Abstract
In contrast to 15 or more validated and/or proposed tick-borne spotted fever group species, only three named medically important rickettsial species are associated with insects. These insect-borne rickettsiae are comprised of two highly pathogenic species, Rickettsia prowazekii (the agent of epidemic typhus) and R. typhi (the agent of murine typhus), as well as R. felis, a species with unconfirmed pathogenicity. Rickettsial association with obligate hematophagous insects such as the human body louse (R. prowazekii transmitted by Pediculus h. humanus) and several flea species (R. typhi and R. felis, as well as R. prowazekii in sylvatic form) provides rickettsiae the potential for further multiplications, longer transmission cycles and rapid spread among susceptible human populations. Both human body lice and fleas are intermittent feeders capable of multiple blood meals per generation, facilitating the efficient transmission of rickettsiae to several disparate hosts within urban/rural ecosystems. While taking into consideration the existing knowledge of rickettsial biology and genomic attributes, we have analyzed and summarized the interacting features that are unique to both the rickettsiae and their vector fleas and lice. Furthermore, factors that underlie rickettsial changing ecology, where native mammalian populations are involved in the maintenance of rickettsial cycle and transmission, are discussed.
Keywords: epidemic and murine typhus, ecology, rickettsial biology, recrudescent typhus
1. INTRODUCTION
Rickettsial diseases are widely distributed throughout the world in endemic foci, with sporadic and often seasonal outbreaks. However, from time to time these infections have re-emerged in epidemic form in human populations. Rickettsiae are transmitted between arthropods and mammals, including humans, classifying them as zoonotic pathogens. Infections with rickettsial pathogens can vary from mild to very severe clinical presentations, with the severity of these diseases associated with pathogen virulence as well as host-related factors (i.e., age, delayed diagnosis) [17]. Prior to the use of antibiotics to treat rickettsial infections, mortality as high as 66% was reported for Rickettsia rickettsii, the agent of Rocky Mountain spotted fever (RMSF). However, increased awareness and proper treatment and care have had a great impact on reducing rickettsial case fatality rates, with modern case fatality rates ranging from 1% to over 30% [17].
The biology of Rickettsia is inextricably linked to its eukaryotic host, and both pathogenic and nonpathogenic rickettsiae cause varying degrees of metabolic stress on their host. Rickettsiae enter host cells through clathrin-coated pits by way of induced phagocytosis, evading destruction by exiting the phagosome before phagolysosomal fusion occurs [43]. Living freely in the cytoplasm of the host cell allows rickettsiae access to host nutrients and affords them some protection from the host’s immune responses. Nearly all members of the spotted fever group (SFG) rickettsiae, all of which that are known are naturally tick-borne, move within and between eukaryotic host cells by way of actin polymerization [21, 25]. This is achieved, in part, by the RickA protein that activates the Arp2/3 complex of eukaryotic cells, a multi-subunit protein that directly regulates rearrangement of the actin cytoskeleton. In contrast, the members of the typhus group (TG) rickettsiae are inefficient in the induction of host cell actin polymerization (e.g., they lack the gene encoding RickA) and thus use an alternative means for intercellular spreading, host cell lysis, to exit and infect neighboring cells [43, 45].
In contrast to 15 or more validated and/or proposed tick-borne SFG species [43, 45] only three named non-SFG rickettsial species are associated with insects (Tab. I). Despite growing reports of unclassified Rickettsia spp. in a wide range of insect taxa (reviewed in [33]), we have focused on the medically-important members of the insect-borne rickettsiae, which presently include two highly pathogenic species (R. prowazekii, the agent of epidemic typhus, and R. typhi, the agent of murine typhus) and one species with, as yet, unconfirmed pathogenicity in humans (R. felis). Rickettsial association with obligate blood-sucking insects such as the human body louse (R. prowazekii transmitted by Pediculus h. humanus) and several flea species (R. prowazekii in sylvatic form, R. typhi and R. felis) provide rickettsiae the potential for further multiplications, longer transmission cycles and rapid spread among susceptible human populations. Both human body lice and fleas are intermittent feeders capable of multiple blood meals per generation, facilitating the efficient transmission of rickettsiae to several disparate hosts within an ecosystem. Consequently, given dire environmental conditions (i.e., war, poverty, famine, catastrophe, etc.), insect-associated rickettsial epidemics readily propagate. For example, outbreaks of epidemic typhus can ensue from rapid transmission of R. prowazekii from human to human by infected lice under suitable circumstances in which close contact under overcrowding and unsanitary conditions allow ample lice exchange. Similarly, infected fleas can maintain R. typhi and R. felis for life, giving them the potential for infecting large numbers of susceptible hosts. In contrast, infected female hard ticks (Ixodidae), which are the principal vectors of SFG rickettsiae, feed to repletion once and, while maintaining rickettsiae transstadially and transovarially, are capable of single rickettsial transmission during their larval, nymphal and adult stages. Feeding interruptions may allow ticks to reattach to different hosts and transmit rickettsiae to multiple hosts; however, the typical life style traits of insects that harbor rickettsiae likely foster a much higher rate of non-vertical transmission as compared to ticks.
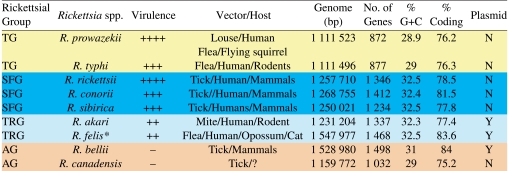
Table I.
Total R. felis genome size: 1 485 148 bp = chromosome; 62 829 bp = pRF. Scheme depicts four main groups of Rickettsia.
This contribution focuses exclusively on insect-borne rickettsiae. For a recent phylogenomic analysis of 10 rickettsial genomes that covers species with tick hosts see [19, 20]. For further information on the biology and clinical attributes of R. prowazekii and R. typhi, as well as other as yet uncultivated and less characterized rickettsial species of sap feeding insects, we recommend that interested readers consult a number of excellent published reviews and books dealing with these fascinating obligate intracellular organisms [1, 5, 13, 31, 33, 40, 41, 44].
2. GENOMIC CHARACTERISTICS OF INSECT-BORNE RICKETTSIAE
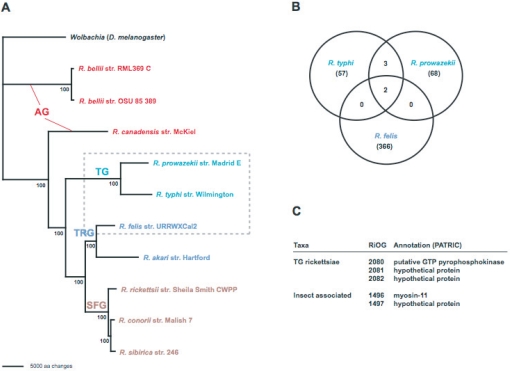
Rickettsia species have traditionally been classified as SFG and TG rickettsiae, with R. bellii considered ancestral to both groups [40, 41]. Based on robust molecular phylogeny estimation, we recently grouped R. bellii (two genome sequences completed) with R. canadensis (formerly of TG rickettsiae) in the ancestral group (AG) rickettsiae, as both taxa branch off basally to all other analyzed species [19, 20]. Thus, the TG rickettsiae now contain only insect-associated R. prowazekii and R. typhi (Fig. 1). Furthermore, phylogenetic analysis of the pRF plasmid-encoded genes of R. felis illustrated an affinity between an R. felis/R. akari clade and the AG rickettsiae [19]. Subsequent phylogenomic analysis further supported the distinction of this transitional group (TRG) rickettsiae from other SFG rickettsiae, as it shares dozens of similar genes, particularly those associated with the mobile gene pool, with AG rickettsiae [20]. Given the non-tick arthropod hosts of its members (R. felis in fleas, R. akari in mites), it is expected that other less characterized Rickettsia species (symbiotic or pathogenic) in non-tick hosts will group within the TRG rickettsiae.
Figure 1.
Phylogenomic characteristics of insect-associated rickettsiae. (A) Estimated phylogeny from an exhaustive search under parsimony of 731 core rickettsial proteins, with branch support from one million bootstrap replicates (redrawn from [22]). Ancestral group (AG) (red on the web figure) rickettsiae, typhus group (TG) (teal) rickettsiae, transitional group (TRG) (blue) rickettsiae, and spotted fever group (SFG) (brown) rickettsiae. Dashed box encloses the three representative insect-associated Rickettsia species. (B) Intersection of the unique R. typhi, R. prowazekii and R. felis genes that are not found in tick-associated Rickettsia. Data are from protein family clustering [22]. (C) List of genes shared exclusively by TG rickettsiae and insect-associated rickettsiae. Further descriptions of the shared RiOGs are posted at the PATRIC website [39].
Despite sharing similar arthropod hosts, the TG and R. felis genomes have strikingly different characteristics (Tab. I). TG genomes are the smallest of the sequenced rickettsial genomes and contain few instances of gene duplication and virtually no presence of products of the bacterial mobile gene pool. On the contrary, R. felis has the largest genome to date for any species of Rickettsia with a completed sequence, and many of its genomic characteristics are similar to the two R. bellii genomes (e.g., many gene rearrangements relative to other Rickettsia, elevated levels of transposases and related elements, presence of DNA transfer genes, etc.). These three genomes, along with the extraordinarily repetitive genome of the scrub typhus agent Orientia tsutsugamushi [15], present an antithesis to the dogma of genome reduction in Rickettsiaceae. Additionally, the rapid increase in the identification of plasmids in many Rickettsia spp. (both with and without completed genome sequences), coupled with the discovery that lateral gene transfer is more common in Rickettsia than previously thought [18, 20], poses newfound challenges for unraveling the mystery of rickettsial pathogenesis.
Our recent phylogenomic study, which analyzed 14 354 predicted ORFs across ten rickettsial genomes, identified only two predicted ORFs shared exclusively by TG rickettsiae and R. felis. Little information was yielded from bioinformatic analysis of these hypothetical proteins, and blastp searches hit mostly eukaryotic sequences but with poor scores. These ORFs have short and truncated traces within other rickettsial genomes, and their presence on the pRF plasmid of R. felis suggests that they are members of the rickettsial mobile gene pool. Additionally, the ORFs are contiguous within the TG genomes as well as on the pRF plasmid, suggesting that they were transferred recently between the lineages. As we did not detect any genes deleted exclusively from the R. felis and TG rickettsiae genomes, these two adjacent poorly characterized ORFs are currently the lone signatures exclusive to the insect-associated Rickettsia.
Given that little insight was gained from phylogenomic analysis, the search for genes involved specifically in insect cell invasion and pathogenesis will likely require the sequencing of genomes from other insect-associated Rickettsia spp., most of which are poorly studied. Perlman et al. [33] list a number of undescribed Rickettsia spp. that cause varying degrees of pathogenesis in a wide range of insect taxa. Their phylogenetic analysis based on 16S rDNA sequences suggested that the majority of these poorly known species are ancestral to even the members of the currently defined AG rickettsiae. Thus, little is known about the origin of pathogenicity within the rickettsial tree, and the mechanisms (if any) defining arthropod vector-specific biology may only come to light with sequencing-based strategies that span the true diversity within Rickettsia.
3. LOUSE-BORNE TYPHUS
Notorious among the members of the genus Rickettsia is R. prowazekii, the causative agent of louse-borne epidemic typhus that has been estimated to be responsible for over 30 million cases of typhus during and immediately after World War I, causing an estimated three million deaths [1, 43]. R. prowazekii has also been responsible for the recent outbreak of louse-borne typhus in refugee camps in Burundi involving thousands of human cases (with mortality exceeding 10%) [43]. This is a reminder that rickettsial diseases can re-emerge in epidemic forms as a result of the catastrophic breakdown of social conditions. Rickettsia prowazekii is now considered a re-emerging pathogen as a result of its increasing prevalence during turbulent times where social and political unrest results in the formation of refugia via the displacement of large human populations, forming overcrowding conditions that ignite the epidemic of louse-borne typhus.
3.1. NATURAL HISTORY OF LOUSE-BORNE TYPHUS
In contrast to other known pathogenic rickettsiae that are all maintained in zoonotic cycles, R. prowazekii maintenance and transmission is very closely associated with human populations. The human body louse, P. h. humanus, is the principal vector for R. prowazekii. Although the head louse, P. h. capitis has been shown to be capable of maintaining R. prowazekii experimentally (reviewed in [1, 36]), its role in transmission is not well established. The human body louse is highly host specific and preferentially spends its entire life cycle from egg to adult on a single human. The louse is an intermittent feeder with a preference for 20 ± 2 °C, which limits its resting sites to areas away from direct contact with the host’s skin, such as the folds of clothing. As an obligate ectoparasite, the body louse requires frequent visits to its host’s skin to obtain a blood meal [1, 3, 8]. The louse imbibes about 1 μL of host blood with each meal and feeds 4–6 times a day. Such intermittent blood feeding behavior allows the louse to acquire rickettsiae from the rickettsemic host. One of the hallmarks of the typhus infection is the onset of high fever. The body louse preference for lower temperature forces it to abandon a feverish patient and seek another host. This attribute is a major factor in the transmission of typhus and is responsible for the flaring of an epidemic within crowded human populations. However, the body louse movement is restricted, and thus close and intimate contact is required for moving from one host to another.
The infection in the louse is initiated after imbibing R. prowazekii-laden blood meals followed by rickettsial entry into the louse midgut epithelial lining. All stages of the louse life cycle can acquire infection. The lysis of infected cells starts to occur a few days after infection, and eventually the heavy loss of gut lining epithelial cells allows the blood meal to enter the body cavity, killing the infected lice within two weeks. Rickettsiae are present in the feces of infected lice as early as three days post infectious blood meal, with the louse feces remaining infectious thereafter [3]. Transmission of R. prowazekii to a new host takes place via scarification of the bite sites with the rickettsiae-laden feces. Although this route of transmission has been a cardinal mechanism for the deposition of rickettsiae into the bite sites, R. prowazekii transmission via louse bites should not be ruled out despite the lack of solid experimental data in support of this view.
3.2. RESERVOIRS OF RICKETTSIA PROWAZEKII
In addition to killing body lice within a few weeks of infection, R. prowazekii is not maintained by vertical transmission in its arthropod host. Therefore, reservoir maintenance is essential for R. prowazekii survival in nature. One critical factor in the maintenance and worldwide distribution of R. prowazekii is that this organism can develop a latent infection within human hosts. Despite the strong, long-lasting immunity that results in survivors of louse-borne typhus, R. prowazekii can sequester itself within the body and remain there presumably for the remainder of the host’s lifetime. Importantly, a recrudescent illness, Brill-Zinsser disease, can develop in individuals with a latent R. prowazekii infection. The symptoms of recrudescent typhus are less pronounced than the initial infection, and the mortality rate of Brill-Zinsser is less than 1%. However, patients with recrudescent typhus can serve as a long-term source R. prowazekii, permitting transmission of rickettsiae via lice for months to years after the primary infection, and these patients can therefore be considered as reservoirs for R. prowazekii. It is possible that recrudescent typhus may have been the mechanism whereby R. prowazekii was disseminated across the globe.
Although many attempts to identify zoonotic cycles of R. prowazekii in areas with louse-borne typhus epidemics such as Ethiopia and Burundi have been unsuccessful, the flying squirrel (Glaucomys volans) has been found to be naturally infected with R. prowazekii in the southern United States. In addition, flying squirrel ectoparasites (lice and fleas) were also implicated in the transmission of R. prowazekii between the squirrels and from squirrels to humans [11]. Since 1976, 39 cases of human infections with R. prowazekii have been documented serologically among residents of rural or suburban areas in the USA [16, 31], one-third of which were associated with flying squirrels. Despite the wide distribution of G. volans from Canada to the entire eastern USA and in isolated areas of Mexico and Central America, the search for a non-human reservoir of R. prowazekii has not been vigorously pursued since the initial earlier investigations. Consequently, the importance of the R. prowazekii and squirrel system remains unclear.
4. MURINE TYPHUS
Murine typhus (endemic and shop typhus) is one of the most widely distributed arthropod-borne infections. Historically, and in the present, its importance has been underestimated worldwide. Murine typhus occurs in epidemics or with high prevalence and is often unrecognized and substantially under reported [42]. Although it may be clinically mild, severe and even fatal cases have been reported [3, 5, 7, 42, 44]. The severity of murine typhus infection has been associated with age, race and delayed diagnosis. If untreated, murine typhus can persist for several months, with ~ 10% of infected adults requiring hospitalization. The mortality rate for all ages is ~ 2% and increases with patient age and the severity of infection due to hepatic and renal dysfunction, CNS abnormalities, and pulmonary compromise [8, 42]. Outbreaks have been reported in Australia and recently in China, Kuwait, Mexico, Spain, Portugal and Thailand [5, 42]. Murine typhus is prevalent throughout the world and accounts for widespread illness in areas infested with rats and their fleas. Recent serosurveys have demonstrated high prevalence of anti-typhus group rickettsiae worldwide, particularly in warm and humid climates (reviewed in [42]).
4.1. NATURAL HISTORY OF MURINE TYPHUS
The classic life cycle of R. typhi involves rats (Rattus rattus and R. norvegicus) and the rat flea, Xenopsylla cheopis [5, 7, 42]. X. cheopis is the main vector, and transmission is affected by contact with rickettsia-containing flea feces during or after blood feeding, as well as via the flea bite [4, 5]. Although Rattus spp. and X. cheopis arose in geographically different parts of the world, they have become cosmopolitan through the agency of humans. Since the geographical distributions of Rattus spp. extend far beyond the range of known human murine typhus, R. typhi may cycle through autochthonous fleas as intermurid vectors [5, 42]. Natural or experimental infection with R. typhi has been reported for over ten species of fleas representing eight genera [5, 42]. In addition to fleas, P. h. humanus, Polyplax lice and bloodsucking mites of rats are also reported to be either naturally or experimentally capable of acquiring and transmitting R. typhi [5, 42]. While the application of PCR technology has facilitated the precise identification of rickettsial species in arthropods, this assay cannot distinguish between transient infections (i.e., accidentally acquired pathogen from rickettsemic vertebrate hosts) and those established in a competent vector (maintain and transmit the rickettsiae).
In many parts of the world murine typhus infection is intimately associated with introduced commensal rodents, such as R. rattus, R. norvegicus, and Mus musculus, and their ectoparasites, particularly fleas. The triad of R. typhi-flea-rat seems to be a good example of true mutualisms in which the rickettsiae exert no harmful effects on the fitness of either the vector or the rat. Using a low passage R. typhi isolate derived from R. rattus in Ethiopia, studies in our laboratory found no observable impact on the fitness of rats and fleas [5, 7, 8, 42]. Additionally, we also demonstrated that many flea species that parasitized rats such as X. cheopis, Leptopsylla segnis, and Echidnophga gallinaceum were capable of acquiring, maintaining and transmitting R. typhi [5]. Although infections with R. typhi in the house mouse M. musculus, and even a shrew, Suncus murinus, have been documented, they do not seem to play a principal role in the transmission of R. typhi to humans. In Myanmar (Burma), of four murine species and one shrew commonly found in buildings, 7% (M. musculus) to 30% (R. rattus) and 38% (Bandicota bengalensis) were seropositive for R. typhi (Tab. II) [5]. In contrast, 62% of R. rattus collected from buildings in Addis Ababa (Ethiopia) and 49% of those collected in Sarawak (Malaysia) were seropositive for murine typhus1. Infection rates in X. cheopis fleas collected from rats in late 1970s varied from 7% to 18% [5]. While infection rates vary considerably among indoor rats and their fleas, murine typhus infection clearly seems associated with indoor rat populations throughout the world. Our field studies revealed how prevalent murine typhus was in several study sites in Ethiopia, Egypt, Israel, Myanmar, Pakistan, and Indonesia, and that urban rats and their fleas were implicated as R. typhi reservoir hosts. However, in the absence of indoor rats, murine typhus infection is maintained in suburban and rural cycles when native animals seek shelter in human habitations where food and hospitable environments are plentiful. Our field work also generated an extensive library of low passage R. typhi isolates from rats. Field studies also revealed that R. typhi infection in R. rattus can persist even in the presence of high anti-typhus antibody titers (≥ 1:1280 IgG) [5]. Most of our rickettsial isolates from rats were actually recovered from whole blood, kidney and spleen of animals exhibiting high anti-R. typhi antibodies. The persistence of R. typhi in otherwise immune hosts was later confirmed in the laboratory after cyclophosphamide (immunosuppressant) treatment of rats 9–12 months post primary infection (unpublished data)1. This observation is reminiscent of R. prowazekii’s recrudescent louse-borne typhus (Brill-Zinsser disease) where, under certain conditions, the rickettsemia occurs years after the recovery from primary infection. Persistent infection in the presence of host immune responses may be an important mechanism that allows TG rickettsiae to perpetuate in nature. While the significance of rickettsial persistence in the absence of zoonotic cycle is obvious in the case of R. prowazekii, its significance for R. typhi/rat model is difficult to assess considering the short life span of R. rattus and R. norvegicus under the field conditions.
Table II.
Selected phenotypic differences between the insect and selected tick borne rickettsiae.
| Rickettsial species | R. prowazekii | R. typhi | R. felis | R. rickettsii | R. conorii |
|---|---|---|---|---|---|
| Intranuclear growth | No | No | No | Yes | Yes |
| Hemolytic activity | Yes | Yes | Yes | No | No |
| Culture attributes: | |||||
| plaque size (Vero) | Small | Small | Large | Large | Large |
| Early escape before host cell death | No | Yes | Yes | Yes | Yes |
| Escape via actin polymerization-derived motility (RickA) | No | No | Yes | Yes | Yes |
| Persistence in humans or mammalian hostsa | Yes | Yes | NR | No (?) | No |
| Maintenance in vectorb | TST only | TST;TOT | TST;TOT | TST; TOT | TST;TOT |
| Major route of transmission to humans | Louse feces | Flea feces | Flea bite | Tick bite | Tick bite |
| Plasmid(s) | No | No | Yes | No | No |
| Type IV Pili | No | No | Yes | No | No |
| Disease in humans: | |||||
| Eschar | No | No | Yes/No | Rare | Yes |
| Rash | Yes | Yes | Yes/No | Yes | Yes |
| Fever | Yes | Yes | Yes | Yes | Yes |
| Mortality Ratec | High | Low | NR | High | Mild |
NR = none reported; ? = Only one instance of R. rickettsii being recovered from a lymph node that was excised one year after the patient treatment and resolution of RMSF [30]. However, despite this exceptional case we are not aware of any instances of recrudescent RMSF like we have seen in Brill-Zinsser disease.
TST = transstadial transmission; TOT = transovarial transmission.
From reference 39 (mortality rate: > 15% high; 2–7% mild/moderate; < 1% low). Note: since fleas and ticks in reality do not “bite”, rickettsiae are released into the host’s skins during the tick probing and feeding.
4.2. CHANGING ECOLOGY OF MURINE TYPHUS IN THE USA
Historically, the urban rat-flea cycle was the primary mode of R. typhi transmission worldwide, being responsible for thousands of human cases that occurred annually in the USA [5, 42]. However, a combination of public health measures, including rodent control and the use of insecticides, resulted in the reduction of murine typhus cases in the USA. Despite this remarkable success in breaking down rat-flea-rat cycle, the current level of reported human cases in the USA is continuing to occur with relatively high prevalence within few R. typhi endemic loci. For example, in 2006, 141 cases of murine typhus were reported in Nueces County in south-central Texas [10]. Of major concern is the changing ecology of murine typhus in both south Texas and southern California wherein the classic cycle of R. typhi, which involves commensal rats and primarily the rat flea (X. cheopis), has been replaced by the Virginia opossum (Didelphis virginiana)/cat flea (Ctenocephalides felis) cycle [10]. Curiously, infected rats and their fleas are hard to document within Texas and California’s murine typhus foci [7]. Additionally, based on serological surveys, R. typhi infections also occur in inland cities where urban and rural dwelling-opossums thrive. Thus, the maintenance of R. typhi in the cat flea/opossum cycle is of potential public health importance and a major health risk considering the distribution of the opossum, which spans within the USA, Mexico, Central America and Canada.
5. FLEA-BORNE RICKETTSIA FELIS
Original investigation of R. felis (originally called the ELB agent) was initiated in 1989 because the cat flea, C. felis, had been suspected in the transmission of Neorickettsia rictici (Ehrlichia rictici), which was killing prize-winning thoroughbred horses in Maryland and elsewhere. We obtained cat fleas from a commercial supplier in California (EL Lab, Soquel, CA, USA) and attempted to infect them with N. rictici. Despite failure to infect with N. rictici, we discovered that the fleas were loaded with Rickettsia-like organisms that reacted with antisera to R. typhi [2, 6]. Follow-up studies via electron microscopy revealed the presence of rickettsiae in the gut epithelial lining, malpighian tubules, muscles, and ovaries of the fleas [2]. Subsequent studies later shed light on the genetic and biological characterizations of the ELB agent and its maintenance via transovarial transmission. Additionally it was found that the infection of the cat fleas was not limited to the EL Lab colony, but also R. felis was found in eight other commercial colonies from various regions within the USA [6, 22]. Surprisingly, these flea colonies were initiated either with fleas from one supplier (EL Lab), in which the ELB agent was first identified, or were started with fleas from stray cats and dogs and later replenished with infected EL fleas from time to time. Infection rates in these colonies ranged from 43% to 93%. In light of these findings, we obtained cat fleas from opossums, cats, dogs, and a bobcat and found that < 10% were infected with this agent [22].
5.1. NATURAL HISTORY OF RICKETTSIA FELIS
The worldwide maintenance of R. felis in nature is unclear. However, recent molecular approaches revealed that R. felis is highly prevalent in cat fleas worldwide and, although numerous reports provide serological evidence for its presence in dogs and cats [14], their role as reservoirs of this bacterium is virtually uninvestigated. From our work [6, 10, 37, 38], we know that opossums play an important role in that they not only seroconvert, but also are heavily infested with the infected cat fleas [32]. Thus, opossums can indirectly serve as a bridge for the transmission of R. felis to humans and their pet cats and dogs. Most of the characterized SFG rickettsiae are maintained via transovarial transmission in ticks, and thus the need for a true “mammalian reservoir” for their maintenance seems unnecessary. However, data are lacking to clearly elucidate the extent by which horizontal transmission contributes to the maintenance and transmission of R. felis. Recent laboratory investigation has yet to demonstrate reciprocal transmission of R. felis in the cat flea-cat model [32]. Within the USA, R. felis is associated with cat fleas and opossums and possibly cats and dogs. Recent serological studies revealed the presence of R. felis in 11% of pet cats and cats from animal shelters and feral colonies [14]. The exposure rate reported is comparable to the 8% R. felis seropositive cats from Northeast USA previously reported by our laboratory. R. felis also circulates in an opossum/cat flea cycle in Brazil [32]. Opossums and other vertebrate hosts may play a role in rickettsial horizontal transmission to other ectoparasites, and this may account for the occasional reports of R. felis infection in other flea species as well as ixodid ticks [32]. The recent report implicating X. cheopis as a vector of R. felis, clearly demonstrates the possible role for the urban and rural rodents, e.g., ship rats (Rattus rattus), in the R. felis cycle, and underscoring its overlap with murine typhus [37]. Of interest is the sympatric existence of both R. typhi and R. felis in Texas and California murine typhus foci where the cat fleas were found to be infected with one but not both species simultaneously [7, 28, 37]. Although the presence of R. felis has been documented in cat flea populations worldwide, the identity of endemic mammalian and/or avian hosts remains to be elucidated.
5.2. RICKETTSIA FELIS AS A HUMAN PATHOGEN
Identification of infected fleas from opossums and cats within several foci of murine typhus in the USA prompted a retrospective investigation for R. felis among human murine typhus patients. In 1994, R. felis was detected by PCR in a blood sample from a hospitalized patient diagnosed with murine typhus [38]. Our initial work was followed by other investigators and the search for this pathogen generated surprises when R. felis was identified among patients from Yucatan, Mexico suspected of having dengue fever [46]. Additionally, clinical, serological and PCR-based evidence of R. felis infection has been reported in patients from Europe, Africa, Asia and South America [32]. Collectively, our published data and these recent reports not only support the pathogenic role of R. felis but also demonstrate its wide geographic distribution and its potential to cause human infections. Unfortunately, no human isolate of R. felis has yet been obtained and, although PCR positive patients demonstrate general symptoms of rickettsial diseases, there is no specific and/or clear picture discriminating R. felis infection from other rickettsioses. The disease in humans is reported as a murine typhus-like illness, and in Texas is known as “Summer Flu”. Laboratory studies in cats that have been used to maintain R. felis-infected flea colonies are reported to be asymptomatic even with rickettsemia lasting over 20 days2. The lack of a human isolate of R. felis is the missing step to define whether this organism is a human pathogen. It is difficult to actively chase “fever of unknown origin” cases in search of rickettsial isolates and, additionally, it is often difficult to obtain rickettsial isolates from patients post antibiotic treatments and/or due to low abundance of rickettsiae in the peripheral blood samples. However, one should not forget that R. felis and likewise R. typhi are intimately associated with household pets and feral animals that live within or in close proximity of human habitations. Such association is conducive to the maintenance of these rickettsial species in fleas and transmission to household inhabitants.
6. CONCLUSIONS AND PERSPECTIVES
In their recent review, Perlman et al. [33] compiled 13 species of insect-associated Rickettsia, of which only the three blood feeding species (the primarily louse-borne R. prowazekii, and the flea-borne R. typhi and R. felis) are known to cause disease in humans. This is in stark contrast to characterized acarine-associated species of Rickettsia, the majority of which have been implicated in vertebrate pathogenesis. In general, there are subtle differences between the tick and insect associated rickettsiae and Table II summarizes a number of their selected biological attributes. Of note is that the major mode of transmission of rickettsiae differs in insect- and tick-borne rickettsioses. While the tick-borne rickettsiae and R. felis are transmitted via bite [26], contact with the infected feces or crushed bodies of fleas and lice on the scarified surfaces of the host serve as a major means of transmission for R. typhi and R. prowazekii. Despite sharing common arthropod hosts, the TG and R. felis have strikingly different genomic characteristics (Tab. I). For instance, the R. prowazekii and R. typhi genomes are the smallest of the sequenced rickettsial genomes and lack mobile gene elements, while R. felis has the largest Rickettsia genome sequenced to date with many gene rearrangements relative to other Rickettsia and elevated levels of transposases and related elements (reviewed in [19, 20]). There are also striking differences between the vectors that harbor pathogenic rickettsiae with regard to transmission and rickettsial maintenance. Both lice and ticks have a hemimetabolous life cycle, in which immature stages resemble the adults and all their post embryonic stages are exclusively blood feeders. Thus, rickettsiae acquired with the blood meal by tick larva will be passed transtadially to nymph, from nymph to adult, and from adult via transovarial transmission to the next progeny. While vertical transmission of rickettsiae is the prominent feature of tick-borne rickettsioses, R. prowazekii is acquired and maintained via horizontal transmission involving both human and human body lice. The flea-borne rickettsioses are acquired and maintained via both horizontal and vertical transmission. For R. typhi, the historic role of rats (R. rattus, R. norvegicus) cannot be overstated, as these successful invasive rodents are known to maintain large populations in nearly every port city in the world. Of concern however, is the spread of rats from portal areas further inland into towns and rural areas where native animals acquire rickettsiae and establish different transmission/maintenance cycles.
In the case of R. felis, we have identified the opossum to play an important role as a host to both R. felis and cat fleas in the USA. However, it is surprising and disconcerting how little we know about the role of vertebrate hosts in the ecology, maintenance and mode of transmission of R. felis despite its worldwide distribution in the cat fleas. Furthermore, detailed experimental studies on the virulence and pathogenicity of R. felis are hampered due to the unavailability of human as well as any other vertebrate-derived isolate. Based on its molecular characteristics, this rickettsial species (formerly known as ELB) was described as a new species and named R. felis [23, see also 12]. However, the original tissue culture isolate of R. felis was contaminated and attempt to produce a sustained culture of ELB strain of R. felis has failed. In 2001, Raoult et al. [35] isolated R. felis from Flea Data Inc. (Freeville, NY, USA) cat fleas using both Vero (Cercopithecus aethiops) and XTC-2 (Xenopus laevis) cell lines. This strain (designated as: Marseille-URRWXCal2 or California-2) was used for sequencing of the R. felis genome [29]. Unfortunately, the California-2 isolate is not currently available and, although it was deposited at the ATCC (ATCC VR-1525), the isolate is currently on “administrative hold.” Recently, two additional strains of R. felis were also isolated from the cat flea; the Pedreira strain came from wild-caught fleas in Brazil, while the LSU strain came from the flea colony maintained at Louisiana State University, USA [24, 34]. These isolates share common insect associated features such as adaptation to lower temperature and other as yet unidentified genomic and phenotypic modifications for reduced virulence and pathogenicity in mammalian hosts that may have occurred resulting from their long maintenance via vertical transmission in the cat flea population.
Surprisingly, despite the earlier and recent pioneering research on rickettsial biology, there are fundamental gaps in our current knowledge that cannot explain the successful maintenance, wide geographic distribution, selectivity in mammalian/arthropod host preference, and highly variable spectrum of virulence and pathogenesis of these obligate intracellular bacteria.
Acknowledgements
The project described was supported by Award Numbers R01AI017828 and R01AI59118 from the National Institute of Allergy and Infectious Diseases (NIAID) to AFA, and through NIAID contract HHSN266200400035C to BSS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the National Institutes of Health.
Footnotes
Azad et al., unpublished data.
Macaluso et al., abstract presented in the 2006 USDA-ASR meeting.
REFERENCES
- 1.Anonymous. The control of lice and louse-borne diseases. Pan American Health Organization/World Health Organization; Washington D.C.: 1973. pp. 1–311. [Google Scholar]
- 2.Adams J.R., Schmidtmann E.T., Azad A.F.. Infection of colonized cat fleas, Ctenocephalides felis with a rickettsia-like microorganism. Am. J. Trop. Med. Hyg. 1990;43:400–409. doi: 10.4269/ajtmh.1990.43.400. [DOI] [PubMed] [Google Scholar]
- 3.Azad A.F. Walker D.H. Biology of rickettsial diseases. CRC; 1988. relationship of vector biology and epidemiology of louse and flea-borne rickettsioses; pp. 51–61. [Google Scholar]
- 4.Azad A.F., Traub R.. Experimental transmission of murine typhus by Xenopsylla cheopis flea bites. Med. Vet. Entomol. 1989;3:429–433. doi: 10.1111/j.1365-2915.1989.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 5.Azad A.F.. Epidemiology of murine typhus. Ann. Rev. Entomol. 1990;35:553–569. doi: 10.1146/annurev.en.35.010190.003005. [DOI] [PubMed] [Google Scholar]
- 6.Azad A.F., Sacci J.B. Jr., Nelson W.M., Dasch G.A., Schmidtman E.T., Carl M.. Genetic characterization and transovarial transmission of a novel typhus-like Rickettsia found in cat fleas. Proc. Natl. Acad. Sci. USA. 1992;89:43–46. doi: 10.1073/pnas.89.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azad A.F., Radulovic S., Higgins J.A., Noden B.H., Troyer M.J.. Flea-borne rickettsioses: some ecological considerations. Emerg. Infect. Dis. 1997;3:319–328. doi: 10.3201/eid0303.970308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azad A.F., Beard C.B.. Interactions of rickettsial pathogens with arthropod vectors. Emerg. Infect. Dis. 1998;4:179–186. doi: 10.3201/eid0402.980205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brill N.E.. An acute infectious disease of unknown origin: A clinical study based on 221 cases. Am. J. Med. Sci. 1910;139:484–502. doi: 10.1016/0002-9343(52)90016-8. [DOI] [PubMed] [Google Scholar]
- 10.Boostrom A.M., Beier S., Macaluso J.A., Macaluso K.R., Sprenger D., Hayes J.. et al. Opossums, cat fleas and rickettsial diseases in Texas. Emerg. Infect. Dis. 2002;8:543–548. doi: 10.3201/eid0806.010350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bozeman F.M., Masiello S.A., Williams M.S., Elisberg B.L.. Epidemic typhus rickettsiae isolated from flying squirrels. Nature. 1975;255:545–547. doi: 10.1038/255545a0. [DOI] [PubMed] [Google Scholar]
- 12.Bouyer D.H., Stenos J., Crocquet-Valdes P., Moron C.G., Popov V.L., Zavala-Velazquez J.E., Foil L.D., Stothard D.R., Azad A.F., Walker D.H.. Rickettsia felis: the molecular characterization of a new member of the spotted fever group. Int. J. Syst. Evol. Microbiol. 2001;51:339–347. doi: 10.1099/00207713-51-2-339. [DOI] [PubMed] [Google Scholar]
- 13.Burgdoerfer W., Anacker R.L. Academic Press; 1981. Rickettsiae and rickettsial diseases; p. 650. [Google Scholar]
- 14.Case J.B., Chomel B., Nicholson W., Foley J.E.. Serological survey of vector-borne zoonotic pathogens in pet cat and cats from animal shelters and feral colonies. J. Feline Med. Surg. 2005;8:111–117. doi: 10.1016/j.jfms.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho N.-H., Kim H.R., Lee J.H., Kim S.Y., Kim J., Cha S.. et al. The Orientia tsutsugamushi, genome reveals massive proliferation of conjugative type IV secretion system and host-cell interaction genes. Proc. Natl. Acad. Sci. USA. 2007;104:7981–7986. doi: 10.1073/pnas.0611553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duma R.J., Sonenshine D.E., Bozeman F.M., Veazey J.M. Jr., Elisberg B.L., Chadwick D.P.. et al. Epidemic typhus in the United States associated with flying squirrels. JAMA. 1981;245:2318–2323. [PubMed] [Google Scholar]
- 17.Dumler J.S., Taylor J.P., Walker D.H.. Clinical and laboratory features of murine typhus in South Texas, 1980 through 1987. JAMA. 1991;266:1365–1370. [PubMed] [Google Scholar]
- 18.Fuxelius H.-H., Darby A.C., Cho N.-H., Andersson S.G.E.. Visualization of pseudogenes in intracellular bacteria reveals the different tracks to gene destruction. Genome Biol. 2008;9:R42. doi: 10.1186/gb-2008-9-2-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillespie J.J., Beier M.S., Rahman M.S., Ammerman N.C., Shallom J.M., Purkayastha A.. et al. Horizontal inheritance of plasmid genes in Rickettsia felis. PLoS One. 2007;2:e266. doi: 10.1371/journal.pone.0000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillespie J.J., Williams K., Shukla M., Snyder E.E., Nordberg E.K., Ceraul S.M.. et al. Rickettsia phylogenomics: unwinding the intricacies of obligate intracellular life. PLoS One. 2008;3:e2018. doi: 10.1371/journal.pone.0002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gouin E., Egile C., Dehoux P., Villiers V., Adams J., Gertler F.. et al. The RickA protein of Rickettsia conorii activates the Arp2/3 complex. Nature. 2004;427:457–461. doi: 10.1038/nature02318. [DOI] [PubMed] [Google Scholar]
- 22.Higgins J.A., Sacci J.P. Jr., Schriefer M.E., Endris R.G., Azad A.F.. Molecular identification of rickettsia-like microorganisms associated with colonized cat fleas (Ctenocephalides felis) Insect Mol. Biol. 1994;3:27–33. doi: 10.1111/j.1365-2583.1994.tb00147.x. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J.A., Radulovic S., Schriefer M.E., Azad A.F.. Rickettsia felis: a new species of pathogenic rickettsia isolated from cat fleas. J. Clin. Microbiol. 1996;34:671–674. doi: 10.1128/jcm.34.3.671-674.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horta M.C., Lubruna M.B., Durigon E.L., Schumaker T.T.S.. Isolation of Rickettsia felis in mosquito cell line C6/36. Appl. Environ. Microbiol. 2006;72:1705–1707. doi: 10.1128/AEM.72.2.1705-1707.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeng R.L., Goley E.D., D’Alessio J.A., Chaga O.Y., Svitkina M., Borisy G.G.. et al. A Rickettsia WASP-like protein activates the Arp2/3 complex and mediates actin-based motility. Cell. Microbiol. 2004;6:761–769. doi: 10.1111/j.1462-5822.2004.00402.x. [DOI] [PubMed] [Google Scholar]
- 26.Macaluso K.R., Pornwiroon W., Popov V.L., Foil L.D.. Identification of Rickettsia felis in the salivary glands of cat fleas. Vector Borne Zoonotic Dis. 2008;8:391–396. doi: 10.1089/vbz.2007.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDade J.E., Shepard C.C., Redus M.A., Newhouse V.F., Smith J.D.. Evidence of Rickettsia prowazekii infections in the United States. Am. J. Trop. Med. Hyg. 1980;29:277–284. doi: 10.4269/ajtmh.1980.29.277. [DOI] [PubMed] [Google Scholar]
- 28.Noden B.H., Radulovic S., Higgins J.A., Azad A.F.. Molecular identification of two closely related rickettsial species, Rickettsia typhi and R. felis, in individual cat fleas, Ctenocephalides felis (Siphonaptera: Pulicidae) J. Med. Entomol. 1998;35:410–414. doi: 10.1093/jmedent/35.4.410. [DOI] [PubMed] [Google Scholar]
- 29.Ogata H., Renesto P., Audic S., Robert C., Blanc G., Fournier P.-E.. et al. The genome sequence of Rickettsia felis identifies the first putative conjugative plasmid in an obligate intracellular parasite. PLoS Biol. 2005;3:e248. doi: 10.1371/journal.pbio.0030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parker R.T., Menon P.G., Merideth A.M., Snyder M.J., Woodward T.E.. Persistence of Rickettsia rickettsii in a patient recovered from Rocky Mountain spotted fever. J. Immunol. 1954;73:383–386. [PubMed] [Google Scholar]
- 31.Perine P.L., Chandler B.P., Krause D.K., McCardle P., Awoke S., Habte-Gabr E., Wisseman C.L. Jr., McDade J.E.. A clinico-epidemiological study of epidemic typhus in Africa. Clin. Infect. Dis. 1992;14:1149–1158. doi: 10.1093/clinids/14.5.1149. [DOI] [PubMed] [Google Scholar]
- 32.Pérez-Osorio C.E., Zavala-Velázquez J.E., Arias León J.J., Zavala-Castro J.E.. Rickettsia felis as emergent global threat for humans. Emerg. Infect. Dis. 2008;14:1019–1023. doi: 10.3201/eid1407.071656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perlman S.J., Hunter M.S., Zchori-Fein E.. The emerging diversity of Rickettsia. Proc. Biol. Sci. 2006;273:2097–2106. doi: 10.1098/rspb.2006.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pornwiroon W., Pourciau S.S., Foil D.L., Macaluso K.R.. Rickettsia felis from cat fleas: isolation and culture in a tick-derived cell line. Appl. Environ. Microbiol. 2006;72:5589–5595. doi: 10.1128/AEM.00532-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raoult D., La Scola B., Enea M., Fournier P.E., Roux V., Fenollar F.. et al. A flea-associated rickettsia pathogenic for humans. Emerg. Infect. Dis. 2001;7:73–81. doi: 10.3201/eid0701.010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson D., Leo N., Prociv P., Barker S.C.. Potential role of head lice, Pediculus humanus capitis, as vector of Rickettsia prowazekii. Parasitol. Res. 2003;90:209–211. doi: 10.1007/s00436-003-0842-5. [DOI] [PubMed] [Google Scholar]
- 37.Schriefer M.E., Sacci J.B. Jr., Higgins J.A., Taylor J.P., Azad A.F.. Murine typhus: updated role of multiple urban components and a second typhus-like rickettsiae. J. Med. Entomol. 1994;31:681–685. doi: 10.1093/jmedent/31.5.681. [DOI] [PubMed] [Google Scholar]
- 38.Schriefer M.E., Sacci J.B. Jr., Dumler J.S., Bullen M.G., Azad A.F.. Identification of a novel rickettsial infection in a patient diagnosed with murine typhus. J. Clin. Microbiol. 1994;32:949–954. doi: 10.1128/jcm.32.4.949-954.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snyder E.E., Kampanya N., Lu J., Nordberg E. K., Karur H.R.. et al. PATRIC: the VBI PathoSystems Resource Integration Center. Nucleic Acids Res. 2007;35:D401–D406. doi: 10.1093/nar/gkl858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stothard D.R., Clark J.B., Fuerst P.A.. Ancestral divergence of Rickettsia bellii from the spotted fever and typhus groups of rickettsia and antiquity of the genus Rickettsia. Int. J. Syst. Bacteriol. 1994;44:798–804. doi: 10.1099/00207713-44-4-798. [DOI] [PubMed] [Google Scholar]
- 41.Stothard D.R., Fuerst P.A.. Evolutionary analysis of the spotted fever and typhus groups of Rickettsia using 16S rRNA gene sequences. Syst. Appl. Microbiol. 1995;18:52–61. [Google Scholar]
- 42.Traub R., Wisseman C.L. Jr., Farhang-Azad A.. The ecology of murine typhus – a critical review. Trop. Dis. Bull. 1978;75:237–317. [PubMed] [Google Scholar]
- 43.Walker D.H., Ismail N.. Emerging and re-emerging rickettsioses: endothelial cell infection and early disease events. Nat. Rev. Microbiol. 2008;6:375–386. doi: 10.1038/nrmicro1866. [DOI] [PubMed] [Google Scholar]
- 44.Wolbach S.B., Todd J.L., Palfrey F.W. The etiology and pathology of typhus. League of Red Cross Societies at the Harvard University Press; Cambridge: 1922. Pathology of typhus in man; pp. 152–221. [Google Scholar]
- 45.Yu X.-J., Walker D.H. Dworkin M., Falkow S., Rosenberg E., Schleifer K.-H., Stackebrandt E. The Prokaryotes. 3rd ed. Springer; 2006. The Order Rickettsiales; pp. 493–528. [Google Scholar]
- 46.Zavala-Velazquez J.E., Ruiz-Sosa J.A., Sanchez-Elias R.A., Becerra-Carmona G., Walker D.H.. Rickettsia felis rickettsiosis in Yucatan. Lancet. 2000;356:1079–1080. doi: 10.1016/S0140-6736(00)02735-5. [DOI] [PubMed] [Google Scholar]