplantation [34], or inherited immunodeficiencies such as severe combined immunodeficiency (SCID) [35], hyperimmunoglobulinemia M [36], and CD40 ligand deficiency [37]. Recently, PML has been reported in patients with Multiple Sclerosis (MS) and Crohn’s disease that are receiving the drug, natalizumab, which blocks leukocyte transport from the gut to the brain [27,38,39]. The most common cause of PML is associated with HIV/AIDS [40,41]. Approximately 5–8% of HIV-infected individuals develop PML as a result of JCV reactivation due to immunosuppression [41], and it usually results in fatality [29,30,31]. There is currently no effective treatment for PML [31].
2.2. JCV Background and Life Cycle
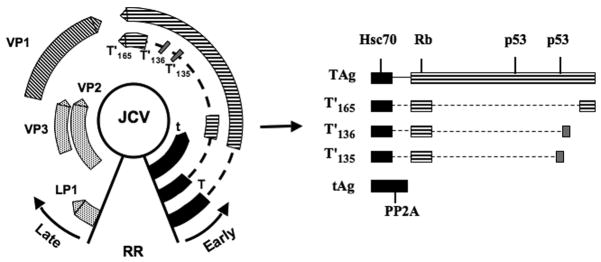
JCV is a small, nonenveloped virus with an icosahedral capsid containing 5- and 6-fold axes of symmetry [42]. The viral capsid is comprised of the major capsid protein, VP1, arranged as 72 pentamers [43] each of which interacts with the C-terminus of one of the minor capsid proteins, VP2 or VP3 [44]. The circular, dsDNA genome is approximately 5.1 kilobase pairs in size and is packaged with cellular histones, forming the viral minichromosome [45], which has structural similarities to cellular host chromatin [46]. The viral genome is divided into three main regions: the early coding region, the late coding region, and the non-coding control region (NCCR) or regulatory region (RR) (Fig. 1). The early coding region encodes for small tumor antigen (t Ag) and large tumor antigen (T Ag), and the late coding region encodes for the viral capsid proteins (or V antigens): VP1, VP2, and VP3, as well as the viral nonstructural protein agnoprotein. The NCCR separates the early and late coding regions and houses the viral enhancers, promoters, and the origin of DNA replication [42].
Fig. 1.
Molecular map of the JCV genome. The JCV genome is a circular, dsDNA genome, approximately 5.1 kb in size. The viral genome is divided into the early coding region and the late coding region, which are separated by the non-coding control region or regulatory region (RR). Transcription of viral genes is temporally regulated and occurs in a bidirectional manner as depicted by the black arrows. Early genes include small t antigen, large T antigen, T′(135), T′ (136), and T′ (165). Late genes include LP1, VP1, VP2, and VP3. The JCV t- and T- antigen and T′ proteins and host-cellular protein interaction domains are shown on the right. Specifically, heat-shock 70 (Hsc70), retinoblastoma (Rb), p53, and PP2A are indicated due to their role in cellular transformation.
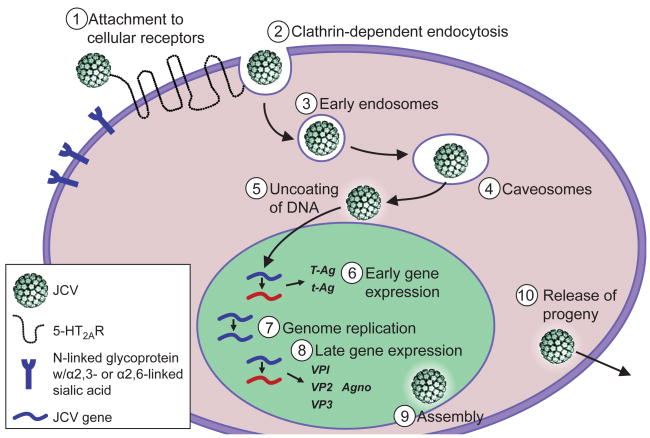
The JC virus life cycle is initiated by JCV attachment to host cellular receptors, and then the virus traffics through cellular compartments to the nucleus where viral transcription and genome replication take place (Fig. 2). Initial studies to define a receptor for JCV suggested that infection is mediated by an N-linked glycoprotein with α-2,3-linked sialic acid [47], while more recent studies uncovered that JCV can use an N-linked glycoprotein with α-2,3- or α-2,6-linked sialic acid [48]. Virus binding to sialic acid is mediated through viral protein 1 (VP1), the pentameric protein of the viral outer capsid [49]. Recent studies to identify a proteinaceous receptor for JCV revealed that serotonin receptor 5-hydroxytryptophan (5-HT)2A facilitates JCV entry into host cells [50]. The serotonin receptor 5-HT2AR is a seven transmembrane-spanning G-protein-coupled receptor (GPCR) that belongs to the family of 5-HT serotonin receptors. 5-HT2AR is abundantly expressed on cells in the brain [[51] including neurons and glial cells [52] and in the kidney [51]. Since the receptor expression is consistent with JCV tropism, it is possible that 5-HT2AR expression is a key host-cell determinant.
Fig. 2.
The JCV life cycle. JCV infection of host cells is initiated by attachment to cellular receptors, α-2,3- or α-2,6-linked sialic acid and serotonin receptor 5-HT2AR. The virus is internalized into cells by clathrin-dependent endocytosis. JCV then traffics through early endosomes and caveosomes to the nucleus. In the nucleus viral early gene transcription occurs, followed by viral DNA replication and late gene transcription. After production of the viral structural proteins VP1, VP2, and VP3, progeny virions are assembled in the nucleus and released.
Following engagement of receptors on the cell surface, JCV enters cells by clathrin-dependent endocytosis [53] through an Eps-15-dependent mechanism [54]. JCV cell entry also requires tyrosine kinase activity and leads to the activation of mitogen activated protein kinases (MAPK) ERK1 and ERK2 [54]. After the virus enters into clathrin-coated pits, it is delivered to early endosomes and caveosomes [55]. JCV entry and infection are dependent on acidic endosomal pH [56] yet a specific role for a pH-dependent step in the JCV life cycle remains unclear. The virus traffics through the cell via the cellular cytoskeleton components: intermediate filaments and microtubules, to the nucleus where the rest of the virus life cycle takes place [56]. JCV VP1 contains a weak nuclear localization signal (NLS) [57] and virus nuclear localization is most efficient in the presence of minor capsid protein VP2 or VP3, both of which also contain a NLS [58,59]. Viral uncoating is thought to begin in the endoplasmic reticulum followed by translocation to the cytosol and eventual transport of the genome to the nucleus.
Once in the nucleus, transcription of the viral genome occurs in a temporally regulated fashion to transcribe the early and late genes. The promoter of the Mad-1 strain contains two characteristic 98-base pair repeats, which function in a bidirectional manner to regulate the expression of early and late genes. First, the viral promoter drives transcription of the early genes. Cellular transcription factors, including AP1, NF-1, NF-kB, NFAT, and YB-1 have been identified to bind to the JCV enhancer/promoter region and play a role in regulating JCV expression through activation or silencing [60,61,62,63,64,65,66,67]. The JCV early mRNA is alternatively spliced into 5 transcripts: large T Ag, small t Ag, T′ (135), T′ (136), and T′ (165) [68]. T Ag is a regulatory protein that directs transcription and viral DNA replication. When T Ag is expressed in abundance it can bind to the origin of DNA replication, unwind the viral DNA through its helicase activity, and recruit the host-cell DNA polymerase to drive replication [69]. The T′ proteins also function to enhance T Ag-driven DNA replication [70]. Furthermore, T Ag suppresses early gene transcription and initiates transcription of the late viral genes VP1, VP2, and VP3, and Agno. The viral capsid proteins VP1, VP2, and VP3 are assembled into viral capsids in the nucleus [57]. Agnoprotein is produced in JCV-infected cells and regulates viral transcription and replication by directly interacting with T Ag [71], yet it is not packaged into new virions [72]. JCV localizes to discrete nuclear regions, ND10 domains, where it is thought that viral assembly takes place [73] prior to release of progeny virions.
2.3. JCV Oncogenic Features
Like other DNA viruses, JCV usurps the host-cell DNA replication machinery in order to drive viral replication. The interplay of viral- and host-cell-factors is necessary for a productive viral replication cycle, but this also presents interesting consequences for the cells. First, in order for the virus to replicate, it must drive the cell cycle from G1 into S phase. The virus achieves this by binding to and inactivating cellular proteins that prevent transition into S phase. The viral T Ag protein binds to and sequesters the retinoblastoma family tumor suppressors Rb [74], p130 [75], p107 [76], and tumor suppressor p53 [77] (Fig. 1), which normally function to regulate the cell cycle. The N-terminal domain of T Ag is required for cellular transformation and contains the sequence LXCXE, the binding domain for Rb family members, and the J domain [78]. T antigen binding to Rb disrupts its ability to regulate cyclin-cyclin dependent kinase (cdk) activity, thereby preventing the cells from exiting the cell cycle [79]. Normally, Rb family members sequester the E2F transcription factors, which are necessary for cell cycle progression. However, when T Ag is bound to Rb, this interaction is prevented, releasing E2F and forcing the cell to enter S phase. The C-terminal region of T Ag contains the p53-binding domain [80]. p53 is a tumor suppressor that can initiate cell cycle arrest, induce DNA repair, and drive apoptosis or cellular senescence. Thus, sequestration of p53 by T Ag promotes cell cycle progression in the presence of DNA damage and inhibits the function of p53 to induce apoptosis, causing uncontrolled cell growth. T Ag has also been shown to interact with insulin receptor substrate-1 (IRS-1) [81] and β-catenin [82]. In JCV T Ag-positive cells, β-catenin expression was increased and localized to the nucleus where it is thought to enhance the expression of c-myc, another regulator of the cell cycle [83]. JCV T Ag can transform cells in culture including rat fibroblasts and baby hamster kidney cells, albeit inefficiently. T Ag-transformed cells exhibit multinucleation, increased doubling time, growth in anchorage dependent-conditions, and subcutaneous growth in nude mice [84,77]. Further, JCV T Ag has been associated with altered chromosomal stability and anueploidy in B lymphocytes and tumors [85,86,87,88]. In addition to T Ag, other viral proteins have been suggested to play a role in cellular transformation. Agnoprotein has been demonstrated to bind to p53 and disrupt the cell cycle by causing cells to arrest at the G2/M phase [89]. In addition, a recent study suggests that JCV small t Ag interacts with protein phosphatase 2A (PP2A), a serine/threonine-specific protein phosphatase that plays a key regulatory role in the mitogen-activated protein kinase (MAPK) signaling pathway [90]. SV40 small t Ag interacts with PP2A, disrupting its ability to inactivate MAPK signaling, leading to uncontrolled cell growth [91]. The oncogenic potential of JCV proteins and their complex interactions with cellular proteins are reviewed in greater detail in this issue.
3. JCV and Tumorigenesis in Animal Models
Although JC virus is not permissive in hosts other than humans, inoculation of some mammalian species results in tumorigenesis. JCV can induce tumor formation in small rodents including hamsters [92–96] and rats [97], and in non-human primates including owl monkeys and squirrel monkeys [98–105] (Table 1). The tumor type, origin, and characteristics vary between host species and also depend on the route of inoculation and strain of virus utilized. Although JCV does not induce tumors in normal mice, expression of the JCV early region in transgenic mouse models causes tumorigenesis.
Table 1.
Tumorigenesis of JCV in animal models.
| Animal | Tumor Type | Reference |
|---|---|---|
| Hamster | Medulloblastoma | Padgett et al. 1977 and Zu Rhein et al. 1979 |
| Glioma | Walker et al. 1973 | |
| Neuroblastoma | Varakis et al. 1978 | |
| Pineocytoma | Padgett et al. 1977 | |
|
| ||
| Rat | Neuroectodermal | Ohsumi et al. 1986 |
|
| ||
| Owl Monkey | Astrocytoma | London et al. 1978 |
| Glial/Neural Origin | ||
| Glioblastoma | Major et al. 1987 | |
|
| ||
| Squirrel Monkey | Astrocytoma | Houff et al. 1983 and London et al. 1983 |
3.1. Small rodent models
3.1.a. Hamsters
Newborn golden Syrian hamsters (Mesocricetus auratus) inoculated with JCV develop tumors within 3–12 months of inoculation. JCV-induced tumors include: medulloblastomas, a malignant brain tumor that originates in the cerebellum [92,93]; gliomas, a brain tumor that arises from glial cells and originates in the brain [94]; neuroblastomas, a neuroendocrine tumor that arises from the neural crest and induces tumors in nerve tissue [95]; and, pineocytomas, which arise from the pineal gland [92]. Hamsters that develop tumors show signs of neurological illness, which rapidly progresses and can result in death [92]. The tumor type, origin, and characteristics are dependent on the JCV strain and route of inoculation. Mad-1, the prototype JCV strain isolated from patients with PML, is neurooncogenic and resultant tumors include gliomas [94] and medulloblastomas [92,93]. Newborn hamsters inoculated intracerebrally and subcutaneously with JCV Mad-1 develop malignant gliomas within 6 months [94] or medulloblastomas within 3–6 months [93]. While newborn hamsters inoculated with JCV Mad-2 strain develop medulloblastomas paralleling the pathogenesis of the Mad-1 strain, hamsters inoculated with the Mad-4 strain develop pineocytomas and medulloblastomas [92]. Taken together, these studies highlight the JCV strain-specific differences in tumor type and origin. Furthermore, collected tumor tissue resuspended and inoculated subcutaneously into newborn hamsters, induced tumors within 1–2 months [94], suggesting that JCV-induced tumors can be subcutaneously passaged in hamsters. On the other hand, hamsters inoculated intraocularly with JCV develop neuroblastomas. These tumors develop after a 6–11 month period of latency and primary tumors arise in the abdominal cavity, pelvis, mediastinum, and neck region. JCV-induced neuroblastomas from intraocular inoculation metastasize causing additional tumors in the liver, bone marrow, and lymph nodes. Neuroblastomas can also be serially transplanted in weanling hamsters and grown in tissue culture [95]. Collectively, newborn hamsters inoculated with JCV develop tumors at a rapid rate, yet the tumor origin and characteristics are dependent on the strain and inoculation site.
3.1.b. Rats and Transgenic Mice
Inoculation of newborn Sprague-Dawley rats with JCV Tokyo-1 strain results in development of neuroectodermal tumors of the cerebrum and olfactory bulb in approximately 80% of animals. The tumors develop within 21–70 weeks post-inoculation. The rats show signs of neurological illness beginning at 21 weeks post-inoculation such as weakness, ataxia, and death. Also, JCV-induced tumors are oncogenic when transplanted into new experimental rats [97]. While there is a strong correlation of JCV-induced tumors in rats, there are few reports that have followed this study.
JCV is not oncogenic in normal mice nor does it transform mouse cells in culture. However, transgenic mice that express the early region of the JCV genome develop tumors in a tissue-specific fashion within 6–8 months after birth. In one study, 4 out of 5 transgenic mice developed primary abdominal tumors, adrenal neuroblastomas, which mestastisized to other tissues including the intestine, stomach, liver, spleen, and brain [106]. Studies to understand how oncogenesis is regulated in JCV-transgenic mice suggest that JCV-induced oncogenesis in mice is controlled not only by T Ag but also the viral regulatory region containing the enhancer and promoter [107]. Transgenic mice expressing the early region of the archetype strain JCV (CY) develop primitive neuroectodermal tumors (PNETs) in the hindbrains, which resemble human medulloblastomas. Biochemical and immunohistochemical analysis of the PNETs revealed that severely ill mice had higher levels of expression of T Ag RNA and protein and that T Ag was expressed in the nuclei of all the tumor tissue analyzed at a rate of 25–75% of cells per sample. These studies suggest that JCV (CY) early region-transgenic mice develop brain tumors and that the JCV promoter region plays a key regulatory role in T Ag expression in the CNS [108]. Moreover, transgenic mice that express the JCV Mad-4 strain T Ag and promoter region develop large tumors in the base of the skull within 1 year in 50% of animals. Through histological and immunohistochemical analysis the tumors were determined to have arisen from the pituitary gland. Furthermore, the authors show that tissues isolated from these tumors express increased levels of p53, which is in complex with viral T Ag [109]. Transgenic mice expressing the JCV early region develop tumors within 1 year of birth. Similar to JCV-induced oncogenesis in hamsters, the type and characteristics of the tumors that develop in transgenic mice are dependent on the strain of JCV utilized and the region of the genome expressed in the mice.
3.2. Non-human Primates
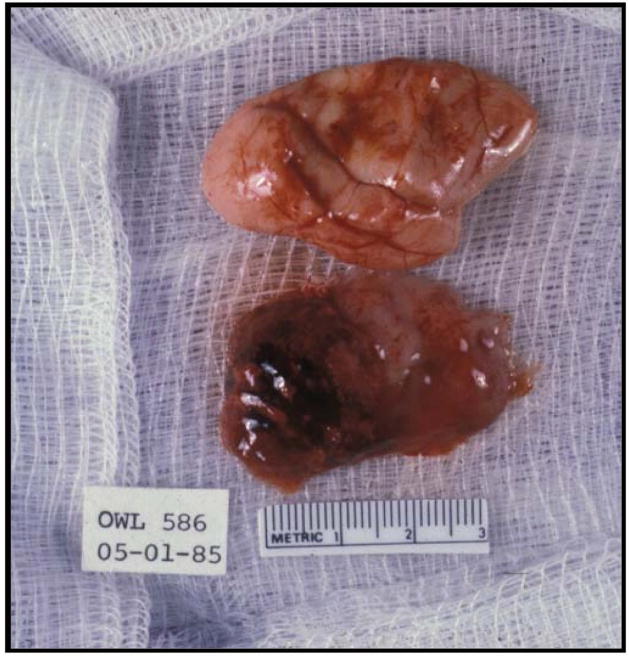
Squirrel monkeys (Saimiri sciureus) and owl monkeys (Aotus trivirgatus), which are both non-permissive to viral infection by JCV, develop fatal brain tumors 14–36 months after being inoculated with JCV [98,104,99,103]. While there is a long period of latency between inoculation time and symptom onset, the tumors progress rapidly causing neurologic impairment, coma, and death [102]. Owl monkeys develop astrocytomas resembling GBM or tumors of glial and neural origin [98,101], while squirrel monkeys develop astrocytomas [99,103]. Tissues isolated from JCV-induced tumors of owl monkeys have been shown to express T Ag [98,100] and contain viral genome integrated into host cells [104,105] but do not produce V antigens [98]. Interestingly, a suspension of tumor tissue isolated from an owl monkey with a JCV-induced astrocytoma [98] and inoculated into a recipient juvenile owl monkey resulted in the development of a glioblastoma [100] (Fig. 3). Further, explanted tumor tissue from the recipient owl monkey grown in culture expressed T Ag in the nucleus and produced infectious JCV. Viral T Ag isolated from the explanted tumor cultures formed a complex with tumor suppressor p53 unlike T Ag isolated from other owl monkey glioblastomas of the Mad-1 and Mad-4 strains suggesting that Mad-1 T Ag complexes with p53 to induce tumorigenesis [100].
Fig. 3.
Glioblastoma in an owl monkey brain. Shown is a representative image of a glioblastoma induced in the right cerebral hemisphere (bottom panel) in the brain of an owl monkey after inoculation with tumor suspensions from an explanted JCV-induced owl monkey astrocytoma. The top panel shows the left cerebral hemisphere free of tumors. The juvenile owl monkey was inoculated intracerebrally with suspensions of JCV-infected primary human fetal glial cells and developed a glioblastoma 28 months later. JCV DNA was recovered from tumor cells and demonstrated homology to JCV Mad-1 yet had a 19-base pair deletion in one of the 98-base pair repeats of the regulatory region. The viral sequence showed similarity to the JCV neurooncogenic strain, Mad-4. This new virus was named JCV-586. Image was kindly provided by Eugene Major.
4. JCV and Associated Human Cancers
The association of JCV with human cancers is controversial. The majority of the human population is seropositive for JC virus with seroconversion occurring during adolescence. Therefore, while JCV DNA can be extracted from human tumor tissue, it is challenging to determine causality due to the ubiquitous nature of the virus. JCV is a tumor-causing virus in species that do not serve as the natural host for the virus such as small rodents and non-human primates (Section 3). Therefore, it is reasonable to hypothesize that JCV infection and T Ag expression in humans could cause aberrant cell growth and tumor formation. Several reports have postulated that JCV infection is associated with various types of human cancers including gastrointestinal cancers: colorectal [110,111], gastric [112,113], and esophageal [114]; brain cancers: glioblastomas [115,116,117,118], oligoastrocytomas [119], oligodendrogliomas [120], and medullablastomas [121,122,123]; and lung cancer [124]. Groups have also reported that there is no association or involvement of JCV in these particular cancers [125,126,127]. Most of the studies designed to test whether JCV is associated with human cancers have utilized PCR to detect viral DNA from tumor tissue and immunohistochemistry to detect viral T Ag protein in tissue samples. Some of the available data are discussed below.
4.1. Cancers of the CNS
Given that JC virus has a restricted tissue tropism for glial cells and astrocytes in the CNS, and that it causes brain tumors in small rodent models and non-human primates, many groups have investigated the role of JCV in human brain tumors. While the role of JCV in brain tumors is still hotly debated, JCV has been reported to have an association with gliomas or glial-derived tumors [128]: oligoastrocytomas [119], glioblastomas [115,118,117,116], oligodendrogliomas [120], as well as medulloblastomas [121,122,123], while other groups report a lack of association between JCV and brain tumors [129,130,131,132,126]. Since there are conflicting views on the association of JCV with human brain tumors, several representative studies are discussed in more detail below. Medulloblastomas, malignant tumors that arise from the cerebellum, are one of the most common types of brain tumors in children [133]. Furthermore, JCV-induced tumors in small animal models resemble medulloblastomas [92,93]. In a study to determine the role of JCV in human medulloblastomas, Krynska et al. reported that DNA from 11 out of 23 medulloblastoma tissues were positive for JCV DNA. PCR analysis revealed that 87% of samples were positive for the N-terminal region of T Ag, 56% were positive for the C-terminal region, and 87% were positive for the VP1 region. Additionally, T Ag was expressed in the nuclei of 4 out of 16 samples as determined by IHC. The authors raise the idea that the majority of samples were positive for PCR corresponding to the N-terminal region of T Ag, which is known to associate with p53, mutation of which has been demonstrated to play a role in 5–10% of medulloblastomas [122]. In another study to assess the role of JCV in medulloblastomas, 20 paraffin-embedded tissue samples were analyzed for the presence of JCV DNA and viral proteins. JCV Agno gene was detected by PCR analysis (69%) and agnoprotein was expressed in the cytoplasm of neoplastic cells in 55% of samples as assessed by IHC. Furthermore, nuclear T Ag was only expressed in some neoplastic cells, while agnoprotein was expressed in the absence of T Ag in some neoplastic cells [134]. The expression of agnoprotein in medulloblastoma samples in the absence of T Ag is interesting and suggests that T Ag expression may not be the only marker of JCV in tumor tissues, especially since agnoprotein has been reported to have oncogenic properties similar to T Ag [89]. This study demonstrates that it is worth analyzing the expression of other JCV proteins in addition to T Ag in IHC analysis of tumor tissues.
Since JCV inoculation of animal models results in a variety of brain tumors, researchers have also examined the role of JCV in other cancers including glioblastoma multiforme (GBM). Pina-Oveido and colleagues present a convincing case for JCV in GBM in a 54-year old immunocompetent individual. Tumor tissue was analyzed for JCV proteins by IHC and revealed T-Ag positive nuclei and expression of agnoprotein in the cytoplasm. T Ag-positive cells were isolated by laser capture microdissection, DNA was isolated, and PCR analysis was performed. JCV DNA was amplified using PCR primers specific to the early, late, and control regions, which was confirmed by Southern blot analysis. Sequence analysis of the JCV coding region revealed that the virus isolated from the tissue was the Mad-1 strain with point mutations in the control region [118]. In another case study, JCV DNA and protein were detected in a GBM from an immunocompromised individual with MS. GBM tumor tissue was positive for nuclear T Ag by IHC, and JCV DNA was amplified by PCR using primers specific to the early and late regions. The JCV DNA was determined to be Mad-1 strain by sequence analysis [135]. These independent case studies indicate an association of JCV in GBM tumor tissues, yet represent a small sample number. Contrary to these findings, a previous report in which 80 tissue samples from patients with GBM were analyzed, JCV DNA was not detected by PCR analysis using primers for either the early or control region [129]. Therefore, while the role of JCV in GBM in the presented case studies suggests a correlation, there is a lack of consistency in studies with larger cohorts. It is also possible that differences in the laboratory techniques utilized can explain the discrepancies. In an interesting study performed by Rollison and Del Valle and colleagues, they sought to determine whether the controversy of JCV and cancer association could be explained by the discrepancies in techniques, experimenters and/or laboratory locations. To address this concern, they performed a study to analyze brain tumors for the presence of JCV DNA using PCR performed in two independent laboratories. The study included tissue from 225 pediatric and adult brain tumors including astrocytomas, gliomas, oligodendrogliomas, and medulloblastomas. Results from the study demonstrate that JCV DNA was rarely detected in the brain tumor tissue and no tumor tissue tested positive in both laboratories [132].
4.2. Colorectal Cancer
JCV spread is thought to occur by fecal-oral transmission, suggesting that JCV contacts intestinal cells in human hosts. Given the ubiquitous nature of JCV and the high frequency of colon cancer, researchers have sought to determine a correlation. Some studies to assess the role of JCV in human colon cancer, have found that JCV T Ag DNA sequences are expressed in neoplastic tissues of the colonic mucosa but sequences were excluded from adjacent non-neoplastic colonic epithelial tissues [111,110]. For instance, in a study by Enam et al., researchers found that of 22 samples from colonic neoplastic tissue 81.5% were positive for the early region of the JCV genome by PCR analysis and 62.9% were positive by immunohistochemistry [83]. However, there are a number of reports demonstrating that JCV DNA can be isolated from adjacent non-neoplastic colon tissue [136], enteric glial cells of the myentric plexus of patients with chronic idiopathic pseudo-obstruction (CIIP) [137], healthy tissue in the GI tract [138,136], and in premalignant lesions of the colon [139,136]. On the other hand, Laghi et al. report that JCV T Ag sequences are found in both normal human colon mucosa and colorectal cancers but that there is an increased number of copies of JCV T Ag in cancer cells versus non-neoplastic colon cells [111]. According to a study by Riccardello et al., JCV Mad-1 strains isolated from colon cancer tissue contained multiple mutations with the most common exhibiting only one 98bp repeat in the control region, rather than the two 98bp repeats normally expressed in the Mad-1 prototype strain. The pmlΔ98 mutant strain exhibited decreased replication rates in PHFG cells but increased transformation in Rat2 fibroblasts in comparison to Mad-1 [140]. Taken together, these reports suggest the presence of JCV genomic DNA and T Ag protein expression in colorectal cancer tissue, yet viral DNA and protein are not excluded from surrounding healthy tissues.
4.3. Other Cancers
The role of JCV in other types of gastrointestinal cancers and lung cancer has been investigated. In a recent study, JCV DNA was isolated from normal and abnormal esophageal tissues. While tissue from healthy patients and patients with esophageal disorders were both positive for JCV DNA, only carcinoma tissue was positive for JCV proteins by immunohistochemistry. Laser capture microdissection was used to isolate cells that were positive by immunohistochemistry and individual cells were further analyzed for JCV DNA using PCR and Southern blot analysis [114]. Additionally, JCV T Ag sequences have been amplified from both normal gastric mucosa and gastric cancer tissue. In one study, DNA sequences specific to T Ag, VP1, and the regulatory region were found in both normal and cancer tissue. However, T Ag protein was only expressed in cancer tissue (39%) and not in non-neoplastic tissue (0%) [113]. However, another study on JCV in gastric cancer reports that JCV T Ag sequence is amplified from 100% of normal mucosa and 86% from gastric cancer tissues. The authors report that there is a higher JCV viral load in cancer tissue compared to normal tissue [112]. Moreover, some researchers have reported that JCV T Ag sequence has been amplified from lung carcinomas at a higher rate than normal lung tissues [124] yet other groups report no such correlation between JCV and lung carcinogenesis [141, 142]. It should be noted that in the study by Zheng and colleagues there was 103 lung carcinoma samples and 18 normal samples. The low number of normal tissue samples may have influenced the results. This is common for a number of studies, as normal tissue may not be as easily obtained.
5. Discussion
JCV is a significant human pathogen with a profound biology. However, a causative role for JCV in human cancers has not been established. What criteria are necessary to establish causality or an association of JCV with human cancers? What criteria have been established for other DNA viruses that are thought to induce cancer? Human papillomavirus (HPV) has clearly been shown to be the major causative agent in cervical cancer. Viral genes E6 and E7 are consistently detected in cervical cancer tissue and can transform cells in culture. Further, viral oncogenes E6 and E7 bind to tumor suppressor p53, providing a molecular basis of cellular transformation and oncogenesis. One of the strongest pieces of evidence that links HPV to cervical cancer is that only certain viral genotypes, considered “high-risk” subtypes, contribute to cervical cancer, while other low-risk subtypes that are widespread do not cause cancer. The finding that only certain HPV genotypes contribute to cervical cancer was important, as this allowed researchers to not be distracted by the other HPV genotypes expressed in cervical tissue that are not oncogenic [143]. Thus, the role of HPV in cervical cancer is clear based on epidemiologic evidence, the consistent presence of viral genes in cancer tissues, a link to specific viral genotypes, and a molecular basis for viral oncogenesis and cellular transformation. The necessary criteria to establish a causal relationship between a virus and cancer include: detection of viral genome or gene products in cancer tissue, a molecular basis for virus-induced oncogenicity, and consistency of the association [144].
Does JCV fit these criteria? Genomic DNA and expression of viral proteins has been demonstrated in a number of tumor tissues. It is evident that JCV can cause tumors in experimental animals and transform cells in culture. Furthermore, JCV has several oncogenic features including T Ag, which directly interacts with tumor suppressors and cell-cycle regulators to disrupt the cell cycle and prevent apoptosis. T Ag is also considered oncogenic based on the fact that expression of the viral T Ag in transgenic mice results in the development of tissue-specific tumors. While these findings are compelling, a consistent association of JCV with a particular type of tumor or human cancer has not been established. Thus far, there is a lack of epidemiological studies that link JCV to human cancers. JCV has been reported to contribute to a wide variety of tumors indicating that there is not a clear correlation of JCV with a particular type of cancer. Moreover, there are relatively few reports that implicate JCV in human cancers, and there are a comparable number of studies that refute these findings. Taken together, a causative role of JCV in human cancers is still unclear. However, since JCV genomic DNA and oncogenic viral proteins have been detected in multiple tumor tissues, it is reasonable to hypothesize that JCV serves as a co-factor in tumorigenesis. For instance, it is possible that JCV initially infects rapidly dividing tumor cells and expression of viral early genes then contributes to oncogenesis. If this type of JCV infection occurs in a cell that is already undergoing aberrant cell growth due to misregulation of the cell cycle or other genetic or environmental components, JCV may provide the “2nd hit” according to Knudson’s two-hit hypothesis of cancer development [145]. Therefore, it is worthwhile to continue to explore the role of JCV in human cancers as a co-factor in oncogenesis especially since ever-changing environmental and social factors play such an important role in disease pathogenesis and cancer.
It should be noted that many human tumor samples used in studies to determine whether JCV plays a role in human cancers have been obtained from HIV-positive individuals and children. This brings to light the question of immunocompetency in the individuals from which samples are obtained and the use of appropriate controls. For example, in one study, 20 samples of medulloblastomas were obtained from HIV-positive individuals including some pediatric patients [134]. Since HIV presents a state of immunosuppression and JCV reactivation in the CNS, a careful examination of the case histories of the patients should be noted. For instance, this particular study may have been strengthened by the addition of control group of 20 individuals with medulloblastomas that were immunocompetent or HIV-negative. Similarly, most of the brain tumors that have been associated with JCV are found in children who do not have completely mature immune systems. This presents an interesting question regarding immune status and JCV pathogenesis. Does immune status determine whether JCV will cause a productive infection, reside latently in tissues such as the brain, or contribute to tumorigenesis? Studies analyzing the association of JCV with human brain tumors in various states of immunocompetency would be worthwhile and may reveal interesting new insights into the role of JCV and human cancers.
Table 2.
Reported associations of JCV and human cancers.
| Tumor Type/Cancer | References |
|---|---|
| Glioblastomas | Del Valle et al. 2000, Del Valle et al. 2001, Boldorini et al. 2003, and Pina-Oviedo et al. 2006 |
| Medulloblastoma | Krynska et al. 1999, Del Valle et al. 2000, Delbue et al. 2005, and Shiramizu et al. 2007 |
| Astrocytoma | Del Valle et al. 2001 and Boldorini et al. 2003 |
| Oligoastrocytoma | Rencic et al. 1996, Caldarelli-Stephano et al. 2000, and Del Valle et al. 2001 |
| Oligodendroglioma | Caldarelli-Stephano et al. 2000, Del Valle et al. 2001, Del Valle et al. 2002, and Boldorini et al. 2003 |
| Ependymoma | Caldarelli-Stephano et al. 2000 and Del Valle et al. 2001 |
| Colorectal cancer | Laghi et al. 1999, Ricciardiello et al. 2001, Enam et al. 2002, Lin et al. 2008, and Jung et al. 2008 |
| Esophageal cancer | Del Valle et al. 2005 |
| Gastric cancer | Shin et al. 2006 and Murai et al. 2007 |
| Lung cancer | Zheng et al. 2007 |
Acknowledgments
We thank Eugene Major and Wendy Virgadamo for preparation of figures and the Atwood Lab for assistance in preparation of the manuscript. This work has been supported by NIH R01CA71878 and NIH R01NS43097.
Abbreviations
- JCV
JC virus
- PML
Progressive Multifocal Leukoencephalopathy
- T Ag
Large tumor antigen
- t Ag
small tumor antigen
Footnotes
Conflict of Interest Statement
The authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Padgett BL, Walker DL, ZuRhein GM, Eckroade RJ, Dessel BH. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971;1(7712):1257–60. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- 2.Major EO, Amemiya K, Tornatore CS, Houff SA, Berger JR. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin Microbiol Rev. 1992;5(1):49–73. doi: 10.1128/cmr.5.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knowles WA, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71(1):115–23. doi: 10.1002/jmv.10450. [DOI] [PubMed] [Google Scholar]
- 4.Bofill-Mas S, Girones R. Excretion and transmission of JCV in human populations. J Neurovirol. 2001;7(4):345–9. doi: 10.1080/13550280152537210. [DOI] [PubMed] [Google Scholar]
- 5.Bofill-Mas S, Pina S, Girones R. Documenting the epidemiologic patterns of polyomaviruses in human populations by studying their presence in urban sewage. Appl Environ Microbiol. 2000;66(1):238–45. doi: 10.1128/aem.66.1.238-245.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato A, et al. Detection of the archetypal regulatory region of JC virus from the tonsil tissue of patients with tonsillitis and tonsilar hypertrophy. J Neurovirol. 2004;10(4):244–9. doi: 10.1080/13550280490468663. [DOI] [PubMed] [Google Scholar]
- 7.Monaco MC, Jensen PN, Hou J, Durham LC, Major EO. Detection of JC virus DNA in human tonsil tissue: evidence for site of initial viral infection. J Virol. 1998;72(12):9918–23. doi: 10.1128/jvi.72.12.9918-9923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monaco MC, Atwood WJ, Gravell M, Tornatore CS, Major EO. JC virus infection of hematopoietic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implications for viral latency. J Virol. 1996;70(10):7004–12. doi: 10.1128/jvi.70.10.7004-7012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chesters PM, Heritage J, McCance DJ. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J Infect Dis. 1983;147(4):676–84. doi: 10.1093/infdis/147.4.676. [DOI] [PubMed] [Google Scholar]
- 10.Dorries K, ter Meulen V. Progressive multifocal leucoencephalopathy: detection of papovavirus JC in kidney tissue. J Med Virol. 1983;11(4):307–17. doi: 10.1002/jmv.1890110406. [DOI] [PubMed] [Google Scholar]
- 11.Dorries K. Molecular biology and pathogenesis of human polyomavirus infections. Dev Biol Stand. 1998;94:71–9. [PubMed] [Google Scholar]
- 12.Dorries K, et al. Association of human polyomavirus JC with peripheral blood of immunoimpaired and healthy individuals. J Neurovirol. 2003;9 (Suppl 1):81–7. doi: 10.1080/13550280390195379. [DOI] [PubMed] [Google Scholar]
- 13.Azzi A, et al. Human polyomaviruses DNA detection in peripheral blood leukocytes from immunocompetent and immunocompromised individuals. J Neurovirol. 1996;2(6):411–6. doi: 10.3109/13550289609146907. [DOI] [PubMed] [Google Scholar]
- 14.Dubois V, et al. Latency and reactivation of JC virus in peripheral blood of human immunodeficiency virus type 1-infected patients. J Clin Microbiol. 1997;35(9):2288–92. doi: 10.1128/jcm.35.9.2288-2292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubois V, et al. Detection of JC virus DNA in the peripheral blood leukocytes of HIV-infected patients. Aids. 1996;10(4):353–8. doi: 10.1097/00002030-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Tornatore C, et al. Detection of JC virus DNA in peripheral lymphocytes from patients with and without progressive multifocal leukoencephalopathy. Ann Neurol. 1992;31(4):454–62. doi: 10.1002/ana.410310426. [DOI] [PubMed] [Google Scholar]
- 17.Atwood WJ, Amemiya K, Traub R, Harms J, Major EO. Interaction of the human polyomavirus, JCV, with human B-lymphocytes. Virology. 1992;190(2):716–23. doi: 10.1016/0042-6822(92)90909-9. [DOI] [PubMed] [Google Scholar]
- 18.Silverman L, Rubinstein LJ. Electron microscopic observations on a case of progressive multifocal leukoencephalopathy. Acta Neuropathol. 1965;5(2):215–24. doi: 10.1007/BF00686519. [DOI] [PubMed] [Google Scholar]
- 19.Zurhein G, Chou SM. Particles Resembling Papova Viruses in Human Cerebral Demyelinating Disease. Science. 1965;148:1477–9. doi: 10.1126/science.148.3676.1477. [DOI] [PubMed] [Google Scholar]
- 20.Dorries K, Vogel E, Gunther S, Czub S. Infection of human polyomaviruses JC and BK in peripheral blood leukocytes from immunocompetent individuals. Virology. 1994;198(1):59–70. doi: 10.1006/viro.1994.1008. [DOI] [PubMed] [Google Scholar]
- 21.Houff SA, et al. Involvement of JC virus-infected mononuclear cells from the bone marrow and spleen in the pathogenesis of progressive multifocal leukoencephalopathy. N Engl J Med. 1988;318(5):301–5. doi: 10.1056/NEJM198802043180507. [DOI] [PubMed] [Google Scholar]
- 22.Astrom KE, Mancall EL, Richardson EP., Jr Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukaemia and Hodgkin’s disease. Brain. 1958;81(1):93–111. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]
- 23.Khalili K, White MK. Human demyelinating disease and the polyomavirus JCV. Mult Scler. 2006;12(2):133–42. doi: 10.1191/135248506ms1264oa. [DOI] [PubMed] [Google Scholar]
- 24.Mark AS, Atlas SW. Progressive multifocal leukoencephalopathy in patients with AIDS: appearance on MR images. Radiology. 1989;173(2):517–20. doi: 10.1148/radiology.173.2.2798883. [DOI] [PubMed] [Google Scholar]
- 25.Henson J, Rosenblum M, Armstrong D, Furneaux H. Amplification of JC virus DNA from brain and cerebrospinal fluid of patients with progressive multifocal leukoencephalopathy. Neurology. 1991;41(12):1967–71. doi: 10.1212/wnl.41.12.1967. [DOI] [PubMed] [Google Scholar]
- 26.Berger JR, Pall L, Lanska D, Whiteman M. Progressive multifocal leukoencephalopathy in patients with HIV infection. J Neurovirol. 1998;4(1):59–68. doi: 10.3109/13550289809113482. [DOI] [PubMed] [Google Scholar]
- 27.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon beta-1a for multiple sclerosis. N Engl J Med. 2005;353(4):369–74. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- 28.Langer-Gould A, Steinman L. Progressive multifocal leukoencephalopathy and multiple sclerosis: lessons from natalizumab. Curr Neurol Neurosci Rep. 2006;6(3):253–8. doi: 10.1007/s11910-006-0013-z. [DOI] [PubMed] [Google Scholar]
- 29.Fong IW, Toma E. The natural history of progressive multifocal leukoencephalopathy in patients with AIDS. Canadian PML Study Group. Clin Infect Dis. 1995;20(5):1305–10. doi: 10.1093/clinids/20.5.1305. [DOI] [PubMed] [Google Scholar]
- 30.Kuchelmeister K, Gullotta F, Bergmann M, Angeli G, Masini T. [Progressive multifocal leukoencephalopathy (PML) in AIDS: morphological and topographical characteristics] Verh Dtsch Ges Pathol. 1991;75:189–90. [PubMed] [Google Scholar]
- 31.Kishida S. [Progressive multifocal leukoencephalopathy--epidemiology, clinical pictures, diagnosis and therapy] Brain Nerve. 2007;59(2):125–37. [PubMed] [Google Scholar]
- 32.Morriss MC, et al. Progressive multifocal leukoencephalopathy in an HIV-infected child. Neuroradiology. 1997;39(2):142–4. doi: 10.1007/s002340050383. [DOI] [PubMed] [Google Scholar]
- 33.Berger JR, et al. Progressive multifocal leukoencephalopathy in HIV-1-infected children. Aids. 1992;6(8):837–41. doi: 10.1097/00002030-199208000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Shitrit D, Lev N, Bar-Gil-Shitrit A, Kramer MR. Progressive multifocal leukoencephalopathy in transplant recipients. Transpl Int. 2005;17(11):658–65. doi: 10.1007/s00147-004-0779-3. [DOI] [PubMed] [Google Scholar]
- 35.Newman JT, Frisque RJ. Identification of JC virus variants in multiple tissues of pediatric and adult PML patients. J Med Virol. 1999;58(1):79–86. [PubMed] [Google Scholar]
- 36.Redfearn A, Pennie RA, Mahony JB, Dent PB. Progressive multifocal leukoencephalopathy in a child with immunodeficiency and hyperimmunoglobulinemia M. Pediatr Infect Dis J. 1993;12(5):399–401. doi: 10.1097/00006454-199305000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Hecht JH, Glenn OA, Wara DW, Wu YW. JC virus granule cell neuronopathy in a child with CD40 ligand deficiency. Pediatr Neurol. 2007;36(3):186–9. doi: 10.1016/j.pediatrneurol.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353(4):375–81. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 39.Van Assche G, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N Engl J Med. 2005;353(4):362–8. doi: 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- 40.Major EO, Ault GS. Progressive multifocal leukoencephalopathy: clinical and laboratory observations on a viral induced demyelinating disease in the immunodeficient patient. Curr Opin Neurol. 1995;8(3):184–90. [PubMed] [Google Scholar]
- 41.Berger JR, Concha M. Progressive multifocal leukoencephalopathy: the evolution of a disease once considered rare. J Neurovirol. 1995;1(1):5–18. doi: 10.3109/13550289509111006. [DOI] [PubMed] [Google Scholar]
- 42.Shah KV, Fields BN, Knipe DM, Howley PM. Polyomaviruses. 3. Vol. 2. Philadelphia: Lippincott-Raven Publishers; 1996. Fields virology; pp. 2027–43. [Google Scholar]
- 43.Stehle T, Gamblin SJ, Yan Y, Harrison SC. The structure of simian virus 40 refined at 3.1 A resolution. Structure. 1996;4(2):165–82. doi: 10.1016/s0969-2126(96)00020-2. [DOI] [PubMed] [Google Scholar]
- 44.Chen XS, Stehle T, Harrison SC. Interaction of polyomavirus internal protein VP2 with the major capsid protein VP1 and implications for participation of VP2 in viral entry. Embo J. 1998;17(12):3233–40. doi: 10.1093/emboj/17.12.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meneguzzi G, Pignatti PF, Barbanti-Brodano G, Milanesi G. Minichromosome from BK virus as a template for transcription in vitro. Proc Natl Acad Sci U S A. 1978;75(3):1126–30. doi: 10.1073/pnas.75.3.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muller U, Zentgraf H, Eicken I, Keller W. Higher order structure of simian virus 40 chromatin. Science. 1978;201(4354):406–15. doi: 10.1126/science.208155. [DOI] [PubMed] [Google Scholar]
- 47.Liu CK, Wei G, Atwood WJ. Infection of glial cells by the human polyomavirus JC is mediated by an N-linked glycoprotein containing terminal alpha(2–6)-linked sialic acids. J Virol. 1998;72(6):4643–9. doi: 10.1128/jvi.72.6.4643-4649.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dugan AS, Gasparovic ML, Atwood WJ. Direct correlation between sialic acid binding and infection of cells by two human polyomaviruses (JC virus and BK virus) J Virol. 2008;82(5):2560–4. doi: 10.1128/JVI.02123-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gee GV, Tsomaia N, Mierke DF, Atwood WJ. Modeling a sialic acid binding pocket in the external loops of JC virus VP1. J Biol Chem. 2004;279(47):49172–6. doi: 10.1074/jbc.M409326200. [DOI] [PubMed] [Google Scholar]
- 50.Elphick GF, et al. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science. 2004;306(5700):1380–3. doi: 10.1126/science.1103492. [DOI] [PubMed] [Google Scholar]
- 51.Bonhaus DW, et al. The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. Br J Pharmacol. 1995;115(4):622–8. doi: 10.1111/j.1476-5381.1995.tb14977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bockaert J, Claeysen S, Becamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006;326(2):553–72. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- 53.Pho MT, Ashok A, Atwood WJ. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J Virol. 2000;74(5):2288–92. doi: 10.1128/jvi.74.5.2288-2292.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Querbes W, Benmerah A, Tosoni D, Di Fiore PP, Atwood WJ. A JC virus-induced signal is required for infection of glial cells by a clathrin- and eps15-dependent pathway. J Virol. 2004;78(1):250–6. doi: 10.1128/JVI.78.1.250-256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Querbes W, O’Hara BA, Williams G, Atwood WJ. Invasion of host cells by JC virus identifies a novel role for caveolae in endosomal sorting of noncaveolar ligands. J Virol. 2006;80(19):9402–13. doi: 10.1128/JVI.01086-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ashok A, Atwood WJ. Contrasting roles of endosomal pH and the cytoskeleton in infection of human glial cells by JC virus and simian virus 40. J Virol. 2003;77(2):1347–56. doi: 10.1128/JVI.77.2.1347-1356.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shishido-Hara Y, et al. Analysis of capsid formation of human polyomavirus JC (Tokyo-1 strain) by a eukaryotic expression system: splicing of late RNAs, translation and nuclear transport of major capsid protein VP1, and capsid assembly. J Virol. 2000;74(4):1840–53. doi: 10.1128/jvi.74.4.1840-1853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang D, Haynes JI, 2nd, Brady JN, Consigli RA. Identification of a nuclear localization sequence in the polyomavirus capsid protein VP2. Virology. 1992;191(2):978–83. doi: 10.1016/0042-6822(92)90276-u. [DOI] [PubMed] [Google Scholar]
- 59.Gasparovic ML, Gee GV, Atwood WJ. JC virus minor capsid proteins Vp2 and Vp3 are essential for virus propagation. J Virol. 2006;80(21):10858–61. doi: 10.1128/JVI.01298-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sadowska B, Barrucco R, Khalili K, Safak M. Regulation of human polyomavirus JC virus gene transcription by AP-1 in glial cells. J Virol. 2003;77(1):665–72. doi: 10.1128/JVI.77.1.665-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amemiya K, Traub R, Durham L, Major EO. Interaction of a nuclear factor-1-like protein with the regulatory region of the human polyomavirus JC virus. J Biol Chem. 1989;264(12):7025–32. [PubMed] [Google Scholar]
- 62.Raj GV, Safak M, MacDonald GH, Khalili K. Transcriptional regulation of human polyomavirus JC: evidence for a functional interaction between RelA (p65) and the Y-box-binding protein, YB-1. J Virol. 1996;70(9):5944–53. doi: 10.1128/jvi.70.9.5944-5953.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Safak M, Gallia GL, Khalili K. A 23-bp sequence element from human neurotropic JC virus is responsive to NF-kappa B subunits. Virology. 1999;262(1):178–89. doi: 10.1006/viro.1999.9886. [DOI] [PubMed] [Google Scholar]
- 64.Manley K, et al. NFAT4 is required for JC virus infection of glial cells. J Virol. 2006;80(24):12079–85. doi: 10.1128/JVI.01456-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Safak M, Gallia GL, Ansari SA, Khalili K. Physical and functional interaction between the Y-box binding protein YB-1 and human polyomavirus JC virus large T antigen. J Virol. 1999;73(12):10146–57. doi: 10.1128/jvi.73.12.10146-10157.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Safak M, Gallia GL, Khalili K. Reciprocal interaction between two cellular proteins, Puralpha and YB-1, modulates transcriptional activity of JCVCY in glial cells. Mol Cell Biol. 1999;19(4):2712–23. doi: 10.1128/mcb.19.4.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Major EO, Amemiya K, Elder G, Houff SA. Glial cells of the human developing brain and B cells of the immune system share a common DNA binding factor for recognition of the regulatory sequences of the human polyomavirus, JCV. J Neurosci Res. 1990;27(4):461–71. doi: 10.1002/jnr.490270405. [DOI] [PubMed] [Google Scholar]
- 68.Trowbridge PW, Frisque RJ. Identification of three new JC virus proteins generated by alternative splicing of the early viral mRNA. J Neurovirol. 1995;1(2):195–206. doi: 10.3109/13550289509113966. [DOI] [PubMed] [Google Scholar]
- 69.Waga S, Bauer G, Stillman B. Reconstitution of complete SV40 DNA replication with purified replication factors. J Biol Chem. 1994;269(14):10923–34. [PubMed] [Google Scholar]
- 70.Prins C, Frisque RJ. JC virus T′ proteins encoded by alternatively spliced early mRNAs enhance T antigen-mediated viral DNA replication in human cells. J Neurovirol. 2001;7(3):250–64. doi: 10.1080/13550280152403290. [DOI] [PubMed] [Google Scholar]
- 71.Safak M, et al. Interaction of JC virus agno protein with T antigen modulates transcription and replication of the viral genome in glial cells. J Virol. 2001;75(3):1476–86. doi: 10.1128/JVI.75.3.1476-1486.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jay G, Nomura S, Anderson CW, Khoury G. Identification of the SV40 agnogene product: a DNA binding protein. Nature. 1981;291(5813):346–9. doi: 10.1038/291346a0. [DOI] [PubMed] [Google Scholar]
- 73.Shishido-Hara Y, Ichinose S, Higuchi K, Hara Y, Yasui K. Major and minor capsid proteins of human polyomavirus JC cooperatively accumulate to nuclear domain 10 for assembly into virions. J Virol. 2004;78(18):9890–903. doi: 10.1128/JVI.78.18.9890-9903.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dyson N, et al. Large T antigens of many polyomaviruses are able to form complexes with the retinoblastoma protein. J Virol. 1990;64(3):1353–6. doi: 10.1128/jvi.64.3.1353-1356.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ludlow JW, Skuse GR. Viral oncoprotein binding to pRB, p107, p130, and p300. Virus Res. 1995;35(2):113–21. doi: 10.1016/0168-1702(94)00094-s. [DOI] [PubMed] [Google Scholar]
- 76.Dyson N, Buchkovich K, Whyte P, Harlow E. The cellular 107K protein that binds to adenovirus E1A also associates with the large T antigens of SV40 and JC virus. Cell. 1989;58(2):249–55. doi: 10.1016/0092-8674(89)90839-8. [DOI] [PubMed] [Google Scholar]
- 77.Bollag B, Chuke WF, Frisque RJ. Hybrid genomes of the polyomaviruses JC virus, BK virus, and simian virus 40: identification of sequences important for efficient transformation. J Virol. 1989;63(2):863–72. doi: 10.1128/jvi.63.2.863-872.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Holman PS, Gjoerup OV, Davin T, Schaffhausen BS. Characterization of an immortalizing N-terminal domain of polyomavirus large T antigen. J Virol. 1994;68(2):668–73. doi: 10.1128/jvi.68.2.668-673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tretiakova A, Krynska B, Gordon J, Khalili K. Human neurotropic JC virus early protein deregulates glial cell cycle pathway and impairs cell differentiation. J Neurosci Res. 1999;55(5):588–99. doi: 10.1002/(SICI)1097-4547(19990301)55:5<588::AID-JNR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 80.Sharma AK, Kumar G. A 53 kDa protein binds to the negative regulatory region of JC virus early promoter. FEBS Lett. 1991;281(1–2):272–4. doi: 10.1016/0014-5793(91)80409-v. [DOI] [PubMed] [Google Scholar]
- 81.Lassak A, et al. Insulin receptor substrate 1 translocation to the nucleus by the human JC virus T-antigen. J Biol Chem. 2002;277(19):17231–8. doi: 10.1074/jbc.M110885200. [DOI] [PubMed] [Google Scholar]
- 82.Gan DD, et al. Involvement of Wnt signaling pathway in murine medulloblastoma induced by human neurotropic JC virus. Oncogene. 2001;20(35):4864–70. doi: 10.1038/sj.onc.1204670. [DOI] [PubMed] [Google Scholar]
- 83.Enam S, et al. Association of human polyomavirus JCV with colon cancer: evidence for interaction of viral T-antigen and beta-catenin. Cancer Res. 2002;62(23):7093–101. [PubMed] [Google Scholar]
- 84.Haggerty S, Walker DL, Frisque RJ. JC virus-simian virus 40 genomes containing heterologous regulatory signals and chimeric early regions: identification of regions restricting transformation by JC virus. J Virol. 1989;63(5):2180–90. doi: 10.1128/jvi.63.5.2180-2190.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Neel JV, et al. Hypothesis: “Rogue cell”-type chromosomal damage in lymphocytes is associated with infection with the JC human polyoma virus and has implications for oncopenesis. Proc Natl Acad Sci U S A. 1996;93(7):2690–5. doi: 10.1073/pnas.93.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ricciardiello L, et al. Induction of chromosomal instability in colonic cells by the human polyomavirus JC virus. Cancer Res. 2003;63(21):7256–62. [PubMed] [Google Scholar]
- 87.Theile M, Grabowski G. Mutagenic activity of BKV and JCV in human and other mammalian cells. Arch Virol. 1990;113(3–4):221–33. doi: 10.1007/BF01316675. [DOI] [PubMed] [Google Scholar]
- 88.Tognon M, et al. Large T antigen coding sequences of two DNA tumor viruses, BK and SV40, and nonrandom chromosome changes in two glioblastoma cell lines. Cancer Genet Cytogenet. 1996;90(1):17–23. doi: 10.1016/0165-4608(96)00067-2. [DOI] [PubMed] [Google Scholar]
- 89.Darbinyan A, et al. Evidence for dysregulation of cell cycle by human polyomavirus, JCV, late auxiliary protein. Oncogene. 2002;21(36):5574–81. doi: 10.1038/sj.onc.1205744. [DOI] [PubMed] [Google Scholar]
- 90.Sariyer IK, Khalili K, Safak M. Dephosphorylation of JC virus agnoprotein by protein phosphatase 2A: inhibition by small t antigen. Virology. 2008;375(2):464–79. doi: 10.1016/j.virol.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sontag E, et al. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993;75(5):887–97. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- 92.Padgett BL, Walker DL, ZuRhein GM, Varakis JN. Differential neurooncogenicity of strains of JC virus, a human polyoma virus, in newborn Syrian hamsters. Cancer Res. 1977;37(3):718–20. [PubMed] [Google Scholar]
- 93.Zu Rhein GM, Varakis JN. Perinatal induction of medulloblastomas in Syrian golden hamsters by a human polyoma virus (JC) Natl Cancer Inst Monogr. 1979;51:205–8. [PubMed] [Google Scholar]
- 94.Walker DL, Padgett BL, ZuRhein GM, Albert AE, Marsh RF. Human papovavirus (JC): induction of brain tumors in hamsters. Science. 1973;181(100):674–6. doi: 10.1126/science.181.4100.674. [DOI] [PubMed] [Google Scholar]
- 95.Varakis J, ZuRhein GM, Padgett BL, Walker DL. Induction of peripheral neuroblastomas in Syrian hamsters after injection as neonates with JC virus, a human polyoma virus. Cancer Res. 1978;38(6):1718–22. [PubMed] [Google Scholar]
- 96.Varakis JN, ZuRhein GM. Experimental pineocytoma of the Syrian hamster induced by a human papovavirus (JC). A light and electron microscopic study. Acta Neuropathol. 1976;35(3):243–64. [PubMed] [Google Scholar]
- 97.Ohsumi S, Motoi M, Ogawa K. Induction of undifferentiated tumors by JC virus in the cerebrum of rats. Acta Pathol Jpn. 1986;36(6):815–25. doi: 10.1111/j.1440-1827.1986.tb03116.x. [DOI] [PubMed] [Google Scholar]
- 98.London WT, et al. Brain tumors in owl monkeys inoculated with a human polyomavirus (JC virus) Science. 1978;201(4362):1246–9. doi: 10.1126/science.211583. [DOI] [PubMed] [Google Scholar]
- 99.London WT, et al. Viral-induced astrocytomas in squirrel monkeys. Prog Clin Biol Res. 1983;105:227–37. [PubMed] [Google Scholar]
- 100.Major EO, Mourrain P, Cummins C. JC virus-induced owl monkey glioblastoma cells in culture: biological properties associated with the viral early gene product. Virology. 1984;136(2):359–67. doi: 10.1016/0042-6822(84)90172-7. [DOI] [PubMed] [Google Scholar]
- 101.Major EO, Vacante DA, Traub RG, London WT, Sever JL. Owl monkey astrocytoma cells in culture spontaneously produce infectious JC virus which demonstrates altered biological properties. J Virol. 1987;61(5):1435–41. doi: 10.1128/jvi.61.5.1435-1441.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Houff SA, et al. Neuroradiological studies of JCV-induced astrocytomas in nonhuman primates. Prog Clin Biol Res. 1983;105:253–9. [PubMed] [Google Scholar]
- 103.Houff SA, et al. New world primates as a model of viral-induced astrocytomas. Prog Clin Biol Res. 1983;105:223–6. [PubMed] [Google Scholar]
- 104.Miller NR, et al. Brain tumors of owl monkeys inoculated with JC virus contain the JC virus genome. J Virol. 1984;49(3):848–56. doi: 10.1128/jvi.49.3.848-856.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miller NR, London W, Padgett BL, Walker DL, Wallen WC. The detection of JC viral genome in owl monkey tumors. Prog Clin Biol Res. 1983;105:271–88. [PubMed] [Google Scholar]
- 106.Small JA, Khoury G, Jay G, Howley PM, Scangos GA. Early regions of JC virus and BK virus induce distinct and tissue-specific tumors in transgenic mice. Proc Natl Acad Sci U S A. 1986;83(21):8288–92. doi: 10.1073/pnas.83.21.8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Feigenbaum L, Hinrichs SH, Jay G. JC virus and simian virus 40 enhancers and transforming proteins: role in determining tissue specificity and pathogenicity in transgenic mice. J Virol. 1992;66(2):1176–82. doi: 10.1128/jvi.66.2.1176-1182.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krynska B, Otte J, Franks R, Khalili K, Croul S. Human ubiquitous JCV(CY) T-antigen gene induces brain tumors in experimental animals. Oncogene. 1999;18(1):39–46. doi: 10.1038/sj.onc.1202278. [DOI] [PubMed] [Google Scholar]
- 109.Gordon J, Del Valle L, Otte J, Khalili K. Pituitary neoplasia induced by expression of human neurotropic polyomavirus, JCV, early genome in transgenic mice. Oncogene. 2000;19(42):4840–6. doi: 10.1038/sj.onc.1203849. [DOI] [PubMed] [Google Scholar]
- 110.Lin PY, et al. Prevalence and genotype identification of human JC virus in colon cancer in Taiwan. J Med Virol. 2008;80(10):1828–34. doi: 10.1002/jmv.21296. [DOI] [PubMed] [Google Scholar]
- 111.Laghi L, et al. JC virus DNA is present in the mucosa of the human colon and in colorectal cancers. Proc Natl Acad Sci U S A. 1999;96(13):7484–9. doi: 10.1073/pnas.96.13.7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murai Y, et al. High JC virus load in gastric cancer and adjacent non-cancerous mucosa. Cancer Sci. 2007;98(1):25–31. doi: 10.1111/j.1349-7006.2006.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shin SK, et al. Oncogenic T-antigen of JC virus is present frequently in human gastric cancers. Cancer. 2006;107(3):481–8. doi: 10.1002/cncr.22028. [DOI] [PubMed] [Google Scholar]
- 114.Del Valle L, et al. Detection of JC virus DNA sequences and expression of viral T antigen and agnoprotein in esophageal carcinoma. Cancer. 2005;103(3):516–27. doi: 10.1002/cncr.20806. [DOI] [PubMed] [Google Scholar]
- 115.Boldorini R, et al. Molecular characterisation of JC virus strains detected in human brain tumours. Pathology. 2003;35(3):248–53. doi: 10.1080/0031302031000123245. [DOI] [PubMed] [Google Scholar]
- 116.Delbue S, et al. Distribution, characterization and significance of polyomavirus genomic sequences in tumors of the brain and its covering. J Med Virol. 2005;77(3):447–54. doi: 10.1002/jmv.20474. [DOI] [PubMed] [Google Scholar]
- 117.Del Valle L, et al. Reactivation of human neurotropic JC virus expressing oncogenic protein in a recurrent glioblastoma multiforme. Ann Neurol. 2000;48(6):932–6. [PubMed] [Google Scholar]
- 118.Pina-Oviedo S, et al. Glioblastoma multiforme with small cell neuronal-like component: association with human neurotropic JC virus. Acta Neuropathol. 2006;111(4):388–96. doi: 10.1007/s00401-006-0050-3. [DOI] [PubMed] [Google Scholar]
- 119.Rencic A, et al. Detection of JC virus DNA sequence and expression of the viral oncoprotein, tumor antigen, in brain of immunocompetent patient with oligoastrocytoma. Proc Natl Acad Sci U S A. 1996;93(14):7352–7. doi: 10.1073/pnas.93.14.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Del Valle L, et al. Detection of JC polyomavirus DNA sequences and cellular localization of T-antigen and agnoprotein in oligodendrogliomas. Clin Cancer Res. 2002;8(11):3332–40. [PubMed] [Google Scholar]
- 121.Shiramizu B, Hu N, Frisque RJ, Nerurkar VR. High prevalence of human polyomavirus JC VP1 gene sequences in pediatric malignancies. Cell Mol Biol (Noisy-le-grand) 2007;53(3):4–12. [PMC free article] [PubMed] [Google Scholar]
- 122.Krynska B, et al. Detection of human neurotropic JC virus DNA sequence and expression of the viral oncogenic protein in pediatric medulloblastomas. Proc Natl Acad Sci U S A. 1999;96(20):11519–24. doi: 10.1073/pnas.96.20.11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Del Valle L, et al. Expression of a human polyomavirus oncoprotein and tumour suppressor proteins in medulloblastomas. Mol Pathol. 2001;54(5):331–7. doi: 10.1136/mp.54.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zheng H, et al. Oncogenic role of JC virus in lung cancer. J Pathol. 2007;212(3):306–15. doi: 10.1002/path.2188. [DOI] [PubMed] [Google Scholar]
- 125.Volter C, Hausen H, Alber D, de Villiers EM. Screening human tumor samples with a broad-spectrum polymerase chain reaction method for the detection of polyomaviruses. Virology. 1997;237(2):389–96. doi: 10.1006/viro.1997.8772. [DOI] [PubMed] [Google Scholar]
- 126.Hayashi H, et al. JC virus large T protein transforms rodent cells but is not involved in human medulloblastoma. Neuropathology. 2001;21(2):129–37. doi: 10.1046/j.1440-1789.2001.00384.x. [DOI] [PubMed] [Google Scholar]
- 127.Lundstig A, et al. No excess risk for colorectal cancer among subjects seropositive for the JC polyomavirus. Int J Cancer. 2007;121(5):1098–102. doi: 10.1002/ijc.22770. [DOI] [PubMed] [Google Scholar]
- 128.Caldarelli-Stefano R, et al. JC virus in human glial-derived tumors. Hum Pathol. 2000;31(3):394–5. doi: 10.1016/s0046-8177(00)80256-7. [DOI] [PubMed] [Google Scholar]
- 129.Arthur RR, et al. Lack of association of human polyomaviruses with human brain tumors. J Neurooncol. 1994;20(1):55–8. doi: 10.1007/BF01057961. [DOI] [PubMed] [Google Scholar]
- 130.Rollison DE, et al. Serum antibodies to JC virus, BK virus, simian virus 40, and the risk of incident adult astrocytic brain tumors. Cancer Epidemiol Biomarkers Prev. 2003;12(5):460–3. [PubMed] [Google Scholar]
- 131.Munoz-Marmol AM, et al. Rarity of JC virus DNA sequences and early proteins in human gliomas and medulloblastomas: the controversial role of JC virus in human neurooncogenesis. Neuropathol Appl Neurobiol. 2006;32(2):131–40. doi: 10.1111/j.1365-2990.2006.00711.x. [DOI] [PubMed] [Google Scholar]
- 132.Rollison DE, et al. Investigation of human brain tumors for the presence of polyomavirus genome sequences by two independent laboratories. Int J Cancer. 2005;113(5):769–74. doi: 10.1002/ijc.20641. [DOI] [PubMed] [Google Scholar]
- 133.Farwell JR, Dohrmann GJ, Flannery JT. Medulloblastoma in childhood: an epidemiological study. J Neurosurg. 1984;61(4):657–64. doi: 10.3171/jns.1984.61.4.0657. [DOI] [PubMed] [Google Scholar]
- 134.Del Valle L, et al. Expression of human neurotropic polyomavirus JCV late gene product agnoprotein in human medulloblastoma. J Natl Cancer Inst. 2002;94(4):267–73. doi: 10.1093/jnci/94.4.267. [DOI] [PubMed] [Google Scholar]
- 135.Del Valle L, et al. Expression of JC virus T-antigen in a patient with MS and glioblastoma multiforme. Neurology. 2002;58(6):895–900. doi: 10.1212/wnl.58.6.895. [DOI] [PubMed] [Google Scholar]
- 136.Theodoropoulos G, et al. Assessment of JC polyoma virus in colon neoplasms. Dis Colon Rectum. 2005;48(1):86–91. doi: 10.1007/s10350-004-0737-2. [DOI] [PubMed] [Google Scholar]
- 137.Selgrad M, et al. JC Virus Infects the Enteric Glia of Patients with Chronic Idiopathic Intestinal Pseudo-obstruction. Gut. 2008 doi: 10.1136/gut.2008.152512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ricciardiello L, et al. JC virus DNA sequences are frequently present in the human upper and lower gastrointestinal tract. Gastroenterology. 2000;119(5):1228–35. doi: 10.1053/gast.2000.19269. [DOI] [PubMed] [Google Scholar]
- 139.Jung WT, Li MS, Goel A, Boland CR. JC virus T-antigen expression in sporadic adenomatous polyps of the colon. Cancer. 2008;112(5):1028–36. doi: 10.1002/cncr.23266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ricciardiello L, et al. Mad-1 is the exclusive JC virus strain present in the human colon, and its transcriptional control region has a deleted 98-base-pair sequence in colon cancer tissues. J Virol. 2001;75(4):1996–2001. doi: 10.1128/JVI.75.4.1996-2001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Giuliani L, et al. Detection of oncogenic viruses SV40, BKV, JCV, HCMV, HPV and p53 codon 72 polymorphism in lung carcinoma. Lung Cancer. 2007;57(3):273–81. doi: 10.1016/j.lungcan.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 142.Hogan TF, Padgett BL, Walker DL, Borden EC, Frias Z. Survey of human polyomavirus (JCV, BKV) infections in 139 patients with lung cancer, breast cancer, melanoma, or lymphoma. Prog Clin Biol Res. 1983;105:311–24. [PubMed] [Google Scholar]
- 143.Munger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63(10):4417–21. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pagano JS, et al. Infectious agents and cancer: criteria for a causal relation. Semin Cancer Biol. 2004;14(6):453–71. doi: 10.1016/j.semcancer.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 145.Knudson AG., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68(4):820–3. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]