Abstract
Haemophilus parasuis is a colonizer of the upper respiratory tract of healthy pigs, but virulent strains can cause a systemic infection characterized by fibrinous polyserositis, commonly known as Glässer’s disease. The variability in virulence that is observed among H. parasuis strains is not completely understood, since the virulence mechanisms of H. parasuis are largely unknown. In the course of infection, H. parasuis has to survive the host pulmonary defences, which include alveolar macrophages, to produce disease. Using strains from different clinical backgrounds, we were able to detect clear differences in susceptibility to phagocytosis. Strains isolated from the nose of healthy animals were efficiently phagocytosed by porcine alveolar macrophages (PAM), while strains isolated from systemic lesions were resistant to this interaction. Phagocytosis of susceptible strains proceeded through mechanisms independent of a specific receptor, which involved actin filaments and microtubules. In all the systemic strains tested in this study, we observed a distinct capsule after interaction with PAM, indicating a role of this surface structure in phagocytosis resistance. However, additional mechanisms of resistance to phagocytosis should be explored, since we detected different effects of microtubule inhibition among systemic strains.
Keywords: phagocytosis resistance, Haemophilus parasuis, alveolar macrophages, strain variability
1. INTRODUCTION
Haemophilus parasuis is a member of the γ-proteobacteria family Pasteurellaceae. Although it was first described as the etiological agent of Glässer’s disease, it is also known as a common inhabitant of the upper respiratory tract of healthy pigs. Besides Glässer’s disease, which is characterized by fibrinous polyserositis and arthritis, it also produces pneumonia and sudden death in swine [24]. H. parasuis strains differ in their virulence from highly virulent to non virulent [23], and it has been shown that they can be genetically divided in two major clades, one of them associated with production of systemic disease [22].
However, little is known about the mechanisms that allow virulent H. parasuis strains to cause disease. Recently, several groups have sought to identify the virulence factors of this bacterium using genomic methods [8, 12, 15, 16], but a direct demonstration of the importance of the selected genes to virulence has not been shown.
Older reports showed a correlation between the degree of virulence and the serotype of the strain [19, 25], suggesting a role of lipooligosaccharide (LOS) and/or other polysaccharides in the virulence of this species. Recent studies on the function of LOS indicate a partial role of this molecule in adhesion to and release of IL-8 and IL-6 by endothelial cells [2, 29]. It is also noteworthy that capsule production was shown to be enhanced after in vivo passage, but it was produced by all strains tested, including non-virulent reference strains [24]. Conversely, a previous report indicated that strains associated with pathological conditions were uncapsulated [17]. Thus, association of capsule with H. parasuis virulence remains a controversial issue due to the difficulty in detecting capsule production and the lack of reproducibility of the findings.
In 1995, Vahle et al. [28] studied the dynamics of infection with H. parasuis after intranasal inoculation with a systemic isolate. In those studies, H. parasuis was recovered from lung tissue before any other systemic organ and prior to systemic disease onset. Recently, our group proposed that serum resistance was a virulence mechanism linked to the ability of virulent strains to produce systemic infection [4]. However, based on the dynamics of infection, H. parasuis has to first reach the lung and survive the host pulmonary defences before invading the blood stream. In the lung, bacteria have to confront alveolar macrophages, whose main function is to clear infection through phagocytosis of invading microorganisms. Since resistance to phagocytosis is a common trait of virulent Pasteurellaceae [7, 10, 11, 20], the aim of this study was to determine if resistance to phagocytosis is also a virulence mechanism of H. parasuis. Here, we report that H. parasuis strains from different clinical origin present different susceptibility to phagocytosis by porcine alveolar macrophages (PAM).
2. MATERIALS AND METHODS
2.1. Porcine alveolar macrophages isolation
All procedures involving animals followed EU normative (Council Directive 86/609/EEC) and were performed with institutional authorization. Prior to the collection of bronchoalveolar fluid, four healthy pigs from different conventional herds were euthanized by intravenous sodium pentobarbital overdose. Bronchoalveolar lavage of the lungs was performed with 100 mL aliquots of sterile PBS containing gentamicin at 70 μg/mL (Sigma-Aldrich, Madrid, Spain). To collect the PAM, lavage fluids were centrifuged at 230 × g for 15 min, and cells were washed twice with Dulbecco’s Modified Eagle’s Medium (DMEM) containing gentamicin (50 μg/mL). Concentration of PAM was adjusted to 1 × 107 cells/mL and aliquots were stored at −150 °C in DMEM with 10% of DMSO, 20% fetal bovine serum (FBS). PAM isolation was confirmed by detection of macrophage markers (SWC3, CD169 and SLAII) in the cells by flow cytometry.
2.2. Fluorescein labelling of H. parasuis strains
Eight H. parasuis field strains and the reference strains Nagasaki (virulent) and SW114 (non-virulent) were used in this study (Tab. I). Strains were grown on chocolate agar at 37 °C and 5% CO2, and the subsequent overnight, non-confluent growth was harvested from the plates and resuspended in PBS at an OD660nm between 0.6 and 1.3, depending on the strain (spectrophotometer VIS 7200; Dinko Instruments, Barcelona, Spain). Two milliliters of these suspensions were centrifuged at 11 000 × g for 5 min and the bacterial pellet was resuspended in 1 mL of PBS containing fluorescein isothiocyanate (FITC, from Sigma-Aldrich) at 100 μg/mL. After incubation for 45 min at 37 °C and 225 r.p.m., labelled bacteria were washed two times with complete DMEM and resuspended to a final volume of 0.5 mL in the same medium. To determine the final bacterial concentration after labelling and washing, ten fold dilutions were plated on chocolate agar. The concentration of the final inoculum was approximately 109 CFU/mL. To confirm fluorescent labelling, 10 μL samples were examined under a fluorescence microscope.
Table I.
Significant characteristics of the H. parasuis strains used in this study.
| Strain | Isolation site | Disease status or lesions | Serotype | Serum susceptibilitya | Reference |
|---|---|---|---|---|---|
| Nagasakib | Systemic | Fibrinous polyserositis | 5 | Resistant | [14] |
| 264/99 | Systemic | Fibrinous polyserositis | 10 | Resistant | [21] |
| ER-6P | Systemic | Fibrinous polyserositis | 15 | Resistant | [22] |
| PC4-6P | Systemic | Fibrinous polyserositis | 12 | Resistant | [22] |
| SW114c | Unknown | Healthy | 3 | Sensitive | [14] |
| F9 | Nose | Healthy | 6 | Sensitive | [22] |
| SC14-1 | Nose | Healthy | 15 | Sensitive | [21] |
| MU21-2 | Nose | Healthy | 7 | Sensitive | [21] |
| 167/03 | Lung | Unknown | 15 | Sensitive | [21] |
| 2757 | Lung | Pneumonia and pleuritis | 1 | Sensitive | [21] |
From [4], except data for F9 which was produced for this study;
virulent reference strain;
non-virulent reference strain.
2.3. Phagocytosis assays
The basic phagocytosis assay was performed as described before [27], with some modifications. PAM were seeded in 6-well plates (NUNC, Fisher Bioblock Scientific, Madrid, Spain) at a concentration of 5 × 105 cells in 3 mL of DMEM supplemented with 10% FBS and 1% L-glutamine (complete DMEM) per well. After attachment of the cells to the plates, duplicate wells were inoculated with FITC-labelled bacteria at a multiplicity of infection (MOI) of 100–200. After 1 h of incubation, plates were transferred to an ice bath to stop phagocytosis and supernatants were removed, centrifuged at 11 000 × g for 5 min and the pellet used for capsule staining (see below). PAM were then washed twice with 3 mL of complete DMEM and once with 3 mL of PBS. After the washes, PAM were scraped into 0.5 mL of 0.1% BSA in PBS and the cell suspensions were analyzed by flow cytometry in an EPICS® XL-MCL™ Flow Cytometer (Beckman Coulter, Madrid, Spain). Assays were repeated using PAM from different animals.
2.3.1. Bacterial survival after phagocytosis
Survival of four of the strains after phagocytosis was examined. PAM were seeded in 24 well plates at 5 × 105 cells/well and bacteria were added to an MOI of 100–200. After 1 h of incubation to allow uptake of bacteria, wells were washed three times and complete DMEM with or without gentamicin (100 μg/mL) and penicillin G (5 μg/mL) was added to the wells. Incubation proceeded for 0, 2 or 5 additional hours. At those times, wells were washed again and PAM were lysed with 500 μL of distilled water and physical disruption by pipetting. Surviving bacteria were counted by plating dilutions on chocolate agar plates.
2.3.2. Opsonization and extracellular detection of H. parasuis
A 1:1 mixture (v/v) of hyperimmune rabbit sera against strains Nagasaki and SW114 [4] was used to opsonize H. parasuis strains before phagocytosis by PAM. As a control for specificity, a H. parasuis non-specific rabbit antiserum, directed against cholera toxoid (TCC), was also used. Opsonization was performed by incubation of the different FITC-labelled bacterial suspensions with the same volume of the inactivated rabbit serum at 37 °C and 200 r.p.m. for 45 min. The opsonized strains were washed to eliminate free antibody before use in the phagocytosis assay. In addition, fresh non-immunized rabbit serum was used to opsonize virulent strains to test the effect of complement opsonization on phagocytosis.
The same mixture of hyperimmune sera was used to verify the internalization of the bacteria by PAM. For extracellular detection of H. parasuis after incubation with PAM, wells were washed to eliminate unbound bacteria and were incubated with a dilution of hyperimmune sera (6 μL sera in 500 μL of 0.1% BSA in PBS). Plates were incubated for 60 min at 225 r.p.m. on ice. After three washes with 0.1% BSA in PBS, cells were incubated with phycoerythrin (PE)-conjugated anti-rabbit IgG (Sigma). After washing, fluorescence associated to the PAM was measured by flow cytometry (FITC, total bacteria and PE, extracellular bacteria).
2.3.3. Microscopy and image analysis
PAM were plated at a concentration of 2 × 105 cells/well in a chamber slide (NUNC Lab-Tek) and were subsequently infected with one nasal (SW114) and three systemic (264/99, ER6-P and PC4-6P) FITC-labelled strains at a MOI = 50–100. After 1 h of incubation, phagocytosis was stopped by chilling the slides on ice. PAM were then washed twice with complete DMEM, once with PBS, fixed with 4% paraformaldehyde for 10 min and permeabilized with 0.5% Triton X-100 in PBS for 25 min, at RT. The cells were then stained with tetramethylrhodamine B isothiocyanate-phalloidin (50 μg/mL), counterstained with DAPI (1 μg/mL) and mounted in Vectashield.
Preparations were viewed on a Nikon eclipse 90i epifluorescence microscope (× 100/NA 1.3 objective). To asses the association of bacteria with PAM, we captured image stacks of cells using a Leica TCS SP2 confocal microscope (× 63/NA 1.4 objective). Z stack images were acquired at intervals of 0.3 μm. Images were processed by using the LCS from Leica and Image J software v3.91 software1.
2.3.4. Phagocytosis inhibitors
To explore the role of actin filaments and microtubules in the phagocytosis process, PAM were incubated with 1 μg/mL of cytochalasin D (cytD) or 10 μM colchicine (both from Sigma-Aldrich) 2 h before and during the phagocytosis assay. Also, bacterial protein synthesis was inhibited by adding 100 μg/mL of chloramphenicol to the phagocytosis assay. In both assays, detection of total and extracellular bacteria was performed as described above.
2.4. Maneval capsule staining
Samples of FITC-labelled strains were examined by Maneval staining for detection of capsule [5] before and after the incubation with PAM. Preparations were observed with a Nikon eclipse 90i microscope equipped with a Nikon DXM 1200F camera.
3. RESULTS
3.1. Virulent strains of H. parasuis avoid phagocytosis
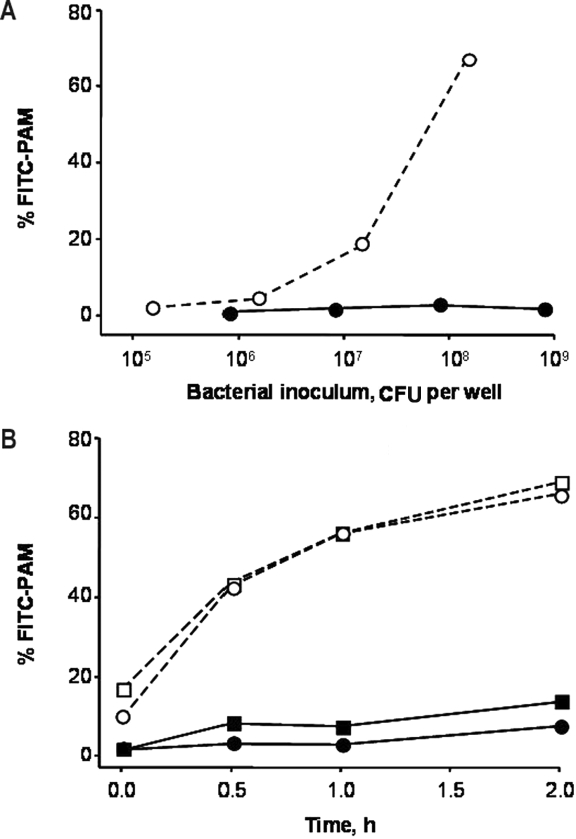
Phagocytosis assays with different concentrations of FITC-labelled Nagasaki and SW114 (virulent and non-virulent reference strains, respectively) indicated a clear difference in the interaction of these two strains with PAM. While the non-virulent strain SW114 associated with a high percentage of PAM, the virulent strain Nagasaki did not associate with PAM (Fig. 1A). Different incubation times were also tested to optimize phagocytosis conditions (Fig. 1B) and the final conditions for the assay were set at 1 h of incubation of approximately 108 CFU of H. parasuis with 5 × 105 PAM per well (MOI = 100−200).
Figure 1.
Optimization of basic conditions for phagocytosis assay. Different quantities of FITC-labelled bacteria (A) and different incubation times of phagocytosis (B) were tested with PAM and virulent (solid lines) or non-virulent (dashed lines) strains. Results are shown as the percentage of macrophages with green-fluorescent bacteria detected by flow cytometry. The strains used were Nagasaki (●), 264/99 (■), SW114 (○) and F9 (□). In (B), bacterial inoculi were 5 × 108 CFU of Nagasaki, 2 × 108 CFU of 264/99, 2 × 107 CFU of SW114 and 2 × 107 CFU of F9.
The differences observed with the reference strains were also detected with field strains from different clinical backgrounds: 3 nasal, 3 systemic and 2 pulmonary (Tab. I). Incubation of nasal strains with PAM resulted in a higher percentage of PAM with associated bacteria than systemic strains: 40–90% PAM with associated nasal strains versus 1–20% PAM with systemic strains (Fig. 2, black bars). Although we observed interexperimental variation with PAM from different pigs, significant differences were found in all experiments between nasal and systemic strains (Student’s T test of independent experiments; P from 0.01 to 2.6 × 10−16). In addition, when we pooled the data from the individual experiments, we still observed a clear difference between nasal and systemic strains (Student’s T test; P = 2.2 × 10−12). Furthermore, PAM inoculated with nasal strains showed 2 to 15 times more FITC-fluorescence intensity compared to the ones incubated with systemic strains.
Figure 2.
Phagocytosis of H. parasuis strains by PAM. PAM (5 × 105 cells/well) were incubated with different FITC-labelled strains of H. parasuis. After 1 h of incubation, extracellular bacteria were detected with a H. parasuis-specific antiserum coupled with a phycoerythrin-secondary antibody. Macrophages were then analysed by flow cytometry. Results are presented as the percentages of cells associated with fluorescent bacteria (black bars), cells with bacteria inside (only green fluorescence; dark gray bars) and cells with bacteria on the surface (green and red fluorescence; light gray bars). Inoculi of bacteria were: 109 CFU/well of Nagasaki; 2–4 × 108 CFU/well of 264/99, ER-6P and PC4-6P; 7–8 × 107 CFU/well of SW114, F9 and SC14-1. Data are means and standard deviations from four independent experiments.
Two of the systemic strains tested (Nagasaki, and ER-6P) showed no association with PAM, but strains 264/99 and PC4-6P consistently showed a low level of association with the macrophages. PAM inoculated with the two pulmonary strains used in this study showed different levels of associated FITC fluorescence. While strain 167/03 showed a high degree of association with PAM similar to that of nasal strains (approximately 50% of PAM with associated 167/03), strain 2757 associated to a lesser degree (approximately 20% of PAM with associated 2757).
The internalization of the FITC-labelled non-virulent strains was confirmed by flow cytometry and the specific antiserum to detect extracellular bacteria (Fig. 2). In the case of strains 264/99 and PC4-6P, the low association with the PAM correlated with the majority of bacteria being internalized, since no significant extracellular bacteria were detected (Fig. 2). Internalization of bacteria was further confirmed by confocal microscopy (Fig. 3; and on-line material only: supplementary information for video available at www.vetres.org).
Figure 3.
Confocal images showing the association of virulent (ER6-P and PC4-6P) and non-virulent (SW114) strains with PAM. The green signals correspond to FITC-labelled H. parasuis. PAM were stained with rhodamine-phalloidin (red) to label the cytoplasm and the nuclei were counterstained with DAPI (blue). Z stack images from two independent experiments were captured. Scale bar, 2 μm. (For a colour version of this figure, please consult www.vetres.org.)
In survival assays, we also detected a greater association (between 20 and 100 times) of nasal strains to PAM after 1 h of phagocytosis (0.8% and 2.0% of inoculum for SW114 and SC14-1, respectively; 0.02% of inoculum for Nagasaki and 264/99 and 0.05% for PC4-6P). Internalized bacteria, independent of the strain, were killed by PAM after 2 h.
Opsonization of virulent H. parasuis strains with specific antibodies increased the association of these bacteria with PAM to 60–80%, similar to levels seen with the nasal strains. Phagocytosis levels of the nasal strains were not affected by antibody-opsonization (not shown). Opsonization using a non-specific antiserum (directed against TCC) or a fresh rabbit pre-immune serum was not successful in promoting phagocytosis of systemic strains (not shown), indicating that non-specific antibodies or complement-opsonization did not have any effect in the phagocytosis of H. parasuis.
3.2. Effect of in vitro passage
We used ER-6P, a virulent strain with a known origin and passage history (ER-6P was a direct isolate from the pericardium of a piglet with Glässer’s disease), to explore the effect of in vitro passage on the phagocytosis susceptibility. To test this, we replated strain ER-6P on chocolate agar every 2 days for 14 consecutive passages. At that time, the culture was stored at −80 °C and was designated ER-6Pp14. When ER-6Pp14 was tested in the phagocytosis assay, we observed that plate passage induced changes in the original strain ER-6P, making it more susceptible to phagocytosis (Fig. 4), i.e. the percentage of PAM with associated bacteria increased noticeably. In addition to the increased susceptibility to phagocytosis of strain ER-6Pp14, we also observed other altered phenotypes, such as larger colonies on chocolate agar and increased auto-agglutination (not shown).
Figure 4.
Effect of plate passage of strain ER-6P. Strain ER-6P and the same strain after 14 passages on agar plates (ER-6Pp14) were tested with PAM for their susceptibility to phagocytosis. Both strains were FITC-labelled and incubated with PAM. Extracellular bacteria were detected with a H. parasuis-specific antiserum coupled with a PE-secondary antibody. Percentages of macrophages with FITC and PE fluorescence were detected by flow cytometry. Figure shows representative results of three independent experiments.
3.3. Inhibition of phagocytosis
The association of nasal strains to PAM was reduced at 4 °C, indicating that the PAM have to be active for the uptake and that probably there is not a specific receptor for attachment of these strains. The lack of specific receptor was also supported by the competition assays with non-labelled bacteria. We tried to competitively inhibit the attachment and uptake of FITC-labelled SW114 (107 CFU) with non-labelled SW114 (107 or 108 CFU) and non-labelled systemic strains (108, 109 or 1010 CFU). We observed that the phagocytosis of the non-virulent strain SW114 by PAM could not be blocked or reduced by homologous or heterologous competition (not shown).
We sought to determine the role of the cytoskeletal components actin and microtubules in the phagocytic uptake of H. parasuis. The addition of the actin filament inhibitor cytD before and during the phagocytosis assay had a clear effect on the internalization of non-virulent strains (Fig. 5), although the inhibition was not complete. Interestingly, uptake of virulent strains PC4-6P and 264/99 was not affected by cytD, indicating that these strains are entering the PAM through a different mechanism than non-virulent strains.
Figure 5.
Effect of cytD on the phagocytosis of H. parasuis strains. FITC-labelled strains of H. parasuis were incubated with PAM with (dashed bars) or without (plain bars) cytD. After 1 h, extracellular bacteria were detected with a H. parasuis-specific antiserum coupled with a phycoerythrin-secondary antibody and macrophages were analysed by flow cytometry. Results are presented as the percentage of macrophages with internalized bacteria (black bars) and surface associated bacteria (grey bars).
Inhibition of microtubules by colchicine partially inhibited the internalization of nasal strains SW114 and F9, but did not affect the phagocytosis and internalization of SC14-1 and MU21-2. Phagocytosis of Nagasaki and ER-6P was not affected by colchicine, but phagocytosis of PC4-6P (Fig. 6) and 264/99 was significatively increased (Student’s T test, P = 0.037 and 0.008, respectively) by the microtubule inhibitor. Overall, these results demonstrate that there are several mechanisms involved in the phagocytosis of H. parasuis by PAM, and that there is a variable susceptibility of H. parasuis strains to this process.
Figure 6.
The effects of colchicine in phagocytosis of strain PC4-6P. FITC-labelled strain PC4-6P was used in phagocytosis assays to test the effects of colchicine. After 1 h, extracellular bacteria were detected with a H. parasuis-specific antiserum coupled with a phycoerythrin-secondary antibody and macrophages were analysed by flow cytometry. Results are presented as the percentage of macrophages with total bacteria (black bars), internalized bacteria (dark grey bars) and surface associated bacteria (light grey bars).
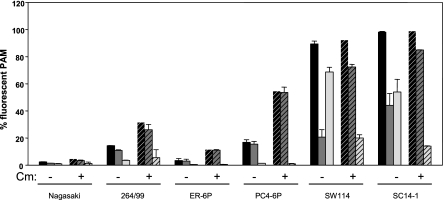
To determine if there was a role for newly synthesized proteins in the evasion process, we used the bacterial protein synthesis inhibitor chloramphenicol to block new protein synthesis. When we used chloramphenicol, we detected significant increase (Student’s T test, P < 0.05) in the susceptibility of the systemic strains to phagocytosis (Fig. 7). However, the level of phagocytosis in the presence of the inhibitor remained moderate. In nasal strains, the level of total phagocytosis was not affected by chloramphenicol, but the level of internalization was significantly increased by the inhibitor (Student’s T test, P < 0.05; Fig. 7).
Figure 7.
Effect of inhibiting bacterial protein synthesis on phagocytosis. FITC-labelled strains of H. parasuis were incubated with PAM in the presence of chloramphenicol (Cm; dashed bars) or without the inhibitor (plain bars). After 1 h, extracellular bacteria were detected with a H. parasuis-specific antiserum coupled with a phycoerythrin-secondary antibody and macrophages were analysed by flow cytometry. Results are presented as the percentage of macrophages with total bacteria (black bars), internalized bacteria (dark grey bars) and surface associated bacteria (light grey bars).
3.4. Capsule production
Figure 8 shows the detection of capsule in Nagasaki and PC4-6P strains before and after incubation with macrophages. Similar results were obtained with ER-6P and 264/99 strains. Capsule was more evident in the systemic strains after incubation with PAM than in their initial inoculi, but capsule was not detected in nasal strains before or after incubation with PAM (not shown).
Figure 8.
Capsule detection by Maneval staining of bacterial smears. Strains Nagasaki (left panels) and PC4-6P (right panels) were examined before (top panels) and after (bottom panels) incubation with PAM. Capsule is observed as a white halo surrounding the bacterial body. (For a colour version of this figure, please consult www.vetres.org.)
4. DISCUSSION
Although little is known about the virulence mechanisms of H. parasuis, the ability of this bacterium to reach and survive in the lung is probably a key feature in producing disease. Thus, resistance to pulmonary innate immune defences, such as phagocytosis by alveolar macrophages, could be a cornerstone for the development of pneumonia or Glässer’s disease. In fact, the incubation of different H. parasuis strains with PAM revealed differences that correlated with the clinical origin of the strains. While strains isolated from systemic lesions were resistant to phagocytosis, nasal strains were efficiently phagocytosed by PAM, and this interaction resulted in subsequent bacterial death. Additionally, resistance to phagocytosis by H. parasuis virulent strains could be linked, at least partially, to capsule production, since production of capsule was only seen in strains that were resistant to phagocytosis.
In agreement with previous reports [27], antibody-mediated (type I) phagocytosis was highly efficient for all strains. Indeed, it is well known that pigs that produce a strong antibody response against H. parasuis can survive a homologous challenge. Thus, bacterial clearance in the lung by type I phagocytosis will prevent systemic infection and therefore, overcome Glässer’s disease. However, complement-mediated (type II) phagocytosis did not result in efficient phagocytosis of virulent strains of H. parasuis. It is known that virulent H. parasuis strains are resistant to serum [4] and, taking into account that bacterial capsule is known to interfere with complement deposition [3], it is possible that opsonization by complement is not effective in H. parasuis and therefore can not mediate phagocytosis for the virulent, encapsulated strains. Consequently, the innate immune response will not be able to stop H. parasuis infection, and a humoral response will be needed. Competition assays showed that there was not a specific receptor for the phagocytosis of H. parasuis, and contrary to other members of the Pasteurellaceae family, such as Haemophilus ducreyi or Histophilus somni [9, 30], virulent strains did not inhibit the ability of the macrophages to phagocytose other susceptible bacteria.
Our results suggest that several cellular processes participate in the phagocytosis of H. parasuis strains. Phagocytosis of non-virulent nasal strains by PAM was efficient and dependent on actin filaments and active phagocytes. However, colchicine only demonstrated strain dependent effects among the nasal strains tested; indicating that there may be more than one mechanism involved. The contribution of bacterial products from the nasal strains on phagocytosis was minor, since the inhibition of bacterial protein synthesis by chloramphenicol resulted in no changes in the overall number of cell associated (internal or external) bacteria, although there was an increase in the internalization.
Conversely, virulent strains resisted phagocytosis, and our data indicate that there may be several mechanisms of evasion. First, the observation of a more prominent capsule after incubation of virulent bacteria with PAM supports a role for capsule in phagocytosis resistance. Previous studies reported controversial results about the presence or absence of capsule in H. parasuis and its possible role in virulence [17, 24]. In our experience, capsule in this bacterium is not easy to demonstrate (we did not detect capsule in H. parasuis grown on agar plates [4]), so the induction of capsule production by incubation with PAM is a significant finding, and may help us to understand the regulation of capsule production. In addition, the slight increase in phagocytosis susceptibility observed with systemic strains after treatment with the bacterial protein inhibitor chloramphenicol may indicate that there is an additional, proteinaceous factor involved in phagocytosis resistance. Concurrently, systemic strains showed two distinct phenotypes: while strains Nagasaki and ER-6P showed negligible association with the phagocytes, strains 264/99 and PC4-6P were internalized to some degree by the PAM. Contrary to what was observed for nasal strains, internalization of PC4-6P and 264/99 by PAM was not affected by inhibition of actin polymerization, indicating that a different mechanism of internalization was involved. Surprisingly, the internalization of systemic strains PC4-6P and 264/99 was increased by inhibition of microtubules by colchicine. These results indicate a possible role of microtubules in the mechanism of resistance of these strains, which may produce an inhibitor that would operate through a microtubule-dependent pathway to block phagocytosis. However, since these strains did not survive inside the phagocytes, internalization of virulent strains was discarded as an invasion mechanism. Alternatively, recent reports point to the existence of self-destructive cooperation in bacteria [1], in which one part of the population “sacrifices” for the survival of the rest. Curiously, the existence of different phenotypic sub-populations in strains of H. parasuis is apparent by the existence of different colony morphologies in some isolates, including strain PC4-6P. The specific mechanisms involved in this phenomenon still remain to be elucidated.
As it is known for other pathogens [6], we detected striking changes in strain ER-6P after in vitro passages. Our results show that adaptation of the strain to laboratory conditions yielded an attenuated phenotype for phagocytosis and alteration of other traits, such as increased autoagglutination. These results reiterate the importance of using field strains with low in vitro passage to study virulence in these bacteria. This may be most important for reproducibility of infection studies in animals, since the number of passages could affect the number of virulent bacteria in the inoculum.
Interestingly, the results obtained in this study with strain 167/03, demonstrate that while this strain was originally taken as a pulmonary isolate, it demonstrated a level of phagocytosis susceptibility similar to nasal strains. This supports previous results that indicate that this strain has characteristics of a non-virulent strain. Strain 167/03 clustered by MLST with nasal strains from healthy pigs from disease-free farms [22] and was found to be serum sensitive at a level similar to that of nasal strains [4]. The clinical data of the strain also support the same conclusion. Strain 167/03 was isolated from the lung of an animal from a farm affected with porcine reproductive and respiratory syndrome virus (PRRSV) and porcine circovirus type 2 (PCV2). PCV2 is well known as an immuno-suppressing virus [13, 26] and, although there is some controversy about the immunosuppressive effects of PRRSV, the fact that this virus replicates primarily in alveolar macrophages could affect the competence of these cells to clear opportunistic pathogens such as non-virulent strains of H. parasuis [18, 27]. Thus, we believe that strain 167/03 was able to survive in the lungs where it was isolated due to the suppressed immune status of the host, not due to pathogenic mechanisms of the bacteria. In contrast, strain 2757, which is also a pulmonary isolate, showed features which differed from those of strain 167/03. Strain 2757 was isolated from a case of pneumonia and pleuritis and was classified by MLST in a cluster of strains isolated from pneumonia cases. Also, strain 2757 was shown to be serum-sensitive [4] but, in this study, we have shown that strain 2757 is resistant to phagocytosis by alveolar macrophages. All these data support the idea that strain 2757 is a true pulmonary strain; capable of producing pneumonia (resistant to phagocytosis by alveolar macrophages) but not able to invade systemically (sensitive to serum). This stresses that using strains isolated from the lung can give misleading results.
In conclusion, we report for the first time that phagocytosis resistance is a virulence mechanism of H. parasuis and that several factors may be involved in this function.
Online material
Confocal images showing the association of virulent (ER6-P and PC4- 6P) and non-virulent (SW114) strains with PAM. The green signals correspond to FITC-labelled H. parasuis. PAM were stained with rhodamine-phalloidin (red) to label the cytoplasm and the nuclei were counterstained with DAPI (blue).
Acknowledgments
The authors are grateful to Núria Galofré (CReSA) for technical support and to Fernando Rodríguez (CReSA) for helpful discussions. This work was funded by the Ministerio de Cienciae Innovación of Spain (grant AGL2007-60432).
Footnotes
References
- 1.Ackermann M., Stecher B., Freed N.E., Songhet P., Hardt W.D., Doebeli M.. Self-destructive cooperation mediated by phenotypic noise. Nature. 2008;454:987–990. doi: 10.1038/nature07067. [DOI] [PubMed] [Google Scholar]
- 2.Bouchet B., Vanier G., Jacques M., Gottschalk M.. Interactions of Haemophilus parasuis and its LOS with porcine brain microvascular endothelial cells. Vet. Res. 2008;39:42. doi: 10.1051/vetres:2008019. [DOI] [PubMed] [Google Scholar]
- 3.Celli J., Finlay B.B.. Bacterial avoidance of phagocytosis. Trends Microbiol. 2002;10:232–237. doi: 10.1016/s0966-842x(02)02343-0. [DOI] [PubMed] [Google Scholar]
- 4.Cerdà-Cuéllar M., Aragon V.. Serum-resistance in Haemophilus parasuis is associated with systemic disease in swine. Vet. J. 2008;175:384–389. doi: 10.1016/j.tvjl.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Corstvet R.E., Gentry M.J., Newman P.R., Rummage J.A., Confer A.W.. Demonstration of age-dependent capsular material on Pasteurella haemolytica serotype 1. J. Clin. Microbiol. 1982;16:1123–1126. doi: 10.1128/jcm.16.6.1123-1126.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fux C.A., Shirtliff M., Stoodley P., Costerton J.W.. Can laboratory reference strains mirror “real-world” pathogenesis? Trends Microbiol. 2005;13:58–63. doi: 10.1016/j.tim.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Harper M., Boyce J.D., Adler B.. Pasteurella multocida pathogenesis: 125 years after Pasteur. FEMS Microbiol. Lett. 2006;265:1–10. doi: 10.1111/j.1574-6968.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 8.Hill C.E., Metcalf D.S., MacInnes J.I.. A search for virulence genes of Haemophilus parasuis using differential display RT-PCR. Vet. Microbiol. 2003;96:189–202. doi: 10.1016/s0378-1135(03)00212-8. [DOI] [PubMed] [Google Scholar]
- 9.Howard M.D., Boone J.H., Buechner-Maxwell V., Schurig G.G., Inzana T.J.. Inhibition of bovine macrophage and polymorphonuclear leukocyte superoxide anion production by Haemophilus somnus. Microb. Pathog. 2004;37:263–271. doi: 10.1016/j.micpath.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Inzana T.J., Ma J., Workman T., Gogolewski R.P., Anderson P.. Virulence properties and protective efficacy of the capsular polymer of Haemophilus (Actinobacillus) pleuropneumoniae serotype 5. Infect. Immun. 1988;56:1880–1889. doi: 10.1128/iai.56.8.1880-1889.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inzana T.J.. Capsules and virulence in the HAP group of bacteria. Can. J. Vet. Res. 1990;54(Suppl):S22–S27. [PubMed] [Google Scholar]
- 12.Jin H., Wan Y., Zhou R., Li L., Luo R., Zhang S.. et al. Identification of gene transcribed by Haemophilus parasuis in necrotic porcine lung through the selective capture of transcribed sequences (SCOTS) Environ. Microbiol. 2008;10:3326–3336. doi: 10.1111/j.1462-2920.2008.01729.x. [DOI] [PubMed] [Google Scholar]
- 13.Kekarainen T., Montoya M., Mateu E., Segales J.. Porcine circovirus type 2-induced interleukin-10 modulates recall antigen responses. J. Gen. Virol. 2008;89:760–765. doi: 10.1099/vir.0.83354-0. [DOI] [PubMed] [Google Scholar]
- 14.Kielstein P., Rapp-Gabrielson V.J.. Designation of 15 serovars of Haemophilus parasuis on the basis of immunodiffusion using heat-stable antigen extracts. J. Clin. Microbiol. 1992;30:862–865. doi: 10.1128/jcm.30.4.862-865.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melnikow E., Dornan S., Sargent C., Duszenko M., Evans G., Gunkel N.. et al. Microarray analysis of Haemophilus parasuis gene expression under in vitro growth conditions mimicking the in vivo environment. Vet. Microbiol. 2005;110:255–263. doi: 10.1016/j.vetmic.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Metcalf D.S., MacInnes J.I.. Differential expression of Haemophilus parasuis genes in response to iron restriction and cerebrospinal fluid. Can. J. Vet. Res. 2007;71:181–188. [PMC free article] [PubMed] [Google Scholar]
- 17.Morozumi T., Nicolet J.. Morphological variations of Haemophilus parasuis strains. J. Clin. Microbiol. 1986;23:138–142. doi: 10.1128/jcm.23.1.138-142.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murtaugh M.P., Xiao Z., Zuckermann F.. Immunological responses of swine to porcine reproductive and respiratory syndrome virus infection. Viral Immunol. 2002;15:533–547. doi: 10.1089/088282402320914485. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen R.. Pathogenicity and immunity studies of Haemophilus parasuis serotypes. Acta Vet. Scand. 1993;34:193–198. doi: 10.1186/BF03548209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noel G.J., Hoiseth S.K., Edelson P.J.. Type b capsule inhibits ingestion of Haemophilus influenzae by murine macrophages: studies with isogenic encapsulated and unencapsulated strains. J. Infect. Dis. 1992;166:178–182. doi: 10.1093/infdis/166.1.178. [DOI] [PubMed] [Google Scholar]
- 21.Olvera A., Calsamiglia M., Aragon V.. Genotypic diversity of Haemophilus parasuis field strains. Appl. Environ. Microbiol. 2006;72:3984–3992. doi: 10.1128/AEM.02834-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olvera A., Cerda-Cuellar M., Aragon V.. Study of the population structure of Haemophilus parasuis by multilocus sequence typing. Microbiology. 2006;152:3683–3690. doi: 10.1099/mic.0.29254-0. [DOI] [PubMed] [Google Scholar]
- 23.Rapp-Gabrielson V.J., Gabrielson D.A., Schamber G.J.. Comparative virulence of Haemophilus parasuis serovars 1 to 7 in guinea pigs. Am. J. Vet. Res. 1992;53:987–994. [PubMed] [Google Scholar]
- 24.Rapp-Gabrielson V., Oliveira S., Pijoan C. Straw B., Zimmerman J., D’Allaire S., Taylor D. (Eds.), Diseases of swine. Iowa State University Press; 2006. Haemophilus parasuis; pp. 681–690. [Google Scholar]
- 25.Rosner H., Kielstein P., Muller W., Rohrmann B.. Relationship between serotype, virulence and SDS-PAGE protein patterns of Haemophilus parasuis. Dtsch. Tierarztl. Wochenschr. 1991;98:327–330. [PubMed] [Google Scholar]
- 26.Segales J., Allan G.M., Domingo M.. Porcine circovirus diseases. Anim. Health Res. Rev. 2005;6:119–142. doi: 10.1079/ahr2005106. [DOI] [PubMed] [Google Scholar]
- 27.Solano G.I., Bautista E., Molitor T.W., Segales J., Pijoan C.. Effect of porcine reproductive and respiratory syndrome virus infection on the clearance of Haemophilus parasuis by porcine alveolar macrophages. Can. J. Vet. Res. 1998;62:251–256. [PMC free article] [PubMed] [Google Scholar]
- 28.Vahle J.L., Haynes J.S., Andrews J.J.. Experimental reproduction of Haemophilus parasuis infection in swine: clinical, bacteriological, and morphologic findings. J. Vet. Diagn. Invest. 1995;7:476–480. doi: 10.1177/104063879500700409. [DOI] [PubMed] [Google Scholar]
- 29.Vanier G., Szczotka A., Friedl P., Lacouture S., Jacques M., Gottschalk M.. Haemophilus parasuis invades porcine brain microvascular endothelial cells. Microbiology. 2006;152:135–142. doi: 10.1099/mic.0.28312-0. [DOI] [PubMed] [Google Scholar]
- 30.Wood G.E., Dutro S.M., Totten P.A.. Haemophilus ducreyi inhibits phagocytosis by U-937 cells, a human macrophage-like cell line. Infect. Immun. 2001;69:4726–4733. doi: 10.1128/IAI.69.8.4726-4733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Confocal images showing the association of virulent (ER6-P and PC4- 6P) and non-virulent (SW114) strains with PAM. The green signals correspond to FITC-labelled H. parasuis. PAM were stained with rhodamine-phalloidin (red) to label the cytoplasm and the nuclei were counterstained with DAPI (blue).