Abstract
BACKGROUND
Prostaglandin E2 (PGE2) has been shown to modulate angiogenesis and tumour progression via the E-series prostanoid-2 (EP2) receptor. Endometrial adenocarcinomas may be exposed to endogenous PGE2 and exogenous PGE2, present at high concentration in seminal plasma.
METHODS
This study investigated fibroblast growth factor 2 (FGF2) mRNA expression and cell signalling in response to seminal plasma or PGE2, using an endometrial adenocarcinoma (Ishikawa) cell line stably expressing the EP2 receptor (EP2 sense cells) and endometrial adenocarcinoma explants.
RESULTS
Seminal plasma and PGE2 induced a significant up-regulation of FGF2 expression in EP2 sense but not parental untransfected Ishikawa (wild-type) cells (P < 0.05). These effects were inhibited by co-treatment with EP2 receptor antagonist or inhibitors of protein kinase A, c-Src, epidermal growth factor receptor (EGFR) kinase or extracellular signal-regulated kinase (ERK) signalling. The treatment of EP2 sense cells with seminal plasma induced cAMP accumulation and phosphorylation of c-Src, EGFR kinase and ERK via the EP2 receptor. Finally, seminal plasma and PGE2 significantly increased FGF2 mRNA expression in endometrial adenocarcinoma tissue explants via the EP2 receptor (P < 0.05).
CONCLUSIONS
Seminal plasma and PGE2 can similarly activate FGF2 expression and EP2 receptor signalling in endometrial adenocarcinoma cells. These data highlight the potential for seminal plasma exposure to facilitate tumorigenesis–angiogenesis in endometrial adenocarcinomas in vivo.
Keywords: endometrial carcinoma, E-series prostanoid receptor 2, fibroblast growth factor 2, seminal plasma
Introduction
Prostaglandins (PGs) are bioactive lipids that have a wide range of physiological and pathological functions in the female reproductive tract. They are synthesized by the actions of cyclooxygenase (COX) enzymes and terminal prostanoid synthase enzymes that are specific to the prostanoids that they produce (Narumiya et al., 1999; Jabbour and Sales, 2004).
There are two major isoforms of the COX enzymes (COX-1 and COX-2), which are regulated by a range of paracrine and autocrine mechanisms. COX-1 is generally considered to be constitutively expressed, whereas COX-2 is rapidly induced by growth factors, oncogenes and other tumorigenic factors (Smalley and DuBois, 1997; Vane et al., 1998). Recent evidence shows that both isoforms are up-regulated in several cancers and play a central role in tumorigenesis, including those of the reproductive tract (Hwang et al., 1998; Jabbour et al., 2001; Kitamura et al., 2002; Sales et al., 2002a; Gupta et al., 2003; Sales and Jabbour, 2003). PGE2 exerts its effect via G-protein-coupled receptors termed E-series prostanoid receptors (EPs). Four EP subtypes have been described, termed EP1–EP4, which use alternate signalling pathways (Narumiya et al., 1999; Sales and Jabbour, 2003).
PGE2 biosynthesis and E-series prostanoid-2 (EP2) receptor expression and signalling are significantly elevated in endometrial adenocarcinoma tissues compared with normal endometrium (Jabbour et al., 2001). In a recent study, we have shown that PGE2 via the EP2 receptor can promote the expression and release of a potent pro-angiogenic factor, vascular endothelial growth factor (VEGF), from endometrial adenocarcinoma cells (Sales et al., 2004). These data suggest an autocrine/paracrine regulation of neoplastic endometrial cell function by PGE2 via the EP2 receptor.
In addition to the regulation of endometrial function by PGE2 produced endogenously by COX enzymes, endometrial tissue may also be exposed to exogenous PGE2 from seminal plasma. Seminal plasma is characterized by a very high PG content with PGE2 levels of ~70 μg/ml, at least 1000-fold higher than those measured in normal endometrium (Templeton et al., 1978; Smith et al., 1981; Lumsden et al., 1983; Bendvold et al., 1987). Emerging evidence suggests that seminal plasma constituents can travel into the endometrium and regulate gene expression (Robertson, 2005). This has prompted the suggestion that, in sexually active women, endometrial pathologies associated with aberrant prostanoid receptor expression may be enhanced following exposure to seminal plasma (Jabbour and Sales, 2004).
This study was designed to investigate the role of seminal plasma in the modulation of neoplastic endometrial cell function via the EP2 receptor using the Ishikawa endometrial adenocarcinoma cell line stably expressing the EP2 receptor (EP2 sense cells) and endometrial adenocarcinoma explants. We found that seminal plasma and PGE2 could up-regulate the expression of the potent mitogenic/pro-angiogenic gene, fibroblast growth factor 2 (FGF2) via the EP2 receptor in EP2 sense cells. This elevation in FGF2 gene expression is mediated via the EP2 receptor in a cAMP-, c-Src-, epidermal growth factor receptor (EGFR)- and extracellular signal-regulated kinase (ERK)-dependent manner. Furthermore, using endometrial adenocarcinoma tissue explants, we have shown that the EP2 receptor and FGF2 co-localize within the glandular epithelial compartment and have confirmed that seminal plasma and PGE2 can up-regulate the expression of FGF2 ex vivo in endometrial adenocarcinoma biopsy explants via the EP2 receptor.
Materials and methods
Culture medium was purchased from Invitrogen (Paisley, Scotland, UK). Fetal calf serum (FCS) and penicillin–streptomycin were obtained from PAA Laboratories (Middlesex, UK). FGF2 goat polyclonal antibody (sc-1360), EGFR rabbit polyclonal antibody (sc-03), p-tyr (PY99) agarose conjugate and myc (9E10) agarose conjugate were purchased from Autogen Bioclear (Wiltshire, UK). Anti-phospho-p44/42 ERK (9101) and anti-p44/42 ERK (9102) were obtained from Cell Signaling Technologies (New England Biolabs, Hertfordshire, UK). EP2 receptor rabbit polyclonal antibody (CAY-101750) was purchased from Axxora (Nottingham, UK). Alkaline phosphatase secondary antibody, indomethacin, phosphate-buffered saline (PBS), bovine serum albumin (BSA) and PGE2 were purchased from Sigma (Dorset, UK). The enhanced chemofluorescence (ECF) system was obtained from Amersham Biosciences (Little Chalfont, Bucks, UK). PD98059, AG1478, PP2, 4-cyano-3-methylisoquinoline (4C3MQ), Anti-c-Src (p-Tyr 418) rabbit polyclonal antibody and Protein A/G agarose were purchased from Merck Biosciences (Nottingham, UK). Doses of chemical inhibitors and dilutions of antibody were determined empirically by titration using the manufacturer’s guidelines.
Patients and tissue collection
Endometrial adenocarcinoma tissue was collected from women who were undergoing hysterectomy, and who had been prediagnosed with adenocarcinoma of the uterus. Hysterectomy specimens were collected from the operating theatre, placed on ice and opened by a gynaecological pathologist with minimal delay. Small samples of adenocarcinoma tissue (5 mm–3 cm) were placed in medium on ice and removed to the laboratory for culture. The diagnosis of adenocarcinoma was confirmed histologically by a specialist pathologist in all cases. The grading of the degree of differentiation was carried out according to the criteria defined by Federation Internationale Obstetrics et Gynaecologie (FIGO; Pecorelli et al., 1999). Pooled human seminal plasma was obtained from healthy young men involved in the ongoing semen donor programme. Seminal plasma was isolated from the pooled ejaculate by Percoll density gradient centrifugation at 500 × g for 20 min. The seminal plasma was pooled and stored at -70°C. The PGE2 concentration in the pooled seminal plasma was determined by enzyme-linked immunosorbent assay (ELISA) as described previously (Sales et al., 2002b) and found to be 43.5 ± 8.7 μg/ml. The seminal plasma was used on the cells at a 1:250 dilution. At this dilution, the PGE2 present in the pooled ejaculate corresponds comparably with the concentration of PGE2 used in this study (100 nM). At this dilution, seminal plasma has been reported to exert no adverse effect on HeLa cell viability (Jeremias et al., 1997). Ethical approval was obtained from Lothian Research Ethics Committee, and written informed consent was obtained from all subjects before tissue collection.
Cell culture
Human Ishikawa endometrial adenocarcinoma cells (European Collection of Cell Culture, Centre for Applied Microbiology, Wiltshire, UK) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) nutrient mixture F-12 with glutamax-1 and pyridoxine supplemented with 10% FCS and 1% antibiotics (stock 500 IU penicillin and 500 μg/ml streptomycin) at 37°C and 5% CO2 (v/v). Stable EP2 transfectant cells (EP2 sense cells) were maintained under the same conditions with the addition of a maintenance dose of 200 μg/ml G418.
EP2 receptor cell characterization
EP2 receptor amplification, transfection into Ishikawa cells and characterization of the EP2 sense cell line used in this study have been described previously (Sales et al., 2004). The EP2 sense cell line was found to express a 4.2 ± 1.8-fold greater level of EP2 receptor than parental untransfected Ishikawa cells (wild-type). This level of EP2 receptor expression in EP2 sense cells is comparable with the levels of EP2 receptor observed in endometrial adenocarcinoma biopsy tissues (Sales et al., 2004).
Taqman quantitative RT–PCR
FGF mRNA expression in EP2 sense and wild-type Ishikawa cells or endometrial adenocarcinomas was measured by quantitative RT–PCR analysis. For expression in EP2 sense and wild-type Ishikawa cells, 2.5 × 105 cells were seeded in six-well plates and allowed to attach and grow for 24 h. Cells were subsequently serum-starved overnight in medium containing 8.4 μM indomethacin. Medium was removed and replaced with DMEM (in the presence of 10% FCS and 8.4 μM indomethacin) containing vehicle, a 1:250 dilution of seminal plasma or 100 nM PGE2, and cells were incubated for 2, 4, 6 and 8 h at 37°C. In subsequent experiments, a 6-h time point was chosen, and EP2 sense cells were incubated with seminal plasma or PGE2 following pretreatment with inhibitors of protein kinase A (PKA; 4C3MQ, 1 μM), c-Src (PP2, 10 μM), EGFR kinase (AG1478, 100 nM) or mitogen-activated protein kinase kinase (MEK; PD98059, 50 μM) for 30 min or the EP2 receptor antagonist AH6809 (10 μM) for 2 min, and RNA was extracted from cells using Total RNA Isolation Reagent (TRIR; Abgene, Epsom, UK) according to the manufacturer’s instructions.
To investigate the effect of seminal plasma on FGF2 expression in intact endometrial adenocarcinoma tissue, biopsy explants (n = 6; two well-differentiated and four moderately differentiated adenocarcinomas) were finely minced and serum-starved overnight at 37°C in the presence of 8.4 μM indomethacin. Samples were divided into four portions and pre-incubated for 30 min in vehicle (two portions) or PD98059, or 2 min in AH6809. Tissue was subsequently treated for 6 h with vehicle, a 1:250 dilution of seminal plasma or 100 nM PGE2. Tissue was homogenized in 1 ml of TRIR, and RNA was extracted according to the manufacturer’s protocol. Quantified RNA samples were reverse transcribed, and quantitative RT–PCR was performed as described previously (Sales et al., 2004). The sequence of the FGF2-specific primers and probe are as follows: forward: 5′-CCGACGGCCGCGTTGAC-3′; reverse: 5′-GACACAACTCCTCTCTCTT-3′ and probe: 5′-FAM-AGAAGAGCGACCCTCACATAMRA-3′. The ribosomal 18S primers and probe sequences are as follows: forward: 5′-CGGCTACCACATCCAAGGAA-3′; reverse: 5′-GCTGGAATTACCGCGGCT-3′ and probe (VIC labelled): 5′-TGCTGGCACCAGACTTGCCCTC-3′ (Sales et al., 2004; Milling Smith et al., 2006). The expression of FGF2 was normalized for RNA loading for each sample using the 18S rRNA as an internal standard. Results are expressed relative to a sample of cDNA from normal endometrium included in every PCR as an internal standard. Fold increase in FGF2 expression was determined by dividing the relative expression of FGF2 in seminal plasma or PGE2-treated samples by the level of expression in vehicle-treated samples at the same time points.
Cyclic AMP assay
Cyclic AMP accumulation was determined in response to treatment with seminal plasma at a dilution of 1:250 in the absence or presence of inhibitors of cell signalling. EP2 sense cells (2.5 × 105) were plated in six-well plates and allowed to attach overnight. The following day the cells were starved by overnight treatment with serum-free medium containing 8.4 μM indomethacin, and thereafter, the cells were incubated for 1 h at 37°C in serum-free medium containing 1 mM 3-isobutyl-1-methylxanthine (Sigma) and 8.4 μM indomethacin in the presence or absence of inhibitors of PKA (4C3MQ, 1 μM), c-Src (PP2, 10 μM), EGFR kinase (AG1478, 100 nM) or MEK (PD98059, 50 μM) or the EP2 receptor antagonist AH6809 (10 μM) for 2 min. Cells were then stimulated with a 1:250 dilution of seminal plasma for the time indicated in the legend to figure 2A or left unstimulated. Following stimulation, cells were lysed in 0.1 M HCl, and cAMP concentration was determined by ELISA using a cAMP kit (Biomol, Affiniti, Exeter, UK) according to the manufacturer’s protocol. Concentrations were normalized to the protein concentration of the lysate measured by the method of Lowry (Bio-Rad, Hemel Hempstead, UK).
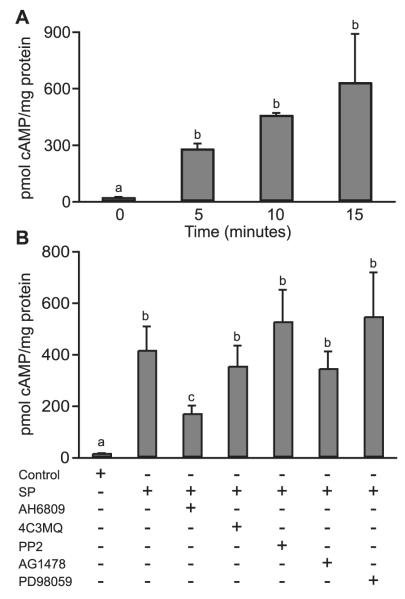
Figure 2.
cAMP accumulation in response to seminal plasma. (A) E-Series prostanoid-2 (EP2) sense cells were treated with a 1:250 dilution of seminal plasma for 5, 10 and 15 min or left untreated (0 min). b is significantly different from a (P < 0.01). (B) EP2 sense cells were treated with seminal plasma for 15 min in the absence/presence of the EP2 receptor antagonist (AH6809) or chemical inhibitors of protein kinase A (4C3MQ), c-Src (PP2), EGFR kinase (AG1478) or MEK (PD98059). b is significantly different from a (P < 0.05); c is significantly different from a and b (P < 0.05). All data are shown as mean ± SEM from three independent experiments.
Phosphorylated ERK, c-Src and EGFR studies
For studies of ERK phosphorylation, 2.5 × 105 cells were seeded in six-well plates, and for c-Src and pEGFR studies, 3 × 106 cells were seeded in 10-cm dishes. On the following day, the cells were washed with PBS and incubated in serum-free culture medium containing penicillin/streptomycin and 8.4 μM indomethacin for at least 16 h. Cells were then pretreated with specific chemical inhibitors of PKA (4C3MQ, 1 μM), c-Src (PP2, 10 μM), EGFR kinase (AG1478, 100 nM) or MEK (PD98059, 50 μM) for 1 h before stimulation with seminal plasma at a dilution of 1:250. The EP2 receptor antagonist (AH6809, 10 μM) was added simultaneously with the seminal plasma treatment. After stimulation with seminal plasma, cells were washed with ice-cold PBS and proteins were extracted with protein lysis buffer [150 mM NaCl, 50 mM Tris–HCl (pH 7.4), 10 mM EDTA, 0.6% nonidet-P40 and 10% glycerol containing protease inhibitors]. Insoluble material was pelleted by centrifugation at 19 000 × g for 20 min at 4°C. The clarified lysate was removed to a new tube, and protein content was quantified as described above.
Transient cell transfections and immunoprecipitation
The role of c-Src, EGFR and ERK in seminal plasma-mediated cell signalling was investigated further by the use of dominant negative (DN) isoforms targeted against MEK, EGFR and c-Src. The DN-MEK and DN-EGFR cDNA constructs were provided by Professor Zvi Naor (Department of Biochemistry, University of Tel Aviv, Israel). The DN-MEK was generated as described previously (Seger et al., 1994; Jaaro et al., 1997) by mutation of lysine 97 to alanine to yield a catalytically inactive enzyme. The myc-tagged ERK mitogen-activated protein kinase construct and DN-c-Src construct was obtained from Professor Robert Millar (MRC Human Reproductive Sciences Unit, Edinburgh, UK). DN-SRC was generated by mutating lysine 295 to methionine to generate a kinase-dead enzyme as described previously (Davidson et al., 2004). The DN-EGFR construct was produced by mutating lysine 721 to alanine as described previously (Benard et al., 2001). EP2 sense cells seeded overnight in 6-cm dishes (7.5 × 106 per dish) were exposed to 2.5 μg of a myc-tagged ERK and 2.5 μg of DN-SRC, DN-EGFR, DN-MEK or control plasmid (pcDNA3; Invitrogen) in the presence of 30 μl of SuperFect (Qiagen, Crawley, UK) in a total volume of 1.2 ml of medium for 3 h and then cultured overnight in complete medium. Thereafter, cells were incubated overnight in serum-free medium containing 8.4 μM indomethacin and subsequently treated with vehicle, a 1:250 dilution of seminal plasma or seminal plasma in the presence of AH6809 for 2 min. Cells were lysed as described above, and myc-tagged ERK or phospho-tyrosine (p-Tyr) were isolated by immunoprecipitation. Briefly, equal amounts of protein were incubated with myc-antibody or p-Tyr antibody conjugated to protein A agarose beads overnight at 4°C with gentle rotation. Beads were washed extensively with lysis buffer, and immune complexes were then solubilized in Laemmli buffer (125 mM Tris–HCl pH 6.8, 4% sodium dodecyl sulphate (SDS), 5% 2-mercaptoethanol, 20% glycerol and 0.05% bromophenol blue) then boiled for 5 min. Thereafter, solutes were subjected to Western blot analysis as described below.
Western blot analysis
For ERK studies, 20 μg of protein was loaded for each sample. Proteins were resolved on 4–12% Bis-Tris gels (Nupage, Invitrogen) and transferred onto polyvinylidene difluoride membrane (Millipore, Watford, UK). Membranes were blocked for 1 h at 25°C in 4% BSA diluted in TBST (50 mM Tris–HCl, 150 mM NaCl and 0.05% v/v Tween-20) and incubated overnight at 4°C with anti-phospho-p44/42 ERK, anti-p44/42 ERK, c-Src or EGFR primary antibodies at a dilution of 1:1000. Thereafter, blots were washed and incubated with alkaline phosphatase-conjugated secondary antibodies at a dilution of 1:40 000. Immunore-active proteins were visualized by the ECF system according to the manufacturer’s instructions. Specific proteins were revealed, quantified and normalized to total protein expression using Typhoon 9400 PhosphorImager and ImageQuant TL software (Molecular Dynamics, Amersham Biosciences). Fold increase in protein expression was determined by dividing the relative level of phosphorylated protein following treatment by the level in vehicle-treated controls. Additionally, phosphorylated cSrc and EGFR blots were normalized against light-chain immunoglobulin G (IgG) on the same blots.
Immunohistochemistry and confocal laser microscopy
EP2 receptor and FGF2 protein expression were co-localized in endometrial adenocarcinomas (n = 12; four moderate, four well-differentiated and four poorly differentiated adenocarcinomas) by dual immunofluorescence immunohistochemistry. Tissue sections were prepared as described previously and blocked using 5% normal horse serum diluted in PBS. Optimal dilution of antibody was determined by titration on serial sections. Subsequently, sections were incubated with goat anti-FGF2 antibody at a dilution of 1:80 for 18 h at 4°C. Control sections were incubated with equivalent concentration of normal goat IgG. Thereafter, sections were washed with PBS and incubated with biotinylated horse anti-goat (DAKO; Dako Corp., High Wycombe, UK) followed by incubation with the fluorochrome streptavidin 488 Alexafluor (Molecular Probes, Eugene, OR, USA) diluted 1:200 in PBS. Sections were re-blocked with 5% normal goat serum diluted in PBS and incubated with rabbit anti-EP2 receptor antibody at a dilution of 1:100 at 4°C for 18 h. Control sections were incubated with equivalent concentration of rabbit IgG. Thereafter, the sections were washed in PBS and incubated with the fluorochrome streptavidin 546 Alexafluor (Molecular Probes) diluted 1 in 200 in PBS at 25°C for 20 min. Sections were counterstained with 1:2000 dilution of To-Pro2 (Molecular Probes) and mounted in permafluor (Immunotech-Coulter), coverslipped, visualized and photographed as described previously (Sales et al., 2005).
Statistics
Data were subjected to statistical analysis with analysis of variance (ANOVA) and Fisher’s protected least significant difference tests (Statview 4.0; Abacus Concepts, Piscataway, NJ, USA) and statistical significance accepted when P < 0.05.
Results
Seminal plasma and PGE2 induce FGF2 expression via activation of EP2 receptor signalling
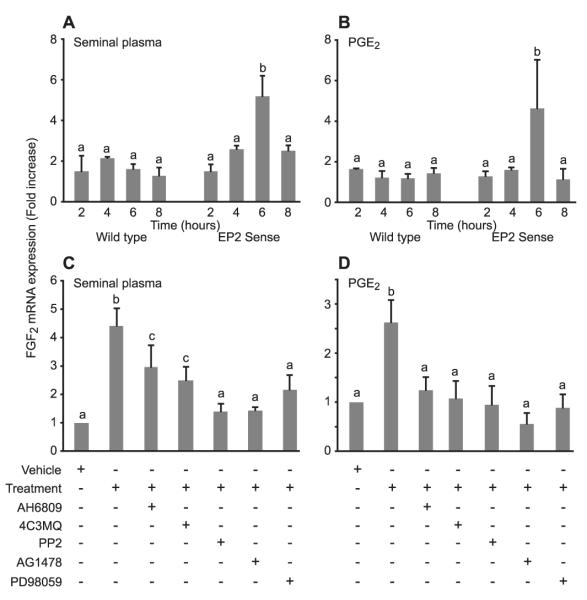
Wild-type Ishikawa cells and EP2 sense cells were treated with seminal plasma (Figure 1A) or 100 nM PGE2 (Figure 1B) for 2, 4, 6 and 8 h. A significant increase in FGF2 mRNA expression was observed in EP2 sense cells after 6 h of treatment with seminal plasma or PGE2 compared with vehicle-treated cells (5.2 ± 0.9- and 4.6 ± 2.3-fold increase for seminal plasma and PGE2, respectively; P < 0.005 and P < 0.05; data are expressed as mean ± SEM). However, no such increase in FGF2 expression was observed in wild-type cells treated with seminal plasma (Figure 1A) or PGE2 (Figure 1B).
Figure 1.
Fibroblast growth factor 2 (FGF2) expression in response to seminal plasma and prostaglandin E2 (PGE2). FGF2 mRNA expression in wild-type and E-series prostanoid-2 (EP2) sense cells measured by real-time RT–PCR after the treatment of cells for 2, 4, 6 and 8 h with a 1:250 dilution of (A) seminal plasma (SP; b is significantly different from a, P < 0.005) or (B) 100 nM PGE2; b is significantly different from a (P < 0.05). FGF2 mRNA expression in EP2 sense cells treated for 6 h with (C) SP or (D) PGE2 in the absence or presence of the EP2 receptor antagonist (AH6809) or chemical inhibitors of protein kinase A (4C3MQ), c-Src (PP2), EGFR kinase (AG1478) or mitogen-activated protein kinase (MEK) (PD98059). b and c are significantly different from a (P < 0.05); c is significantly different from b (P < 0.05). Data are shown as mean ± SEM from three independent experiments.
Because FGF2 expression was unaltered by the treatment of wild-type Ishikawa cells with either seminal plasma or PGE2 within the 8-h time frame, subsequent experiments were performed using EP2 sense cells alone. The co-treatment of EP2 sense cells with AH6809, 4C3MQ, PP2, AG1478 or PD98059 significantly reduced the expression of FGF2 induced by seminal plasma (Figure 1C; P < 0.05) or PGE2 (Figure 1D; P < 0.05).
Seminal plasma promotes cAMP accumulation via the EP2 receptor
The incubation of EP2 sense cells with seminal plasma for 5, 10 or 15 min induced a significant time-dependent increase in cAMP accumulation compared with untreated cells (Figure 2A; 269.6± 30.7, 448.3± 14.2 and 621.4± 261.1 pmoles cAMP/mg protein for 5, 10 and 15 min, respectively compared with 11.7 + 5.4 pmoles cAMP/mg protein for untreated cells at 0 min; P < 0.01). cAMP levels were significantly reduced in the presence of the EP2 receptor antagonist (Figure 2B; P < 0.05). There was no significant difference in cAMP accumulation in EP2 sense cells in response to seminal plasma in the presence of 4C3MQ, PP2, AG1478 or PD98059 (Figure 2B).
Seminal plasma induces activation of ERK phosphorylation via the EP2 receptor
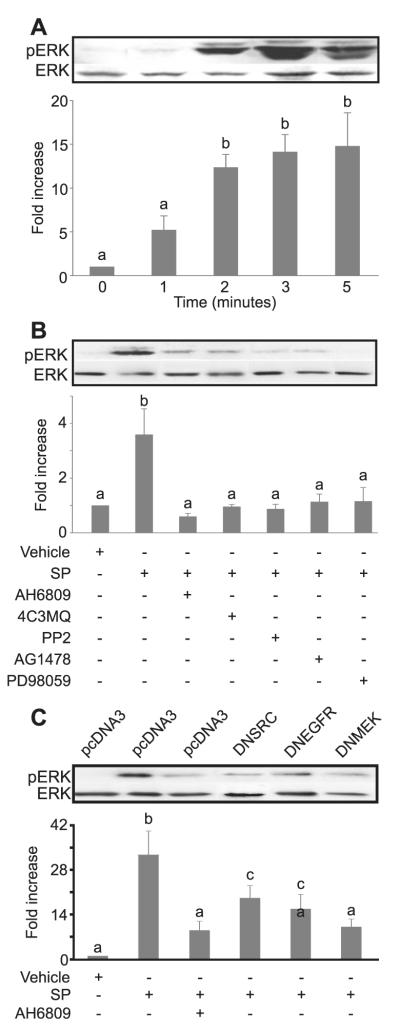
Following serum starvation of EP2 sense cells and in the absence of ligand or growth factors, minimal basal levels of ERK phosphorylation were observed (Figure 3A, time zero).
Figure 3.
Extracellular signal-regulated kinase (ERK) phosphorylation in E-series prostanoid-2 (EP2) sense cells in response to seminal plasma. (A) ERK phosphorylation in EP2 sense cells treated with a 1: 250 dilution of seminal plasma for 0–3 and 5 min. (B) ERK phosphorylation in EP2 sense cells treated with seminal plasma for 2 min in the absence/presence of the EP2 receptor antagonist (AH6809) or chemical inhibitors of protein kinase A (4C3MQ), c-Src (PP2), EGFR kinase (AG1478) or MEK (PD98059). b is significantly different from a (P < 0.05). (C) ERK phosphorylation in EP2 sense cells co-transfected with a myc-tagged ERK cDNA construct together with a dominant negative cDNA isoform targeted against c-Src (lane 4), the EGFR (lane 5), MEK (lane 6) or empty vector (lanes 1–3) and stimulated with seminal plasma for 2 min either alone (lanes 2, 4–6) or in the presence of AH6809 (lane 3). The tagged ERK construct was immunoprecipitated with an anti-myc-antibody and immunoblotted. ERK phosphorylation levels are shown as mean ± SEM from three independent experiments. b and c are significantly different from a (P < 0.05); c is significantly different from b (P < 0.05). Data are presented as representative blots together with semi-quantitative analysis of ERK phosphorylation shown as mean ± SEM from three independent experiments. b and c are significantly different from a (P < 0.05); c is significantly different from b (P < 0.05).
The treatment of EP2 sense cells with the panel of chemical inhibitors on their own showed no significant alteration to the basal levels of ERK phosphorylation observed following serum starvation at time zero (data not shown); however, the co-treatment of EP2 sense cells with seminal plasma and the chemical inhibitors significantly inhibited ERK phosphorylation in response to treatment with seminal plasma for 2 min (Figure 3B; P < 0.05).
The treatment of empty vector-transfected EP2 sense cells with seminal plasma for 2 min resulted in a significant phosphorylation of ERK compared with vehicle-treated cells (Figure 3C). This seminal plasma-induced elevation in ERK phosphorylation was inhibited by the co-treatment of empty vector-transfected EP2 sense cells with AH6809 or by the co-transfection of cells with DN-SRC, DN-EGFR or DN-MEK cDNAs (Figure 3C; P < 0.05).
Seminal plasma induces phosphorylation of c-Src and EGFR via the EP2 receptor
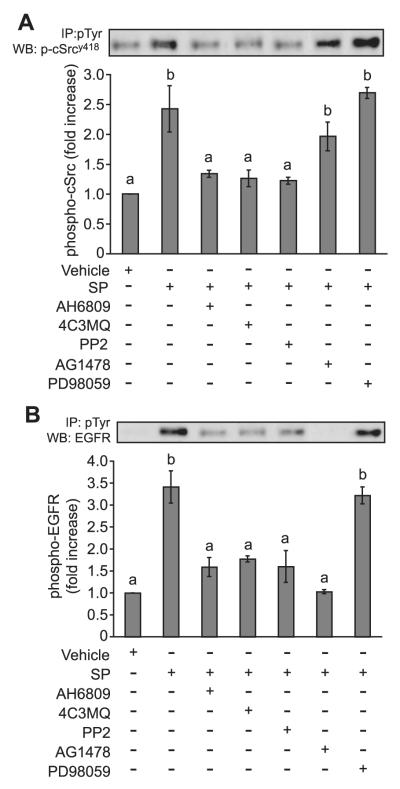
The phosphorylation of c-Src (Figure 4A) and EGFR (Figure 4B) was significantly increased following treatment of EP2 sense cells with seminal plasma (P < 0.05). This seminal plasma-induced phosphorylation of c-Src and EGFR was abolished by the co-incubation of EP2 sense cells with AH6809, 4C3MQ and PP2. However, the co-incubation of EP2 sense cells with AG1478 reduced the seminal plasma-induced phosphorylation of EGFR but not c-Src, suggesting that c-Src phosphorylation is upstream of EGFR phosphorylation. These data are in agreement with our previous observations for PGE2 signalling via the EP2 receptor (Sales et al., 2004) and demonstrate that seminal plasma activates EP2 receptor signalling in a similar manner to that proposed for endogenously secreted PGE2.
Figure 4.
Phosphorylation of c-Src and epidermal growth factor receptor (EGFR). (A) E-Series prostanoid-2 (EP2) sense cells were treated with a 1:250 dilution of seminal plasma for 2 min in the absence/presence of the EP2 receptor antagonist (AH6809) or chemical inhibitors of protein kinase A (4C3MQ), c-Src (PP2), EGFR kinase (AG1478) or mitogen-activated protein kinase kinase (MEK) (PD98059). Cell lysates were immunoprecipitated with an anti-phosphotyrosine antibody and immunoblotted for phosphorylated c-Src. (B) Immunoblots were stripped and reprobed for EGFR phosphorylation. The figure shows a representative blot together with semi-quantitative analysis of c-Src or EGFR phosphorylation shown as mean ± SEM from three independent experiments; b is significantly different from a (P < 0.05).
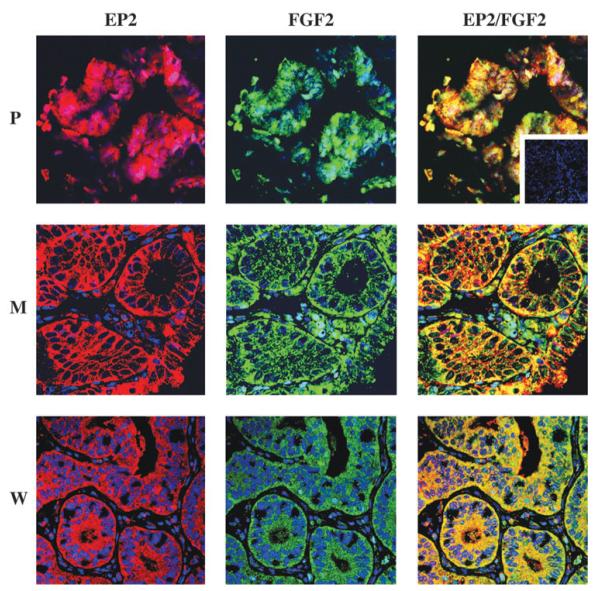
EP2 receptor and FGF2 co-localize in endometrial adenocarcinoma
Laser confocal immunofluorescence microscopy showed the EP2 receptor (red channel) and FGF2 (green channel) were co-localized (merged, yellow) in the neoplastic epithelial cells of poorly, moderately and well-differentiated endometrial adenocarcinoma tissues, with minimal stromal cell immunoreactivity observed (Figure 5). The substitution of the primary antibody with a control IgG from the host species resulted in the loss of the specific immunostaining.
Figure 5.
Confocal immunofluorescent localization of E-series prostanoid-2 (EP2) receptor and fibroblast growth factor 2 (FGF2) in endometrial adenocarcinoma. Localization of the site of expression of the EP2 receptor (red; left panel), FGF2 (green; central panel) and co-localization of EP2 receptor and FGF2 (merged yellow; right panel) in human endometrial adenocarcinoma. P, poorly differentiated; M, moderately differentiated and W, well-differentiated adenocarcinomas; inset, negative control section.
Seminal plasma and PGE2 induce FGF2 expression in endometrial adenocarcinoma explants
Seminal plasma and PGE2 treatment induced a 3.1 ± 0.4- and 2.2 ± 0.2-fold increase in FGF2 mRNA expression, respectively (P < 0.05). The pretreatment of tissue explants with AH6809 significantly reduced the seminal plasma and PGE2-induced elevation in FGF2 (Figure 6; P < 0.05).
Figure 6.
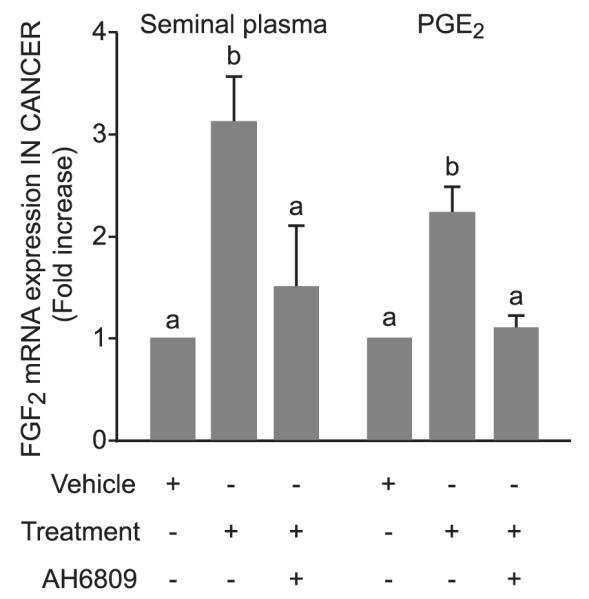
Effect of seminal plasma and prostaglandin E2 (PGE2) on fibroblast growth factor 2 (FGF2) mRNA expression in endometrial adenocarcinoma. Real-time RT–PCR analysis of FGF2 mRNA expression in endometrial adenocarcinoma tissue treated with a 1:250 dilution of seminal plasma (lane 2) or 100 nM PGE2 (lane 5) alone or in the presence of the E-series prostanoid-2 receptor antagonist (AH6809; lanes 3 and 6 for seminal plasma and PGE2, respectively). Data are shown as mean ± SEM from three independent experiments; b is significantly different from a (P < 0.05).
Discussion
COX enzymes, prostanoids and their receptors are now established as major factors involved in reproductive tract pathology (Jabbour et al., 2001; Sales and Jabbour, 2003; Jabbour and Sales, 2004). COX-1 and COX-2, together with the EP2 receptor, are up-regulated in reproductive tract carcinomas, including carcinoma of the endometrium (Tong et al., 2000; Jabbour et al., 2001; Ferrandina et al., 2002) and have been shown to up-regulate the expression of tumorigenic and angiogenic genes such as Ang-1 and Ang-2 (Tsujii et al., 1998; Sales et al., 2002a) and VEGF (Gupta et al., 2003; Sales et al., 2004) and down-regulate the production of anti-angiogenic genes (Perchick and Jabbour, 2003). Moreover, PGs have been shown to up-regulate COX-2 expression and PG biosynthesis, thereby establishing a positive feedback loop to amplify the angiogenic and tumorigenic signal (Tjandrawinata et al., 1997; Jabbour et al., 2005; Sales et al., 2005).
The EP2 receptor is a Gαs-coupled seven-transmembrane receptor, which largely initiates intracellular signalling via the cAMP-dependent PKA second messenger system (Regan, 2003). In endometrial adenocarcinomas, EP2 receptor signalling to cAMP is elevated compared with normal tissue (Jabbour et al., 2001), indicating a possible autocrine/paracrine regulation of neoplastic endometrial cell function via the PGE2–EP2–PKA axis. Indeed, utilizing the endometrial adenocarcinoma cell model system overexpressing the EP2 receptor to the levels observed in endometrial adenocarcinomas (EP2 sense cells), we have previously ascertained a role for PGE2-mediated activation of EP2 receptor signalling in the promotion of angiogenic factor release via the cAMP-, c-Src- and EGFR-mediated phosphorylation of ERK (Sales et al., 2004).
It is well established that PGE2 is present at ~10 000-fold higher concentration in seminal plasma than that produced locally at the site of inflammation, and at least 1000-fold higher than normal endometrium (Templeton et al., 1978; Smith et al., 1981; Lumsden et al., 1983; Bendvold et al., 1987). The reported concentration in the seminal plasma of humans for PGE2 and 19-R hydroxy PGE2 (a selective agonist for EP2 receptor) are 70 μg/ml and 250 μg/ml, respectively (Taylor and Kelly, 1975; Templeton et al., 1978; Bendvold et al., 1987), ~20-fold greater than that of PGF2α (Bendvold et al., 1987). Although it has been previously thought that the cervix may provide a barrier to the passage of seminal plasma into the uterine cavity, there is now increasing evidence that the effects of seminal plasma extend to the uterus, with in vivo studies demonstrating that active seminal plasma constituents are carried, together with sperm, into the higher tract facilitated by rapid and sustained peristaltic contractions of the uterus (Robertson, 2005).
In this study, we have demonstrated for the first time that seminal plasma can activate intracellular signalling in endometrial adenocarcinoma cells via the EP2 receptor. The data reported herein demonstrate that the seminal plasma can activate the c-Src, EGFR and ERK pathways in endometrial adenocarcinoma cells (EP2 sense), in a similar manner to PGE2 via the EP2 receptor (Sales et al., 2004), because c-Src, EGFR and ERK phosphorylation in EP2 sense cells were inhibited by an EP2 receptor antagonist (AH6809). Although the EP2 receptor antagonist AH6809 was previously reported to be an antagonist of the EP1 receptor, a study by Woodward et al. (1995) has shown that AH6809 acts as a potent antagonist of the EP2 receptor with substantial competition for PGE2 at concentrations up to 10 μM (Woodward et al., 1995), and PGF2α at >100 μM (Abramovitz et al., 2000). We failed to detect any EP1 receptor and found no difference in the levels of EP3, EP4 or FP receptors in Ishikawa wild-type and EP2 sense cells (data not shown) and thus interpret the actions of AH6809 in this study as being an antagonist of the EP2 receptor.
ERK is a key signalling mechanism involved in the control of gene transcription and regulates many processes such as cellular transformation and growth. Our previous data (Sales et al., 2004) demonstrate that the PGE2-mediated activation of ERK via the EP2 receptor occurs via the cAMP- and c-Src-mediated transactivation of the EGFR. In the earlier study, we showed that a cell-permeable cAMP analogue, dibutryl cAMP, could transphosphorylate the EGFR and phosphorylate ERK in a similar manner to PGE2 via the EP2 receptor in EP2 sense cells; however, the ability of EGF to phosphorylate the EGFR and ERK was independent of cAMP and c-Src (Sales et al., 2004). In this study, we show that seminal plasma can also transphosphorylate the EGFR and phosphorylate ERK in a similar manner to PGE2, via the EP2 receptor. From these data and our previous observations of cell signalling in the EP2 sense cell line, it would appear that the phosphorylation of c-Src precedes that of EGFR, because both chemical inhibitors of c-Src and EGFR kinase inhibited EGFR phosphorylation; however, only the inhibitor of c-Src inhibited c-Src phosphorylation. As the inhibitor of MEK failed to inhibit either c-Src or EGFR phosphorylation, this implies that c-Src and EGFR phosphorylation precedes that of ERK phosphorylation. These data are consistent with evidence from several studies that support the sequence of G-protein-coupled receptor signalling to downstream ERK via the c-Src-mediated transactivation of the EGFR (Luttrell et al., 1999; Pierce et al., 2001; Pai et al., 2002; Buchanan et al., 2003; Sales et al., 2004; Sales et al., 2005). These observations indicate that this pathway can be activated by endogenously synthesized PGs, and following exposure to exogenous prostanoids such as those present in seminal plasma.
FGF2 is a potent mitogenic and angiogenic factor, causing endothelial cell proliferation and migration, extracellular matrix degradation and modulation of adhesion factors (Presta et al., 2005). FGF2 expression is elevated in endometrial carcinoma, and it has been suggested to promote the vascularization and invasiveness of carcinomas (Yoshida et al., 2002; Billottet et al., 2004). Moreover, FGF2 overexpression in endomentrial adenocarcinoma cells can promote tumour growth when implanted s.c. in nude mice (Giavazzi et al., 2003). This study has demonstrated that seminal plasma and PGE2 can up-regulate the expression of FGF2 mRNA in EP2 sense cells, but not wild-type Ishikawa cells, and endometrial adenocarcinoma tissues. Furthermore, we have shown that this seminal plasma- and PGE2-mediated expression of FGF2 occurs via the EP2 receptor following the sequential activation of cAMP-dependent PKA, c-Src, EGFR kinase and ERK signalling.
Thus, pathologies of the endometrium, associated with an elevation in the expression of prostanoid receptors, may be exacerbated by exposure to seminal plasma. This study has demonstrated a mechanism whereby seminal plasma may promote endometrial carcinoma in sexually active women via up-regulation of gene expression of potent growth factors such as FGF2. The data presented herein also highlight the potential advantage of combining COX enzyme inhibitors together with EP2 receptor antagonists or EGFR or ERK signalling pathway inhibitors as therapeutic agents in endometrial adenocarcinoma. These agents would target the actions of both endogenously produced PGs and the exogenous prostanoids present in seminal plasma.
Acknowledgements
The authors thank Ms Joan Creiger for patient recruitment and sample collection.
References
- Abramovitz M, Adam M, Boie Y, Carriere M, Denis D, Godbout C, Lamontagne S, Rochette C, Sawyer N, Tremblay NM, et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483:285–293. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Benard O, Naor Z, Seger R. Role of dynamin Src, and Ras in the protein kinase C-mediated activation of ERK by gonadotropin-releasing hormone. J Biol Chem. 2001;276:4554–4563. doi: 10.1074/jbc.M006995200. [DOI] [PubMed] [Google Scholar]
- Bendvold E, Gottlieb C, Svanborg K, Bygdeman M, Eneroth P. Concentration of prostaglandins in seminal fluid of fertile men. Int J Androl. 1987;10:463–469. doi: 10.1111/j.1365-2605.1987.tb00220.x. [DOI] [PubMed] [Google Scholar]
- Billottet C, Elkhatib N, Thiery JP, Jouanneau J. Targets of fibroblast growth factor 1 (FGF-1) and FGF-2 signaling involved in the invasive and tumourigenic behavior of carcinoma cells. Mol Biol Cell. 2004;15:4725–4734. doi: 10.1091/mbc.E04-04-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem. 2003;278:35451–35457. doi: 10.1074/jbc.M302474200. [DOI] [PubMed] [Google Scholar]
- Davidson L, Pawson AJ, De Maturana RL, Freestone SH, Barran P, Millar RP, Maudsley S. Gonadotropin-releasing hormone-induced activation of diacylglycerol kinase–zeta and its association with active c-src. J Biol Chem. 2004;279:11906–11916. doi: 10.1074/jbc.M310784200. [DOI] [PubMed] [Google Scholar]
- Ferrandina G, Legge F, Ranelletti FO, Zannoni GF, Maggiano N, Evangelisti A, Mancuso S, Scambia G, Lauriola L. Cyclooxygenase-2 expression in endometrial carcinoma: correlation with clinicopathologic parameters and clinical outcome. Cancer. 2002;95:801–807. doi: 10.1002/cncr.10736. [DOI] [PubMed] [Google Scholar]
- Giavazzi R, Sennino B, Coltrini D, Garofalo A, Dossi R, Ronca R, Tosatti MP, Presta M. Distinct role of fibroblast growth factor-2 and vascular endothelial growth factor on tumour growth and angiogenesis. Am J Pathol. 2003;162:1913–1926. doi: 10.1016/S0002-9440(10)64325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RA, Tejada LV, Tong BJ, Das SK, Morrow J, Dey SK, DuBois RN. Cyclooxygenase-1 is overexpressed and promotes angiogenic growth factor production in ovarian cancer. Cancer Res. 2003;63:906–911. [PubMed] [Google Scholar]
- Hwang D, Scollard D, Byrne J, Levine E. Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst. 1998;90:455–460. doi: 10.1093/jnci/90.6.455. [DOI] [PubMed] [Google Scholar]
- Jaaro H, Rubinfeld H, Hanoch T, Seger R. Nuclear translocation of mitogen-activated protein kinase kinase (MEK1) in response to mitogenic stimulation. Proc Natl Acad Sci USA. 1997;94:3742–3747. doi: 10.1073/pnas.94.8.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour HN, Sales KJ. Prostaglandin receptor signalling and function in human endometrial pathology. Trends Endocrinol Metab. 2004;15:398–404. doi: 10.1016/j.tem.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Jabbour HN, Milne SA, Williams AR, Anderson RA, Boddy SC. Expression of COX-2 and PGE synthase and synthesis of PGE2 in endometrial adenocarcinoma: a possible autocrine/paracrine regulation of neoplastic cell function via EP2/EP4 receptors. Br J Cancer. 2001;85:1023–1031. doi: 10.1054/bjoc.2001.2033. [DOI] [PubMed] [Google Scholar]
- Jabbour HN, Sales KJ, Boddy SC, Anderson RA, Williams AR. A positive feedback loop that regulates cyclooxygenase-2 expression and prostaglandin F2α synthesis via the F-series-prostanoid receptor and extra-cellular signal-regulated kinase 1/2 signaling pathway. Endocrinology. 2005;146:4657–4664. doi: 10.1210/en.2005-0804. [DOI] [PubMed] [Google Scholar]
- Jeremias J, David SS, Toth M, Witkin SS. Induction of messenger RNA for the 70 kDa heat shock protein in HeLa cells and the human endocervix following exposure to semen: implications for antisperm antibody production and susceptibility to sexually transmitted infections. Hum Reprod. 1997;12:1915–1919. doi: 10.1093/humrep/12.9.1915. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Kawamori T, Uchiya N, Itoh M, Noda T, Matsuura M, Sugimura T, Wakabayashi K. Inhibitory effects of mofezolac, a cyclooxygenase-1 selective inhibitor, on intestinal carcinogenesis. Carcinogenesis. 2002;23:1463–1466. doi: 10.1093/carcin/23.9.1463. [DOI] [PubMed] [Google Scholar]
- Lumsden MA, Kelly RW, Baird DT. Primary dysmenorrhoea: the importance of both prostaglandins E2 and F2α. Br J Obstet Gynaecol. 1983;90:1135–1140. doi: 10.1111/j.1471-0528.1983.tb06460.x. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol. 1999;11:177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- Smith OP Milling, Battersby S, Sales KJ, Critchley HO, Jabbour HN. Prostacyclin receptor up-regulates the expression of angiogenic genes in human endometrium via cross talk with epidermal growth factor receptor and the extracellular signaling receptor kinase 1/2 pathway. Endocrinology. 2006;147:1697–1705. doi: 10.1210/en.2005-1073. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8:289–293. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- Pecorelli S, Benedet JL, Creasman WT, Shepherd JH. FIGO staging of gynaecologic cancer. FIGO committee on Gynecologic Oncology. International Federation of Gynecology and Obstetrics. Int J Gynaecol Obstet. 1999;65:5–10. doi: 10.1016/s0020-7292(98)00234-3. [DOI] [PubMed] [Google Scholar]
- Perchick GB, Jabbour HN. Cyclooxygenase-2 overexpression inhibits cathepsin D-mediated cleavage of plasminogen to the potent antiangiogenic factor angiostatin. Endocrinology. 2003;144:5322–5328. doi: 10.1210/en.2003-0986. [DOI] [PubMed] [Google Scholar]
- Pierce KL, Luttrell LM, Lefkowitz RJ. New mechanisms in heptahelical receptor signaling to mitogen activated protein kinase cascades. Oncogene. 2001;20:1532–1539. doi: 10.1038/sj.onc.1204184. [DOI] [PubMed] [Google Scholar]
- Presta M, Dell’Era P, Mitola S, Moroni E, Ronca R, Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003;74:143–153. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Robertson SA. Seminal plasma and male factor signalling in the female reproductive tract. Cell Tissue Res. 2005;322:43–52. doi: 10.1007/s00441-005-1127-3. [DOI] [PubMed] [Google Scholar]
- Sales KJ, Jabbour HN. Cyclooxygenase enzymes and prostaglandins in reproductive tract physiology and pathology. Prostaglandins Other Lipid Mediat. 2003;71:97–117. doi: 10.1016/s1098-8823(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Sales KJ, Katz AA, Howard B, Soeters RP, Millar RP, Jabbour HN. Cyclooxygenase-1 is up-regulated in cervical carcinomas: autocrine/paracrine regulation of cyclooxygenase-2, prostaglandin E receptors, and angiogenic factors by cyclooxygenase-1. Cancer Res. 2002a;62:424–432. [PMC free article] [PubMed] [Google Scholar]
- Sales KJ, Katz AA, Millar RP, Jabbour HN. Seminal plasma activates cyclooxygenase-2 and prostaglandin E2 receptor expression and signalling in cervical adenocarcinoma cells. Mol Hum Reprod. 2002b;8:1065–1070. doi: 10.1093/molehr/8.12.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales KJ, Maudsley S, Jabbour HN. Elevated prostaglandin EP2 receptor in endometrial adenocarcinoma cells promotes vascular endothelial growth factor expression via cyclic-3′,5′-adenosine monophosphate-mediated transactivation of the epidermal growth factor receptor and extracellular signal-regulated kinase 1/2 signaling pathways. Mol Endocrinol. 2004;18:1533–1545. doi: 10.1210/me.2004-0022. [DOI] [PubMed] [Google Scholar]
- Sales KJ, List T, Boddy SC, Williams AR, Anderson RA, Naor Z, Jabbour HN. A novel angiogenic role for prostaglandin F2α–FP receptor interaction in human endometrial adenocarcinomas. Cancer Res. 2005;65:7707–7716. doi: 10.1158/0008-5472.CAN-05-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger R, Seger D, Reszka AA, Munar ES, Eldar-Finkelman H, Dobrowolska G, Jensen AM, Campbell JS, Fischer EH, Krebs EG. Overexpression of mitogen-activated protein kinase kinase (MAPKK) and its mutants in NIH 3T3 cells. Evidence that MAPKK involvement in cellular proliferation is regulated by phosphorylation of serine residues in its kinase subdomains VII and VIII. J Biol Chem. 1994;269:25699–25709. [PubMed] [Google Scholar]
- Smalley WE, DuBois RN. Colorectal cancer and nonsteroidal anti-inflammatory drugs. Adv Pharmacol. 1997;39:1–20. doi: 10.1016/s1054-3589(08)60067-8. [DOI] [PubMed] [Google Scholar]
- Smith SK, Abel MH, Kelly RW, Baird DT. Prostaglandin synthesis in the endometrium of women with ovular dysfunctional uterine bleeding. Br J Obstet Gynaecol. 1981;88:434–442. doi: 10.1111/j.1471-0528.1981.tb01009.x. [DOI] [PubMed] [Google Scholar]
- Taylor PL, Kelly RW. The occurrence of 19-hydroxy F prostaglandins in human semen. FEBS Lett. 1975;57:22–25. doi: 10.1016/0014-5793(75)80143-8. [DOI] [PubMed] [Google Scholar]
- Templeton AA, Cooper I, Kelly RW. Prostaglandin concentrations in the semen of fertile men. J Reprod Fertil. 1978;52:147–150. doi: 10.1530/jrf.0.0520147. [DOI] [PubMed] [Google Scholar]
- Tjandrawinata RR, Dahiya R, Hughes-Fulford M. Induction of cyclo-oxygenase-2 mRNA by prostaglandin E2 in human prostatic carcinoma cells. Br J Cancer. 1997;75:1111–1118. doi: 10.1038/bjc.1997.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong BJ, Tan J, Tajeda L, Das SK, Chapman JA, DuBois RN, Dey SK. Heightened expression of cyclooxygenase-2 and peroxisome proliferator-activated receptor-delta in human endometrial adenocarcinoma. Neoplasia. 2000;2:483–490. doi: 10.1038/sj.neo.7900119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Woodward DF, Pepperl DJ, Burkey TH, Regan JW. 6-Isopropoxy-9-oxoxanthene-2-carboxylic acid (AH6809), a human EP2 receptor antagonist. Biochem Pharmacol. 1995;50:1731–1733. doi: 10.1016/0006-2952(95)02035-7. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Harada T, Iwabe T, Taniguchi F, Fujii A, Sakamoto Y, Yamauchi N, Shiota G, Terakawa N. Induction of hepatocyte growth factor in stromal cells by tumour-derived basic fibroblast growth factor enhances growth and invasion of endometrial cancer. J Clin Endocrinol Metab. 2002;87:2376–2383. doi: 10.1210/jcem.87.5.8483. [DOI] [PubMed] [Google Scholar]