Abstract
In cattle, the lymphoid rich regions of the rectal-anal mucosa at the terminal rectum are the preferred site for Escherichia coli O157:H7 colonisation. All cattle infected by rectal swab administration demonstrate long-term E. coli O157:H7 colonisation, whereas orally challenged cattle do not demonstrate long-term E. coli O157:H7 colonisation in all animals. Oral, but not rectal challenge of sheep with E. coli O157:H7 has been reported, but an exact site for colonisation in sheep is unknown. To determine if E. coli O157:H7 can effectively colonise the ovine terminal rectum, in vitro organ culture (IVOC) was initiated. Albeit sparsely, large, densely packed E. coli O157:H7 micro-colonies were observed on the mucosa of ovine and control bovine terminal rectum explants. After necropsy of orally inoculated lambs, bacterial enumeration of the proximal and distal gastrointestinal tract did suggest a preference for E. coli O157:H7 colonisation at the ovine terminal rectum, albeit for both lymphoid rich and non-lymphoid sites. As reported for cattle, rectal inoculation studies were then conducted to determine if all lambs would demonstrate persistent colonisation at the terminal rectum. After necropsy of E. coli O157:H7 rectally inoculated lambs, most animals were not colonised at gastrointestinal sites proximal to the rectum, however, large densely packed micro-colonies of E. coli O157:H7 were observed on the ovine terminal rectum mucosa. Nevertheless, at the end point of the study (day 14), only one lamb had E. coli O157:H7 micro-colonies associated with the terminal rectum mucosa. A comparison of E. coli O157:H7 shedding yielded a similar pattern of persistence between rectally and orally inoculated lambs. The inability of E. coli O157:H7 to effectively colonise the terminal rectum mucosa of all rectally inoculated sheep in the long term, suggests that E. coli O157:H7 may colonise this site, but less effectively than reported previously for cattle.
Keywords: E. coli O157:H7, IVOC, lymphoid, terminal rectum, ovine
1. INTRODUCTION
Enterohaemorrhagic Escherichia coli (EHEC) O157:H7 is a major cause of human gastrointestinal disease that can lead to life-threatening illnesses, such as haemorrahagic colitis and renal failure [13, 24]. The main transmission routes for human infection are through faecal contamination of foods and direct animal contact. Ruminants, especially cattle, are cited as the main reservoir, although small ruminants, such as sheep, have also been implicated [2, 3, 9, 14, 23].
As a necessity for the development of optimal intervention strategies, various ruminant studies, mostly in cattle, have reported E. coli O157:H7 tissue tropisms in the ruminant gastrointestinal tract (GIT). Indeed Grauke et al. [12] first suggested that the lower GIT of orally inoculated cattle and sheep, including the colon, was one location associated with E. coli O157:H7 persistent colonisation. More recently, Naylor et al. [23] showed the lymphoid follicle-dense mucosa at the terminal rectum, as the primary site for E. coli O157:H7 localisation in young calves following oral inoculation. This hypothesis was further assessed by Sheng et al. [26] who demonstrated that rectal swab administration of E. coli O157:H7 resulted in persistent long-term colonisation, thus strongly supporting the terminal rectum mucosa as the preferred site for E. coli O157:H7 colonisation in cattle. A recent feedlot E. coli O157:H7 infection study generated supportive data suggesting that “super-shedder” status is linked to greater E. coli O157:H7 colonisation of the terminal rectum mucosa [5]. Furthermore, necropsy of cattle that were defined as long-term colonised by E. coli O157:H7, revealed that of all GIT sites examined, only the terminal rectum was culture positive for E. coli O157:H7 [17]. Collectively, these data are compelling that in cattle, E. coli O157:H7 colonises the lower GIT effectively, but that long term and high level shedding is dependent upon colonisation of the terminal rectum mucosa.
Significantly less is known about the primary site of colonisation for E. coli O157:H7 in sheep, should a single site exist. Interpretation of data from the various oral inoculation studies conducted [6, 7, 12, 15, 28] have not identified the terminal rectum as the primary site of colonisation. Indeed, small sparse attaching and effacing (A/E) lesions induced by E. coli O157:H7 have been identified in the ovine colon more readily than at other sites [28]. Using the same E. coli O157:H7 isolate, our and other laboratories have investigated and hypothesized the role of various E. coli O157:H7 virulence factors in the well established conventionally weaned 6-week-old lamb model [15, 27, 29]. Indeed, the E. coli O157:H7 isolate (NCTC12900nalr) previously reported from orally challenged 6-week-old lambs by our laboratory has demonstrated persistent shedding in the faeces [15, 28], but detailed bacterial enumeration of the ovine gastrointestinal tract has not been undertaken to determine if the terminal rectum is preferentially colonised. For the study to be presented here, in vitro organ culture (IVOC) was initiated to determine if our E. coli O157:H7 isolate could colonise the ovine terminal rectum mucosa. Since E. coli O157:H7 rectal inoculation of cattle did demonstrate long-term colonisation in all experimentally infected animals, comparative rectal and oral inoculation studies that encompassed detailed characterisation of the ovine gastrointestinal tract were performed to determine if indeed the terminal rectum was the primary localisation site associated with E. coli O157:H7 persistence in the ovine host.
2. MATERIALS AND METHODS
2.1. Bacterial strain, inocula and reagents
E. coli O157:H7 strain NCTC12900nalr is a naturally occurring strain and was previously described and characterised using relevant in vitro and in vivo models [1, 15]. It does not possess stx1 and stx2, but is does induce attaching and effacing (A/E) rearrangements on HEp-2, and porcine cell lines and in vivo ovine models. NCTC12900nalr was stored in heart infusion broth (HIB) supplemented with 30% glycerol (v/v) at −80 °C and was streaked to single colonies on 5% sheep blood agar when required. For bovine and ovine IVOC assays and ovine in vivo studies, bacterial inocula was prepared by growth in 20 mL or 100 mL LB broths for 16 h, at 37 °C with shaking (225 rpm). For IVOC assays, 25 μL of overnight culture, corresponding to approximately 1 × 107 CFU, was inoculated directly onto each tissue explant. For in vivo studies, overnight LB broth cultures, corresponding to approximately 1 × 109 CFU/mL (1 × 1010 inocula) were administered as described below.
2.2. Experimental animals
For ovine and bovine IVOC, two healthy 6-week-old cross-bred lambs and two 12-week-old Holestein bull calves were used on two separate occasions. For ovine in vivo studies, a total of thirty, healthy 5-week-old cross bred lambs were used. Lambs were separated into groups and each group was housed in a separate room with a cement floor, maintained at negative pressure. For experiment 1, 12 5-week-old cross-bred conventionally reared lambs were randomly separated into two equal groups (A and B). For experiment 2, 18 cross-bred conventionally reared 5-week-old lambs were randomly divided into two groups, group C comprising of twelve animals and group D comprising of six animals. All groups were adapted to feed and housing conditions for at least one week prior to E. coli O157:H7 inoculation. Throughout these studies all lambs were fed pelleted feed twice daily and had free access to drinking water. Prior to challenge faecal samples were taken from all lambs and cattle used in all studies and confirmed negative for E. coli O157:H7 by immunomagnetic separation (IMS) and culture.
2.3. Bovine and ovine IVOC
The bovine and ovine intestinal IVOC system was adapted from the bovine and porcine intestinal IVOC systems described previously [10, 11]. Briefly, the rectal anal tissue of the terminal rectum and the last 2 cm of the rectum immediately before the terminal rectum, termed rectum-1, were excised and placed in cold complete RPMI medium without D-mannose. Approximately 12 mm2 explants were biopsied and placed mucosal side up onto biopsy foam pads, in sterile 24-well Nunclon Delta surface tissue culture plates, with 4 explants on each biopsy foam pad. Complete RPMI 1640 medium (10% Fetal Bovine serum, 0.25% lactalbumin hydrolsylate, 0.2 μg/mL hydrocortisone, 0.1 μg/mL insulin, 75 mM β-mercaptoethanol, 2 mM L-glutamine, 2 mM L-aspartate) was added to each well (1 mL). Explants were then placed on a see-saw rocker and maintained at 37 °C (5% CO2) for 8 h. Complete RPMI medium was replaced after the first 2 h post incubation and hourly thereafter.
2.4. In vivo challenge methods and collection of faeces
Two separate in vivo studies were performed with lambs challenged at 6-weeks of age. For experiment 1, all lambs in group A were orally challenged with 1 × 1010 CFU of E. coli O157:H7 isolate NCTC12900nalr. Inocula were delivered in a 10 mL volume using a worming gun (Novartis Animal Health) ensuring that the whole inoculum was delivered directly to the pharynx. Group B lambs acted as a negative control for analysis of immune cell populations in the epithelial cells of the terminal rectum. Rectal faecal samples were taken by digital insertion from all lambs in group A on days 3, 5 and 7 after inoculation. For experiment 2, all lambs in group C were dosed per rectum following manual faecal evacuation with 1 × 1010 CFU of E. coli O157:H7. Inocula were delivered in a 10 mL volume using a syringe and flexible catheter tube (Agrihealth, lamb reviver). Lambs were restrained for 2 min to ensure the inocula remained in the rectum. All group D lambs were orally challenged with 1 × 1010 CFU of E. coli O157:H7 as described for groups A and B of experiment 1. Faecal samples were taken per rectum by digital insertion daily for fourteen days post inoculation. For both experiment 1 and 2, necropsies were performed on selected lambs (Sect. 2.5). All procedures were conducted under the jurisdiction of Home Office licence 70/6250 granted under the Animals (Scientific Procedures) Act (1986).
2.5. Necropsy of E. coli O157:H7 infected lambs
All lambs from groups A and B from experiment 1 and from group C of experiment 2 were euthanased and necropsied as described previously [6]. Briefly, for experiment 1, two lambs were examined at days 3, 5 and 7 after inoculation, respectively. Tissue samples, either for bacterial enumeration or immunohistochemistry (IHC), were collected from the duodenum, jejunum, ileum, caecum, ascending colon, spiral colon, the last 2 cm of the distal rectum and the terminal rectum. For experiment 2, three lambs from group C were examined at day 1 and day 3 after inoculation and the remaining six animals were examined on day 14 after inoculation. Tissue samples for bacterial enumeration, IHC or confocal analysis, were collected from the duodenum, jejunum, ileum, caecum, ascending colon, spiral colon, terminal rectum and from six sites excised at approximately 2 cm apart measured from the terminal rectum (rectum-1), along the rectum toward the distal colon (rectum-2 to rectum-6) [15]. Group D lambs did not undergo necropsy since bacterial enumeration data for orally challenged animals was collated in experiment 1. However, an orally challenged group was still required for experiment 2, firstly, as a control for E. coli O157:H7 recovery in faecal samples and secondly, all six animals were required for effective statistical analyses.
2.6. Bacterial enumeration
For experiments 1 and 2, all E. coli O157 isolates were detected using methods described previously [15]. Briefly, lamb faeces (1 g) and necropsy tissue samples (1 g) were separately re-suspended in 9 mL of BPW, mashed with sterile forceps and vortexed. Ten-fold-serial dilutions were plated directly onto sorbitol MacConkey agar (SMAC) plates supplemented with nalidixic acid (15 μg/mL). If no direct counts were observed after overnight incubation of the plates at 37 °C, the BPW homogenates were enriched by incubation at 37 °C for 6 h, and then plated onto SMAC supplemented with nalidixic acid (15 μg/mL). The serogroup of bacteria recovered was verified by E. coli O157-specific latex agglutination (Oxoid, Basingstoke, UK).
2.7. Microscopy
Preparation of tissues taken at necropsy for subsequent visualisation by light and confocal microscopy was performed as described previously [15].
2.8. Statistical analysis
For oral and rectal shedding data from in vivo experiment 2, analysis of variance (ANOVA) was calculated on the log2 (counts). The data was used to calculate the difference between the bacterial counts for the oral and rectally E. coli O157:H7 inoculated lambs for each day and a p-value and 95% confidence intervals were generated.
3. RESULTS
3.1. Interaction of E. coli O157:H7 NCTC12900nalr with ovine IVOC
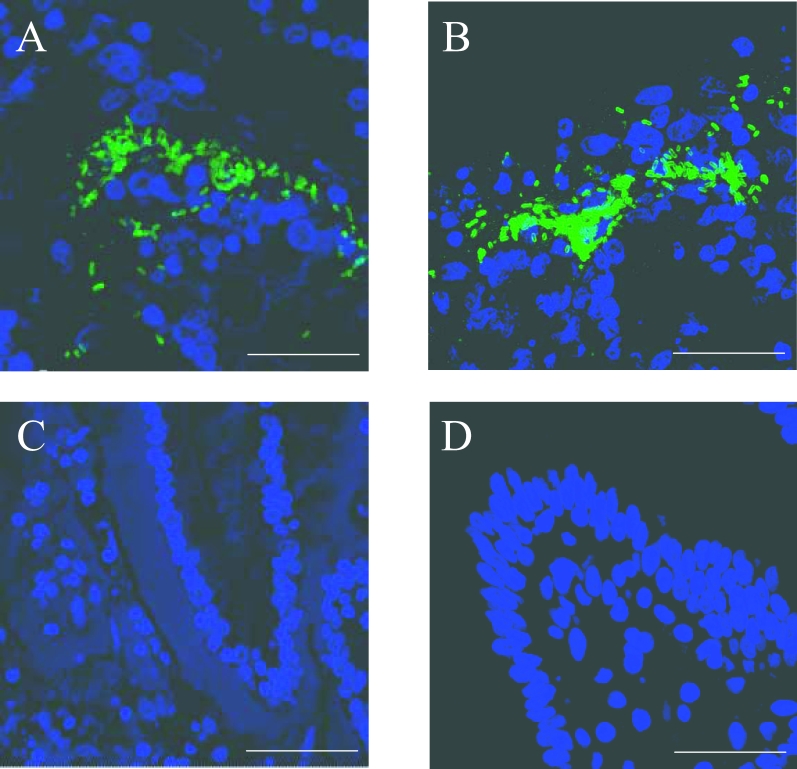
The ability of E. coli O157:H7 NCTC12900nalr to associate intimately with ovine and bovine rectum-1 and terminal rectum explants was assessed. After staining with anti-O157-FITC and analysis by confocal microscopy, small clusters of E. coli O157 (< 10 bacteria) were observed, although diffuse adherence patterns were also noted throughout all explant types. Albeit sparsely, larger, more densely packed micro-colonies of E. coli O157 were associated with the mucosa of terminal rectum explants (Fig. 1), but not the mucosa of rectum-1 explants (data not shown). Similar findings were made for the bovine control tissues.
Figure 1.
Observation of E. coli O157:H7 densely packed micro-colonies on ovine (A) and bovine (B) terminal rectum explants as visualised by confocal microscopy. Dapi (blue) = nuclei. FITC (green) = E.coli O157. (C) and (D) are uninfected ovine and bovine terminal rectum explants, respectively. E. coli O157:H7 was incubated with explants for 8 h with changing of the media occurring every hour after the first 2 h. Bar (A) = 32.17 μm, (B) = 27.5 μm, C = 49.26 μm, D = 31.77 μm.
3.2. Localisation of E. coli O157:H7 in the ovine gastrointestinal tract after oral inoculation of lambs
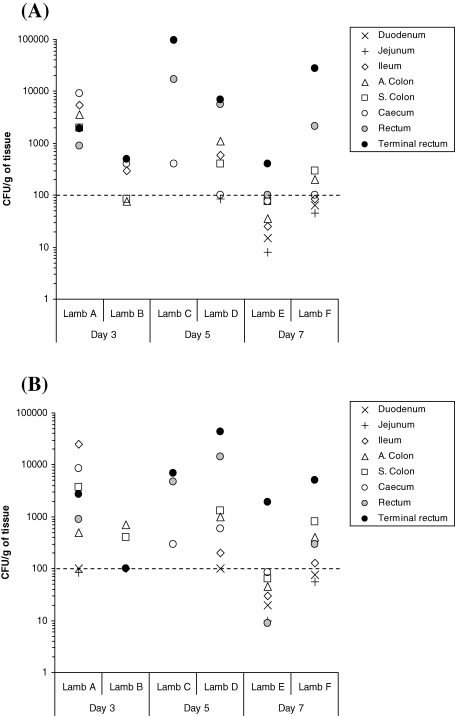
For experiment 1, lymphoid and non-lymphoid intestinal tissues from two, randomly selected orally inoculated 6-week-old lambs were taken at necropsy on days 3, 5 and 7 after oral inoculation. Samples were analysed by bacterial enumeration and tissues were also stained for CD3 (T-cell), CD79a (B-cell) and CD68 (monocyte macrophage) cells. Bacterial enumeration of homogenised tissues removed at necropsy did reveal that although all lambs were E. coli O157:H7 positive, not all lambs were positive for every tissue cultured (Fig. 2). Furthermore, there was variation in the numbers of E. coli O157:H7 recovered from each lamb at each time point. However, on days 5 and 7 after inoculation, approximately a 100-fold more E. coli O157:H7 were recovered from the rectum-1 and terminal rectum sites compared with all other GIT sites cultured. Comparison of lymphoid and non-lymphoid tissue was inconclusive with higher numbers recovered from lymphoid tissues in lambs C and F and from non-lymphoid tissues in lambs D and E. The highest numbers of E. coli O157:H7 were recovered consistently from the faeces of lamb D.
Figure 2.
Evaluation of E. coli O157:H7 tropisms to lymphoid (A) and non-lymphoid (B) regions of the GIT in young lambs orally inoculated with approximately 1 × 1010 CFU, as determined by bacterial enumeration after necropsy. All data points on or above the dotted line were obtained after direct plating. All data points below the dotted line were obtained after enrichment of faecal samples negative for growth after direct plating. These enrichment data points are only nominal and could represent any value between 1 and 99 CFU/g faeces−1. Samples where no E. coli O157:H7 growth was detected after enrichment are not shown. N.B.: Non-lymphoid ileum was actually sampled from a part of the ileum where less dense lymphoid tissue reside, as the majority of ovine ileum exhibits lymphoid rich sites.
Analysis of immune-cell infiltration in all lambs from days 5 and 7 after inoculation, revealed similar levels of T-cell, B-cell and monocyte macrophage specific staining in terminal rectum associated lymphoid tissue of lambs infected with E. coli O157:H7 as in unaffected controls (data not shown).
3.3. Duration and extent of E. coli O157:H7 faecal shedding from lambs inoculated rectally and orally
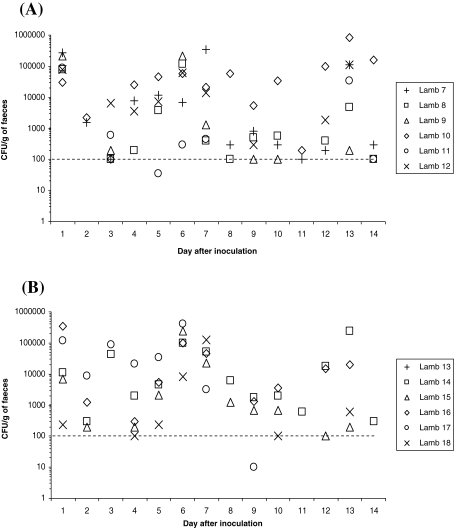
For experiment 2, faecal samples were taken daily over a 2-week period from 6 orally and 6 rectally inoculated 6-week-old lambs, and plated onto the appropriate media. The faecal shedding data from each individual lamb are shown in Figure 3. Twenty-four hours after rectal and oral inoculation, 6 and 5 lambs were positive, respectively, with recoveries at 104–105 and 102–105 CFU/g faeces−1, respectively. Throughout the 2-week period of the study, variable shedding of E. coli O157:H7 was noted with only one significant difference (p = 0.044) between groups observed at day 1 after inoculation in which recovery of E. coli O157:H7 from animals inoculated rectally was higher. Over the 2-week period of the study, 66/84 and 56/84 faecal samples were culture-positive for E. coli O157:H7 from rectally and orally inoculated lambs, respectively. At the end point of the study (day 14) four rectally inoculated lambs were shedding E. coli O157:H7, whereas only one lamb from the orally inoculated group was shedding E. coli O157:H7.
Figure 3.
The distribution of E. coli O157:H7 shedding over time after the inoculation of 6-week-old lambs either (A) rectally or (B) orally with approximately 1 × 1010 CFU. All data points shown are from direct plating only. E. coli O157:H7 was not detected from the faeces of lamb 21 during the fourteen day sampling period. Samples where no E. coli O157:H7 growth was detected after enrichment are not shown.
3.4. Recovery of E. coli O157:H7 NCTC12900nalr from tissues of the gastrointestinal tract of rectally inoculated lambs
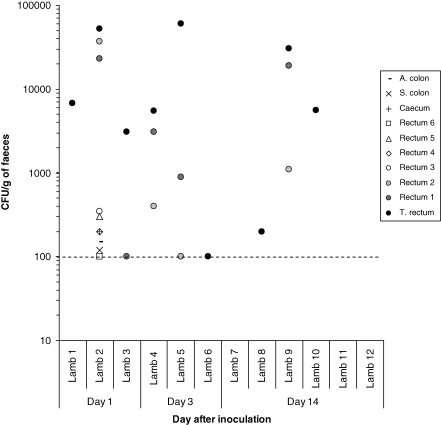
For experiment 2, necropsies were performed after rectal inoculation on three lambs on day 1, three lambs on day 3 and six lambs on day 14 after inoculation, respectively. The numbers of E. coli O157:H7 recovered from different sites along the distal GIT are shown in Figure 4. Recovery of E. coli O157:H7 from the faecal samples taken immediately before necropsy is also shown. Only one lamb was culture-positive for E. coli O157:H7 for all rectum, spiral and ascending colon, and caecum samples, although numbers were low, and that was on day 1. Otherwise, E. coli O157:H7 was not recovered from the colon, caecum and proximal rectum samples from any of the remaining 11 animals. No lambs were culture positive for E. coli O157:H7 recovery from the duodenum, jejunum and ileum. However, the terminal rectum was positive in 9 of 12 animals examined whereas rectum 1 and rectum 2 samples were positive in 5 of 12 and 4 of 12 animals, respectively.
Figure 4.
Recovery of E. coli O157:H7 from the gastrointestinal tract of young lambs rectally inoculated with approximately 1 × 1010 CFU. All data points shown on or above the dotted line are from direct plating only. No E. coli O157:H7 growth was detected after enrichment and zero values are not shown.
It was observed that tissues cultured from 3 lambs necropsied had more than 1 × 104E. coli O157:H7 CFU/g per terminal rectum shed E. coli O157:H7 up to 106 CFU/g of faeces. By contrast, six lambs with less than 1 × 104E. coli O157:H7 CFU/g per terminal rectum only shed lower numbers.
3.5. Visualisation of E. coli O157:H7 associated with the terminal rectum mucosa
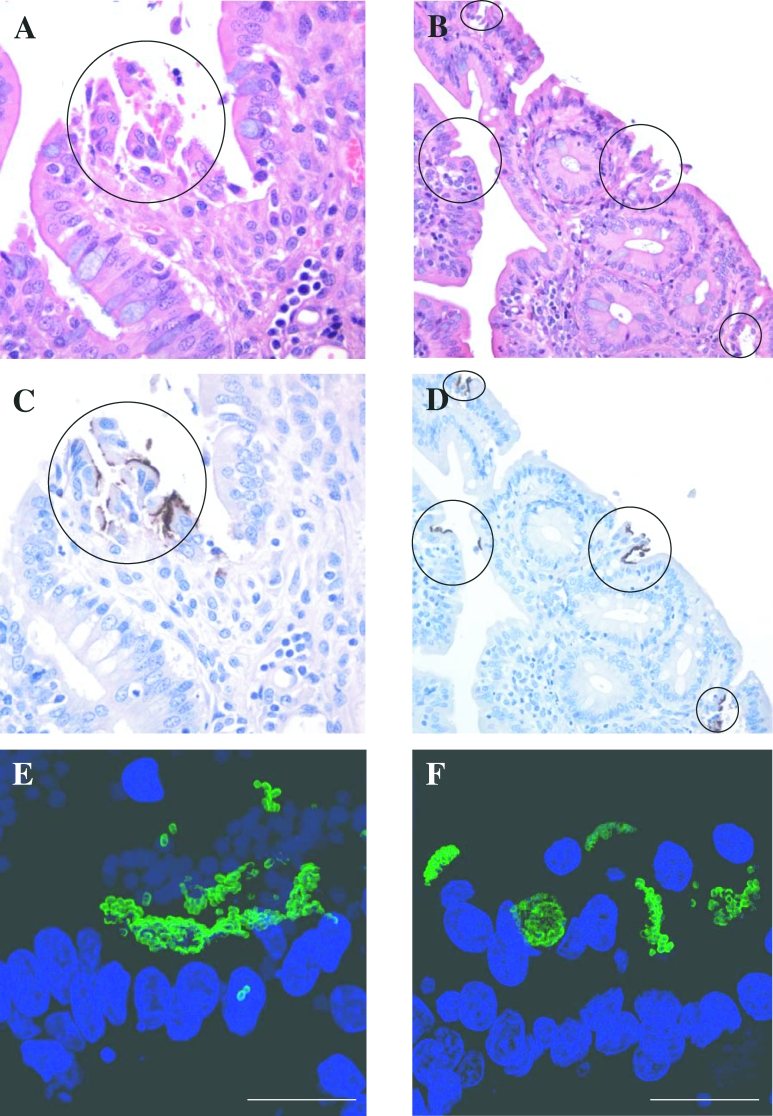
From experiment 2, duodenum, jejunum, ileum, spiral colon, ascending colon, caecum, rectum and terminal rectum tissue sections taken from the gastrointestinal tract were initially fixed in formalin for subsequent staining and analysis by microscopy. Taking into consideration the bacteriological data at necropsy, only tissues from rectum-1 and terminal rectum were selected for staining with anti-O157-FITC for examination by confocal microscopy (Tab. I). Seven of the 12 animals examined exhibited tissues that showed diffuse adherence and small clusters (< 10 bacteria) of E. coli O157:H7 to rectum-1 and terminal rectum. Of these, two exhibited large, densely packed micro-colonies at the terminal rectum mucosa (Tab. I and Fig. 5). By day 14, only 1 of the 6 rectally inoculated animals exhibited large, densely packed micro-colonies colonised at the rectum-1 and terminal rectum sites by E. coli O157:H7.
Table I.
Summary of adherence patterns of E. coli O157:H7 with the rectum-1 and terminal rectum of rectally inoculated young lambs as observed by confocal microscopy.
| Necropsy day after E. coli O157:H7 rectal inoculation |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
3 |
14 |
||||||||||
| DA |
LA |
DA |
LA |
DA |
LA |
|||||||
| Lamb | R1 | TR | R1 | TR | R1 | TR | R1 | TR | R1 | TR | R1 | TR |
| 1 | ✓ | - | - | - | ||||||||
| 2 | ✓ | ✓ | ✓ | ✓+ | ||||||||
| 3 | ✓ | ✓ | - | - | ||||||||
| 4 | ✓ | ✓ | - | ✓+ | ||||||||
| 5 | ✓ | ✓ | - | ✓+++ | ||||||||
| 6 | - | ✓ | - | - | ||||||||
| 7 | - | - | - | - | ||||||||
| 8 | - | - | - | - | ||||||||
| 9 | ✓ | ✓ | ✓+ | ✓+++ | ||||||||
| 10 | - | - | - | - | ||||||||
| 11 | - | - | - | - | ||||||||
| 12 | - | - | - | - | ||||||||
DA, diffuse adherence; LA, localised adherence; R1, rectum-1; TR, terminal rectum; X, diffuse adherence pattern observed; ✓+, small clusters of E. coli O157:H7 observed; ✓+++, large densely packed clusters of E. coli O157:H7; -, no E. coli O157:H7 detected.
Figure 5.
Light and confocal microscopy of E. coli O157:H7 localised adherence and micro-colony formation at the terminal rectum mucosa of rectally inoculated young lambs at day 3 (Lamb 5: A, C and E) and 14 (Lamb 9: B, D and F) after inoculation. Open circles denote localised adherence. Staining: (A and B) = Haematoxylin and Eosin; (C and D) = Meyer’s haematoxylin (terminal rectum-blue), immunoperoxidase (E. coli O157-brown); (E and F) Dapi (blue) = nuclei; FITC (green) = E.coli O157. Magnification (AandC) = 400×, (B and D) = 200×. Bar (E) = 20 μm, (F) = 18 μm.
4. DISCUSSION
Using previously described IVOC techniques [10, 11] we have demonstrated for the first time that E. coli O157:H7 can adhere and associate intimately, albeit sparsely as large, densely packed micro-colonies, on the mucosal surface of explants taken from the terminal rectum of 6-week-old lambs. That the explants are not under the same physiological conditions as in vivo, and the epithelial cell cycle and the metabolism of the explants may have be disrupted, may in part explain why few micro-colonies were observed. Indeed, epithelial cell rate proliferation, which will cease in biopsy, may be linked to the number of E. coli O157:H7 micro-colonies formed as indicated by previous cell-proliferation studies [18]. Since micro-colonies were only observed at the mucosa of the ovine terminal rectum, but not the rectum-1 mucosa, this may suggest a tissue tropism for this phenotype. However, the numbers of samples evaluated for the present study were limited and care should be taken in drawing firm conclusions. Nevertheless, a previous bovine IVOC study did demonstrate the development of micro-colonies at the bovine terminal rectum mucosa, although foci of intimately attached E. coli O157:H7 were observed at the ileum and colon [11].
Oral inoculation studies in sheep have suggested that the lower GIT is the primary location for E. coli O157:H7 colonisation [12, 28]. Since the E. coli O157:H7 used presently was Stx-negative, we undertook a detailed bacterial enumeration of ovine tissues from orally challenged lambs with a focus towards the terminal rectum and showed that on days 5 and 7 after inoculation, greater numbers of E. coli O157:H7 were recovered from the terminal rectum, as compared to other tissues of the GIT. Unlike Naylor et al. [22] for cattle, there was no conclusive evidence from our bacterial enumeration data, that E. coli O157:H7 preferentially associated with lymphoid follicles at the terminal rectum in sheep. Indeed, in some lambs more E. coli O157:H7 were associated with non-lymphoid tissue, suggesting no clear-cut tropism for lymphoid follicles. Also, analysis of immune cell infiltration did not reveal any heightened response to the presence of E. coli O157:H7 at the terminal rectum. In contrast, cattle studies have reported a local IgA response to E. coli O157:H7 colonisation of the terminal rectum [16, 21]. However, this local response did not clear E. coli O157:H7 from this primary colonisation site in cattle. Previous studies have suggested that during the first seven days after oral inoculation of sheep, E. coli O157:H7 may be passing through the digesta rather than colonising specific regions of the GIT [12, 26]. Nevertheless, the necrospy data presented here does indicate that on day 5 and 7, but not on day 3 after inoculation, E. coli O157:H7 did exhibit a tissue tropism towards the distal GIT. Therefore, a modified rectal administration methodology was initiated to test this hypothesis.
Rectal administration of E. coli O157:H7 using a cylindrical sponge previously demonstrated enhanced long-term persistence in cattle [26]. Since oral inoculation involves dispensing the inoculum into the pharynx, we wished to administer the inoculum in a similar manner onto the distal end of the rectum, so that the only experimental variable was the passing of E. coli O157:H7 through the GIT. At necropsy of E. coli O157:H7 rectally inoculated lambs, the terminal rectum was colonised with E. coli O157:H7 most frequently, and in some lambs was the only associated tissue at days 1, 3 and 14 after rectal inoculation. Rectum-1 was colonised with E. coli O157:H7, but in fewer lambs even though the inoculum was delivered proximal to this site. Nevertheless, confocal microscopy revealed that not all lambs that were E. coli O157:H7 culture-positive for terminal rectum, demonstrated E. coli O157:H7 micro-colonies on the terminal rectum mucosa. At the end point of this study, only one lamb which generally shed E. coli O157:H7 in higher numbers than all other lambs, exhibited large, densely packed micro-colonies. That five other remaining lambs at this time point did not demonstrate E. coli O157:H7 micro-colony formation with the terminal rectum, does suggest that within this cohort of animals, preferential colonisation of this site did not occur. Furthermore, if indeed the terminal rectum was the primary E. coli O157:H7 site, we may have noted rapid colonisation of this site as reported for cattle [5, 17, 26]. To further support the necropsy data, no significant differences in E. coli O157:H7 faecal shedding of 6 rectally and 6 orally inoculated lambs were noted from day 2 to day 14 after inoculation. That greater numbers were recovered from the faeces of rectally inoculated lambs on day 1 after inoculation is an unsurprising finding given the oral inoculum has to travel through the GIT before being shed.
Previous oral inoculation sheep studies from other laboratories have used mature, fully ruminating sheep to monitor E. coli O157:H7 persistent colonisation. Grauke et al. [12] did isolate the highest numbers of E. coli O157:H7 from the lower GIT, but the recovery of positive cultures was inconsistent and only occurred during the first week after oral inoculation. However, after an abrupt dietary change 42 days after inoculation, E. coli O157:H7 was recovered from the rectal tissue of one animal. A subsequent rectal administration model of E. coli O157:H7 colonisation in adult ruminants did further support the findings of Naylor et al. [22] that the terminal rectum mucosa is the primary colonisation site in cattle [26]. Interestingly, the authors of the latter study discuss E. coli O157:H7 oral and rectal challenge of fully mature cattle, but only the oral challenge of fully mature sheep. Whether the authors did rectally swab fully mature sheep with E. coli O157:H7 and a poor take was observed was not discussed. Importantly, the rectal administration method used presently involved applying a flush of E. coli O157:H7 in liquid rather than inserting a saturated sponge swab. As discussed previously [26], by its’ very nature, rubbing a saturated sponge against the terminal rectum mucosa will have contributed to the enhanced colonisation of E. coli O157:H7 and in part may explain why consistent colonisation of the ovine terminal rectum was not observed. Nevertheless, the literature does suggest that locating the primary E. coli O157:H7 colonisation site in adult sheep is thwarted with difficulties.
Intermittent E. coli O157:H7 colonisation of the terminal rectum may be in part explained by the differences in the mechanics of ovine and bovine faeces formation. Despite ovine and bovine species having physiological similarities with respect to the spiral colon, in the ovine host this region of the GIT is the only site of faecal pellet formation. In sheep, this distinctiveness is coupled with a permanent and localised contractile activity to give faeces in the form of pellets, whereas in cattle, faeces are formed during 5 min phases of continuous slow migrating activity away from the proximal GIT (aborally) [25]. These mechanical and physiological differences between the faecal types (hard versus soft) may imply that E. coli O157:H7 in the digesta of the GIT, become encased within the hard faecal pellets at the ovine spiral colon, thus reducing the chances of E. coli O157:H7 colonisation at the terminal rectum mucosa.
Whether Shiga-toxin plays a significant role in colonisation of the terminal rectum remains unclear, although Naylor et al. [22] did demonstrate that a naturally occurring Stx-negative human outbreak E. coli O157:H7 strain did preferentially colonise the terminal rectum mucosa. Furthermore, Cornick et al. [8] has reported that Shiga-toxin does not facilitate E. coli O157:H7 colonisation in sheep.
In conclusion, the studies presented here are the first to report that E. coli O157:H7 can form micro-colonies on ovine terminal rectum explants and colonise the terminal rectum of rectally inoculated young lambs. Although, the in vivo data does suggest that the ovine terminal rectum may be colonised by E. coli O157:H7, colonisation is less efficient than in cattle. Furthermore, and in contrast to rectally inoculated cattle [5, 17, 26] the numbers of E. coli O157:H7 shed in ovine faeces over the time-course of the study were not significantly different to those of orally inoculated lambs. We suggest that a more comprehensive understanding of the factors that promote effective colonisation at the ovine terminal rectum requires further analysis in order to assess the appropriateness of intervention strategies [4, 19, 20] for use in controlling E. coli O157:H7 in the field.
Acknowledgements
The authors would like to thank the ASU and Histopathology staff who assisted with this study and Defra, UK, for funding much of the work reviewed in this manuscript (Grant OZO713).
REFERENCES
- 1.Best A., La Ragione R.M., Clifford D., Cooley W.A., Sayers A.R., Woodward M.J.. A comparison of Shiga-toxin negative Escherichia coli O157 aflagellate and intimin deficient mutants in porcine in vitro and in vivo models of infection. Vet. Microbiol. 2006;113:63–72. doi: 10.1016/j.vetmic.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 2.Borczyk A.A., Karmali M.A., Lior H., Duncan L.M.. Bovine reservoir for verotoxin-producing Escherichia coli O157:H7. Lancet. 1987;1:98. doi: 10.1016/s0140-6736(87)91928-3. [DOI] [PubMed] [Google Scholar]
- 3.Chapman P.A., Cerdan-Maló A.T., Ellin M., Ashton R.. Escherichia, coli O157 in cattle and sheep at slaughter, on beef and lamb carcasses and in raw beef and lamb products in South Yorkshire, UK. Int. J. Food Microbiol. 2001;64:139–150. doi: 10.1016/s0168-1605(00)00453-0. [DOI] [PubMed] [Google Scholar]
- 4.Chase-Topping M.E., McKendrick I.J., Pearce M.C., MacDonald P., Mathews L., Halliday J.. et al. Risks factors for the presence of high-level shedding of Escherichia coli O157 on Scottish farms. J. Clin. Microbiol. 2007;45:1594–1603. doi: 10.1128/JCM.01690-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cobbold R.N., Hancock D.D., Rice D.H., Berg J., Stilborn R., Hovde C.J., Besser T.E.. Rectoanal junction colonisation of feedlot cattle by Escherichia coli O157:H7 and its association with supershedders and excretion dynamics. Appl. Environ. Microbiol. 2007;73:1563–1568. doi: 10.1128/AEM.01742-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cookson A.L., Wales A.D., Roe J.M., Hayes C.M., Pearson G.R., Woodward M.J.. Variation in the persistence of Escherichia coli O157:H7 in experimentally inoculated 6-week-old conventional lambs. J. Med. Microbiol. 2002;12:1032–1040. doi: 10.1099/0022-1317-51-12-1032. [DOI] [PubMed] [Google Scholar]
- 7.Cornick N.A., Booher S.L., Casey T.A., Moon H.W.. Persistent colonisation of sheep by Escherichia coli O157:H7 and other E. coli pathotypes. Appl. Environ. Microbiol. 2000;66:4926–4934. doi: 10.1128/aem.66.11.4926-4934.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornick N.A., Helgerson A.F., Sharma V.. Shiga toxin and Shiga toxin-encoding phage do not facilitate Escherichia coli O157:H7 colonization in sheep. Appl. Environ. Microbiol. 2007;73:344–346. doi: 10.1128/AEM.01328-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gansheroff L.J., O’Brien A.D.. Escherichia coli O157:H7 in beef cattle presented for slaughter in the U.S.: higher prevalence rates than previously estimated. Proc. Natl. Acad. Sci. USA. 2000;97:2959–2961. doi: 10.1073/pnas.97.7.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girard F., Batisson I., Frankel G., Harel J., Fairbrother J.M.. Interaction of Enteropathogenic and shiga toxin-producing Escherichia coli and porcine intestinal mucosa: role of intimin and tir in adherence. Infect. Immun. 2005;73:6005–6016. doi: 10.1128/IAI.73.9.6005-6016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girard F., Dziva F., van Diemen P., Phillips A.D., Stevens M.P., Frankel G.. Adherence of enterohemorrhagic Escherichia coli O157, O26 and O111 strains to bovine intestinal explants ex vivo. Appl. Environ. Microbiol. 2007;73:3084–3090. doi: 10.1128/AEM.02893-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grauke L.J., Kudva I.T., Won Yoon J., Hunt C.W., Williams C.J., Hovde C.J.. Gastrointestinal tract location of Escherichia coli O157:H7 in ruminants. Appl. Environ. Microbiol. 2002;68:2269–2277. doi: 10.1128/AEM.68.5.2269-2277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karmali M.A., Petric M., Lim C., Flemming P.C., Steele P.T.. Escherichia coli cytotoxin, haemolytic-uraemic syndrome, and haemorrhagic colitis. Lancet. 1983;2:1299–1300. doi: 10.1016/s0140-6736(83)91167-4. [DOI] [PubMed] [Google Scholar]
- 14.Kudva I.T., Hatfield P.G., Hovde C.J.. Escherichia coli O157:H7 in the microbial flora of sheep. J. Clin. Microbiol. 1995;34:431–433. doi: 10.1128/jcm.34.2.431-433.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.La Ragione R.M., Best A., Clifford D., Weyer U., Johnson L., Marshall R.N.. et al. Influence of colostrum deprivation and concurrent Cryptosporidium parvum infection on the colonisation and persistence of Escherichia coli O157:H7 in young lambs . Med. Microbiol. 2006;55:819–828. doi: 10.1099/jmm.0.46469-0. [DOI] [PubMed] [Google Scholar]
- 16.Li J., Hovde C.J.. Expression profiles of bovine genes in the rectoanal junction mucosa during colonisation with Escherichia coli O157:H7. Appl. Environ. Microbiol. 2007;73:2380–2385. doi: 10.1128/AEM.02262-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim J.Y., Li J., Sheng H., Besser T.H., Potter K., Hovde C.J.. Escherichia coli O157:H7 colonisation at the rectoanal junction of long-duration culture-positive cattle. Appl. Environ. Microbiol. 2007;73:1380–1382. doi: 10.1128/AEM.02242-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnuson B.A., Davis M., Hubele S., Austin P.R., Kudva I.T., Williams C.J.. et al. Ruminant gastrointestinal cell proliferation and clearance of Escherichia coli O157:H7. Infect. Immun. 2000;68:3808–3814. doi: 10.1128/iai.68.7.3808-3814.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mathews L., Low J.C., Gally D.L., Pearce M.C., Mellor D.I., Heesterbeek J.A.P.. et al. Heterogenous shedding of Escherichia coli O157 in cattle and its implications for control. Proc. Natl. Acad. Sci. USA. 2006;103:547–552. doi: 10.1073/pnas.0503776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathews L., McKendrick I.J., Ternent H., Gunn G.J., Synge B., Woolhouse M.E.J.. Super-shedding cattle and the transmission dynamics of Escherichia coli O157. Epidemiol. Infect. 2006;134:131–142. doi: 10.1017/S0950268805004590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nart P., Holden N., McAteer S.P., Wang D., Flockhart A.F., Naylor S.W.. et al. Mucosal antibody responses of colonised cattle to Escherichia coli O157-secreted proteins, flagellin, outer membrane proteins and lipopolysaccharide. FEMS Immunol. Med. Microbiol. 2007;52:59–68. doi: 10.1111/j.1574-695X.2007.00341.x. [DOI] [PubMed] [Google Scholar]
- 22.Naylor S.W., Low J.C., Besser T.E., Mahajan A., Gunn G.J., Pearce M.C.. et al. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonisation of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 2003;71:1505–1512. doi: 10.1128/IAI.71.3.1505-1512.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogden I.D., Hepburn N.F., MacRae M., Strachan N.J.C., Fenlon D.R., Rusbridge S.M., Penningtion T.H.. Long-term survival of Escherichia coli O157 on the pasture following an outbreak associated with sheep at a scout camp. Lett. Appl. Microbiol. 2002;34:100–104. doi: 10.1046/j.1472-765x.2002.01052.x. [DOI] [PubMed] [Google Scholar]
- 24.Riley L.W., Remis R.S., Helgerson S.D., McGee H.B., Wells J.G., Davis B.R.. et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 25.Ruckebusch Y., Fioramonti J.. Motor profile of the ruminant colon: hard vs. soft faeces production. Experientia. 1980;36:1184–1185. doi: 10.1007/BF01976116. [DOI] [PubMed] [Google Scholar]
- 26.Sheng H., Davis M.A., Knecht H.J., Hovde C.J.. Rectal administration of Escherichia coli O157:H7: novel model for colonisation of ruminants. Appl. Environ. Microbiol. 2004;70:4588–4595. doi: 10.1128/AEM.70.8.4588-4595.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Torres A.G., Milflores-Flores L., Gerardo Garcia-Gallegos J., Patel S.D., Best A., La Ragione R.M.. et al. Environmental regulation and colonisation attributes of long polar fimbriae (LPF) of Escherichia coli O157:H7. Int. J. Med. Microbiol. 2007;297:177–185. doi: 10.1016/j.ijmm.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Woodward M.J., Best A., Sprigings K.A., Pearson G.A., Skuse A.M., Wales A.D.. et al. Non-toxigenic Escherichia coli O157:H7 strain NCTC12900 causes attaching-effacing lesions and eae-dependent persistence in weaned sheep. Int. J. Med. Microbiol. 2003;293:299–308. doi: 10.1078/1438-4221-00264. [DOI] [PubMed] [Google Scholar]
- 29.Vlisidou I., Dziva F., La Ragione R.M., Best A., Garmendia J., Hawes P.. et al. Role of intimin-tir interactions and the tir-cytoskeleton coupling protein in the colonization of calves and lambs by Escherichia coli O157:H7. Infect. Immun. 2006;74:758–764. doi: 10.1128/IAI.74.1.758-764.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]