Abstract
Increased expression of cyclooxygenase-2 (COX-2) and subsequent prostaglandin production is an important event in several human malignancies, including colorectal cancer. COX-2 mediated prostanoid synthesis has been shown to play a key role in tumor progression with prostaglandin E2 (PGE2) being shown to promote tumor growth, invasion and angiogenesis. The role of the other prostaglandins produced by COX-2 in tumors remains poorly understood. We have shown that colorectal tumor cells produce prostaglandin F2α (PGF2α) and provide evidence that PGF2α may play an important role in colorectal tumorigenesis. Our data show that PGF2α is secreted by both colorectal adenoma and carcinoma-derived cell lines at levels in excess of those detected for PGE2. These cell lines were also found to express the PGF2α receptor (FP) indicating potential autocrine effects of PGF2α. This finding is further supported by an in vivo immunohistochemical study of FP expression in resected colon tissue. These data show epithelial expression of FP in normal colorectal mucosa and also in colorectal adenomas and carcinomas. We compared the relative abilities of PGF2α and PGE2 to induce cell motility in vitro in colorectal tumor cell lines and show the first evidence of prostaglandin-induced cell motility in colorectal adenoma cell lines. PGF2α induced cell motility with equivalent potency to PGE2 in all the cell lines tested and was also shown to increase the invasion of carcinoma-derived cells into reconstituted basement membrane. These data show that PGF2α may play an important role in the malignant progression of colorectal tumors.
Keywords: colon, cancer, adenoma, prostaglandin, motility, invasion
Colorectal cancer remains a major cause of cancer deaths globally. The inducible form of cyclooxygenase (COX-2), the key enzyme in prostanoid biosynthesis, is overexpressed in over 80% of colorectal cancers.1 Furthermore, COX-2 is frequently expressed during the premalignant adenoma stages of colorectal tumorigenesis and shows a size dependent increase in expression indicating a role in tumor progression.2
The importance of COX-2 expression in colorectal tumor progression has been highlighted by studies both in vitro and in vivo, and its effects are mediated through its prostanoid products.3 COX-2 catalyses the formation of prostaglandin H2 (PGH2) from arachidonic acid and PGH2 is subsequently converted to several structurally related primary prostanoids, namely prostaglandin E2 (PGE2), PGD2, PGI2, Thromboxane A2 and prostaglandin F2α (PGF2α) by the action of specific synthases. Increased levels of PGE2 have been observed in colorectal cancers when compared with histologically normal tissue.4-6 Subsequent studies using in vitro models have shown that PGE2 is able to promote tumor cell growth,7,8 modulate apoptosis9 and increase cell motility.8 COX-2 expression and subsequent PGE2 production has also been shown to enhance the production of angiogenic factors.10-12
Studies of prostaglandin production in colorectal tumors have also identified the production of other primary prostanoids including PGF2α.4-6 In 1 study on intestinal tissue from familial adenomatous polyposis (FAP) patients, the detected levels of PGF2α were 30-fold higher than for PGE2.13
Although widely studied in other tissues, the potential role of PGF2α in colorectal cancer remains to be elucidated. The most extensively studied area of PGF2α biology is its role in the regression of the corpus luteum during pregnancy.14 Interestingly, PGF2α stimulates the proliferation of Swiss mouse 3T3 cells with greater efficacy than PGE2.15,16
PGF2α is thought to largely act through the FP G-protein-coupled receptor, although the expression of this receptor has not been determined in colorectal cancer. Because of the importance of COX-2 overexpression, the aim of the study presented here was to investigate the potential role of PGF2α in colorectal tumorigenesis. To this end, we determined the expression of the FP receptor in normal and neoplastic colorectal tissue, and a panel of adenoma and carcinoma-derived cell lines. The production of PGF2α was examined in parallel with that of PGE2 in the cell lines. Finally, we assessed the ability of both PGF2α and PGE2 to promote cell motility and invasion which represent important hallmarks of malignant progression in these tumors.
Material and methods
Cell lines and culture conditions
AA/C1 is a clonogenic, adenoma cell line derived from a 3 to 4 cm polyp from the descending colon of a familial adenomatous polyposis (FAP) patient17 and is cultured in conditioned medium as described by Williams et al.18 RG/C2 is a clonogenic cell line derived from a sporadic tubular adenoma of the sigmoid colon of 1–2 cm in diameter and is cultured in DMEM supplemented with 20% (vol/vol) FBS (Autogen Bioclear, Calne, UK).19 Both of these adenoma-derived cell lines are anchorage-dependent and are nontumorigenic in athymic nude mice. HCA7 was established from a moderately well differentiated mucinous carcinoma of the colon20 and was a kind gift from Dr. Sue Kirkland (London, UK). HCA7-col29 was sub-cloned from the parental line21 and will be hereafter referred to as HCA7. SW480 was derived from a sporadic colonic adenocarcinoma.22 The carcinoma lines were cultured in DMEM supplemented with 10% (vol/vol) FBS, and all cell lines were cultured as adherent cells in 25 cm2 tissue culture flasks.
For growth response assays, cells were seeded at 106 per 25 cm2 flask and allowed to recover for 48 hr. The medium was then changed to DME-F12 (Invitrogen, Paisley, UK), supplemented with 2% FBS, containing the appropriate amount of prostaglandin F2α (PGF2α) (Sigma, Poole, UK) or vehicle control. A range of prostaglandin concentrations from 0.1 to 10 μM was tested and compared with vehicle and untreated control. Treatments were made from prepared stocks so that a 1:1,000 dilution of 95% ethanol was added to each treated flask. Following a 72 hr treatment period, attached cell yield and the proportion of apoptotic cells that had detached from the culture substrate were quantified using a haemocytometer. The level of apoptosis in cultured colonic cells can be assessed by measuring the proportion of cells that detach from the flask and float in the medium.23,24 Apoptosis was confirmed in these ‘floating’ cells by morphology (following acridine orange staining).
Prostaglandin production assay
PGF2α and prostaglandin E2 (PGE2) enzyme immunoassay kits (Cayman Chemical Company, Ann Arbor, MI) were used to assay the release of PGF2α and PGE2 into the growth medium by the cells. The lower limit of detection of the assays was 5.5 pg/ml for PGF2α and 36 pg/ml for PGE2, and the upper limit of detection was 64 pg/ml and 250 pg/ml for PGF2α and PGE2, respectively. Cells were grown to ~70% confluency and the standard culture medium replaced with serum-free DMEM-F12 (Invitrogen, Paisley, UK) for a period of 72 hr whereupon the medium was removed, centrifuged and decanted, snap-frozen in liquid nitrogen and stored at −70°C prior to analysis. These samples were diluted appropriately so that readings fell within the detection limits of the assay. PGF2α and PGE2 production was normalized according to the number of adherent cells present in the particular culture at the time of sampling. The results are expressed as picograms of prostaglandin/106 cells and represent the average of 2 independent experiments performed in duplicate.
In vitro motility and invasion assays
Cell motility assays were carried out using a transwell filter motility assay as previously described.25 For quantitative analysis of cell motility 8 μm pore size insert filters (Becton Dickinson, Oxford, UK) were coated with 10 μg ml−1 Vitrogen Type I collagen (Cohesion, Palo Alto, CA). For invasion assays, 8 μm transwell filters precoated with Matrigel (Becton Dickinson, Oxford, UK) were used. For both types of analysis 1 × 105 cells were seeded per well in calcium-free DMEM containing 0.1% FBS (CF-DMEM) and allowed to adhere for 4 hr.
Initial experiments using a range of doses of prostaglandins from 0.1 to 10 μM [PGF2α or PGE2 (Sigma, Poole, UK) diluted 1:1,000 from a 1 mM stock made up in 95% ethanol, or vehicle (1:1,000 dilution of 95% ethanol)] were performed, 1 μM was selected as it was found to stimulate significant motility.
Following a 24 hr incubation, cells were removed from the upper filter surface with a cotton swab. The filters were fixed and stained with haematoxylin. Cells that had moved to the lower filter surface were counted in 10 fields at 20× magnification. Three independent experiments were carried out in triplicate, and the data are expressed as the mean ± SEM. Statistical analysis of this data was performed using the Student’s t test. Differences were considered significance when the p value was <0.05.
Western blot analysis
Samples of 2 × 106 cells were prepared for Western blotting as described previously.26 The FP receptor was detected using a rabbit polyclonal antibody (1:1,000 dilution; Cayman Chemical Cayman, Ann Arbor, MI) and the Lumiglo ECL detection system (Amersham Biosciences, Little Chalfont, UK). Ishikawa endometrial carcinoma cells stably overexpressing the FP receptor were included as a positive control.27 Anti α-tubulin (diluted 1:1,000; Sigma, Poole, UK) was used as a loading control.
Immunohistochemistry
Immunohistochemical analysis of FP receptor expression was carried out as previously described.27 Following approval from the local research ethics committee, anonymised paraffin-embedded, formalin-fixed archival material was retrieved from the files of the Department of Histopathology, Bristol Royal Infirmary for use in this study. A total of 15 colorectal adenomas were examined. These included 5 small (≤5 mm) tumors, 5 medium-sized (5–10 mm) tumors and 5 large (≥10 mm) tumors. We also examined 10 invasive adenocarcinomas taken from colorectal resection specimens. Five samples of histologically normal mucosa were also included in the study.
The FP receptor was detected using a FP-specific affinity purified rabbit polyclonal antibody (diluted 1:500; Cayman, Ann Arbor, MI) and a biotinylated swine anti-rabbit secondary antibody (diluted 1:500; Dako Cytomation, Ely, UK). The specificity of the antibody was confirmed by prior overnight incubation at 4°C with blocking peptide (diluted 1:50; Cayman, Ann Arbor, MI). This preblocking was used as a negative control as shown in Figure 2b. A standard avidin-biotin immunoperoxidase technique (Dako Cytomation, Ely, UK) was employed and the immunoreaction visualized by means of the diamino benzidine reaction with haemotoxylin counterstaining. Antigen retrieval was performed by treating sections for 5 min in a pressure cooker in 0.1% citrate buffer (pH 6.0). Sections were examined for FP immunoreactivity.
Figure 2.
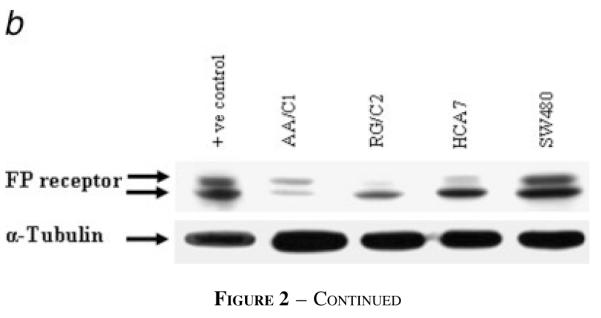
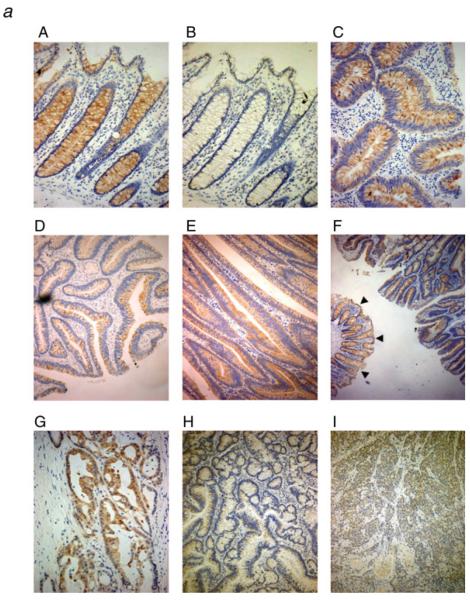
The FP receptor is expressed in resected colorectal adenomas, carcinomas and tumor derived cell lines. (a) Sections of resected human tissue were immunohistochemically stained with FP-specific antibody and digitally photographed on a light microscope. This figure shows representative views selected from data for: 15 colorectal adenomas [5 small (≤5 mm), 5 medium (5–10 mm) and 5 large (≥10 mm) tumors]; 10 invasive adenocarcinomas (3 well differentiated, 3 moderately differentiated and 4 poorly differentiated); and 5 samples of normal mucosa taken from diverticulitis patients. A: Normal (×200 magnification); B: normal—corresponding negative control (preabsorbed over-night with FP blocking peptide) (×200); C: small adenoma (×200); D: medium adenoma (×100); E: large adenoma (×100); F: large adenoma, low magnification showing normal (arrows) and adenoma from the same surgical resection (×25); G: moderately differentiated carcinoma (×200); H: well differentiated carcinoma (×100); I: poorly differentiated carcinoma (×100). (b) Expression of the FP receptor in colorectal tumor cell lines. Western blots were carried out on samples from the colorectal tumor cell lines using an FP-specific antiserum. The FP receptor runs as a doublet in the FP-overexpressing endometrial carcinoma cell line used as a positive control, with the lower band at ~64 kDa.25 The presence of the FP receptor was detected in all of the cell lines examined. The blot was reprobed with α-tubulin to control for equal loading of samples. Expression analysis was carried out in 3 repeated experiments and the results shown are representative.
Results
Prostaglandin F2α is produced by colorectal adenoma and carcinoma cells
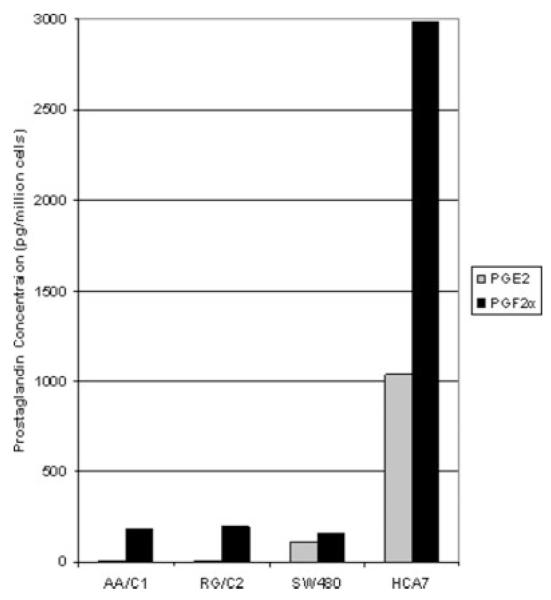
Samples of media were taken following 72 hr incubation of the adenoma-derived cell lines AA/C1 and RG/C2, and from the carcinoma-derived cell lines HCA7 and SW480. For this culture period, serum-free media was used and these samples were assayed for both PGE2 and PGF2α content using enzyme immunoassay. Prostaglandin concentrations were calculated in pg per million cells. Figure 1 shows the mean value obtained, and Table I the range of results from 2 independent experiments performed in duplicate.
Figure 1.
Colorectal tumor cells produce PGF2α. Graphical representation of PGF2α and PGE2 produced by colorectal adenoma and carcinoma cell lines. Enzyme immunoassays for PGF2α and PGE2 were performed on serum-free media from each cell line following 72 hr incubation. AA/C1 and RG/C2 are adenoma-derived cell lines, whereas SW480 and HCA7 are carcinoma-derived. Prostaglandin production was calculated as picogrammes per million cells and these data represent the mean of 2 experiments performed in duplicate.
TABLE I.
COLORECTAL TUMOR CELLS PRODUCE PGF2α
| Cell line |
||||
|---|---|---|---|---|
| AA/C1 | RG/C2 | SW480 | HCA7 | |
| Prostaglandin | ||||
| PGE2 (pg/million cells) | 5.20–8.18 | 5.12–9.86 | 86.21–123.69 | 910.39–1157.61 |
| PGF2α (pg/million cells) | 157.14–203.10 | 175.52–217.96 | 139.35–178.69 | 2777.26–3193.16 |
The table gives a numerical representation of the data presented in Figure 1. These data represent the range of values obtained from 2 experiments performed in duplicate. AA/C1 and RG/C2 are adenoma-derived cell lines, whereas SW480 and HCA7 are carcinoma-derived.
Although relatively low amounts of PGE2 were found in the adenoma-derived cell lines AA/C1 and RG/C2 (6.69 and 7.49 pg/million cells, respectively), both produced PGF2α at a level comparable with that detected in SW480 carcinoma cells. The HCA7 carcinoma line produces very high levels of PGE2 due to the relatively high levels of cyclooxygenase-2 (COX-2) expression in these cells caused by stabilization of the COX-2 mRNA.28 It is interesting to note that HCA7 also produced higher levels of PGF2α than PGE2.
The FP prostaglandin receptor is expressed in colorectal tumors and in tumor-derived cell lines
The major receptor for PGF2α has been designated FP and has been shown to be a Gq-linked G-protein coupled receptor which mediates its effects through modulating intracellular calcium and inositol phosphate.29 To our knowledge, no previous studies have reported the expression of the FP receptor in normal human colon tissue, colorectal tumors or in colorectal adenoma and carcinoma cell lines. In vivo, prostaglandins can be produced by both the epithelial and stromal components of the tumor and may act in an autocrine or paracrine fashion on neighboring cell types, eliciting a range of biological effects. To determine the possible target tissues of PGF2α in colorectal tumors we used immunohistochemistry on resected human tissues to determine the expression pattern of the FP receptor in vivo (Fig. 2). The data presented are representative of 5 samples of normal colonic epithelium resected from patients with diverticulitis; 15 samples of benign colonic adenoma and 10 samples of colorectal adenocarcinoma. The colorectal adenomas were divided according to size (small = <5 mm; medium = 5–10 mm; large = >10 mm).
Strong positive immunoreactivity to the FP receptor was seen in the epithelial component of normal colonic mucosa (100% (n = 5) Fig. 2a panel A) compared with the negative control (Fig. 2a panel B), yet no staining was detected in stromal cells. All of the colorectal adenomas (15/15) displayed positive epithelial immunoreactivity for FP but no expression was observed in any stromal tissues (Fig. 2a panels C–F).
The results in colorectal carcinomas were more varied. Carcinomas 7/10 displayed positive immunoreactivity for FP in the epithelium with no staining detected in stromal constituents (Fig. 2a panels G–I). Positive immunoreactivity for the FP receptor was detected in all of the normal and adenoma samples, and the majority of the carcinomas, both the intensity and distribution of the FP staining were observed to be similar when comparing normal with tumor tissue.
Having shown that the FP receptor was expressed in normal and tumor tissue in vivo we then studied the expression of the FP receptor in adenoma and carcinoma-derived cell lines by Western blotting. Representative results of 3 repeated experiments are shown in Figure 2b. Ishikawa endometrial carcinoma cells stably overexpressing the FP receptor were included as a positive control.27 Both of the adenoma cell lines and all 3 carcinoma cell lines show expression of the FP receptor. A doublet of bands was seen for each cell line and it is not possible, using the reagents currently available, to determine whether these represent 2 distinct isoforms of the receptor or some form of post-translational modification.27 However, these data do show the expression of the FP receptor in normal and neoplastic colorectal epithelium, suggesting a possible autocrine role for PGF2α secreted by the cells.
Prostaglandins F2α and E2 stimulate cell motility in colorectal adenoma-derived cell lines
Previous studies have shown that PGE2 confers broad ranging tumor-promoting effects in colorectal cancer yet nothing is currently known about the role of PGF2α in this disease. Having shown that colorectal tumor cells produce PGF2α and that the FP receptor is expressed by colorectal epithelial cells in vitro and in vivo, we investigated the potential role of PGF2α in colorectal tumorigenesis using in vitro models.
Previous studies have reported that exogenous addition of PGF2α stimulated cell growth in the colorectal carcinoma cell line SW1116,7 but produced no significant effects on growth in HCT8 and HT29.30 Studies in this laboratory have shown that cell lines derived from colorectal adenomas31 and carcinomas32 are growth stimulated by exogenous addition of PGE2. We conducted PGF2α growth response experiments on the cell lines studied above (adenomas: AA/C1, RG/C2; carcinomas: SW480, HCA7) with a range of concentrations from 0.1 to 10 μM. No significant change in cell growth or apoptosis was detected in any of the cell lines studied over a 72 hr period (data not shown).
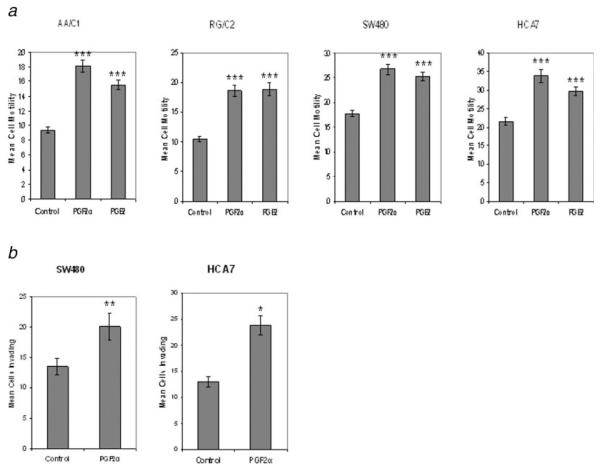
Previous work has shown that PGE2 has multiple effects on colorectal carcinoma cells, including increasing cell motility.8,33 There are no previous reports of PGF2α inducing motility in tumor cells and, importantly, no studies of prostaglandin-induced motility in colorectal adenomas. We compared the ability of exogenous PGF2α and PGE2 to influence the motility of colorectal adenoma (AA/C1, RG/C2) and carcinoma (SW480, HCA7) cells using a Boyden-chamber transwell-filter cell motility assay, compared to solvent control. Initial experiments were performed using a range of doses of prostaglandins (0.1–10 μM) and 1 μM was selected as it was found to stimulate significant motility. Cells were treated and incubated for 24 hr to allow movement through the collagen-coated filters. Figure 3a shows the average results of 3 independent experiments performed in triplicate. As would be expected, the adenoma derived cell lines AA/C1 and RG/C2, which represent earlier stages of colorectal tumor progression, were less motile than their carcinoma counterparts, which display a ~2-fold higher rate of motility (Fig. 3a).
Figure 3.
PGF2α-induced motility and invasion in colorectal tumor cells. (a) The effects of 1 μM PGF2α or PGE2 on the motility of colorectal adenoma- (AA/C1 & RG/C2) and carcinoma-derived (SW480 & HCA7) cell lines as measured by collagen-coated transwell filter assay after 24 hr of treatment, compared to solvent control. The data are expressed as the total cells observed to have migrated from 10 fields of view (±SEM) and represent 3 separate experiments performed in triplicate. *p < 0.05; **p < 0.01; ***p < 0.001 prostaglandin vs. solvent control. (b) The effects of 1 μM PGF2α on the invasion of colorectal carcinoma cell lines into Matrigel™ after 24 hr of treatment, compared to solvent control. The data are expressed as the total cells observed to have invaded from 10 fields of view (±SEM) and represent 3 separate experiments performed in triplicate. *p < 0.05; **p < 0.01; ***p < 0.001 prostaglandin vs. control.
Both PGF2α and PGE2 significantly increased cell motility in adenoma- (AA/C1 and RG/C2, p = <0.001) and carcinoma-derived (HCA7 and SW480, p = <0.001) cell lines. These are the first data showing the ability of prostaglandins to increase motility in adenoma cells which represent the earlier, nonmalignant stages of colorectal tumor development.
Prostaglandin F2α stimulates invasion in carcinoma cells
The process by which tumor cells break out from their site of origin and metastasise to distant sites requires, in addition to motility, an ability to invade through the basement membrane and underlying mesenchymal cells. This process can be modeled in vitro by coating transwell filters (as used for the motility assay above) with reconstituted basement membrane, in this instance Matrigel™. PGE2 has been shown previously to stimulate invasion by colorectal cancer cells in this type of assay.34 The adenoma-derived cell lines have been shown previously to be noninvasive [Ref. 35 and Qualtrough and Paraskeva, unpublished observations] in keeping with their premalignant nature. Adoption of an invasive phenotype is a key distinction between benign and malignant colorectal tumors.
The data presented in Figure 3b clearly shows that 1 μM PGF2α significantly stimulates invasion of HCA7 and SW480 colon carcinoma cells into a reconstituted basement membrane (HCA7, p = <0.05; SW480, p = <0.01). The figures show a 1.8-fold stimulation of invasion in HCA7 and a 1.5-fold increase in SW480 with PGF2α compared with control.
Discussion
The overexpression of COX-2 is an important event in colorectal cancer. It has also become clear that it is the prostanoid products of COX-2 activity that mediate major effects on tumor cell behavior by promoting growth, survival, invasive behavior and inducing angiogenesis.36 Much of the focus of prostanoid research in the area of colorectal cancer has fallen on PGE2, widely accepted to be the major prostanoid product of COX-2 in these tumors.31 COX-2 overexpression also leads to the production of other primary prostanoids such as PGD2, PGI2 and PGF2α, although the role of these in colorectal tumorigenesis remains poorly understood.36 Several researchers have detected PGF2α production in colorectal tumor tissue, and in 1 study the levels detected were found to be in excess of those for PGE2.13 The purpose of the study presented here was to determine the potential role of PGF2α in colorectal tumorigenesis.
The data presented here clearly shows readily detectable levels of PGF2α produced by cell lines derived from both colorectal adenomas and carcinomas. Considering the known importance of PGE2 in colorectal tumorigenesis, it is of interest that the levels of PGF2α found were in excess of those of PGE2, suggesting that PGF2α may be of biological importance in these tumors.
The primary prostanoids have been shown to function through specific prostanoid receptors which, in the case of PGF2α, has been designated FP.27,37 Although there are no published studies on the expression of FP in the human lower gastrointestinal tract, 1 previous study showed FP expression in the epithelium of the stomach.38 We have now shown for the first time that the FP receptor is expressed in the normal colonic epithelium, and also in the epithelial component of colorectal adenomas and carcinomas. Interestingly, no significant discernible difference was observed in the intensity of FP immunoreactivity between normal and tumor samples. However, the greatly altered signaling context of the neoplastic tissue could elicit different cellular responses to PGF2α. Consistent with these in vivo studies, we showed the expression of the FP receptor in cell lines derived from both colorectal adenomas and carcinomas. The presence of the PGF2α ligand and the expression of the FP receptor in colorectal tumor epithelial cells imply a functionality to this pathway. To determine the potential role of PGF2α in colorectal tumorigenesis we used an in vitro model system. We found no significant effects on cell growth or apoptosis in response to PGF2α in either the adenoma or carcinoma cell lines tested. This finding is in relation with another published study on the HCT8 and HT29 cell lines,30 where no growth stimulation was observed. Similarly, Qiao et al. also showed no proliferative response in HT29 cells to PGF2α, whereas SW116 were growth stimulated, suggesting that the cellular response to PGF2α may be tumor specific.7
The acquisition of a motile phenotype is a hallmark of colorectal tumor progression. Previous studies have shown that PGE2 can stimulate motility in the colorectal carcinoma cell line LS174T.8 No previous study has examined the ability of prostaglandins to promote motility in colorectal adenoma cells and there are no reports of PGF2α affecting cell motility in colonic epithelial cells. We compared the effects of PGF2α and PGE2 on cell motility in both adenoma and carcinoma-derived cell lines. We report the first evidence of prostaglandin-induced cell motility in adenoma cells which responded to both PGF2α and PGE2. Our data also show that PGF2α significantly stimulates cell motility in colorectal carcinoma cell lines, as well as adenomas, and is comparable with PGE2 in its ability to do so. These data show an important biological effect of PGF2α in colonic tumor cells and also suggest that prostaglandins may contribute to the progression of the adenoma to carcinoma sequence by increasing cell motility.
The process of tumor invasion (and therefore malignancy) requires cell motility but also alterations in cell adhesion and the secretion of enzymes to degrade basement membrane and matrix components. Pai et al. have reported that PGE2 potentiates invasiveness in the colorectal carcinoma cell lines SW480 and LoVo although the effects of PGF2α remain unreported.34 We have now shown that PGF2α can also increase the invasiveness of the SW480 and HCA7 carcinoma cell lines in vitro, with the SW480 data being comparable with that previously published for PGE2.34 These increases were statistically significant in both of the cell lines and demonstrate a potentially important and novel tumorpromoting effect of PGF2α in colorectal cancer. Because of the complexity of the invasion process, it is possible that PGF2α may also stimulate the production of matrix remodeling enzymes and alter cell adhesion complexes, in addition to stimulating cell motility. PGE2 has been shown to stimulate colon cancer cell invasion through transactivation of the epidermal growth factor receptor (EGFR).34 We have not seen EGFR activation by PGF2α in our system (data not shown), suggesting alternate mechanisms of action for this prostaglandin which represents an interesting area for potential future study.
In conclusion, the data presented here show that PGF2α may be an important product of COX-2 overexpression in colorectal tumors, by promoting tumor progression and potentially metastasis. The use of high doses of NSAIDs in colorectal cancer prevention and therapy has recently come under close scrutiny due to the adverse cardiovascular effects associated with long term use of Rofecoxib and the subsequent withdrawal of this drug from clinical use.39 Although this does not preclude the use of this type of drug in an adjuvant setting, it does highlight the need for more targeted approaches.40,41 To facilitate this, it is of key importance that we increase our understanding of the pleiotropic effects of the various prostanoid products of the COX-2 enzyme. The data presented here show that PGF2α, as well as the widely-studied PGE2 needs to be considered in our understanding of colorectal cancer.
Acknowledgements
We wish to thank Dr. Sharon Battersby (MRC Human Reproductive Sciences Unit, Edinburgh) for expert assistance with the immunohistochemistry.
Grant sponsors: Cancer Research UK and Citrina Foundation.
References
- 1.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–8. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 2.Elder DJ, Baker JA, Banu NA, Moorghen M, Paraskeva C. Human colorectal adenomas demonstrate a size-dependent increase in epithelial cyclooxygenase-2 expression. J Pathol. 2002;198:428–34. doi: 10.1002/path.1232. [DOI] [PubMed] [Google Scholar]
- 3.Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23:2840–55. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 4.Rigas B, Goldman IS, Levine L. Altered eicosanoid levels in human colon cancer. J Lab Clin Med. 1993;122:518–23. [PubMed] [Google Scholar]
- 5.Giardiello FM, Spannhake EW, DuBois RN, Hylind LM, Robinson CR, Hubbard WC, Hamilton SR, Yang VW. Prostaglandin levels in human colorectal mucosa: effects of sulindac in patients with familial adenomatous polyposis. Dig Dis Sci. 1998;43:311–16. doi: 10.1023/a:1018898120673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang VW, Shields JM, Hamilton SR, Spannhake EW, Hubbard WC, Hylind LM, Robinson CR, Giardiello FM. Size-dependent increase in prostanoid levels in adenomas of patients with familial adenomatous polyposis. Cancer Res. 1998;58:1750–3. [PubMed] [Google Scholar]
- 7.Qiao L, Kozoni V, Tsioulias GJ, Koutsos MI, Hanif R, Shiff SJ, Rigas B. Selected eicosanoids increase the proliferation rate of human colon carcinoma cell lines and mouse colonocytes in vivo. Biochim Biophys Acta. 1995;1258:215–23. doi: 10.1016/0005-2760(95)00100-q. [DOI] [PubMed] [Google Scholar]
- 8.Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem. 2001;276:18075–81. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- 9.Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–6. [PubMed] [Google Scholar]
- 10.Ben-Av P, Crofford LJ, Wilder RL, Hla T. Induction of vascular endothelial growth factor expression in synovial fibroblasts by prostaglandin E and interleukin-1: a potential mechanism for inflammatory angiogenesis. FEBS Lett. 1995;372:83–7. doi: 10.1016/0014-5793(95)00956-a. [DOI] [PubMed] [Google Scholar]
- 11.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–16. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 12.Cianchi F, Cortesini C, Bechi P, Fantappie O, Messerini L, Vannacci A, Sardi I, Baroni G, Boddi V, Mazzanti R, Masini E. Up-regulation of cyclooxygenase 2 gene expression correlates with tumor angiogenesis in human colorectal cancer. Gastroenterology. 2001;121:1339–47. doi: 10.1053/gast.2001.29691. [DOI] [PubMed] [Google Scholar]
- 13.Nugent KP, Spigelman AD, Phillips RK. Tissue prostaglandin levels in familial adenomatous polyposis patients treated with sulindac. Dis Colon Rectum. 1996;39:659–62. doi: 10.1007/BF02056946. [DOI] [PubMed] [Google Scholar]
- 14.Diaz FJ, Anderson LE, Wu YL, Rabot A, Tsai SJ, Wiltbank MC. Regulation of progesterone and prostaglandin F2α production in the CL. Mol Cell Endocrinol. 2002;191:65–80. doi: 10.1016/s0303-7207(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 15.de Asua LJ, Clingan D, Rudland PS. Initiation of cell proliferation in cultured mouse fibroblasts by prostaglandin F2α. Proc Natl Acad Sci USA. 1975;72:2724–8. doi: 10.1073/pnas.72.7.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sauane M, Correa L, Rogers F, Krasnapolski M, Barraclough R, Rudland PS, de Asua LJ. Prostaglandin F2α induces cyclin D1 expression and DNA synthesis via early signalling mechanisms in swiss mouse 3T3 cells. Biochem Biophys Res Commun. 2000;270:11–16. doi: 10.1006/bbrc.2000.2383. [DOI] [PubMed] [Google Scholar]
- 17.Paraskeva C, Buckle BG, Sheer D, Wigley CB. The isolation and characterisation of colorectal epithelial cell lines at different stages in malignant transformation from familial polyposis coli patients. Int J Cancer. 1984;34:49–56. doi: 10.1002/ijc.2910340109. [DOI] [PubMed] [Google Scholar]
- 18.Williams AC, Harper SJ, Paraskeva C. Neoplastic transformation of a human colonic epithelial cell line: in vitro evidence for the adenoma-to-carcinoma sequence. Cancer Res. 1990;50:4724–30. [PubMed] [Google Scholar]
- 19.Paraskeva C, Finerty S, Mountford RA, Powell SC. Specific cytogenetic abnormalities in two new human colorectal adenoma-derived epithelial cell lines. Cancer Res. 1989;49:1282–6. [PubMed] [Google Scholar]
- 20.Kirkland SC. Dome formation by a human colonic adenocarcinoma cell line (HCA-7) Cancer Res. 1985;45:3790–5. [PubMed] [Google Scholar]
- 21.Marsh KA, Stamp GWH, Kirkland SC. Isolation and characterisation of multiple cell types from a single human colonic carcinoma: tumorigenicity of these cell types in a xenograft system. J Pathol. 1993;170:441–50. doi: 10.1002/path.1711700407. [DOI] [PubMed] [Google Scholar]
- 22.Hague A, Manning AM, Hanlon KA, Huschtscha LI, Hart D, Paraskeva C. Sodium butyrate induces apoptosis in human colonic tumour cell lines in a p53-independent pathway: implications for the possible role of dietary fibre in the prevention of large bowel cancer. Int J Cancer. 1993;55:498–505. doi: 10.1002/ijc.2910550329. [DOI] [PubMed] [Google Scholar]
- 23.Hague A, Bracey TS, Hicks DJ, Reed JC, Paraskeva C. Decreased levels of p26-Bcl-2, but not p30 phosphorylated Bcl-2, precede TGFβ1-induced apoptosis in colorectal adenoma cells. Carcinogenesis. 1998;19:1691–5. doi: 10.1093/carcin/19.9.1691. [DOI] [PubMed] [Google Scholar]
- 24.Leibovitz A, Stinson JC, McCombs WB, 3rd, McCoy CE, Mazur KC, Mabry ND. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976;36:4562–9. [PubMed] [Google Scholar]
- 25.Efstathiou JA, Liu D, Wheeler JM, Kim HC, Beck NE, Ilyas M, Karayiannakis AJ, Mortensen NJ, Kmiot W, Playford RJ, Pignatelli M, Bodmer WF. Mutated epithelial cadherin is associated with increased tumorigenicity and loss of adhesion and of responsiveness to the motogenic trefoil factor 2 in colon carcinoma cells. Proc Natl Acad Sci USA. 1999;96:2316–21. doi: 10.1073/pnas.96.5.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams AC, Collard TJ, Paraskeva C. An acidic environment leads to p53 dependent induction of apoptosis in human adenoma and carcinoma cell lines: Implications for clonal selection during colorectal carcinogenesis. Oncogene. 1999;18:3199–204. doi: 10.1038/sj.onc.1202660. [DOI] [PubMed] [Google Scholar]
- 27.Sales KJ, List T, Boddy SC, Williams ARW, Anderson RA, Naor Z, Jabbour HN. A novel angiogenic role for prostaglandin F2α-FP receptor interaction in human endometrial carcinomas. Cancer Res. 2005;65:7707–16. doi: 10.1158/0008-5472.CAN-05-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao J, Sheng H, Inoue H, Morrow JD, DuBois RN. Regulation of constitutive cyclooxygenase-2 expression in colon carcinoma cells. J Biol Chem. 2000;275:33951–6. doi: 10.1074/jbc.M002324200. [DOI] [PubMed] [Google Scholar]
- 29.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: subtypes and signalling. Ann Rev Pharmacol Toxicol. 2001;41:661–90. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 30.Cassano G, Gasparre G, Susca F, LIppe C, Guanti G. Lack of effect by prostaglandin F2alpha on the proliferation of the HCT8 and HT29 human adenocarcinoma cell lines. Oncol Rep. 2000;7:183–6. doi: 10.3892/or.7.1.183. [DOI] [PubMed] [Google Scholar]
- 31.Chell SD, Witherden IR, Dobson RR, Moorghen M, Herman AA, Qualtrough D, Williams AC, Paraskeva C. Increased EP4 receptor expression in colorectal cancer progression promotes cell growth and anchorage independence. Cancer Res. 2006;66:3106–13. doi: 10.1158/0008-5472.CAN-05-3702. [DOI] [PubMed] [Google Scholar]
- 32.Hull MA, Ko SC, Hawcroft G. Prostaglandin EP receptors: targets for treatment and prevention of colorectal cancer? Mol Cancer Ther. 2004;3:1031–9. [PubMed] [Google Scholar]
- 33.Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem. 2003;278:35451–7. doi: 10.1074/jbc.M302474200. [DOI] [PubMed] [Google Scholar]
- 34.Pai R, Nakamura T, Moon WS, Tarnawski AS. Prostaglandins promote colon cancer cell invasion; signaling by cross-talk between two distinct growth factor receptors. FASEB J. 2003;17:1640–7. doi: 10.1096/fj.02-1011com. [DOI] [PubMed] [Google Scholar]
- 35.Brunton VG, Ozanne BW, Paraskeva C, Frame MC. A role for epidermal growth factor receptor, c-Src and focal adhesion kinase in an in vitro model for the progression of colon cancer. Oncogene. 1997;14:283–93. doi: 10.1038/sj.onc.1200827. [DOI] [PubMed] [Google Scholar]
- 36.Gupta RA, DuBois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 37.Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol. 2004;36:1187–205. doi: 10.1016/j.biocel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Hasumoto K, Sugimoto Y, Gotoh M, Segi E, Yamasaki A, Yamaguchi M, Honda H, Hirai H, Negishi M, Kakizuka A, Ichikawa A. Characterisation of the mosue prostaglandin F receptor gene: a transgenic mouse study of a regulatory region that controls its expression in the stomach and kidney but not in the ovary. Genes Cells. 1997;2:571–80. doi: 10.1046/j.1365-2443.1997.1420340.x. [DOI] [PubMed] [Google Scholar]
- 39.Graham D, Campen D, Hui R, Spence M, Cheetham C, Levy G, Shoor S, Ray W. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet. 2005;365:475–81. doi: 10.1016/S0140-6736(05)17864-7. [DOI] [PubMed] [Google Scholar]
- 40.Chell S, Patsos HA, Qualtrough D, H-Zadeh AM, Hicks DJ, Kaidi A, Witherden IR, Williams AC, Paraskeva C. Prospects in NSAID-derived chemoprevention of colorectal cancer. Biochem Soc Trans. 2005;33:667–71. doi: 10.1042/BST0330667. [DOI] [PubMed] [Google Scholar]
- 41.Chell S, Kaidi A, Williams AC, Paraskeva C. Mediators of PGE2 synthesis and signalling downstream of COX-2 represent potential targets for the prevention/treatment of colorectal cancer. Biochim Biophys Acta. 2006;1766:104–19. doi: 10.1016/j.bbcan.2006.05.002. [DOI] [PubMed] [Google Scholar]