Abstract
Shedding of prions via faeces may be involved in the transmission of contagious prion diseases. Here, we fed hamsters 10 mg of 263K scrapie brain homogenate and examined the faecal excretion of disease-associated prion protein (PrPTSE) during the course of infection. The intestinal fate of ingested PrPTSE was further investigated by monitoring the deposition of the protein in components of the gut wall using immunohistochemistry and paraffin-embedded tissue (PET) blotting. Western blotting of faecal extracts showed shedding of PrPTSE in the excrement at 24–72 h post infection (hpi), but not at 0–24 hpi or at later preclinical or clinical time points. About 5% of the ingested PrPTSE were excreted via the faeces. However, the bulk of PrPTSE was cleared from the alimentary canal, most probably by degradation, while an indiscernible proportion of the inoculum triggered intestinal infection. Components of the gut-associated lymphoid tissue (GALT) and the enteric nervous system (ENS) showed progressing accumulation of PrPTSE from 30 days post infection (dpi) and 60 dpi, respectively. At the clinical stage of disease, substantial deposits of PrPTSE were found in the GALT in close vicinity to the intestinal lumen. Despite an apparent possibility of shedding from Peyer’s patches that may involve the follicle-associated epithelium (FAE), only small amounts of PrPTSE were detected in faeces from clinically infected animals by serial protein misfolding cyclic amplification (sPMCA). Although excrement may thus provide a vehicle for the release of endogenously formed PrPTSE, intestinal clearance mechanisms seem to partially counteract such a mode of prion dissemination.
Keywords: prion, oral infection, Peyer’s patches, enteric nervous system, faeces
1. INTRODUCTION
Transmissible spongiform encephalopathies (TSE), also termed prion diseases [1], such as scrapie in sheep and goats, bovine spongiform encephalopathy (BSE), chronic wasting disease (CWD) of elk and deer, or human Creutzfeldt-Jakob disease (CJD) and its variant form vCJD are neurodegenerative and ultimately fatal diseases of the central nervous system (CNS). Characteristically, TSE are associated with the deposition in the brain and spinal cord of a pathological form of the prion protein (PrP) with an abnormally altered folding and/or aggregation structure. The prion hypothesis holds that the causative and transmissible agents of TSE consist in proteinaceous infectious particles (“prions”) that are composed essentially – if not entirely – of misfolded PrP, designated as PrPSc [36, 37]. Disease-associated prion protein, in the following referred to as PrPTSE [8, 13], can be detected in samples from affected animals or humans by analytical methods such as immunohistochemistry (IHC) [22, 33, 47], Western blotting [5, 40, 52], or paraffin-embedded tissue (PET) blotting [41].
In naturally acquired scrapie, CWD, BSE, and vCJD, infection seems to occur most often by ingestion of TSE agents and subsequent invasion of the host via the alimentary tract [8, 28]. Scrapie of sheep and goats and CWD of cervids are contagious diseases that are transmitted horizontally in the field. Contaminated placenta, decomposed carcasses, saliva, urine or skin have been suggested as the contagion of these diseases [15, 30, 34, 38, 42, 46], but faeces provide a further candidate vehicle for the release of prions into the environment [29, 39].
In the past, hamsters orally challenged with scrapie have been established as a relevant rodent model to study the intestinal pathophysiology of alimentary prion infections by taking advantage of PrPTSE as a biochemical marker for infectious prions [7, 8, 33].
Here, we used the hamster model of oral scrapie infection in an attempt to further elucidate pathophysiological events in the intestinal tract following ingestion of prions. In this context, special attention was given to the intestinal fate of ingested PrPTSE, the course of PrPTSE accumulation in the GALT and the ENS, and the shedding of PrPTSE in faeces.
2. MATERIALS AND METHODS
2.1. Animal inoculation
All animal experiments were performed using outbred Syrian hamsters. The studies in animals complied with German legal regulations and were approved by the responsible review boards. Hamsters were fed food pellets doused each with 100 μL of a 10% (w/v) hamster-brain homogenate derived from terminally ill donors infected with 263K scrapie agent, and control animals were mock challenged with brain homogenate from normal hamsters as described previously [3].
2.2. Biochemical detection of PrPTSE in faeces
For sampling of faeces, hamsters were placed in metabolic cages (Harvard Apparatus, Holliston, USA) with free access to food and drinking water. Animals were transferred into metabolic cages once they had ingested the infectious inoculum, and faecal samples were collected at 0–24 hpi, 24–48 hpi, 48–72 hpi, 72–96 hpi, at 70, 100, and 130 dpi prior to the onset of scrapie symptoms, and when the animals were in advanced clinical stages of the disease between 145 and 160 dpi (see Fig. 1). Faeces from individual animals (n > 8 for each investigated time point) sampled before they were perorally challenged with scrapie agent served as negative controls.
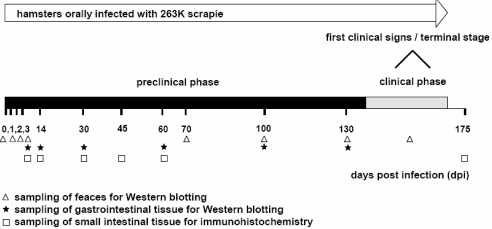
Figure 1.
Time scale displaying the preclinical and clinical phases of incubation of hamsters orally infected with 263K scrapie and indicating the time points at which samples from the animals were collected and tested for PrPTSE. Faeces samples collected at 0–24, 24–48, 48–72 and 72–96 hpi were referred to in the figure as specimens from 0, 1, 2, and 3 dpi, respectively.
A 10% (w/v) faecal homogenate, adjusted with normal hamster brain homogenate as carrier material to a final concentration of 0.125% (w/v) brain tissue, was prepared with an ultraturrax in TBS buffer (50 mM Tris/HCl, 150 mM NaCl, pH 7.2). Extraction of PrPTSE from the faecal homogenate was performed by using a previously published protocol [4] with modifications. In brief: A total of 2 mL sample homogenate (containing 200 mg of faeces) was distributed equally on two 1.5 mL centrifuge vials. All centrifugations were performed in a TLA-55 rotor (Beckman). Samples were centrifuged at 5 000 rpm (1 500 × g) and 4 °C for 3 min, the supernatants were collected and the pellets resuspended in 0.5 mL TBS each. These suspensions were submitted to a second centrifugation at 5 000 rpm (1 500 × g, 4 °C, 3 min), and the resulting supernatants were collected and mixed with the supernatants from the previous centrifugation and centrifuged again for 40 min at 20 000 rpm (25 000 × g) and 4 °C.
The resulting pellets were resuspended in 600 μL 1% (w/v) sarcosyl in TBS using an ultrasonic probe and centrifuged for 40 min at 20 °C and 20 000 rpm (25 000 × g). The resulting supernatants were pooled and submitted to a further centrifugation at 45 000 rpm (125 000 × g) and 4 °C for 2.5 h. The resulting pellet was resuspended in 1 mL 0.1% sarcosyl in TBS, homogenized by ultrasonification, and incubated with constant shaking at 37 °C in the presence of 12.5 μg/mL proteinase K (PK) for 15 min. After a final centrifugation for 2.5 h at 45 000 rpm (125 000 × g) and 4 °C, the resulting pellet was suspended in 20 μL of 2 × Laemmli sample buffer, boiled for 5 min and stored at −80 °C until further processing. The final pellet contained PrPTSE in the form of its PK-resistant core PrP27-30, which was visualized by Western blotting using CDP-Star solution as described elsewhere [44].
A subset of faecal samples from clinically-ill scrapie hamsters was subjected to serial protein misfolding cyclic amplification (sPMCA) for detection of PrPTSE. Here, extraction of PrPTSE was performed as described above with the following modifications: Treatment with PK was omitted during the extraction, and the resulting pellet of the final centrifugation (2.5 h at 45 000 rpm and 4 °C) was resuspended directly in 10% (w/v) normal brain homogenate in PMCA conversion buffer (phosphate buffered saline containing complete protease inhibitor cocktail by Boehringer-Ingelheim, Mannheim, Germany, 4 mM EDTA and 1% Triton-X-100). Serial PMCA was performed as described recently [43].
Faecal samples spiked with 263K scrapie hamster brain homogenate served as positive controls and were used to assess the detection limit of our assay. If not otherwise stated, samples representing 200 mg of faeces in the starting homogenates were loaded onto gels for Western blotting of unknown and control faecal specimens.
In order to visualize the immunological cross-reactivity of crude faecal material 100 mg of normal hamster faeces were homogenized in 400 μL 2 × Laemmli sample buffer, boiled for 10 min and centrifuged at 13 000 rpm (13 000 × g). Ten microliters of the resulting supernatant (representing 2 mg of faeces in the starting homogenate) were then loaded onto the gel for mAb 3F4 Western blotting.
2.3. Western blot analysis of gastrointestinal tissue for PrPTSE
For Western blot analyses of gastrointestinal tissue samples, hamsters (n > 3 animals for each examined time point) were sacrificed by CO2 asphyxiation at 3, 14, 30, 60, 100, and 130 dpi (see Fig. 1). The entire small intestine was removed, carefully processed to eliminate residual content, and subdivided into 20 segments of about 2 cm in length weighing between 50 and 100 mg. The tissue samples were frozen immediately in liquid nitrogen and stored at −80 °C until further processing. After digestion by collagenase and further tissue disintegration by ultrasonification, PrPTSE was extracted in the form of PrP27-30 as described elsewhere [4]. Samples from the small intestine of parallel mock-infected hamsters served as negative controls; samples from uninfected hamsters spiked with 263K scrapie hamster brain homogenate containing 10−6 g of scrapie brain tissue were used as positive controls (not shown).
At 60, 70, 80, 100, and 130 dpi (n = 1, 1, 1, 2, and 4 hamsters, respectively) caeca and large intestines as well as the stomachs and oesophagi were removed from sacrificed animals. These tissue specimens, after removal of residual content, were dissected into pieces of between 50 and 100 mg and subjected to extraction and Western blotting of PrP27-30. Negative and positive controls were prepared as described above.
2.4. Immunohistochemistry and PET blotting for PrpTSE detection in intestinal samples
For immunohistochemical detection of intestinal PrPTSE deposition in situ, hamsters were transcardially perfused with periodate lysine paraformaldehyde (PLP) [31] immediately after CO2 euthanasia performed at 3 dpi (n = 2), and 14, 30, 45 and 60 dpi (n = 6 for each time point), or at the terminal stage of clinical scrapie (n = 3) (see Fig. 1). The distal half of the small intestine was removed and subdivided into 10 segments of about 2 cm in length. Segments from mock-infected animals served as negative controls while terminally ill animals provided intestinal specimens for positive controls. After washing in TBS and postfixation for 5 h in PLP, the samples were dehydrated in ethanol and embedded in paraffin-wax. For microscopic investigation, 6 μm microtome sections were treated with formic acid and immunostained using the monoclonal antibody (mAb) 3F4 [27] and diaminobenzidine as described elsewhere [32]. Normal mouse serum replaced mAb 3F4 in adjacent sections as the internal control for staining specificity. Additionally, 6 μm microtome slices were mounted onto nitrocellulose membranes and processed for PrPTSE detection by PET blotting as described previously [41, 45].
The time scale in Figure 1 displays the preclinical and clinical phases of incubation of hamsters orally infected with 263K scrapie (see also [46]), and indicates the time points at which samples from the animals were tested for PrPTSE.
3. RESULTS
3.1. Western blot detection of PrPTSE in faeces
3.1.1. Specificity, sensitivity and yield of the extraction method
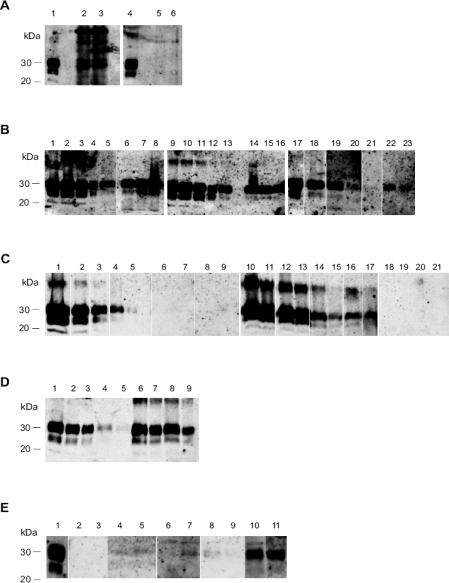
The monoclonal antibody 3F4 has long since been established as an exquisite immunological probe for Western blot detection of normal and pathological prion protein of hamsters. However, as shown in Figure 2A (lanes 2 and 3), direct PrP analysis of raw hamster faeces by Western blotting is not feasible due to substantial unspecific cross-reactivity with various stool components.
Figure 2.
(A) Immunological cross-reactivity of hamster faeces in the Western blot (labeling with mAb 3F4). Lanes 1 and 4: PK digested 263K scrapie hamster brain homogenate containing 5 × 10−7 g of brain tissue. Lanes 2 and 3: Crude homogenates from 2 mg of normal hamster faeces. Lanes 5 and 6: Purified extracts from 100 mg of normal hamster faeces. (B) Semi-quantitative Western blot assessment of the extraction recovery of PrP27-30, the PK-resistant core of PrPTSE, from hamster faeces spiked with 263K scrapie brain homogenate. Lanes 1–5, 9–13 and 17–21: Serially diluted 263K scrapie hamster brain homogenate containing 1 × 10−6 g (lanes 1 and 9), 7 × 10−7 g (lanes 2 and 10), 5 × 10−7 g (lanes 3 and 11), 2.5 × 10−7 g (lanes 4, 12 and 17), 1 × 10−7 g (lanes 5, 13 and 18), 5 × 10−8 g (lane 19), 2 × 10−8 g (lane 20) and 1 × 10−8 g (lane 21) tissue. Lanes 6-8: Extracts from 200 mg normal hamster faeces spiked with 5 × 10−6 g of homogenized 263K scrapie brain tissue before purification. Lanes 14-16: Extracts from 200 mg normal hamster faeces spiked with 3 × 10−6 g of homogenized 263K scrapie brain tissue before purification. Lanes 22–23: Extracts from 200 mg normal hamster faeces spiked with 5 × 10−7 g of homogenized 263K scrapie brain tissue before purification. (C) Western blot detection of PrP27-30, the PK-resistant core of PrPTSE, in faeces of hamsters fed with 263K scrapie brain homogenate. Lanes 1–5: Serially diluted 263K scrapie hamster brain homogenate containing 5 × 10−6 g (lane 1), 1 × 10−6 g (lane 2), 2.5 × 10−7 g (lane 3), 1 × 10−7 g (lane 4) and 2.5 × 10−8 g (lane 5) tissue. Lanes 6-21: Extracts from 200 mg faeces sampled at 0–24 hpi (lanes 6-9), 24–48 hpi (lanes 10-13), 48–72 hpi (lanes 14–17) and 72–96 hpi (lanes 18–21) from 4 different animals. (D) Western blot detection of PrP27-30, the PK-resistant core of PrPTSE, in faeces of hamsters fed with 263K scrapie brain homogenate – Adjustment of dilutions for semi-quantitative assessment of PrPTSE excretion. Lanes 1–5: Serially diluted 263K scrapie hamster brain homogenate containing 5 × 10−7 g (lane 1), 2.5 × 10−7g (lane 2), 1 × 10−7 g (lane 3), 5 × 10−8g (lane 4) and 2 × 10−8 g (lane 5) tissue. Lanes 6–9: 1:2 (lanes 6 and 8) and 1:5 (lanes 7 and 9) diluted extracts from 200 mg faeces sampled at 24–48 hpi (dilutions were prepared from the extracts represented in lanes 10 and 12 of Fig. 2C). (E) Western blot detection of PrP27-30, the PK-resistant core of PrPTSE, after serial protein misfolding cyclic amplification (sPMCA) of PrPTSE in faeces extracts from clinically diseased scrapie hamsters fed with 263K scrapie agent. Lane 1: 263K scrapie hamster brain homogenate containing 5 × 10−7 g tissue. Lanes 2–11: sample signals of extracts from 200 mg faeces of two donor animals after 200 (lanes 2 and 3), 250 (lanes 4 and 5), 300 (lanes 6 and 7), 350 (lanes 8 and 9) and 400 (lanes 10 and 11) cycles of sPMCA.
Our protocol for the extraction of PrPTSE from faeces removed such interfering components (Fig. 2A, lanes 5 and 6) and reliably allowed detection of PrP27-30 in positive control samples of 200 mg faeces spiked with 5 × 10−7 g of homogenized 263K scrapie brain tissue (Fig. 2B, lanes 22 and 23). The staining intensities of the extracts in lanes 22 and 23 were comparable to that of the reference sample in lane 20 (representing 2 × 10−8 g of scrapie brain tissue). This indicates that our extraction method recovered in the final extract ~4% of the faecal PrPTSE spike. The staining intensities of extracts from samples spiked with 3 × 10−6 g of homogenized 263K scrapie brain tissue (Fig. 2B, lanes 14–16) resembled those of the reference signals in lanes 13 and 18 (each representing 1 × 10−7 g of scrapie brain tissue), suggesting an extraction yield of ~3%. Higher amounts of spiking material (i.e. 5 × 10−6g; Fig. 2B, lanes 6–8) resulted in slightly increased retrieval rates of > 5%.
3.1.2. Faecal shedding of PrPTSE
After feeding scrapie brain homogenate to our model animals, faecal excretion of PrPTSE could be observed consistently in samples collected at 24–48 hpi and 48–72 hpi (Fig. 2C, lanes 10–13 and 14–17, respectively). In contrast, no PrP27-30, the PK-resistant core of PrPTSE, was detected by Western blotting of extracts from specimens collected at 0–24 hpi (Fig. 2C, lanes 6–9), 72–96 hpi (Fig. 2C, lanes 18–21), or at later preclinical time points that were examined in our study, i.e. at 70, 100, and 130 dpi, as well as at the clinical stage of disease (not shown). Thus, the observed retention period of the inoculum in the gastrointestinal tract was found to be < 3 days. However, PMCA increased the sensitivity of PrPTSE detection in faeces and revealed that small amounts of the protein were present in faecal extracts from 2 out of 4 clinically-ill scrapie hamsters that were tested (Fig. 2E). Extracts from normal hamster faeces consistently produced negative results after sPMCA (not shown).
3.1.3. Fate of ingested PrPTSE
Hamsters shed between 2–4 g of faeces per day during the first 96 h after infection. Together with this observation, a semi-quantitative comparison of test- and reference samples in Figures 2C and 2D allowed to assess the proportion of ingested PrPTSE that had been faecally excreted. In the following, such an assessment is exemplified for one animal for which the faecal extracts from samples collected at 0–24 hpi, 24–48 hpi, 48–72 hpi and 72–96 hpi have been blotted in lanes 6, 10, 14 and 18 of Figure 2C, respectively. For 0–24 hpi and 72–96 hpi no PrPTSE could be detected. However, the staining intensity observed for 24–48 hpi (Fig. 2C, lane 10) corresponded approximately to that of 1 × 10−6 g scrapie hamster brain tissue (Fig. 2C, lane 2). Therefore, taking the extraction yield determined above (~4%) into account, it can be concluded that the examined faecal sample (200 mg) contained an amount of PrPTSE which was equivalent to that present in 2.5 × 10−5 g of homogenized scrapie hamster brain. For the interval of 48–72 hpi the staining intensity of the faeces sample from the animal (Fig. 2C, lane 14) resembled that of 2.5 × 10−7 g scrapie hamster brain tissue (Fig. 2C, lane 3), suggesting that the amount of PrPTSE in this sample (again 200 mg) corresponded to that in 6.25 × 10−6 g scrapie hamster brain tissue. Accordingly, with about 3 g faeces shed per day, the animal excreted an amount of PrPTSE equivalent to that present n approximately 15 × 2.5 × 10−5g + 15 × 6.25 × 10−6g = 4.7 × 10−4 g of scrapie hamster brain tissue, i.e. about 4.7% of the ingested PrPTSE. Similar assessments performed for the samples of the three other examined animals displayed in Figure 2C (lanes 7–9, 11–13, 15–17 and 19–21), and with further dilutions of faecal extracts as shown in Figure 2D, indicate that on average 5% of the orally administered PrPTSE had been shed via the faeces.
3.2. Western blot detection of PrPTSE in tissue specimens of the gastrointestinal tract
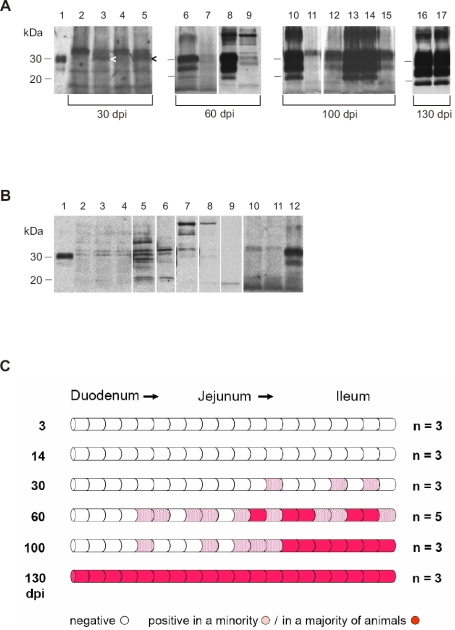
Western blot examination of gut segments from the small intestine failed to detect PrPTSE at 3 and 14 dpi (Fig. 3C). Initial detection of the protein was achieved at 30 dpi in individual segments of the distal jejunum and ileum in 1 out of 3 animals examined (Figs. 3A and 3C). At 60 dpi, Western blot signals for PrP27-30 became more prominent and widespread, and isolated foci of deposition were also observed in the border region of the distal duodenum and proximal jejunum (Figs. 3A and 3C). At 100 dpi, positive signals in the distal jejunum and ileum showed increasing confluence, and at 130 dpi the entire wall of the small intestine exhibited prominent deposition of PrPTSE in the majority of examined animals (Figs. 3A and 3C). First immunoblot detection of focal PrPTSE in caudal intestinal regions (caecum, colon) occurred at 100 dpi; cranially, initial detectable deposition of PrPTSE was found in stomach samples at 130 dpi in 4 hamsters; in 2 out of these 4 animals also the oesophagi were observed to show onset of PrPTSE deposition (data not shown).
Figure 3.
(A) Western blot detection of PrP27-30, the PK-resistant core of PrPTSE, in segments of the small intestine from hamsters fed with 263K scrapie brain homogenate. Lane 1: Blot control, 263K scrapie hamster brain homogenate containing 1 × 10−7 g of brain tissue. Lanes 2 to 5: Samples collected at 30 dpi (weak specific immunostaining signals for PrPTSE in lanes 3 and 5 are marked by arrowheads). Lanes 6 to 9: Samples collected at 60 dpi (adjacent intestinal samples were blotted in lanes 6 and 7, and in lanes 8 and 9). Lanes 10 to 15: Samples collected at 100 dpi (adjacent intestinal samples were blotted in lanes 10 and 11, and in lanes 12–15; the pattern shown in lanes 12–15 would be compatible with bi-directional spread of infectivity in the wall of the intestinal tract. Lanes 16 and 17: Samples collected 130 dpi. Note: Positive samples at 100 and 130 dpi also show strong staining of PrP27-30 dimers. (B) Range of western-blot signals from different segments of small intestine from uninfected hamsters after PrPTSE purification and staining with mAb 3F4. Lane 1: Blot control, 263K scrapie hamster brain homogenate containing 1 × 10−7 g of brain tissue. Lanes 2–11: Unspecific immunostaining in extracts from different segments of the small intestine of uninfected hamsters. Lane 12: Positive control; tissue sample collected at 60 dpi from the small intestine of a hamster orally infected with 263K scrapie. (C) Initial sites, foci, and spread of PrPTSE-deposition in the small intestine of hamsters fed with 263K scrapie brain homogenate. Schematic summary of Western blot findings in screened segments of small intestines collected 3, 14, 30, 60, 100 and 130 dpi. n = number of animals investigated.
Extracts from gut specimens of uninfected hamsters showed a variety of unspecific staining signals with mAb 3F4 in the Western blot (Fig. 3B) which could all be clearly differentiated from PrP27-30. These unspecific signals may have resulted from heterogeneous compounds in the resorptive tissue, depending on the status of the uptake and processing of nutrients, and on differences in the exact tissue composition of the various specimens from the gut wall.
3.3. Immunohistochemical and PET blot detection of PrPTSE in tissue specimens from the distal small intestine
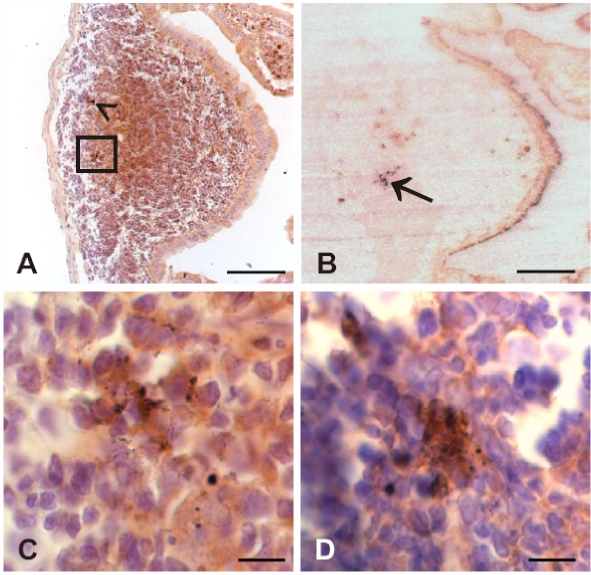
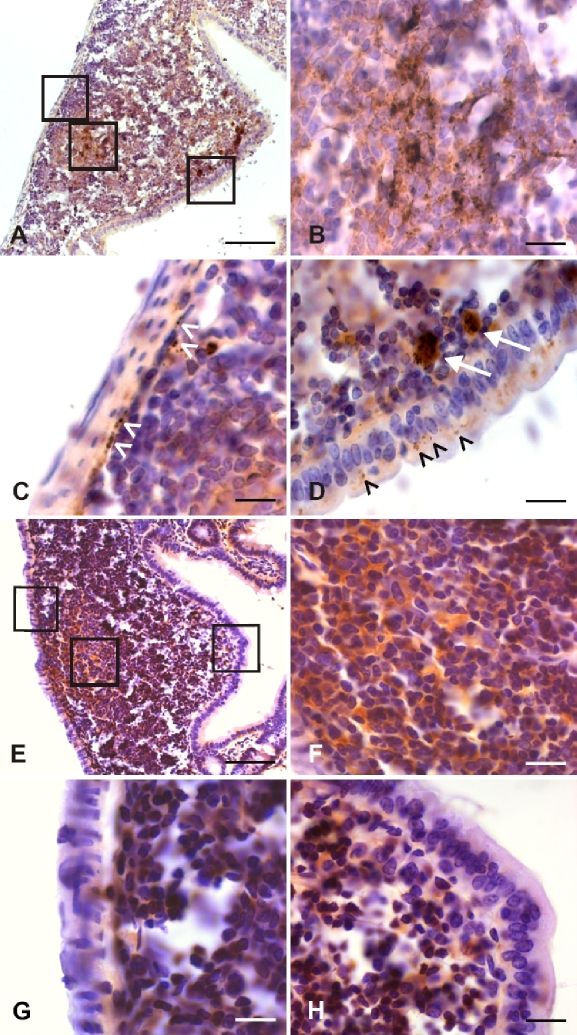
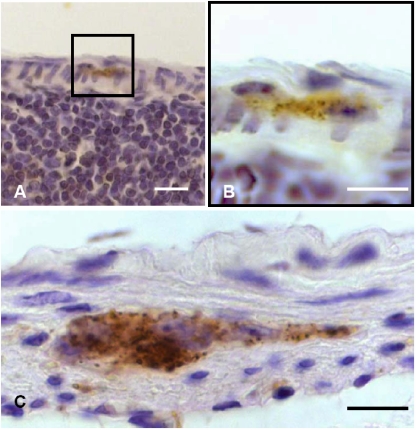
Since prion invasion of alimentary tract tissue showed its initial manifestation (in terms of the deposition of PrPTSE) in the distal small intestine of our model animals, this gut region was further scrutinized by immunohistochemistry and PET blotting for the detection of PrPTSE in GALT and ENS components. Initial foci of PrPTSE deposition were detected at 30 dpi (in 5 out of 6 animals, Fig. 4D and Tab. I) and at 45 dpi (in all 6 animals investigated, Figs. 4A–4C and Tab. I) in germinal centers of Peyer’s patches. Immunohistochemical observations at 45 dpi (Fig. 4A) could be confirmed by PET blotting of adjacent sections for PrPTSE, which visualized PrPTSE by granular, blue-magenta staining (Fig. 4B). In contrast, the ENS did not show deposition of detectable PrPTSE at 30 or 45 dpi. At 60 dpi PrPTSE was found more frequently and widespread in lymphoid follicles in all 6 animals investigated (Figs. 5A–5D and Tab. I) where the protein also often showed co-localisation with macrophages of the dome (Fig. 5D, white arrows). In 1 of the 6 animals showing PrPTSE deposition in the GALT at 60 dpi, PrPTSE was also detected in association with submucosal nerve-like structures of a lymphoid follicle (Fig. 5C, white arrowheads), and with cells of the follicle associated epithelium (FAE, Fig. 5D, black arrowheads). At the clinical stage of disease the GALT, and particularly Peyer’s patches, consistently showed strong immunolabeling for PrPTSE in all examined intestinal samples (not shown). ENS ganglia were positive for PrPTSE in only 2 out of 6 animals investigated at 60 dpi (Tab. I, Figs. 6A and 6B), but at the terminal stage of scrapie, PrPTSE was abundant in submucosal and myenteric plexuses in the small intestine (Fig. 6C). The results obtained in our immunohistochemical examinations from 3 to 60 dpi are summarized in Table I.
Figure 4.
Photomicrographs displaying granularly stained PrPTSE detected by immunohistochemical labeling (A, C, D) or PET blotting (B) at 30 or 45 dpi in the GALT of hamsters fed with 263K scrapie brain homogenate. PrPTSE appears as brown (A, C, D) or blue-magenta (B) granular material. (A) Ileal lymphoid follicle at 45 dpi showing PrPTSE in the germinal centre (arrowhead and inset in A). (B) PET blot in adjacent section to the specimen displayed in A (PrPTSE is indicated by arrow). (C) Higher magnification of inset in A. (D) Detection of PrPTSE in the germinal center of an ileal lymphoid follicle at 30 dpi. Scale bars in A and B, 40 μm; in C and D, 5 μm. (For a colour version of this figure, please consult www.vetres.org.)
Table I.
Immunohistochemical monitoring of PrPTSE deposition in the GALT and ENS of the distal small intestine from hamsters fed with 263K scrapie brain homogenate.
| No. of animals tested positive for PrPTSE/No. of animals in group |
|||||
|---|---|---|---|---|---|
| 3 dpi | 14 dpi | 30 dpi | 45 dpi | 60 dpi | |
| GALT | 0 / 2 | 0 / 6 | 5 / 6 | 6 / 6 | 6 / 6 |
| ENS | 0 / 2 | 0 / 6 | 0 / 6 | 0 / 6 | 2 / 6 |
Figure 5.
Photomicrographs displaying PrPTSE detected by immunohistochemical staining at 60 dpi in the GALT of a hamster fed with 263K scrapie brain homogenate (A–D). Lymphoid follicle from the distal jejunum showing granular deposits of PrPTSE in the germinal center (A, middle inset; B, higher magnification of middle inset in A), in submucosal nerve-like structures (A, left inset; C, higher magnification of left inset in A, white arrowheads), in cells of the FAE and in macrophages of the dome (A, right inset; D, higher magnification of right inset in A, black arrowheads and white arrows, respectively). Scale bars in A, 40 μm; in B, C and D, 5 μm. Photomicrographs of a lymphoid follicle from the distal ileum of an uninfected hamster labeled immunohistochemically with mAb 3F4 (E–G). Unspecific homogeneous brown staining in the germinal center (E, middle inset; F, higher magnification of middle inset in E). Granular immunostaining for PrPSc deposits is absent in the submucosal region (E, left inset; G, higher magnification of left inset in E), or in the FAE (E, right inset; H, higher magnification of right inset in E). Scale bars in E, 40 μm; in F, G and H, 5 μm. (For a colour version of this figure, please consult www.vetres.org.)
Figure 6.
Photomicrographs displaying PrPTSE detected by immunohistochemical staining at 60 dpi (A, B) and at the clinical stage of scrapie (160 dpi, C) in myenteric ganglia of the ileal ENS of hamsters fed with 263K scrapie brain homogenate. Scale bars 5 μm. (For a colour version of this figure, please consult www.vetres.org.)
4. DISCUSSION
4.1. Intestinal fate of ingested PrPTSE
To address the intestinal fate of ingested PrPTSE, we first examined the shedding of PrPTSE in the excrement of orally challenged hamsters around the time-point of infection and the presence of PrPTSE in components of the gut wall at 3 and 14 dpi. We consistently detected pathological prion protein in faeces of the animals at 24–48 h and 48–72 h after peroral uptake of infectious scrapie brain homogenate within the limits of our test sensitivity, but not at 0–24 hpi or later than 72 hpi. Our findings showed that the animals shed on average 5% of the perorally inoculated PrPTSEin the faeces. On the contrary, PrPTSE could not be detected by Western blotting, PET blotting or immunohistochemistry in specimens of the gut wall at 3 dpi and 14 dpi. Thus, a large proportion (i.e. about 95%) of the originally inoculated PrPTSE apparently disappeared from the intestinal tract.
This clearance seems most likely to have been mediated by degradation, which would be consistent with previous observations by Jeffrey et al. [25] that scrapie-associated PrPTSE is readily digested by fluids of the alimentary tract from sheep. Resorption and conveyance to extra-intestinal sites such as peripheral lymph nodes [21] or other LRS components could provide an alternative explanation for the observed PrPTSE clearance. However, in our model animals drainage of inoculum to LRS components outside the gut may be of lower importance for the intestinal clearance than degradation, since infectivity and PrPTSE were found only relatively late (i.e. subsequently to invasion of the brain and spinal cord) in the spleen of hamsters orally challenged with scrapie [5]. Apart from the shed or cleared proportions of the ingested PrPTSE, an indiscernible fraction of the perorally administered inoculum triggered intestinal infection as evidenced by progressing PrPTSE accumulation in components of the gut wall.
4.2. Course of PrPTSE deposition in the GALT and ENS
In order to identify the regions of initial PrPTSE deposition in the gastrointestinal tract of orally challenged hamsters we screened samples from the oesophagus, stomach, small intestine, caecum and large intestine by Western blotting. This revealed the distal jejunum and ileum as the most prominent sites containing initial foci of PrPTSE deposition. Such foci, which were observable at 30 dpi in our model animals, probably did not represent the original inocula (since no PrPTSE could be detected by Western blotting at 3 and 14 dpi) but most plausibly resulted from replication of the agent at sites of primary infection. In contrast, detectable PrPTSE deposition in tested segments from the large intestine, stomach or oesophagus was found to start significantly later, i.e. between 100 and 130 dpi. This was rather indicative of secondary infection that may have occurred by spread within the wall of the alimentary tract or from other parts of the body.
In the distal small intestine, primary infection of tissue with ingested inoculum may have been particularly facilitated by the relatively abundant presence of Peyer’s patches [17], and by an increased contact of infectious inoculum with the luminal surface of the intestinal wall due to a physiologically delayed ileal transit time [20]. Peyer’s patches of the caudal jejunum and ileum were also described to accumulate PrPTSE early in the pathogenesis of natural sheep scrapie [2, 51]. Similarly, in BSE-infected cattle, where the presence of infectious agent is essentially confined to the brain, spinal cord and retina [12], the BSE agent was detected in the distal ileum during preclinical and clinical phases of incubation after experimental peroral challenge [19, 53, 54].
According to the results of our Western blot study, and building on the course of infection in the GALT and ENS of perorally infected hamsters that had been established in previous studies for the period of time between 69 dpi and terminal disease [7, 33], we focused on the distal small intestine to further probe the early PrPTSE deposition in the gut wall by immunohistochemistry and PET blotting at 3, 14, 30, 45 and 60 dpi. For our immunohistochemical analysis, only staining signals of clearly recognizable granular deposits (but not homogeneous brown immunolabeling) were considered as indicative of PrPTSE [6].
Our examinations provided negative results at 3 and 14 dpi, but demonstrated the presence of PrPTSE in Peyer’s patches of the GALT in the majority of animals at 30 and 45 dpi (Tab. I). At 30 dpi the immunohistochemical staining for PrPTSE was often restricted to only one or two 6 μm sections, indicating that a rather limited number of follicular dendritic cells (FDC) was involved in the detected initial deposition of the protein. At 60 dpi IHC revealed the presence of PrPTSE not only in the FDC network but also associated with membranous epithelial cells (possibly M cells) of the FAE [7], macrophages of the dome und submucosal nerve-like structures (Figs. 5C and 5D). In contrast to the GALT, the onset of PrPTSE deposition in ENS components such as myenteric plexuses were found not earlier than 60 dpi (Tab. I, Figs. 6A and 6B). At the terminal stage of scrapie, PrPTSE was consistently present in both GALT and ENS, as has already been established in previous studies that found progressing accumulation of PrPTSE in these intestinal tissues of hamsters orally challenged with scrapie from 69 dpi (the earliest time point available) until clinical disease [7, 8, 33].
Recently, Bergström et al. [9] reported immunohistochemical detection of PrPTSE in Peyer’s patches already 2, 4 and 8 days after oral infection of hamsters with 263K scrapie agent. In this study, a tenfold higher dose of agent than that used by us was administered to the animals which may account for the difference in the time points for the initial detection of GALT-related PrPTSE found by these authors and us. Bergström et al. [9] concluded that “Peyer’s patches constitute at least one of the primary entry sites of PrPSc after oral exposure to scrapie”. By examination of the ENS additionally to the GALT, our study sheds further light on the sequence of PrPTSE deposition in these intestinal tissue components. The findings described above show that at intestinal sites of putatively primary infection the onset of detectable PrPTSE deposition in lymphoid tissue clearly preceded that in submucosal or myenteric ganglia. If the observed temporal-spatial pattern of PrPTSE deposition in the examined intestinal tissue samples correctly reflects the spread of infection, this would be indicative of the GALT being involved in both, establishment of primary intestinal infection and propagation of infection to the ENS in our model animals. Whether blood or lymph is involved in the spread of infection to the ENS has not yet been elucidated and would require further examination.
Findings in other murine models suggest that neuroinvasion can also occur in the absence of detectable lymphoid infection, e.g. after oral challenge by a high dose of agent or exposure to highly neuroinvasive TSE strains [8]. Furthermore, although comprehensive studies in Texel sheep naturally infected with scrapie [50] suggested that the proximity of Peyer’s patches and plexuses of the ENS may facilitate neuroinvasion, in sheep carrying the genotype PrPVRQ/ARR CNS invasion is reported to occur without preceding infection of lymphoid tissue [51]. Also, a subset of sheep orally infected with BSE showed invasion of the CNS without detectable PrPTSE in the lymphoid system [24]. Thus, it has to be noted that the intestinal routing of prions after ingestion of infectivity may considerably vary depending on the combination of the prion strain, dose of the agent and host species.
4.3. Shedding of endogenously formed PrPTSE in faeces
The deposition of substantial amounts of PrPTSE, particularly associated with Peyer’s patches, in close vicinity to the lumen of the small intestine found here and in other studies both before and after the onset of clinical symptoms [9, 49, 53] is suggestive of a further role of the GALT in the pathogenesis after oral scrapie infection. This role would be to provide a source for dissemination of replicated prions from the gut wall into faeces. We found PrPTSE in membranous epithelial cells of the FAE directly adjacent to the intestinal lumen, and release of viruses from the apical (or luminal) surface of polarized intestinal epithelial cells has been described in a variety of reports [14, 48]. Deposition of PrPTSE in FAE components has been observed already previously in orally infected hamsters [7] and in BSE-infected lemurs [10]. However, so far, FAE components such as M cells are discussed primarily as candidate sites of prion entry into the gut wall [7, 18]. Our findings point to a second or alternative role of the FAE in the pathogenesis of orally acquired scrapie and indicate that the FAE may serve as a site for prion release from the host. Theoretically, such dissemination may also arise from other intestinal tissues such as infected ENS, but with respect to their load of PrPTSE and anatomical location Peyer’s patches would appear as the prime candidate source of intestinal prion release.
However, after the initial shedding observed at 24–72 hpi we first failed to detect PrPTSE by Western blotting of faecal extracts at any time point tested during the preclinical or clinical stage of infection. Our analytical extraction procedure, which recovered ~4% of the faecal PrPTSE spike in the final extract, was able to detect PrPTSE when an amount of 5 × 10−7 g of scrapie hamster brain, i.e. ~50 pg of PrPTSE [5], was added to 200 mg of faeces and an extract representing 200 mg of spiked faeces was loaded onto the gel for Western blotting. As known from previous titrations [3, 5], such spikes contained about 0.5–1.5 × 103 intracerebral (i.c.) infective doses (LD50i.c.).Accordingly, unknown faecal samples (of which extracts representing 200 mg of starting material were loaded for Western blotting) should have produced positive results if they had contained an amount of PrPTSE corresponding to > 5 × 10−7 g of scrapie hamster brain per 200 mg of faeces (i.e. > 2.5 × 10−6 g of scrapie hamster brain per 1 g of faeces). When the exogenous spiking material is representative of endogenous PrPTSE in faeces, our observations suggest that the concentration of PrPTSE in faeces from negatively tested donors was at least 400 000-fold lower than that in the brain. If, furthermore, in faeces PrPTSE correlates with infectivity in a similar ratio to that observed previously in the CNS [3, 5], this would indicate that infectivity in the faeces, if present, would be lower than 2.5 to 7.5 × 103 LD50 per gram.
In order to examine whether excretion of PrPTSE by shedding from infected Peyer’s patches (as also recently suggested by Safar et al. [39]) or other contaminated components of the gut wall occurred at low levels not detectable by direct Western blotting when intestinal PrPTSE deposition had been established, we performed serial PMCA of faecal extracts from clinically-ill scrapie hamsters. This revealed the presence of small amounts of PrPTSE that eluded detection when such extracts were analysed without in vitro amplification of the pathological prion protein. Excrement may thus also provide a vehicle for the release of endogenously formed PrPTSE. In the light of the data available so far, this release seems to occur predominantly from Peyer’s patches, possibly under involvement of the FAE. However, the rather low level of contamination found in faeces from clinically-ill animals suggests that such a mode of prion dissemination is at least partially counteracted by the observed ability of the digestive tract to clear substantial amounts of PrPTSE from the alimentary canal.
4.4. Previous reports on the excretion and detection of prions in faeces
A recent study on the excretion of scrapie and BSE-associated PrPTSE in stools of intragastrically challenged mice [29] reported remarkably similar results to our observations with respect to the shedding kinetics of PrPTSE around the time-point of infection, despite differences in the route of inoculation, dose and strain of the agent, and host species. This may be indicative of common characteristics in the alimentary processing of ingested PrPTSE. However, the mouse study did not address the intestinal fate of ingested PrPTSE in detail and failed to detect the protein in samples from mice clinically affected with scrapie.
Safar et al. [39] recently addressed the transmission and detection of prions in faeces from hamsters orally challenged with Sc237 scrapie agent (presumably identical to 263K scrapie). These authors found that faecal shedding of PrPTSE, as determined by conformation dependent immunoassay (CDI), peaked at 2 days after infection and then gradually decreased for the next 16 days. From 20 dpi until the onset of scrapie symptoms, the CDI detected fluctuating levels of PrPTSE in the excrement. The highest infectivity levels in faeces, as determined by bioassay, were detected at 2 and 8 dpi, and excretion of prions then continued at lower levels throughout the entire asymptomatic phase of incubation. The pronounced faecal shedding of PrPTSE and infectious prions observed by Safar et al. [39] may have resulted from the very high dose of agent perorally administered in this experiment (the animals were fed one-half of a scrapie hamster brain which corresponds to about 500 mg of brain tissue). Such a dose possibly “overwhelmed” the digestive system [11] and compromised its ability to clear PrPTSE as efficiently from the alimentary tract as observed when hamsters were fed a lower amount (i.e. 10 mg) of scrapie brain homogenate.
Using different doses for peroral infection, the study by Safar et al. and our work produced complementary findings. By administering a relatively low dose of agent, we were able to elucidate the fate of the ingested PrPTSE in more detail. Furthermore, our immunohistochemical and PET blot examinations of the gut wall experimentally substantiated the notion discussed by Safar et al. [39] that contamination of faeces with prions may be mediated by shedding from infected Peyer’s patches.
4.5. The possible role of faeces in the transmission of contagious prion diseases
With respect to previously raised assumptions that contaminated excrements may be involved in the horizontal transmission of natural sheep scrapie in endemically affected flocks [2], several different aspects have to be considered: First, ruminants have a specific LRS component at the recto-anal junction, (sometimes referred to as “RAMALT”) which accumulates PrPTSE, and from where possibly contaminated macrophages may be released into the very distal gut lumen [16] and shed into the environment. Second, ruminants shed relatively large quantities of faeces, and binding of prions to soil may substantially enhance the efficiency of peroral scrapie transmission [26]. Third, specific factors could possibly enhance dissemination of PrPTSE from the gut wall. This holds particularly true for injuries that may lead to the shedding of intestinal tissue debris, parasitic- or other co-infections [23, 35], and inflammatory processes [42]. Apart from faeces, other vehicles have been discussed for TSE transmissions in the field, e.g. contaminated placenta, decomposed carcasses, saliva, urine or skin [15, 30, 34, 38, 42, 46]. Our protocol for the detection of PrPTSE in faeces combined with sPMCA can help to test excrements from ovines, caprines or cervids in future studies to further pinpoint the sources of infection in contagious prion diseases of animals.
Acknowledgements
We thank Marion Joncic, Ramona Famulla, Patrizia Reckwald and Manuela Friedrich for excellent technical assistance. This work was supported by the EU-funded Network of Excellence “NeuroPrion”, the Deutsche Forschungsgemeinschaft (DFG, TH 1376/2-1) and the German Bundesministerium für Bildung und Forschung (BMBF, FKZ 01KO0515). Dominique Krüger dedicates this article to his mentor, the nutritionist and Director and Professor Rolf Grossklaus.
REFERENCES
- 1.Aguzzi A., Polymenidou M.. Mammalian prion biology: one century of evolving concepts. Cell. 2004;116:313–327. doi: 10.1016/s0092-8674(03)01031-6. [DOI] [PubMed] [Google Scholar]
- 2.Andréoletti O., Berthon P., Marc D., Sarradin P., Grosclaude J., Van Keulen L.. et al. Early accumulation of PrPTSE in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J. Gen. Virol. 2000;81:3115–3126. doi: 10.1099/0022-1317-81-12-3115. [DOI] [PubMed] [Google Scholar]
- 3.Baldauf E., Beekes M., Diringer H.. Evidence for an alternative direct route of access for the scrapie agent to the brain bypassing the spinal cord. J. Gen. Virol. 1997;78:1187–1197. doi: 10.1099/0022-1317-78-5-1187. [DOI] [PubMed] [Google Scholar]
- 4.Beekes M., Baldauf E., Caßens S., Diringer H., Keyes P., Scott A.C.. et al. Western blot mapping of disease-specific amyloid in various animal species and humans with transmissible spongiform encephalopathies using a high-yield purification method. J. Gen. Virol. 1995;76:2567–2576. doi: 10.1099/0022-1317-76-10-2567. [DOI] [PubMed] [Google Scholar]
- 5.Beekes M., Baldauf E., Diringer H.. Sequential appearance and accumulation of pathognomonic markers in the central nervous system of hamsters orally infected with scrapie. J. Gen. Virol. 1996;77:1925–1934. doi: 10.1099/0022-1317-77-8-1925. [DOI] [PubMed] [Google Scholar]
- 6.Beekes M., McBride P.A., Baldauf E.. Cerebral targeting indicates vagal spread of infection in hamsters fed with scrapie. J. Gen. Virol. 1998;79:601–607. doi: 10.1099/0022-1317-79-3-601. [DOI] [PubMed] [Google Scholar]
- 7.Beekes M., McBride P.A.. Early accumulation of pathological PrP in the enteric nervous system and gut-associated lymphoid tissue of hamsters orally infected with scrapie. Neurosci. Lett. 2000;278:181–184. doi: 10.1016/s0304-3940(99)00934-9. [DOI] [PubMed] [Google Scholar]
- 8.Beekes M., McBride P.A.. The spread of prions through the body in naturally acquired transmissible spongiform encephalopathies. FEBS J. 2007;274:588–605. doi: 10.1111/j.1742-4658.2007.05631.x. [DOI] [PubMed] [Google Scholar]
- 9.Bergström A.-L., Jensen T.K., Heegaard P.M.H., Cordes H., Hansen V.B., Laursen H., Lind P.. Short-term study of the uptake of PrPTSE by the Peyer’s patches in hamsters after oral exposure to scrapie. J. Comp. Pathol. 2006;134:126–133. doi: 10.1016/j.jcpa.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Bons N., Mestre-Frances N., Belli P., Cathala F., Gajdusek D.C., Brown P.. Natural and experimental oral infection of nonhuman primates by bovine spongiform encephalopathy agents. Proc. Natl. Acad. Sci. USA. 1999;96:4046–4051. doi: 10.1073/pnas.96.7.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosque P.J., Tyler K.L.. Prions’ travels – feces and transmission of prion diseases. J. Infect. Dis. 2008;198:8–9. doi: 10.1086/588194. [DOI] [PubMed] [Google Scholar]
- 12.Bradley R.. Bovine spongiform encephalopathy and its relationship to the variant form of Creutzfeldt-Jakob disease. Contrib. Microbiol. 2004;11:146–185. doi: 10.1159/000077055. [DOI] [PubMed] [Google Scholar]
- 13.Brown P., Cervenakova L.. A prion lexicon (out of control) Lancet. 2005;365:122. doi: 10.1016/S0140-6736(05)17700-9. [DOI] [PubMed] [Google Scholar]
- 14.Compans R.W.. Virus entry and release in polarized epithelial cells. Curr. Top. Microbiol. Immunol. 1995;202:209–219. doi: 10.1007/978-3-642-79657-9_14. [DOI] [PubMed] [Google Scholar]
- 15.DeJoia C., Moreaux B., O’Connell K., Bessen R.A.. Prion infection of oral and nasal mucosa. J. Virol. 2006;80:4546–4556. doi: 10.1128/JVI.80.9.4546-4556.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzales L., Jeffrey M., Siso S., Martin S., Bellworthy S., Stack M.J.. et al. Diagnosis of preclinical scrapie in samples of rectal mucosa. Vet. Rec. 2005;156:846–847. doi: 10.1136/vr.156.26.846-b. [DOI] [PubMed] [Google Scholar]
- 17.Griebel P.J., Hein W.R.. Expanding the role of Peyer’s patches in B-cell ontogeny. Immunol. Today. 1996;17:30–39. doi: 10.1016/0167-5699(96)80566-4. [DOI] [PubMed] [Google Scholar]
- 18.Heppner F.L., Christ A.D., Klein M.A., Prinz M., Fried M., Kraehenbuhl J.-P., Aguzzi A.. Transepithelial prion transport by M cells. Nat. Med. 2001;7:976–977. doi: 10.1038/nm0901-976. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann C., Ziegler U., Buschmann A., Weber A., Kupfer L., Oelschlegel A.. et al. Prions spread via the autonomic nervous system from the gut to the central nervous system in cattle incubating bovine spongiform encephalopathy. J. Gen. Virol. 2007;88:1048–1055. doi: 10.1099/vir.0.82186-0. [DOI] [PubMed] [Google Scholar]
- 20.Holgate A.M., Read N.W.. Relationship between small bowel transit time and absorption of a solid meal. Dig. Dis. Sci. 1983;28:812–819. doi: 10.1007/BF01296904. [DOI] [PubMed] [Google Scholar]
- 21.Huang F.-P., Farquhar C.F., Mabbot N.A., Bruce M.E., MacPherson G.G.. Migrating intestinal dendritic cells transport PrPTSE from the gut. J. Gen. Virol. 2002;83:267–271. doi: 10.1099/0022-1317-83-1-267. [DOI] [PubMed] [Google Scholar]
- 22.Ironside J.W., McCardle L., Horsburgh A., Lim Z., Head M.W.. Pathological diagnosis of variant Creutzfeldt-Jakob disease. APMIS. 2002;110:79–87. doi: 10.1034/j.1600-0463.2002.100110.x. [DOI] [PubMed] [Google Scholar]
- 23.Jeffrey M., Martin S., Thomson J.R., Dingwall W.S., Begara-McGorum I., Gonzáles L.. Onset and distribution of tissue prp accumulation in scrapie-affected suffolk sheep as demonstrated by sequential necropsies and tonsillar biopsies. J. Comp. Pathol. 2001;125:48–57. doi: 10.1053/jcpa.2001.0476. [DOI] [PubMed] [Google Scholar]
- 24.Jeffrey M., Ryder S., Martin S., Hawkins S.A., Terry L., Berthelin-Baker C., Bellworthy S.J.. Oral inoculation of sheep with the agent of bovine spongiform encephalopathy (BSE). 1. Onset and distribution of disease-specific PrP Accumulation in brain and viscera. J. Comp. Pathol. 2001;124:280–289. doi: 10.1053/jcpa.2001.0465. [DOI] [PubMed] [Google Scholar]
- 25.Jeffrey M., González L., Espenes A., Press C.M., Martin S., Chaplin M.. et al. Transportation of prion protein across the intestinal mucosa of scrapie-susceptible and scrapie-resistant sheep. J. Pathol. 2006;209:4–14. doi: 10.1002/path.1962. [DOI] [PubMed] [Google Scholar]
- 26.Johnson C.J., Phillips K.E., Schramm P.T., McKenzie D., Aiken J.M., Joel A., Pedersen J.A.. Prions adhere to soil minerals and remain infectious. PloS Pathog. 2006;2:e32. doi: 10.1371/journal.ppat.0020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kascsak R.J., Rubenstein R., Merz P.A., Tonna-Demasi M., Fersko R., Carp R.I.. et al. Mouse polyclonal and monoclonal anibody to scrapie-associated fibril protein. J. Virol. 1987;61:3688–3693. doi: 10.1128/jvi.61.12.3688-3693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mabbott N.A., MacPherson G.G.. Prions and their lethal journey to the brain. Nat. Rev. Microbiol. 2006;4:201–211. doi: 10.1038/nrmicro1346. [DOI] [PubMed] [Google Scholar]
- 29.Maluquer de Motes C., Grassi J., Simon S., Herva M.E., Torres J.M., Pumarola M., Girones R.. Excretion of BSE and scrapie prions in stools from murine models. Vet. Microbiol. 2008;131:205–211. doi: 10.1016/j.vetmic.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Mathiason C.K., Powers J.G., Dahmes S.J., Osborn D.A., Miller K.V., Warren R.J.. et al. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science. 2006;314:133–136. doi: 10.1126/science.1132661. [DOI] [PubMed] [Google Scholar]
- 31.McBride P.A., Wilson M.I., Eikelenboom P., Tunstall A., Bruce M.E.. Heparan sulfate proteoglycan is associated with amyloid plaques and neuroanatomically targeted PrP pathology throughout the incubation period of scrapie-infected mice. Exp. Neurol. 1998;149:447–454. doi: 10.1006/exnr.1997.6740. [DOI] [PubMed] [Google Scholar]
- 32.McBride P.A., Beekes M.. Pathological PrP is abundant in sympathetic and sensory ganglia of hamsters fed with scrapie. Neurosci. Lett. 1999;265:135–138. doi: 10.1016/s0304-3940(99)00223-2. [DOI] [PubMed] [Google Scholar]
- 33.McBride P.A., Schultz-Schaeffer W.J., Donaldson M., Bruce M., Diringer H., Kretzschmar H.A., Beekes M.. Early spread of scrapie from the gastrointestinal tract to the central nervous system involves autonomic fibers of the splanchnic and vagus nerves. J. Virol. 2001;75:9320–9327. doi: 10.1128/JVI.75.19.9320-9327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller M.W., Williams E.S., Hobbs N.T., Wolfe L.L.. Environmental sources of prion transmission in mule deer. Emerg. Infect. Dis. 2004;10:1003–1006. doi: 10.3201/eid1006.040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Donoghue P.J.. Cryptosporidium and cryptosporidiosis in man and animals. Int. J. Parasitol. 1995;25:139–195. doi: 10.1016/0020-7519(94)e0059-v. [DOI] [PubMed] [Google Scholar]
- 36.Prusiner S.B.. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 37.Prusiner S.B.. Prions. Proc. Natl. Acad. Sci. USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Race R., Jenny A., Sutton D.. Scrapie infectivity and proteinase K-resistant prion protein in sheep placenta, brain, spleen, and lymph node: implications for transmission and antemortem diagnosis. J. Infect. Dis. 1998;178:949–953. doi: 10.1086/515669. [DOI] [PubMed] [Google Scholar]
- 39.Safar J.G., Lessard P., Tamgüney G., Freyman Y., Deering C., Letessier F., DeArmond S.J., Prusiner S.B.. Transmission and detection of prions in feces. J. Infect. Dis. 2008;198:81–89. doi: 10.1086/588193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaller O., Fatzer R., Stack M., Clark J., Cooley W., Biffiger K.. et al. Validation of a western immunoblotting procedure for bovine PrP(Sc) detection and its use as a rapid surveillance method for the diagnosis of bovine spongiform encephalopathy (BSE) Acta Neuropathol. 1999;98:437–443. doi: 10.1007/s004010051106. [DOI] [PubMed] [Google Scholar]
- 41.Schulz-Schaeffer W., Tschöke S., Kranefuss N., Dröse W., Hause-Reitner D., Giese A.. et al. The paraffin-embedded tissue blot detects PrP(Sc) early in the incubation time in prion diseases. Am. J. Pathol. 2000;156:51–56. doi: 10.1016/S0002-9440(10)64705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seeger H., Heikenwälder M., Zeller N., Kranich J., Schwarz P., Gaspert A.. et al. Coincident scrapie infection and nephritis lead to urinary prion excretion. Science. 2005;310:324–326. doi: 10.1126/science.1118829. [DOI] [PubMed] [Google Scholar]
- 43.Seidel B., Thomzig A., Buschmann A., Groschup M.H., Peters R., Beekes M., Terytze K.. Scrapie agent (strain 263K) can transmit disease via the oral route after persistence in soil over years. PLoS ONE. 2007;2:e435. doi: 10.1371/journal.pone.0000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomzig A., Kratzel C., Lenz G., Krüger D., Beekes M.. Widespread PrPTSE accumulation in muscles of hamsters orally infected with scrapie. EMBO Rep. 2003;4:530–533. doi: 10.1038/sj.embor.embor827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomzig A., Schulz-Schaeffer W., Kratzel C., Mai J., Beekes M.. Preclinical deposition of pathological prion protein PrPTSE in muscles of hamsters orally exposed to scrapie. J. Clin. Invest. 2004;113:1465–1472. doi: 10.1172/JCI21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomzig A., Schulz-Schaeffer W., Wrede A., Wemheuer W., Brenig B., Kratzel C.. et al. Accumulation of pathological prion protein PrPTSE in the skin of animals with experimental and natural scrapie. PLoS Pathog. 2007;3:659–667. doi: 10.1371/journal.ppat.0030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thuring C.M., van Keulen L.J., Langeveld J.P., Vromans M.E., van Zijderveld F.G., Sweeney T.. Immunohistochemical distinction between preclinical bovine spongiform encephalopathy and scrapie infection in sheep. J. Comp. Pathol. 2005;132:59–69. doi: 10.1016/j.jcpa.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Tucker S.P., Thornton C.L., Wimmer E., Compans R.W.. Vectorial release of poliovirus from polarized human intestinal epithelial cells. J. Virol. 1993;67:4274–4282. doi: 10.1128/jvi.67.7.4274-4282.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Keulen L.J.M., Schreuder B.E.C., Vromans M.E.W., Langeveld J.P.M., Smits M.A.. Scrapie-associated prion protein in the gastrointestinal tract of sheep with natural scrapie. J. Comp. Pathol. 1999;121:55–63. doi: 10.1053/jcpa.1998.0300. [DOI] [PubMed] [Google Scholar]
- 50.Van Keulen L.J.M., Schreuder B.E.C., Vromans M.E.W., Langeveld J.P.M., Smits M.A.. Pathogenesis of natural scrapie in sheep. Arch. Virol. Suppl. 2000;16:57–71. doi: 10.1007/978-3-7091-6308-5_5. [DOI] [PubMed] [Google Scholar]
- 51.Van Keulen L.J.M., Vromans M.E.W., van Zijderveld F.G.. Early and late pathogenesis of natural scrapie infection in sheep. APMIS. 2002;110:23–32. doi: 10.1034/j.1600-0463.2002.100104.x. [DOI] [PubMed] [Google Scholar]
- 52.Wadsworth J.D., Joiner S., Hill A.F., Campbell T.A., Desbruslais M., Luthert P.J., Collinge J.. Tissue distribution of protease resistant prion protein in variant Creutzfeldt-Jakob disease using a highly sensitive immunoblotting assay. Lancet. 2001;358:171–180. doi: 10.1016/s0140-6736(01)05403-4. [DOI] [PubMed] [Google Scholar]
- 53.Wells G.A., Dawson M., Hawkins S.A., Green R.B., Dexter I., Francis M.E.. et al. Infectivity in the ileum of cattle challanged orally with bovine spongiform encephalopathy. Vet. Rec. 1994;135:40–41. doi: 10.1136/vr.135.2.40. [DOI] [PubMed] [Google Scholar]
- 54.Wells G.A., Hawkins S.A., Green R.B., Austin A.R., Dexter I., Spencer Y.I.. et al. Preliminary observations on the pathogenesis of experimental bovine spongiform encephalopathy (BSE): an update. Vet. Rec. 1998;142:103–106. doi: 10.1136/vr.142.5.103. [DOI] [PubMed] [Google Scholar]