Abstract
Prostacyclin (PGI2) synthesis and function in the human uterus has been implicated in the regulation of the process of normal and dysfunctional menstruation. PGI2 synthesis is elevated during normal menstruation and is also associated with blood loss in women who suffer from heavy menses. This study was designed to outline further the role of PGI2 in menstruation by investigating the temporal pattern and site of expression of prostaglandin I synthase (PGIS) and the prostacyclin receptor (IP receptor) in the non-pregnant human endometrium across the menstrual cycle. Quantitative RT-PCR demonstrated increased expression of PGIS and IP receptor during the menstrual phase of the cycle compared with all other phases (P < 0.05). Furthermore, PGIS and IP receptor were localised to the glandular epithelium, stromal and endothelial cells in the basal and functional layers of the endometrium. Functionality of the IP receptor in the human endometrium was assessed by measuring cAMP generation following treatment with 100 nmol l−1 of the PGI2 analogue, iloprost. cAMP generation was significantly higher in endometrial tissue collected during the proliferative compared with the secretory phase of the menstrual cycle (P < 0.05).
In conclusion, this study has confirmed increased expression and signalling of PGIS and IP receptor during the menstrual phase and outlines a potential autocrine/paracrine role for PGI2 on several cellular compartments in the endometrium including the endothelium. This may underscore a pivotal role for PGI2 receptor signalling in normal and dysfunctional menstruation.
Introduction
Prostacyclin (PGI2) is a member of the prostanoid family of lipid mediators and is the main prostanoid synthesised by vascular endothelium (Smyth & FitzGerald 2002). Cyclooxygenase (COX) enzyme is the key enzyme in the synthesis of PGI2 from arachidonic acid via the common intermediate in prostaglandin (PG) synthesis, PGH2. In turn, PGH2 is rapidly converted to PGI2 by the action of prostaglandin I synthase (PGIS) (Kniss 1999). There are two predominant isoforms of COX enzymes, COX-1, which is constitutively expressed in many cell types and COX-2, which is induced by factors including cytokines and tumour promoters (Kniss 1999). PGI2 elicits its effects on target cells by interaction with its G protein-coupled receptor (IP receptor), which has a typical seven-transmembrane structure (Narumiya et al. 1999). The IP receptor can stimulate both Gs and Gq species of G proteins causing an increase in cAMP generation and in phosphatidylinositol response (Narumiya et al. 1999).
Temporal expression of other prostaglandins such as PGE2 and PGF2α and their receptors (EP2/EP4 and FP receptors) has been demonstrated in the human endometrium and has been shown to vary with the phase of the menstrual cycle (Lumsden et al. 1986, Smith & Kelly 1988, Milne et al. 2001, Milne & Jabbour 2003). Maximal expression and signalling of these receptors are detected during the mid-late proliferative phase of the menstrual cycle. Recent data implicate a role for PGE2 and PGF2α in the proliferation of glandular epithelial cells via diverse signalling pathways (Jabbour & Boddy 2003, Milne & Jabbour 2003). In contrast, little is known of the expression pattern and function of the IP receptor in human endometrium, although PGIS and IP receptor expression have been demonstrated in pregnant and non-pregnant myometrium (Moonen et al. 1986, Chegini & Rao 1988, Giannoulias et al. 2002).
PGI2 is the main prostanoid synthesised by the vascular endothelium and it causes blood vessel dilatation and inhibition of platelet aggregation (Smyth & FitzGerald 2002). Moreover, PGI2 is known to act as a smooth muscle relaxant (Wilhelmsson et al. 1981, Lumsden & Baird 1986, Dyal & Crankshaw 1988). The effects of PGI2 on platelet aggregation and vascular tone have outlined its potential role in menstruation (Baird et al. 1996). This is supported by observations of elevated PGI2 levels in uterine venous blood during menstruation as compared with other phases of the menstrual cycle (Goodfellow et al. 1982). Moreover, PGI2 synthesis is increased in women suffering from heavy menses compared with those who show normal blood loss (Smith et al. 1981b, Makarainen & Ylikorkala 1986, Cameron et al. 1987). It has also been suggested that menstrual disorders can be the result of a shift in the ratio of different prostaglandins. Heavy menses has been associated with increased synthesis of PGI2 relative to thromboxane A2 and PGE2 relative to PGF2α (Smith et al. 1981a, Makarainen & Ylikorkala 1986). Heavy menses with no known uterine pathology affects 10% of women of child bearing age and is defined as blood loss in excess of 80 ml per menstrual cycle (Prentice 2000).
The objectives of the present study were to investigate the expression of PGIS (the terminal enzyme responsible for the generation of PGI2) and IP receptor in non-pregnant human endometrium and myometrium across the menstrual cycle. Furthermore, the role of PGI2 in endometrial cell signalling has been assessed by investigating the effect of the PGI2 analogue, iloprost, on cAMP generation. A better understanding of IP receptor signalling and function in the human endometrium may ultimately lead to the development of novel therapies in the treatment of menstrual disorders that may be associated with elevated PGI2 synthesis.
Materials and Methods
Patients and tissue collection
Endometrial biopsies at different stages of the menstrual cycle were obtained from women with regular menstrual cycles (25–35 days), who had not received a hormonal preparation in the 3 months preceding biopsy collection. Samples were collected either with an endometrial suction curette (Pipelle, Laboratoire CCD, Paris, France) or as full thickness endometrial biopsies (including the functional layer and basal-myometrial junction) from women undergoing hysterectomy for benign gynaecological indications. Shortly after collection, tissue was either snap frozen in dry ice and stored at −70 °C (for RNA extraction), fixed in neutral buffered formalin (NBF) and wax embedded (for immunohistochemical analyses), or placed in RPMI 1640 (containing 2 mmol l−1 l-glutamine, 100 U penicillin and 100 μg ml−1 streptomycin) and transported to the laboratory for in vitro culture.
Biopsies were classified within a 28-day cycle range using stated last menstrual period (LMP) and histological assessment according to the criteria of Noyes and co-workers (1975). Furthermore, circulating oestradiol and progesterone concentrations at the time of biopsy were consistent for both stated LMP and histological assignment of menstrual cycle stage. Samples were divided according to the phase of the menstrual cycle as: menstrual (days 1–4), early to mid proliferative (EP-MP; days 5–10), late proliferative to ovulatory (LP-Ov; days 11–14), early secretory (ES; days 15–18) and mid to late secretory (MS-LS; days 19–28). Ethical approval was obtained from the Lothian Research Ethics Committee and written informed consent was obtained from all subjects before tissue collection.
Taqman quantitative RT-PCR
RNA was extracted from endometrial biopsies obtained from across the menstrual cycle (n = 35) using Tri Reagent (Sigma, Poole, Dorset, UK) following the manufacturer's instructions. RNA samples were quantified and were reverse transcribed using 5.5 mmol l−1 MgCl2, 0.5 mmol l−1 of each deoxynucleotide triphosphate (dNTP), 2.5 μmol l−1 random hexamers, ribonuclease inhibitor (0.4 U μl−1) and 1.25 U μl−1 Multiscribe reverse transcriptase (all from Applied Biosystems, Warrington, Cheshire, UK). RNA (400 ng) was added to each reverse transcription reaction and samples were incubated for 90 min at 25 °C, 45 min at 48 °C and 5 min at 95 °C. The reaction mix for the PCR consisted of 1 × mastermix, ribosomal 18S forward and reverse primers, ribosomal 18S probe (50 nmol l−1; all from Applied Biosystems), forward and reverse primers for PGIS or IP receptor (300 nmol l−1) and PGIS or IP receptor probe (200 nmol l−1) (all from Biosource UK, Nivelles, Belgium). The reaction mix (48 μl) was aliquoted into tubes and 2 μl cDNA were added. Duplicate 24μl samples plus positive and negative controls were placed in a PCR plate and wells were sealed with optical caps. The PCR reactions were carried out using an ABI Prism 7700 (Applied Biosystems). All primers and probes were designed using the PRIMER express program (Applied Biosystems). The sequences of PGIS primers and probe were: PGIS forward primer, 5′-ACGCAGATGTGGAGATCCCT-3′; reverse, 5′-GTCGTGTTCCGGCTGCA-3′; and probe (6-carboxy fluoroscein labelled) 5′-CCTCAGCAGGTACGGCTTCGGTCTG-3′. The sequences of the IP receptor primers and probe were: IP receptor forward primer, 5′-GCCCTCCCCCTCTACCAA-3′; reverse, 5′-TTTTCCAATAACTGTGGTTTTTGTG-3′; and probe (6-carboxy fluoroscein labelled) 5′-CCAAGAGCCAGCCCCCTTTCTGC-3′. The sequences of 18S primers and probe have been described previously (Milne et al. 2001). Data were analysed and processed using Sequence Detector version 1.6.3 (Applied Biosystems) according to the manufacturer's instructions. Results were expressed relative to an internal positive standard (cDNA obtained from a single sample of endometrial tissue) included in all reactions.
In situ hybridisation
A custom synthesised oligonucleotide double fluoroscein isothiocyanate (FITC)-labelled cDNA probe for IP receptor was obtained from Biognostik (Göttingen, Germany). Sections (5 μm) from full thickness human endometrial biopsies collected across the menstrual cycle (n = 12) were cut onto gelatin-coated slides. Sections were dewaxed and rehydrated and then treated with proteinase K (50 μgml−1 in 100 mmol l−1 Tris–HCl pH 7.6, containing 50 mmol l−1 EDTA) for 15 min at 37 °C to enhance cDNA probe access. Sections were washed in diethylpyrocarbonate-treated water and prehybridised for 4 h at 30 °C with 25 μl of the hybridisation buffer supplied with the probe, which had previously been heated to 95 °C. The sections were then hybridised overnight at 30 °C with the cDNA probe at 6 U μl−1 in hybridisation buffer. Following hybridisation, sections were washed for 2 × 5 min in 1 × SSC at room temperature and 2 × 15 min in 0.1 × SSC at 39 °C. After rinsing in tris buffered saline (TBS), endogenous peroxidase activity was quenched with 10% (v/v) H2O2 in methanol at room temperature. The FITC-labelled probe was detected using standard immunohistochemical reagents with an additional amplification step (TSA Biotin System, NEN Life Science Products, Hounslow, Middlesex, UK). Sections were incubated with blocking buffer for 30 min. Conjugated anti-FITC-horseradish peroxidase (Roche, Diagnostics Ltd, Lewes, E Sussex, UK) was added at a dilution of 1 in 200 in blocking buffer and the sections were incubated for 30 min. After washing, biotinyl tyramide amplification reagent (1 in 50) was applied to each slide and incubated for 15 min. Streptavidin-horseradish peroxidase (1 in 100) was applied after washing and incubated for 30 min; probe localisation was visualised with 3,3′-diaminobenzidine (DAB) substrate. Control sections were treated with a double FITC-labelled oligonucleotide probe containing the same proportion of cysteine (C) and guanine (G) bases as the IP receptor probe to assess background hybridisation. All treatments were carried out at room temperature unless otherwise specified.
Immunohistochemistry
Endometrial sections (5 μm) from across the menstrual cycle (n = 12) were dewaxed in xylene and rehydrated using decreasing grades of ethanol. Antigen retrieval was performed by treating sections for 5 min in a pressure cooker in boiling 0.1% citrate buffer, pH 3.0. Endogenous peroxidase activity was quenched with 10% (v/v) H2O2 in methanol at room temperature. Non-immune swine serum (20% serum in TBS) was applied for 1 h before overnight incubation at 4 °C with rabbit anti-human IP receptor at a dilution of 1 in 500 or rabbit anti-bovine PGIS (shown to cross-react with human PGIS) (Alexis Corporation, Nottingham, UK) at the same concentration. An avidin-biotin peroxidase detection system was then applied (DAKO Ltd, Cambridge, UK) with DAB as the chromagen. The antibody to IP receptor has been described previously (Fortier et al. 2001). Non-immune rabbit serum and antibody pre-absorbed with IP receptor peptide were used as controls for IP receptor, and non-immune rabbit serum was used as a control for PGIS. Immunoreactivity was negligible with pre-absorbed antibody, and with non-immune rabbit serum there was occasional generalised pale brown cross reactivity over the epithelial glands and the endothelium.
Cyclic AMP assay
Endometrial biopsies from the proliferative and secretory phases of the menstrual cycle (n = 8) were minced finely with scissors and divided into three portions. The tissue was incubated overnight at 37 °C in a humidified 5% CO2 incubator in 2 ml RPMI medium (Sigma) containing 2 mmol l−1 l-glutamine, 100 IU penicillin and 100 μg streptomycin, and 3 μgml−1 indomethacin (Sigma). Following overnight treatment, the tissue was incubated in the same medium containing 1-methyl-3-isobutylxanthine (Sigma) at 37 °C for 30 min. It was then treated with control medium or 100 nmol l−1 iloprost (a gift from Schering Health Care, Burgess Hill, W Sussex, UK) for 10 min at 37 °C and lysed in 0.1 mol l−1 HCl and frozen until assayed. Cyclic AMP concentration was measured by ELISA (Biomol, Affiniti, Exeter, Devon, UK) according to the manufacturer's instructions and normalised to the protein concentration determined by a modification of the method of Lowry (Bio-Rad, Hemel Hempstead, Herts, UK). Data are presented as the fold induction of cAMP after treatment with iloprost, where fold induction was calculated relative to the control samples.
Statistics
Where appropriate, data were subjected to statistical analysis with ANOVA and Fisher's protected least significant difference tests (Statview 4.0; Abacus Concepts Inc., Piscataway, NJ, USA) and statistical significance was accepted when P < 0.05.
Results
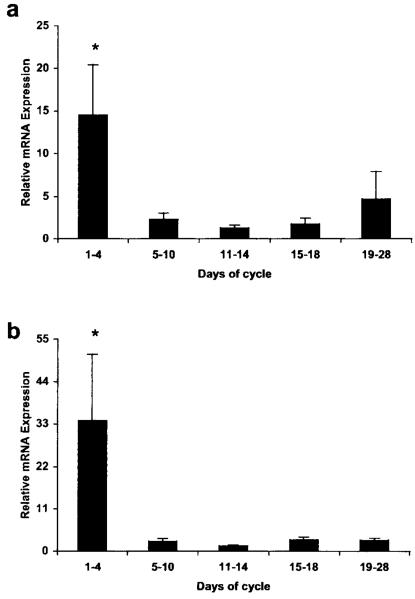
The temporal pattern of PGIS mRNA expression in the human endometrium across the menstrual cycle was studied by quantitative RT-PCR. PGIS mRNA was detected in all samples of human endometrium examined (Fig. 1a). Relative expression was significantly higher in the menstrual phase of the cycle compared with all other phases (14.49 ± 5.92 vs 2.28 ± 0.74 for EP-MP; 1.25 ± 0.33 for LP-Ov; 1.75 ± 0.67 for ES and 4.7 ± 3.15 for MS-LS; P < 0.05 for all comparisons). Similarly, the temporal pattern of IP receptor mRNA expression across the menstrual cycle was assessed by quantitative RT-PCR and IP receptor mRNA was detected in all samples of human endometrium examined (Fig. 1b). Relative expression was significantly higher in the menstrual phase of the cycle compared with all other phases (33.94 ± 17.2 vs 2.62 ± 0.7 for EP-MP; 1.36 ± 0.25 for LP-Ov; 2.95 ± 0.76 for ES and 2.87 ± 0.53 for MS-LS; P < 0.001 for all comparisons).
Figure 1.
Quantitative RT-PCR of (a) PGIS and (b) IP receptor in the human endometrium across the menstrual cycle. Results are expressed as the mean ± S.E.M. of relative mRNA expression levels. *P < 0.05.
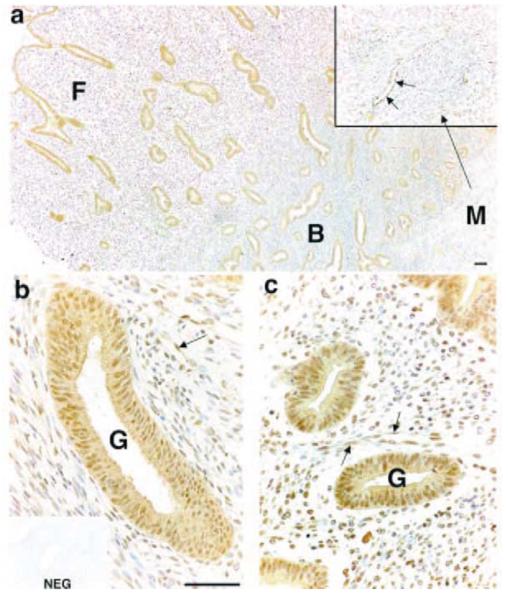
Immunohistochemical staining for PGIS was performed in full thickness human endometrial biopsies collected from across the menstrual cycle. A similar pattern of staining was observed in tissue sections from proliferative and secretory phases (Fig. 2 is a representative sample of endometrial tissue collected during the proliferative phase). Cytoplasmic and nuclear immunoreactivity was detected in glandular epithelial cells in the basal (Fig. 2b) and functional (Fig. 2c) layers. Stromal cell reactivity was also present in both layers, but with greater reactivity in the functional than in the basal layer (Fig. 2a). Endothelial cell staining was detected in the microvasculature in both endometrial layers and reactivity was also present in myometrial smooth muscle cells (enlarged area in Fig. 2a).
Figure 2.
Immunohistochemical localisation of PGIS in human endometrial tissue (functional layer and basal-myometrial junction) collected in the proliferative phase of the menstrual cycle. Strong staining was detected in the glandular epithelial cells (G) in basal (B) and functional (F) layers (a-c) and in smooth muscle cells in the myometrium (M) (enlarged area in inset to a). Stromal staining was detected in the basal (b) and the functional layer (c) and endothelial cell reactivity was present in blood vessels (indicated by arrows) in all layers. NEG, negative control; scale bars = 50 μm.
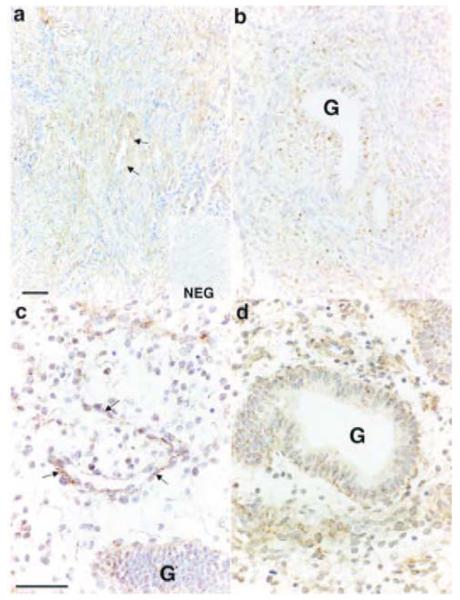
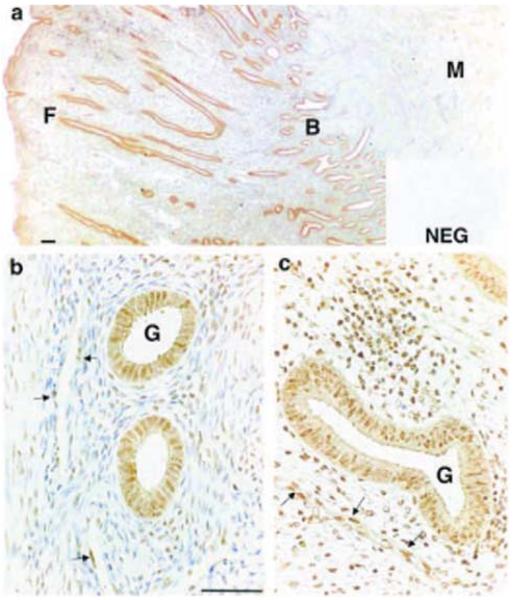
The site of expression of the IP receptor was studied by in situ hybridisation and immunohistochemistry. IP receptor mRNA expression was detected in endometrial samples collected during the menstrual and proliferative phases of the menstrual cycle. IP receptor expression was localised to glandular epithelial and stromal cells in the basal and functional layers of the endometrium (Fig. 3b-d). In addition, expression was present in myometrial smooth muscle cells (Fig. 3a) and in endothelial cells lining vessels in the functional and basal layers of the endometrium and at the basal-myometrial junction (Fig. 3a and c). Immunohistochemical staining for IP receptor protein was detected throughout the menstrual cycle in the cytoplasm and nuclei of glandular epithelial cells in both basal and functional layers (Fig. 4a-c). Similar to PGIS immunoreactivity, IP receptor stromal cell staining was stronger and more widespread in the functional layer (Fig. 4c) compared with the basal layer (Fig. 4b). IP receptor protein expression was also localised to endothelial cells throughout the microvasculature and was present in myometrial smooth muscle cells.
Figure 3.
In situ hybridisation of IP receptor in sections of human endometrial tissue collected in the proliferative phase of the menstrual cycle. IP receptor was expressed in smooth muscle cells in the myometrium (a) and reactivity was also present in glandular epithelial cells (G) and in stromal cells in both the basal layer (b) and the functional layer (c and d). IP receptor was also expressed in vascular endothelial cells (indicated by arrows in a and c). NEG, negative control; scale bars = 50 μm.
Figure 4.
Immunohistochemical localisation of IP receptor in human endometrial tissue (functional layer and basal-myometrial junction) collected in the proliferative phase of the menstrual cycle. Glandular epithelial staining (G) was present in both basal (B) and functional (F) layers and reactivity was detected in smooth muscle cells in the myometrium (M) (a-c). Stromal cell staining was present throughout the endometrium, but was stronger in the functional layer (c) compared with the basal layer (b). Endothelial cells throughout the endometrium and at the basal-myometrial junction also exhibited positive reactivity for the IP receptor (indicated by arrows in b and c). NEG, negative control; scale bars = 50 μm.
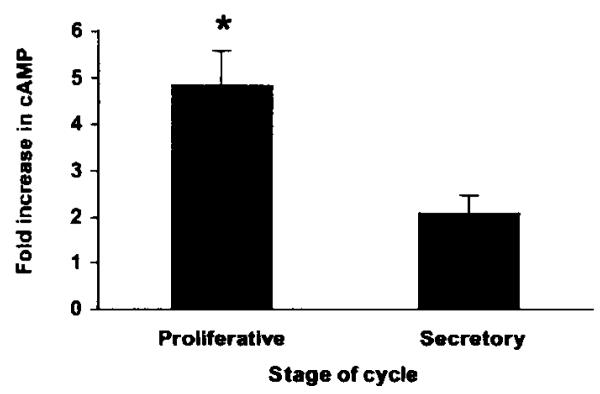
To investigate the functionality of the IP receptor, cAMP generation in response to iloprost (a PGI2 analogue) treatment was assessed in endometrial biopsy tissue collected across the menstrual cycle (Fig. 5). Cyclic AMP generation in response to 100 nmol l−1 iloprost was significantly higher in endometrial samples collected during the proliferative phase compared with the secretory phase (4.83 ± 0.74 fold increase vs 2.07 ± 0.39 fold increase; n = 4 for each group; P < 0.05.)
Figure 5.
Fold increase in cAMP generation in endometrial tissue collected from the proliferative (n = 4) and secretory (n = 4) phases of the menstrual cycle in response to 100 nmol l−1 iloprost. Results are expressed as the mean ± S.E.M. of fold cAMP induction. *P < 0.05.
Discussion
This study demonstrates the expression of PGIS and IP receptor genes in the human endometrium and shows significant up-regulation of both during menstruation. PGIS is the terminal enzyme that leads to synthesis of PGI2 in target tissues (Kniss 1999). The higher expression level of PGIS during menstruation supports previous observations reporting a temporal pattern of PGI2 secretion by the human endometrium across the menstrual cycle; PGI2 concentrations are maximal in uterine venous blood during menstruation (Goodfellow et al. 1982). The elevated expression of IP receptor during menstruation suggests that PGI2 synthesis and IP receptor are temporally regulated to induce their effects on target cells. This is supported by the maximal iloprost-induced cAMP response observed in the endometrium collected during the menstrual/proliferative phases. The IP receptor has been shown to couple to Gs proteins which are linked to increases in cAMP generation (Narumiya et al. 1999). Although only the protein kinase A pathway was investigated in this study, it is predicted that the IP receptor may activate other signalling pathways in the human endometrium, as has been shown recently for other prostaglandin receptors (Jabbour & Boddy 2003, Milne & Jabbour 2003). Such diverse signalling pathways may lead to differential activation of target genes that can promote various phenotypic changes on target cells. In addition to the classical G protein-coupled IP receptor, recent evidence suggests that prostacyclin functions via the nuclear peroxisome proliferator-activated receptor delta (PPARδ) (Lim & Dey 2002). The importance of this mechanism in prostacyclin activity in non-pregnant endometrium is not known. However, it is unlikely to be the predominant signalling pathway, since expression of PPARδ in the normal human endometrium is minimal (Tong et al. 2000).
The factors that regulate the expression of the PGIS and IP receptor during menstruation in the human endometrium are not clear. It is likely that this temporal expression is regulated by steroid hormones as has been postulated for other prostanoids and their receptors (Milne et al. 2001, Milne & Jabbour 2003). Oestradiol-17β has been shown to stimulate the secretion of PGI2 in endometrial stromal cells (Levin et al. 1992). Whether this is associated with up-regulation in the expression of the IP receptor is unclear. Expression of the IP receptor may also be regulated by PGI2 or other prostaglandins. Expression of PGIS and synthesis of PGI2 is induced by COX-2 (Caughey et al. 2001), which is up-regulated during the time of menstruation (Jones et al. 1997). It is also plausible that local mediators within the endometrium may play a role in the regulation of PGI2 synthesis and/or expression of its receptor. For instance, PGI2 is a mediator of the protective effects of vascular endothelial growth factor (VEGF) on the vasculature (Zachary 2001) and PGI2 biosynthesis is up-regulated by VEGF via ERK-mediated cytosolic phospholipase A2 activation and arachidonic acid mobilisation (Zachary & Gliki 2001).
PGIS and IP receptor expression have been co-localised to multi-cellular compartments of the human endometrium. These include stromal, glandular epithelial, endothelial and smooth muscle cells. This suggests that PGI2 acts in an autocrine/paracrine manner within the human endometrium to induce its cellular effects. PGIS and IP receptor immunoreactivities have been demonstrated previously in myocytes, vascular smooth muscle cells and endothelial cells in pregnant and non-pregnant human myometrium (Moonen et al. 1986, Chegini & Rao 1988, Giannoulias et al. 2002). To our knowledge, however, this the first report of localisation of PGIS and IP receptor in glandular epithelial and stromal cells within the human endometrium. Previous studies using autoradiography with [3H]PGI2 on human uterine tissue failed to demonstrate PGI2 binding sites in epithelial cells (Chegini & Rao 1988), although in that study binding sites were demonstrated in myometrial smooth muscle. This inconsistency with findings presented herein may reflect differences in the sensitivity of the methods used. Interestingly, stromal expression of PGIS and IP receptor was highest in the functional layer of the endometrium. In premenopausal women, the human endometrium undergoes phases of proliferation and apoptosis during successive menstrual cycles. These phases are observed predominantly in the functional layer of the endometrium, which is shed at menstruation before regenerating during the proliferative phase of the subsequent menstrual cycle. Hence, this spatio-temporal expression of PGIS and IP receptor may be crucial for, and in keeping with, its predicted role in menstruation.
Baird et al. (1996) have postulated a role for PGI2 in menstruation based on its myometrial smooth muscle and vascular relaxation effects and inhibition of platelet aggregation. This would counteract the effects of other prostaglandins such as PGF2α, which causes vasoconstriction and myometrial smooth muscle contraction (Crankshaw & Dyal 1994). It is also likely that PGI2 is involved in the repair of the vascular bed, since it has protective effects on the endothelium by inhibiting vascular smooth muscle proliferation and enhancing endothelial cell survival (Zachary 2001). The increased expression of PGIS and IP receptor during menstruation is also consistent with a role for PGI2 in the aetiology of menorrhagia. Evidence for this has been provided previously by studies of dysfunctional menstrual bleeding, which have demonstrated increased prostaglandin synthesis including PGI2 (Smith et al. 1981b, Cameron et al. 1987) or increased synthesis of PGI2 relative to thromboxane A2 (Makarainen & Ylikorkala 1986b) in uterine tissue from women with excessive blood loss relative to controls. Whether IP receptor expression and signalling are also elevated in the endometrium of women with dysfunctional menstruation remains to be established.
In summary, this study has demonstrated temporal expression of PGIS and IP receptor in the non-pregnant human endometrium. Expression of both genes is highest during the menstrual phase and is localised to multi-cellular compartments. The function of PGI2 in the human endometrium is linked to the protein kinase A pathway during menstruation. Future studies will elucidate the exact role of PGI2 in menstruation and the mechanisms of its signalling in the human endometrium.
Acknowledgements
The authors would like to acknowledge Mrs Sheila Boddy for assistance with cAMP assays and Mrs Catherine Murray for patient recruitment. This research was funded by the Medical Research Council awarded to H N J (Unit Support) and H O D C (programme grant no. 0000066).
References
- Baird DT, Cameron ST, Critchley HO, Drudy TA, Howe A, Jones RL, Lea RG, Kelly RW. Prostaglandins and menstruation. European Journal of Obstetric Gynecology and Reproductive Biology. 1996;70:15–17. doi: 10.1016/s0301-2115(96)02568-7. [DOI] [PubMed] [Google Scholar]
- Cameron IT, Leask R, Kelly RW, Baird DT. Endometrial prostaglandins in women with abnormal menstrual bleeding. Prostaglandins Leukotrienes and Medicine. 1987;29:249–257. doi: 10.1016/0262-1746(87)90014-x. [DOI] [PubMed] [Google Scholar]
- Caughey GE, Cleland LG, Penglis PS, Gamble JR, James MJ. Roles of cyclooxygenase (COX)-1 and COX-2 in prostanoid production by human endothelial cells: selective up-regulation of prostacyclin synthesis by COX-2. Journal of Immunology. 2001;167:2831–2838. doi: 10.4049/jimmunol.167.5.2831. [DOI] [PubMed] [Google Scholar]
- Chegini N, Rao CV. The presence of leukotriene C4- and prostacyclin-binding sites in nonpregnant human uterine tissue. Journal of Clinical Endocrinology and Metabolism. 1988;66:76–87. doi: 10.1210/jcem-66-1-76. [DOI] [PubMed] [Google Scholar]
- Crankshaw DJ, Dyal R. Effects of some naturally occurring prostanoids and some cyclooxygenase inhibitors on the contractility of the human lower uterine segment in vitro. Canadian Journal of Physiology and Pharmacology. 1994;72:870–874. doi: 10.1139/y94-123. [DOI] [PubMed] [Google Scholar]
- Dyal R, Crankshaw DJ. The effects of some synthetic prostanoids on the contractility of the human lower uterine segment in vitro. American Journal of Obstetric Gynecology. 1988;158:281–285. doi: 10.1016/0002-9378(88)90138-x. [DOI] [PubMed] [Google Scholar]
- Fortier I, Patry C, Lora M, Samadfan R, de Brum-Fernandes AJ. Immunohistochemical localization of the prostacyclin receptor (IP) human bone. Prostaglandins Leukotrienes and Essential Fatty Acids. 2001;65:79–83. doi: 10.1054/plef.2001.0292. [DOI] [PubMed] [Google Scholar]
- Giannoulias D, Alfaidy N, Holloway AC, Gibb W, Sun M, Lye SJ, Challis JR. Expression of prostaglandin I2 synthase, but not prostaglandin E synthase, changes in myometrium of women at term pregnancy. Journal of Clinical Endocrinology and Metabolism. 2002;87:5274–5282. doi: 10.1210/jc.2002-020521. [DOI] [PubMed] [Google Scholar]
- Goodfellow CF, Paton RC, Salmon JA, Moncada S, Clayton JK, Davies JA, McNicol GP. 6-Oxo-prostaglandin F1α and thromboxane B2 in uterine vein blood - a possible role in menstrual bleeding. Thrombosis and Haemostasis. 1982;48:9–12. [PubMed] [Google Scholar]
- Jabbour HN, Boddy SC. Prostaglandin E2 induces proliferation of glandular epithelial cells of the human endometrium via extra-cellular regulated kinase 1/2-mediated pathway. Journal of Clinical Endocrinology and Metabolism. 2003;88:4481–4487. doi: 10.1210/jc.2003-030297. [DOI] [PubMed] [Google Scholar]
- Jones RL, Kelly RW, Critchley HO. Chemokine and cyclooxygenase-2 expression in human endometrium coincides with leukocyte accumulation. Human Reproduction. 1997;12:1300–1306. doi: 10.1093/humrep/12.6.1300. [DOI] [PubMed] [Google Scholar]
- Kniss DA. Cyclooxygenases in reproductive medicine and biology. Journal of the Society for Gynecological Investigations. 1999;6:285–292. doi: 10.1016/s1071-5576(99)00034-9. [DOI] [PubMed] [Google Scholar]
- Levin JH, Stanczyk FZ, Lobo RA. Estradiol stimulates the secretion of prostacyclin and thromboxane from endometrial stromal cells in culture. Fertility and Sterility. 1992;58:530–536. doi: 10.1016/s0015-0282(16)55258-3. [DOI] [PubMed] [Google Scholar]
- Lim H, Dey SK. A novel pathway of prostacyclin signaling - hanging out with nuclear receptors. Endocrinology. 2002;143:3207–3210. doi: 10.1210/en.2002-220159. [DOI] [PubMed] [Google Scholar]
- Lumsden MA, Baird DT. The effect of intrauterine administration of prostacyclin on the contractility of the non-pregnant uterus in vivo. Prostaglandins. 1986;31:1011–1022. doi: 10.1016/0090-6980(86)90205-4. [DOI] [PubMed] [Google Scholar]
- Lumsden MA, Kelly RW, Abel MH, Baird DT. The concentrations of prostaglandins in endometrium during the menstrual cycle in women with measured menstrual blood loss. Prostaglandins Leukotrienes and Medicine. 1986;23:217–227. doi: 10.1016/0262-1746(86)90189-7. [DOI] [PubMed] [Google Scholar]
- Makarainen L, Ylikorkala O. Primary and myoma-associated menorrhagia: role of prostaglandins and effects of ibuprofen. British Journal of Obstetrics and Gynaecology. 1986;93:974–978. doi: 10.1111/j.1471-0528.1986.tb08019.x. [DOI] [PubMed] [Google Scholar]
- Milne SA, Jabbour HN. Prostaglandin PGF2α receptor expression and signaling in human endometrium: role of PGF2α in epithelial cell proliferation. Journal of Clinical Endocrinology and Metabolism. 2003;88:1825–1832. doi: 10.1210/jc.2002-021368. [DOI] [PubMed] [Google Scholar]
- Milne SA, Perchick GB, Boddy SC, Jabbour HN. Expression, localization, and signaling of PGE2 and EP2/EP4 receptors in human nonpregnant endometrium across the menstrual cycle. Journal of Clinical Endocrinology and Metabolism. 2001;86:4453–4459. doi: 10.1210/jcem.86.9.7856. [DOI] [PubMed] [Google Scholar]
- Moonen P, Klok G, Keirse MJ. Distribution of prostaglandin endoperoxide synthase and prostacyclin synthase in the late pregnant uterus. British Journal of Obstetrics and Gynaecology. 1986;93:255–259. doi: 10.1111/j.1471-0528.1986.tb07903.x. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiology Reviews. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. American Journal of Obstetrics and Gynecology. 1975;122:262–263. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- Prentice A. Health burdens of menstrual disorders. In: O'Brien S, Cameron I, Maclean A, editors. Disorders of the Menstrual Cycle. London: RCOG Press; 2000. pp. 13–23. [Google Scholar]
- Smith SK, Kelly RW. The release of PGF2 alpha and PGE2 from separated cells of human endometrium and decidua. Prostaglandins Leukotrienes and Essential Fatty Acids. 1988;33:91–96. doi: 10.1016/0952-3278(88)90146-9. [DOI] [PubMed] [Google Scholar]
- Smith SK, Abel MH, Kelly RW, Baird DT. Prostaglandin synthesis in the endometrium of women with ovular dysfunctional uterine bleeding. British Journal of Obstetrics and Gynaecology. 1981a;88:434–442. doi: 10.1111/j.1471-0528.1981.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Smith SK, Abel MH, Kelly RW, Baird DT. A role for prostacyclin (PGI2) in excessive menstrual bleeding. Lancet. 1981b;1:522–524. doi: 10.1016/s0140-6736(81)92862-2. [DOI] [PubMed] [Google Scholar]
- Smyth EM, FitzGerald GA. Human prostacyclin receptor. Vitamins and Hormones. 2002;65:149–165. doi: 10.1016/s0083-6729(02)65063-0. [DOI] [PubMed] [Google Scholar]
- Tong BJ, Tan J, Tajeda L, Das SK, Chapman JA, DuBois RN, Dey SK. Heightened expression of cyclooxygenase-2 and peroxisome proliferator-activated receptor-delta in human endometrial adenocarcinoma. Neoplasia. 2000;2:483–490. doi: 10.1038/sj.neo.7900119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsson L, Wikland M, Wiqvist N. PGH2, TxA2 and PGI2 have potent and differentiated actions on human uterine contractility. Prostaglandins. 1981;21:277–286. doi: 10.1016/0090-6980(81)90145-3. [DOI] [PubMed] [Google Scholar]
- Zachary I. Signaling mechanisms mediating vascular protective actions of vascular endothelial growth factor. American Journal of Physiology. Cell Physiology. 2001;280:C1375–C1386. doi: 10.1152/ajpcell.2001.280.6.C1375. [DOI] [PubMed] [Google Scholar]
- Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovascular Research. 2001;49:568–581. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]