Abstract
The spermatogonial stem cell initiates and maintains spermatogenesis in the testis. To perform this role, the stem cell must self replicate as well as produce daughter cells that can expand and differentiate to form spermatozoa. Despite the central importance of the spermatogonial stem cell to male reproduction, little is known about its morphological or biochemical characteristics. This results, in part, from the fact that spermatogonial stem cells are an extremely rare cell population in the testis, and techniques for their enrichment are just beginning to be established. In this investigation, we used a multiparameter selection strategy, combining the in vivo cryptorchid testis model with in vitro fluorescence-activated cell sorting analysis. Cryptorchid testis cells were fractionated by fluorescence-activated cell sorting analysis based on light-scattering properties and expression of the cell surface molecules α6-integrin, αv-integrin, and the c-kit receptor. Two important observations emerged from these analyses. First, spermatogonial stem cells from the adult cryptorchid testis express little or no c-kit. Second, the most effective enrichment strategy, in this study, selected cells with low side scatter light-scattering properties, positive staining for α6-integrin, and negative or low αv-integrin expression, and resulted in a 166-fold enrichment of spermatogonial stem cells. Identification of these characteristics will allow further purification of these valuable cells and facilitate the investigation of molecular mechanisms governing spermatogonial stem cell self renewal and hierarchical differentiation.
Stem cells are unique cells within the mammalian body with the capacity for self replication and tissue regeneration. Epidermis, intestinal epithelium, hematopoietic cells, and testis seminiferous tubule epithelium are tissues that are constantly in flux, as transit cells either undergo apoptosis or terminally differentiate. Maintenance of these tissues depends on the presence of tissue-specific stem cells that produce daughter cells committed to differentiating along a pathway determined by the stem cell and the surrounding tissue. Among self-renewing tissues, spermatogenesis and hematopoiesis are considered the most productive. Whereas two to four amplifying divisions are required to generate differentiated epidermis from a single stem cell, it is estimated that nine to eleven amplifying divisions occur before the initiation of meiosis in the testis (1). As a consequence, stem cells in the testis are very rare, possibly comprising as few as 2 in 104 testis cells (2, 3), which complicates the study of stem cell biology and necessitates development of methods for their enrichment.
The frequency of stem cells in the hematopoietic system is also very low, probably 1 in 104 to 105 cells (4–6). However, the development of functional assays, such as the spleen colony assay (7) and long-term reconstitution assay (8), enabled the isolation and study of hematopoietic stem cells (HSC). Although functional assays were essential for definitive identification of stem cell activity in endogenous or enriched cell populations, fluorescence-activated cell sorting (FACS) proved critical in the final determination of cell surface markers present on HSC. Purification of hematopoietic stem cells from partially enriched cell populations is now routinely accomplished by using FACS analysis to separate the HSC based on several characteristics, including cell size, complexity, and surface antigens (9). Through these multiple enrichment steps, it is possible to isolate single stem cells from bone marrow (10, 11), thus facilitating the molecular analysis of their capacity for self renewal and multilineage differentiation.
In contrast, biochemical and molecular characteristics of spermatogonial stem cells have not been described, because a functional assay has not been available to unequivocally identify these cells. We recently developed a technique to transplant donor cells into the seminiferous tubules of an infertile recipient testis (12, 13), in which the transplanted cells proliferate on the basement membrane and establish colonies of spermatogenesis (14). Because spermatogonial stem cells are the only cell type that can produce such a colony, this functional assay provides the necessary confirmation to assess different strategies for enriching spermatogonial stem cells. In recent reports, by using the transplantation assay as a functional endpoint, we demonstrated that spermatogonial stem cells preferentially bind to the extracellular matrix component laminin; selection of testis cells on laminin leads to a 5-fold enrichment of stem cells compared with wild-type testis (15). On the basis of this observation, we subsequently identified α6- and β1-integrins as surface markers on spermatogonial stem cells, because these molecules comprise a known receptor for laminin. Immunoselection of cells expressing these integrins led to a 5- to 10-fold enrichment of stem cells relative to wild-type controls (15). In addition, we found that the in vivo experimental C57BL/6 cryptorchid mouse model resulted in testis cells being enriched approximately 25-fold for spermatogonial stem cells compared with wild-type controls (16).
Using this information, we initiated this study to identify cellular characteristics of spermatogonial stem cells and to use these characteristics as selection criteria. The partially enriched population of cryptorchid testis cells was subjected to FACS analysis, and characteristics that might identify spermatogonial stem cells were evaluated by using principles and selection techniques that have proven valuable for HSC. Our FACS results demonstrated that spermatogonial stem cells are characterized by low side scatter (a measure of intracellular complexity) compared with the whole testis cell population, expression of α6-integrin, negative or low c-kit expression, and negative or low αv-integrin (CD51) expression. By using these selection criteria, a population of testis cells enriched 152- to 166-fold could be produced routinely
Materials and Methods
Donor Mice and Cell Collection.
Donor cells were isolated from the B6; 129S-Gtrosa26 transgenic mouse line (The Jackson Laboratory), designated ROSA26. These mice express the Escherichia coli lacZ (lacZ) structural gene in many cell types, including all stages of spermatogenesis (17). LacZ-expressing cells can be stained blue after incubation with the substrate 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal). Experimental cryptorchid testes were produced by suturing the testis fat pad to the abdominal wall at 6–8 weeks of age, and these testes were used to provide donor cells 2–3 months after surgery (16, 18). Single-cell suspensions from wild-type and cryptorchid testes were prepared by enzymatic digestion, as previously described (19). All cells were kept at 5°C throughout the procedure.
Cell Staining with Antibodies.
The dissociated testis cells were suspended (5 × 106 cells/ml) in PBS containing 0.5% FBS (PBS/FBS). Cells then were incubated with primary antibodies for 20 min on ice, washed twice with excess PBS/FBS, and used for FACS analysis. Primary antibodies used in this investigation were R-phycoerythrin (PE)-conjugated anti-α6-integrin (GoH3), allophycocyanin (APC)-conjugated anti-c-kit (2B8), and biotinylated anti-αv-integrin (H9.2B8; all from PharMingen). For experiments using secondary reagents, cells were further incubated for 20 min with APC-conjugated streptavidin to detect biotinylated antibody. All antibodies or secondary reagents were used at 5 μg/ml. Control cells were not treated with antibodies. After the final wash, cells were resuspended (107 cells/ml) in 2 ml PBS/FBS containing 1 μg/ml propidium iodide (Sigma), filtered into a tube through a 35-μm pore-size nylon screen (Falcon 2235), and kept in the dark on ice until analysis.
FACS.
Cell sorting was performed by a dual-laser FACStar Plus (Becton Dickinson) equipped with 488-nm argon (200 mW) and 633-nm helium neon (35 mW) laser made available through the shared users group at the University of Pennsylvania. An argon laser was used to excite PE and propidium iodide, and emissions were collected with a 575 DF 26 filter for PE and a 610 DF 20 filter for propidium iodide. A neon laser was used to excite APC, and emission was detected with a 675 DF 20 filter. Dead cells were excluded by eliminating propidium iodide-positive events at the time of data collection. Cells were sorted into 5-ml polystyrene tubes (Falcon 2058) containing 2 ml of ice-cold Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS (DMEM/FBS). Approximately 106 cells/fraction were collected for the wild-type (Fig. 1A) and α6-integrin (Fig. 2A) experiments, and 105 cells/fraction were collected for the c-kit (Fig. 3A) and αv-integrin (Fig. 4A) experiments. The sides of the collection tubes were rinsed before and after sorting with DMEM/FBS. Viability of the isolated cells was greater than 95% as assessed by trypan blue exclusion. The sorted cells were transferred to a 16-ml plastic tube (Falcon), centrifuged, and resuspended in 3 ml of cold DMEM/FBS. The plastic tube was gassed with 5% CO2 (to prevent pH change of the medium) and stored overnight at 5°C.
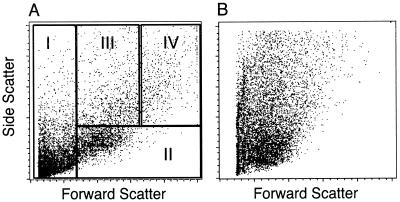
Figure 1.
Comparison of light-scattering properties of dispersed testis cells from adult wild-type (A) and cryptorchid (B) mice by FACS analysis. Relative FSC and SSC are measured in arbitrary units on a linear scale and are determined by cell size and complexity, respectively. Each dot represents the FSC and SSC value for a single testis cell. Cryptorchid cells (B) are devoid of many large cells seen in fraction IV of wild-type testis cells (A). Cells from A were sorted into four fractions based on their light-scattering properties and transplanted into infertile mouse testes to evaluate their functional ability to generate spermatogenesis (see Table 1). Cell distribution in percent of wild-type cells fractionated by FACS: fraction I, 47 ± 3; fraction II, 32 ± 2; fraction III, 6 ± 0.3; fraction IV, 11 ± 1 (mean ± SEM, n = 3).
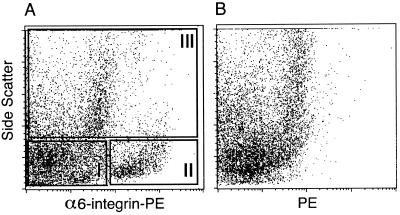
Figure 2.
Flow cytometric analysis of dispersed testis cells from an experimental cryptorchid mouse and separation based on expression of α6-integrin. PE (log scale) fluorescence is plotted against relative SSC (linear scale) for cryptorchid testis cells stained with PE-conjugated anti-α6-integrin antibody (A) or unstained cryptorchid testis cells (B). Cryptorchid testis cells were divided into three populations based on differences in α6-integrin staining and SSC values. Cells in fraction I were characterized by negative to low α6-integrin reactivity and low SSC (α6loSSClo); fraction II cells displayed higher α6-integrin reactivity and low SSC (α6hiSSClo); and cells in fraction III exhibited high SSC values (SSChi). Cell distribution in percent of cryptorchid cells fractionated by FACS: fraction I, 50 ± 2; fraction II, 15 ± 2; fraction III, 28 ± 4 (mean ± SEM, n = 4). Cells from each fraction were transplanted into infertile mouse testes to evaluate their functional ability to generate spermatogenesis (see Table 2).
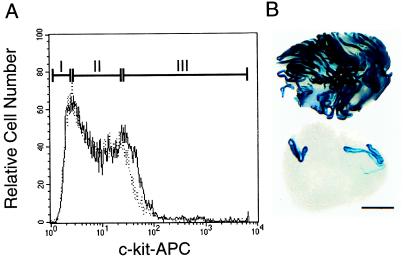
Figure 3.
Flow cytometric analysis of cryptorchid α6hiSSClo cells for c-kit expression. (A) Cryptorchid α6hiSSClo cells (Fig. 2A, fraction II) were stained with an APC-conjugated antibody to the c-kit receptor and examined in a flow cytometer. The distribution of stained (solid line) and unstained control (dotted line) cells is shown. Cells were sorted into three groups based on c-kit immunoreactivity. Fraction I was designated c-kit negative (c-kit−); in fraction II, the solid and dotted lines mostly coincide, and this population was designated c-kit low (c-kitlo); fraction III contained cells with high c-kit immunoreactivity (c-kit+). Cell distribution in percent of cryptorchid cells fractionated by FACS: Fraction I, 2.3 ± 0.1; fraction II, 6.4 ± 0.4; fraction III, 1.8 ± 0.1 (mean ± SEM, n = 3). Cells from each fraction were transplanted into infertile mouse testes to evaluate their functional ability to generate spermatogenesis (see Table 3). (B) Comparison of recipient testes 2 months after transplantation of a mixed population of cryptorchid α6hiSSCloc-kit− and cryptorchid α6hiSSCloc-kitlo cells (105 cells injected, top) or unselected wild-type donor testis cells (105 cells injected, bottom). Individual blue tubules indicate colonies of spermatogenesis arising from donor stem cells. Stain: X-Gal. Bar = 2 mm.
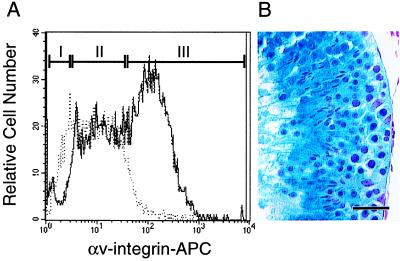
Figure 4.
Flow cytometric analysis of cryptorchid α6hiSSClo cells for αv-integrin expression. (A) Cryptorchid α6hiSSClo cells (Fig. 2A, fraction II) were stained with biotinylated anti-αv-integrin antibody, detected with an APC-conjugated Streptavidin and examined in a flow cytometer. The distribution of stained (solid line) and unstained control (dotted line) cells is shown. Cells were sorted into three groups based on αv-integrin immunoreactivity. Fraction I was designated αv-integrin negative (αv−); in fraction II, the solid and dotted lines mostly coincide, and this population was designated αv-integrin low (αvlo); and fraction III contained cells with high αv-integrin expression (αv+). Cell distribution in percent of cryptorchid cells fractionated by FACS: fraction I, 2.6 ± 0.2; fraction II, 2.7 ± 0.2; fraction III, 8.8 ± 1.2 (mean ± SEM, n = 5). Cells from each fraction were transplanted into infertile mouse testes to evaluate their functional ability to generate spermatogenesis (see Table 4). (B) Histological section showing donor cell-derived spermatogenesis 2 months after transplantation of cryptorchid α6hiSSCloαv− cells. Blue color indicates origin of spermatogenesis from transgenic donor stem cells. Note normal appearance and organization of germ cells and presence of spermatozoa. Stain: X-Gal followed by nuclear fast red. Bar = 50 μm.
Testis Cell Transplantation.
On the day of injection, lacZ-expressing donor cells were resuspended in 100 μl of DMEM/FBS. Each cell suspension (107 cells/ml for wild-type fractions from Fig. 1A and 106 cells/ml for all other fractions) was transplanted into the testes of immunologically compatible C57BL/6 × 129/SvCP F1 hybrid (129/B6) male mice that had been treated with busulfan (50 mg/kg) at 4–6 weeks of age (12, 13). Viability, measured by trypan blue exclusion, was greater than 90%. Unsorted cryptorchid testis cells (5 × 106/ml) of the same age were used as controls. Approximately 10 μl of the cell suspension could be introduced into the seminiferous tubules of a busulfan-treated mouse, resulting in 75–85% filling of the tubules in each testis (19).
Analysis of Recipient Testes.
Recipient testes were collected 2 months after donor cell transplantation and analyzed by X-Gal staining, as described previously (14, 15, 20). Appearance of individual blue-stained stretches of seminiferous tubules in recipient testes represents colonization and spermatogenesis from donor-derived stem cells. Other types of testis cells do not generate spermatogenesis, and endogenous recipient testis cells do not stain with X-Gal because they do not carry the lacZ transgene. The most useful parameters to assess donor cell colonization were the number of colonies and total colonized area (mm2). Colony number was counted manually by using a dissecting microscope, and colonized area was determined by using a computer imaging system (15, 20). Because the number of cells that could be recovered and injected in each experiment varied, colony number and colonized area were normalized to reflect donor cells injected at a concentration of 107 cells/ml (10 μl or 105 cells injected/testis). Statistical analyses were performed by using ANOVA, and significant differences between means were determined by using Tukey's HSD multiple comparisons test (Systat 7.0; SPSS, Chicago).
Results
Stem Cell Activity in Wild-Type Testis Cell Populations.
In our first attempt to purify spermatogonial stem cells, wild-type adult testis cells were separated on the basis of their light-scattering characteristics by using FACS. Forward scatter (FSC) and side scatter (SSC) of incident light in an unstained wild-type population of testis cells are shown in Fig. 1A. Cell size is roughly estimated by FSC, and SSC is determined by cell shape and complexity. FACS separated cells were arbitrarily divided into four fractions according to FSC and SSC. To determine stem cell activity, cells from each fraction (I to IV) were microinjected into the seminiferous tubules of busulfan-treated recipient mice (Table 1). Although the majority of stem cell activity seemed to reside in fraction II, this cell population was not enriched compared with the wild-type control (Table 1). Indeed, the FACS procedure resulted in a 68% reduction in stem cell activity (P < 0.02, Table 1). Our inability to enrich spermatogonial stem cells from the wild-type testis on the basis of light-scattering properties could result from a selective loss of stem cells during the sorting procedure, as has been reported in other systems (21), or could be caused by the fact that the aggregate testis cell population was too complex and consisted of too many cell types. Therefore, although these results indicate that stem cells have high FSC and low SSC values among the wild-type testis cell population, this procedure did not enrich stem cells.
Table 1.
Transplantation/colonization analysis of wild-type mouse testis cells after FACS analysis
| Fraction* | Testes colonized† | Colonies/testis‡ | Fold§ (colony) | Area/testis, mm2‡ | Fold§ (area) |
|---|---|---|---|---|---|
| WT | 8/11 | 3.1 ± 0.9 | 1 | 1.1 ± 0.4 | 1 |
| I (FSClo) | 1/9 | 0.1 ± 0.1 | 0.03 | 0.1 ± 0.1 | 0.09 |
| II (FSChi SSClo) | 5/10 | 1.0 ± 0.4 | 0.32 | 0.4 ± 0.2 | 0.36 |
| III (FSCmid SSChi) | 0/11 | 0 | NA | 0 | NA |
| IV (FSChi SSChi) | 0/12 | 0 | NA | 0 | NA |
Unsorted wild-type (WT) testis cells from mice 4–6 wks of age were used as controls. Fractions (I–IV) from Fig. 1A.
Number of testes colonized/number of testes analyzed.
Results (mean ± SEM) from three separate experiments normalized to 105 cells injected/testis.
Fold enrichment compared to wild-type control. For example, for fraction I, 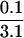 = 0.03-fold enrichment.
= 0.03-fold enrichment.
NA, Not applicable.
In Vivo Enrichment of Spermatogonial Stem Cells by Using the Experimental Cryptorchid Model.
To reduce the number of nonstem cells in the initial cell population, the experimental cryptorchid model, which eliminates most differentiated germ cells, was used. In the present study, the cryptorchid Rosa donors were on a different genetic background (B6; 129S-Gtrosa26) than used in our previous report (C57BL/6J-Gtrosa26; ref. 16); therefore, the efficacy of the in vivo cryptorchid enrichment was reevaluated. Similar to our previous report, the cryptorchid procedure resulted in a 23-fold enrichment of spermatogonial stem cells on the basis of pooled data from experiments presented in Tables 3 and 4. Transplantation of wild-type testis cells, age matched to the cryptorchid controls, resulted in the formation of 0.85 ± 0.1 colonies/testis/105 cells (mean ± SEM, n = 27; data not shown) compared with 19.4 ± 2.7 colonies/testis/105 cells in recipients of cryptorchid cells (Tables 3 and 4). Light-scattering properties of cryptorchid cells display a different pattern than wild-type testis cells and characteristically lack fraction IV (compare Fig. 1 A and B).
Table 3.
Spermatogenic potential of cryptorchid α6hi SSClo testis cells after FACS separation by c-kit expression
| Fraction* | Testes colonized† | Colonies/testis‡ | Fold§ (colony) | Area/testis, mm2‡ | Fold§ (area) |
|---|---|---|---|---|---|
| Cryptorchid | 11 /11 | 18 ± 4 | 1 | 9.4 ± 2.7 | 1 |
| I (c-kit−) | 11 /12 | 118 ± 22 | 6.6 | 54 ± 10 | 5.7 |
| II (c-kitlo) | 11 /12 | 88 ± 20 | 4.9 | 39 ± 8 | 4.1 |
| III (c-kit+) | 2 /11 | 1.8 ± 1.2 | 0.1 | 0.5 ± 0.4 | 0.05 |
Unsorted cryptorchid testes from mice 14–20 wks of age were used as control. Fractions (I–III) from Fig. 3A.
Number of testes colonized/number of testes analyzed.
Reported as described in Table 1 (mean ± SEM).
Fold enrichment compared to cryptorchid control (calculated as described in Table 1).
Table 4.
Spermatogenic potential of cryptorchid α6hi SSClo testis cells FACS separated by αv-integrin expression
| Fraction* | Testes colonized† | Colonies/testis‡ | Fold§ (colony) | Area/testis, mm2‡ | Fold§ (area) |
|---|---|---|---|---|---|
| Cryptorchid | 17 /17 | 20 ± 4 | 1 | 9.5 ± 2.3 | 1 |
| I (αv−) | 14 /14 | 144 ± 11 | 7.2 | 59 ± 5 | 6.2 |
| II (αvlo) | 17 /17 | 86 ± 10 | 4.3 | 32 ± 5 | 3.4 |
| III (αv+) | 2 /18 | 1.1 ± 0.8 | 0.06 | 0.2 ± 0.2 | 0.02 |
Unsorted cryptorchid testes from mice 14–20 wks of age were used as control. Fractions (I–III) from Fig. 4A.
Number of testes colonized/number of testes analyzed.
Reported as described in Table 1 (mean ± SEM).
Fold enrichment compared to cryptorchid control (calculated as described in Table 1).
Analysis of α6-Integrin Expressing Cells from Experimental Cryptorchid Testes.
In previous experiments, we demonstrated that α6-integrin immunoselection was more effective for stem cell enrichment than β1-integrin selection (15). Therefore, cryptorchid testis cells were stained with PE-conjugated anti-α6-integrin antibodies and examined by FACS analysis. A distinct population of cells (Fig. 2A, fraction II) with high-intensity staining for α6-integrin and a low SSC value is evident, which was not observed in FACS of unstained cells (Fig. 2B). The α6-integrin-stained cryptorchid testis cells were divided into three fractions (I–III) to determine the relative stem cell activity (Fig. 2A). Evaluation of recipient testes revealed that most stem cell activity was found in fraction II (α6hiSSClo). The average number of colonies generated from the fraction II cell population was 41, and colonized area was 27 mm2 (Table 2). Compared with cryptorchid controls, a spermatogonial stem cell enrichment of 3.7 (colony number)- and 4.3 (colonized area)-fold was obtained in fraction II (P < 0.01, Table 2).
Table 2.
Spermatogenic potential of cryptorchid testis cells fractionated by α6-integrin expression and SSC
| Fraction* | Testes colonized† | Colonies/testis‡ | Fold§ (colony) | Area/testis, mm2‡ | Fold§ (area) |
|---|---|---|---|---|---|
| Cryptorchid | 12 /13 | 11 ± 3 | 1 | 6.3 ± 2.2 | 1 |
| I (α6lo SSClo) | 5 /12 | 0.8 ± 0.4 | 0.07 | 0.4 ± 0.2 | 0.06 |
| II (α6hi SSClo) | 13 /13 | 41 ± 6 | 3.7 | 27 ± 5 | 4.3 |
| III (SSChi) | 3 /11 | 0.3 ± 0.1 | 0.03 | 0.2 ± 0.1 | 0.03 |
Unsorted cryptorchid testes from mice 14–20 wks of age were used as control. Fractions (I–III) from Fig. 2A.
Number of testes colonized/number of testes analyzed.
Reported as described in Table 1 (mean ± SEM).
Fold enrichment compared to cryptorchid control (calculated as described in Table 1).
Spermatogonial Stem Cells Do Not Express c-kit.
The enriched cryptorchid cell population (α6hiSSClo) was examined by FACS for the expression of c-kit and displayed a bimodal pattern in which most of the cells had negative to low expression (Fig. 3A). For transplantation assay, cryptorchid α6hiSSClo cells were arbitrarily fractionated into three subpopulations based on c-kit expression (fractions I–III, Fig. 3A). The results shown in Table 3 demonstrate that spermatogonial stem cell activity was highest in c-kit− (fraction I) cells and lowest in c-kit+ (fraction III) cells. On the basis of the comparison of stained cells (solid line) with unstained cells (dotted line) in Fig. 3A, only a small percentage of the cryptorchid α6hiSSClo population exhibited positive c-kit expression. Perhaps this result should be expected because most differentiated germ cells were removed by the cryptorchid procedure. Nonetheless, the cryptorchid α6hiSSCloc-kit− cell population was enriched 6.6-fold (colony number) and 5.7-fold (colonized area) for spermatogonial stem cells compared with cryptorchid enrichment alone (P < 0.03, Table 1). This enrichment results in part from the fact that spermatogonial stem cells do not express c-kit, but it must also reflect the fact that these cells have lower endogenous fluorescence than most other cells in the cryptorchid testis population. It is also clear that few, if any, spermatogonial stem cells reside in the c-kit+ fraction (fraction III), as stem cell activity in this population was reduced by 90% (colony number) and 95% (colonized area) compared with cryptorchid controls. The dramatic enrichment of stem cells in c-kit− and c-kitlo populations is striking when compared with the unsorted wild-type testis cell transplants (Fig. 3B). These data provide strong evidence that c-kit is not expressed on spermatogonial stem cells.
Fractionation of Cryptorchid α6hiSSClo Cells Based on αv-Integrin Expression.
Expression of three integrin molecules (α5, αv, and β3) reported to be present on primordial germ cells (PGCs; ref. 22) was examined in cryptorchid α6hiSSClo cells. Although these antibodies showed somewhat similar staining patterns (data not shown), we chose anti-αv-integrin selection for further study, because it appeared to divide the cryptorchid α6hiSSClo cells more clearly than the other two antibodies. Indeed, selection based on αv-integrin expression provided a better fractionation of the cryptorchid α6hiSSClo population than separation based on c-kit expression (compare Figs. 3A and 4A). Therefore, we separated the cryptorchid α6hiSSClo cells into three fractions (I–III, Fig. 4A) based on αv-integrin expression. Microscopic examination after FACS analysis revealed that cryptorchid α6hiSSCloαv− cells were relatively uniform, exhibiting active blebbing and characteristic pseudopods (data not shown).
Compared with the cryptorchid enrichment alone, colony number and colonized area increased significantly after transplantation of cryptorchid α6hiSSCloαv− and cryptorchid α6hiSSCloαvlo selected cells (P < 0.01, Table 4). In the αv− population, the enrichment factors for colony number and colonized area were 7.2 and 6.2, respectively. Similarly, the αvlo population was enriched 4.3-fold (colony number) and 3.4-fold (colonized area) compared with the cryptorchid control. Although both populations were enriched compared with the cryptorchid testis, αv− selection was significantly more effective for enriching stem cells than the αvlo selection (P < 0.01). Histological examination of transplanted testes confirmed the presence of normal appearing spermatogenesis (Fig. 4B). Similar to our observations for c-kit+ cells, αv+ selection resulted in a 94% reduction in colony number and a 98% reduction in colonized area compared with the cryptorchid control (Table 4), suggesting that αv-integrin is not expressed on spermatogonial stem cells. Therefore, the absence of αv-integrin and c-kit expression were both useful parameters for enriching stem cells from the cryptorchid α6hiSSClo cell population.
Discussion
Ideally a pure population of stem cells could be isolated based on expression of specific and unique surface markers. Because such markers have not been described for spermatogonial stem cells, we used a multiparameter strategy, combining the in vivo cryptorchid testis model with in vitro FACS analysis, to separate cells based on several characteristics simultaneously. Similar strategies have been successfully used to enrich and purify hematopoietic stem cells (9). The ability to isolate pure hematopoietic stem cell populations depends, in large part, on the availability of a functional assay to confirm the presence of stem cells and the use of FACS analysis to separate cells based on expression of several cell surface markers. Until recently, the lack of a functional assay made it impossible to use a similar approach to purify spermatogonial stem cells. Therefore, the relatively few reports involving FACS analysis to study testis cells are limited to separations based on DNA content (ploidy), light-scattering properties, and mitochondrial respiratory activity (23–26). These reports suggest the presence of several distinct cell populations within the adult testis, but without a functional assay, identification of spermatogonial stem cells was not possible. Our initial attempts to separate cells from the wild-type testis based on cell size (FSC) and intracellular complexity (SSC) did not establish a population enriched for stem cells, perhaps because the initial cell population was too complex. Therefore, cryptorchid testes that lack differentiated germ cells were subjected to FACS analysis, which allowed identification of a distinct cell population significantly enriched for spermatogonial stem cells.
Using our transplantation assay as a functional endpoint, we first extended our previous observations and demonstrated that stem cells from the cryptorchid mouse testis were found predominantly in the population expressing high levels of α6-integrin. The stem cell rich population also was characterized by low SSC values and low or no expression of the c-kit receptor (α6hiSSCloc-kit−). C-kit is expressed on premeiotic germ cells, and several studies suggest its importance in spermatogonial migration, proliferation, and early differentiation (27, 28). However, controversy continues regarding the presence or function of c-kit in spermatogonial stem cell biology. Although c-kit immunoselection has been used to establish an enriched population of spermatogonia (29), it was not determined whether spermatogonial stem cells were present, because no functional test was used. The results reported here clearly show that spermatogonial stem cells, defined by their functional ability to generate and maintain spermatogenic colonies, express little or no c-kit. This observation is in agreement with a recent report demonstrating expression of c-kit by differentiating, but not undifferentiated, type A spermatogonia in the vitamin A-deficient mouse model (30). In addition, undifferentiated spermatogonia continue to proliferate when the c-kit receptor is blocked with antibodies, but more mature spermatogonia that express c-kit are destroyed (27). However, these results do not necessarily suggest that spermatogonial stem cells never express c-kit. Steel mutant mice that lack the membrane-bound form of Steel factor (kit ligand) have fewer stem cells than wild-type mice (16), which suggests that the c-kit receptor is involved in some aspects of the biology of spermatogonial stem cells. Perhaps c-kit is not required on normal stem cells that are in maintenance mode but is induced in stem cells that are expanding their numbers after irradiation or other damage to the testis stem cell compartment. Interestingly, this occurs in the hematopoietic system, where the c-kit receptor is not expressed on dormant stem cells (31).
PGCs, from which spermatogonial stem cells arise, are characterized by expression of c-kit and several other markers, such as alkaline phosphatase (32), stage-specific embryonic antigen-1 (SSEA-1, ref. 33) and EMA-1 (40) which make it possible to isolate highly purified PGC populations (34, 35). Unfortunately, these specific markers disappear from stem cells by the time they appear in the adult testis (36) and cannot be used for identification. However, PGCs also express several members of the integrin family, including α3, α5, α6, αv, and β1 (22). Both α6- and β1-integrin are present on spermatogonial stem cells (15), and of the other three, αv offered the best possibility to further fractionate the stem cell-rich α6hiSSClo cell population. The transplantation analysis revealed that stem cells express little or no αv-integrin, and the α6hiSSCloαv− cell population was greatly enriched for stem cells. Isolation of highly purified stem cells should now facilitate the identification of other shared surface molecules. Because stem cells are migratory in many organs, a variety of cell adhesion molecules might be present on spermatogonial stem cells. For example, N-CAM and E-cadherin expression have been reported in PGCs and/or gonocytes (37–39). Therefore, it will be useful to determine the level of expression of these and other surface molecules on PGCs and spermatogonial stem cells, not only to achieve further enrichment, but also to understand development and commitment of germ line stem cells.
In the current study, the cryptorchid procedure resulted in a 23-fold enrichment of spermatogonial stem cells compared with wild-type controls. In addition, cryptorchid α6hiSSCloc-kit− and cryptorchid α6hiSSCloαv− testis cells were demonstrated to be enriched 6.6- and 7.2-fold (colony number) respectively, compared with cryptorchid selection alone. Therefore these cell populations represent 152-fold (6.6 × 23) or 166-fold (7.2 × 23) enrichment of spermatogonial stem cells compared with wild-type testis. Because the adult mouse testis is thought to contain about 1 stem cell in 5,000 total testis cells based on morphological studies (2, 3), the spermatogonial stem cell concentration in the cryptorchid α6hiSSCloc-kit− and cryptorchid α6hiSSCloαv− populations would be 1 in 33 (5,000/152) or 1 in 30 (5,000/166), respectively. However, it can be calculated from Tables 3 and 4 that cryptorchid α6hiSSCloc-kit− and cryptorchid α6hiSSCloαv− populations actually produced 1 spermatogenic colony for each 847 (105 cells/118 colonies) or 694 (105 cells/144 colonies) transplanted cells, respectively. How can we explain this difference? In these experiments, control wild-type testis cell populations, age-matched to the cryptorchid donors, produced 0.85 colonies per 105 cells transplanted (see results) or 23.5 times fewer colonies (stem cells) than would be predicted from morphological studies (20 stem cells/105 total testis cells). The low efficiency of colonization in this and previous studies (14, 20) is thought to result from: (i) differences between morphological and functional criteria used for identifying stem cells; (ii) inefficient seeding of stem cells on the basement membrane of the seminiferous tubules; and/or (iii) selective loss of stem cell activity during testis cell collection and handling. Taking into account this level of colonization efficiency, the cryptorchid α6hiSSCloc-kit− and cryptorchid α6hiSSCloαv− populations contained a stem cell concentration of 1 in 36 (847/23.5) or 1 in 30 (694/23.5), respectively. Thus, both methods of calculation indicate that cell populations with a stem cell concentration of 1 in 30–40 total cells can be obtained.
The development of the spermatogonial transplantation technique has established a functional assay for male germ line stem cells, which now allows definitive analysis and quantification of spermatogonial stem cells in any cell population. Using techniques and approaches similar to those applied to bone marrow to identify hematopoietic stem cells, we have begun to characterize spermatogonial stem cells and have found that these cells are α6hiSSClo and have negative or low c-kit expression as well as negative or low αv-integrin expression. A cryptorchid testis cell population with these characteristics is at least 152-fold enriched for spermatogonial stem cells. These purified cell populations now can be routinely isolated and subsequently examined for other molecular and genetic characteristics. Furthermore, extension of the approaches described here will allow pure populations of spermatogonial stem cells to be obtained.
Acknowledgments
We thank Drs. N. Avadhani, L. Karagenc, M. Kotlikoff, M. Nagano, and E. Sandgren for critical evaluation of the manuscript and A. Mouschauer for help in cell sorting. In addition, we are grateful to C. Freeman and R. Naroznowski for assistance with animal maintenance and experimentation and to J. Hayden for help with photography. T.S. was supported by the Japan Society for Promotion of Science. Financial support for the research was from the National Institute of Health (National Institute of Child Health and Human Development grant no. 36504), Commonwealth and General Assembly of Pennsylvania, and the Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation.
Abbreviations
- FACS
fluorescence-activated cell sorting
- APC
allophycocyanin
- DMEM
Dulbecco's modified Eagle's medium
- HSC
hematopoietic stem cell
- LacZ
Escherichia coli lacZ gene
- PE
R-phycoerythrin
- PGC
primordial germ cell
- X-Gal
5-bromo-4-chloro-3-indolyl β-d-galactoside
- FSC
forward scatter
- SSC
side scatter
References
- 1.Potten C S. In: Oxford Textbook of Pathology. McGee J O, Isaacson P G, Wright N A, editors. Oxford: Oxford Univ. Press; 1992. pp. 43–52. [Google Scholar]
- 2.Meistrich M L, van Beek M E A B. In: Cell and Molecular Biology of the Testis. Desjardins C, Ewing L L, editors. New York: Oxford Univ. Press; 1993. pp. 266–295. [Google Scholar]
- 3.Tegelenbosch R A J, de Rooij D G. Mutat Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- 4.Micklem H S, Lennon J E, Ansell J D, Gray R A. Exp Hematol. 1987;15:251–257. [PubMed] [Google Scholar]
- 5.Harrison D E, Astle C M, Lerner C. Proc Natl Acad Sci USA. 1988;85:822–826. doi: 10.1073/pnas.85.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boggs D R, Boggs S S, Saxe D F, Gress L A, Canfield D R. J Clin Invest. 1982;70:242–253. doi: 10.1172/JCI110611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Till J E, McCulloch E A. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 8.Harrison D E. Blood. 1980;55:77–81. [PubMed] [Google Scholar]
- 9.Spangrude G J. Immunol Today. 1989;10:344–350. doi: 10.1016/0167-5699(89)90192-8. [DOI] [PubMed] [Google Scholar]
- 10.Smith L G, Weissman I L, Heimfeld S. Proc Natl Acad Sci USA. 1991;88:2788–2792. doi: 10.1073/pnas.88.7.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osawa M, Hanada K, Hamada H, Nakauchi H. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 12.Brinster R L, Avarbock M R. Proc Natl Acad Sci USA. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinster R L, Zimmermann J W. Proc Natl Acad Sci USA. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagano M, Avarbock M R, Brinster R L. Biol Reprod. 1999;60:1429–1436. doi: 10.1095/biolreprod60.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shinohara T, Avarbock M R, Brinster R L. Proc Natl Acad Sci USA. 1999;96:5504–5509. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinohara T, Avarbock M R, Brinster R L. Dev Biol. 2000;220:401–411. doi: 10.1006/dbio.2000.9655. [DOI] [PubMed] [Google Scholar]
- 17.Nagano M, Brinster R L. APMIS. 1998;106:47–57. doi: 10.1111/j.1699-0463.1998.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 18.Nishimune Y, Aizawa S, Komatsu T. Fertil Steril. 1978;29:95–102. doi: 10.1016/s0015-0282(16)43045-1. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa T, Aréchaga J M, Avarbock M R, Brinster R L. Int J Dev Biol. 1997;41:111–122. [PubMed] [Google Scholar]
- 20.Dobrinski I, Ogawa T, Avarbock M R, Brinster R L. Mol Reprod Dev. 1999;53:142–148. doi: 10.1002/(SICI)1098-2795(199906)53:2<142::AID-MRD3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 21.Hunt S V. Eur J Immunol. 1979;9:853–859. doi: 10.1002/eji.1830091105. [DOI] [PubMed] [Google Scholar]
- 22.Anderson R, Fässler R, Georges-Labouesse E, Hynes R O, Bader B L, Kreidberg J A, Schaible K, Heasman J, Wylie C. Development (Cambridge, UK) 1999;126:1655–1664. doi: 10.1242/dev.126.8.1655. [DOI] [PubMed] [Google Scholar]
- 23.Janca F C, Jost L K, Evenson D P. Biol Reprod. 1986;34:613–623. doi: 10.1095/biolreprod34.4.613. [DOI] [PubMed] [Google Scholar]
- 24.Mays-Hoopes L L, Bolen J, Riggs A D, Singer-Sam J. Biol Reprod. 1995;53:1003–1011. doi: 10.1095/biolreprod53.5.1003. [DOI] [PubMed] [Google Scholar]
- 25.Petit J M, Ratinaud M H, Cordelli E, Spano M, Julien R. Cytometry. 1995;19:304–312. doi: 10.1002/cyto.990190404. [DOI] [PubMed] [Google Scholar]
- 26.Malkov M, Fisher Y, Don J. Biol Reprod. 1998;59:84–92. doi: 10.1095/biolreprod59.1.84. [DOI] [PubMed] [Google Scholar]
- 27.Yoshinaga K, Nishikawa S, Ogawa M, Hayashi S, Kunisada T, Fujimoto T, Nishikawa S. Development (Cambridge, UK) 1991;113:689–699. doi: 10.1242/dev.113.2.689. [DOI] [PubMed] [Google Scholar]
- 28.Vincent S, Segretain D, Nishikawa S, Nishikawa S, Sage J, Cuzin F, Rassoulzadegan M. Development (Cambridge, UK) 1998;125:4585–4593. doi: 10.1242/dev.125.22.4585. [DOI] [PubMed] [Google Scholar]
- 29.von Schonfeldt V, Krishnamurthy H, Foppiani L, Schlatt S. Biol Reprod. 1999;61:582–589. doi: 10.1095/biolreprod61.3.582. [DOI] [PubMed] [Google Scholar]
- 30.Schrans-Stassen B H G J, van de Kant H J G, de Rooij D G, van Pelt A M. Endocrinology. 1999;140:5894–5900. doi: 10.1210/endo.140.12.7172. [DOI] [PubMed] [Google Scholar]
- 31.Ortiz M, Wine J W, Lohrey N, Ruscetti F W, Spence S E, Keller J R. Immunity. 1999;10:173–182. doi: 10.1016/s1074-7613(00)80018-7. [DOI] [PubMed] [Google Scholar]
- 32.Chiquoine A D. Anat Rec. 1954;118:135–145. doi: 10.1002/ar.1091180202. [DOI] [PubMed] [Google Scholar]
- 33.Solter D, Knowles B B. Proc Natl Acad Sci USA. 1978;75:5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCarrey J R, Hsu K C, Eddy E M, Klevecz R R, Bolen J L. J Exp Zool. 1987;242:107–111. doi: 10.1002/jez.1402420116. [DOI] [PubMed] [Google Scholar]
- 35.Abe K, Hashiyama M, Macgregor G, Yamamura K, Abe K. Dev Biol. 1996;180:468–472. doi: 10.1006/dbio.1996.0320. [DOI] [PubMed] [Google Scholar]
- 36.Cooke J E, Godin I, Ffrench-Constant C, Heasman J, Wylie C C. Methods Enzymol. 1993;225:37–58. doi: 10.1016/0076-6879(93)25006-n. [DOI] [PubMed] [Google Scholar]
- 37.Munro S B, Blaschuk O W. Biol Reprod. 1996;55:822–827. doi: 10.1095/biolreprod55.4.822. [DOI] [PubMed] [Google Scholar]
- 38.Orth J M, Jester W F., Jr J Androl. 1995;16:389–399. [PubMed] [Google Scholar]
- 39.Bendel-Stenzel M R, Gomperts M, Anderson R, Heasman J, Wylie C. Mech Dev. 2000;91:143–152. doi: 10.1016/s0925-4773(99)00287-7. [DOI] [PubMed] [Google Scholar]
- 40.Hahnel A C, Eddy E M. J Reprod Immunol. 1987;10:89–110. doi: 10.1016/0165-0378(87)90069-6. [DOI] [PubMed] [Google Scholar]