Abstract
The content of episodic memory consists of representations of unique past events. Episodic memories are grounded in a temporal framework (i.e., we remember when an event occurred). It has recently been argued that episodic-like memory in rats is qualitatively different from human episodic memory because, rather than remembering when an earlier past event occurred, rats used the cue of how long ago it occurred. We asked, therefore, whether rats remember the time of day at which they encountered a distinctive event, in addition to what occurred and where it happened. Rats were tested in the morning and afternoon, on separate days. A distinctive flavor (chocolate) was replenished at a daily-unique location at only one of these times. The interval between first and second daily opportunities to eat (study and test, respectively) was constant. Rats adjusted their revisits to the chocolate location at different times of day by using time of day rather than the cue of how long ago an event occurred. Two lines of evidence suggest that rats remembered the time at which the distinctive event occurred. First, under conditions in which the time of test (but not time of study) was novel, rats immediately transferred their knowledge of the chocolate contingency to the new test time. Second, under conditions in which predictions for study and test times were put in conflict, rats again used study time. Our results suggest that, at the time of memory assessment, rats remember when a recent episode occurred, similar to human episodic memory.
People remember when a past event occurred within the time frame of hours, days, or years (1). It has been argued that retrieval of episodic memories is analogous to traveling back in time to experience specific events from one's personal past (2–4). An earlier definition focused on the content of episodic memory, that is, answering 3 questions about a specific event: what happened, where did it take place, and when did it transpire (5)? We refer to this type of content as what-where-when memory. Clayton and Dickinson (6) introduced the term episodic-like memory to emphasize that behavioral studies in animals evaluate the content of episodic memory rather than subjective experiences.
Recent studies with nonhuman animals (6–18) suggest that animals remember specific episodes from their past (i.e., what-where-when memories). However, controversy has emerged about the comparability of episodic-like memory in rodents and episodic memory in humans (17). Roberts et al. (2, 17) suggested that memory for when an event occurred suggests an ability akin to mentally traveling in time to locate an event within a temporal framework; such an ability would be similar to human episodic memory, in which people reconstruct past experiences using an absolute temporal dimension (1, 19, 20). By contrast, a judgment of how long ago an event occurred is quite different from human episodic memory. Such a judgment could also be solved by simpler alternative mechanisms (e.g., timing an interval since a distinctive event occurred or assessing relative familiarity of temporally distant events). Remembering the time of day at which an event occurred is different from an assessment of how long ago it occurred (21). An animal's sense of time of day depends upon an internal circadian clock (19, 22). Because animals have an internal circadian clock that provides information about time of day, their knowledge about time of day does not depend on external stimuli such as light onset or other environmental cues. Thus, we use the term time of day synonymously with the phase of a circadian oscillator (i.e., the proportion within a 24-h cycle).
The objective of this research was to determine, at the time of memory assessment, whether rats remember when (i.e., the time of day at which) an earlier distinctive event occurred, in addition to what occurred and where it happened. Rats can use how-long-ago cues under conditions in which both when and how-long-ago cues are available (17). Therefore, we sought behavioral evidence for remembering when an event occurred by eliminating the usefulness of how long ago an event occurred as a temporal cue. This demonstration is important because development of a rodent model of episodic memory may improve our understanding of human disorders of memory (23).
Rats were placed in an 8-arm radial maze twice per day. Upon first exposure, rats obtained their first opportunity to eat regular rat chow and a preferred food type, chocolate. We refer to the first feeding opportunity as first helpings. After a delay, rats were returned to the maze. However, to obtain their second opportunity to eat chow (i.e., second helpings), they needed to avoid revisiting locations where they obtained their first helpings earlier that same day because the old locations no longer provided chow. To obtain their second helpings of chocolate, they had to revisit the same location that provided chocolate earlier in the day, but the chocolate location replenished (or failed to replenish) according to a temporal rule. Consequently, obtaining chocolate at second helpings required the rat to remember when and where they found the chocolate (what) during their first helpings, documenting the use of what-where-when memory. We refer to the first helpings of the day as a study phase and the second helpings as a test phase; the first phase provides an opportunity to study the initial food locations and the second phase is a test of memories of first-helpings locations because the rats obtain additional food by visiting the locations that were not visited in the study phase. The delay between study and test phases (i.e., first and second helpings) is referred to as a retention interval.
We investigated whether rats remembered the time of day at which they had recently encountered a distinctive food type (chocolate) using a group of rats that served in 4 experiments in sequence. Rats were trained in the morning and afternoon, on separate days, but chocolate replenished at a daily-unique location at only 1 of these times (counterbalanced across rats). The rats searched for food (i.e., their first helpings of the day) in an initial study phase. Subsequently, the rats searched again for food in a test phase (i.e., their second helpings of the day) on the same day; the delay between study and test phases was 2 min. Chocolate was found in the maze at a randomly selected location in their first helpings each day; for rats to obtain chocolate at their second helpings of the day, they needed to remember where they encountered the chocolate during their first helpings (i.e., chocolate was only available at the same location that provided chocolate in their first helpings). We made the delay between first and second helpings irrelevant to finding chocolate by using a constant 2-min retention interval in both morning and afternoon sessions. Rats were given only one opportunity to obtain first and second helpings per day, and both of these feedings occurred either in the morning or in the afternoon in Experiment 1. Availability of chocolate during the second helpings on any one day depended on whether rats obtained their first-helpings (study phase) in the morning or in the afternoon on that day. By contrast, locations baited with chow during first helpings never replenished at second helpings. If rats use what-where-when memories, then they should be able to revisit the chocolate location at second helpings at the correct time of day (and limit revisits at the incorrect time of day); what-where-when memories are implicated, in this case, because the rat would need to remember when and where they found the chocolate (what) during their first helpings (study phase).
Rats selectively adjusted their revisits to the chocolate location (where) at the times of day (when) at which these locations were scheduled to replenish chocolate (what; Experiment 1), consistent with the use of what-where-when memory. Next, we used a phase shift of light onset in the colony (24, 25) to determine whether the rats used time of day (i.e., circadian phase) or an interval cue (i.e., the interval since light onset) to find their second helpings (Experiment 2). Under conditions in which predictions for circadian time of day and an interval cue were dissociated, we observed revisits to the chocolate location based on circadian time of day. Finally, we used 2 techniques to determine whether, at the time of second helpings, the rats remembered when the first helpings occurred, rather than discriminating the time of day at second helpings. First, we introduced 7-h retention intervals that maintained the familiar times of day at first helpings but used novel times of day at second helpings (Experiment 3). The rats immediately transferred (i.e., without feedback) their chocolate revisit strategy to the novel situation. Thus, the rats used the familiar time of day at their first helpings to guide chocolate revisits. Second, we conducted a conflict test. Because early and late sessions overlapped in time in Experiment 3, we were able to begin a session with a late opportunity for first helpings and end with an early opportunity for second helpings in the conflict test (Experiment 4). Under these conditions, predictions for using the time of day at first and second helpings were dissociated. In both Experiments 3 and 4, revisits to the chocolate location were guided by the time of day at first helpings. Our results suggest that, at the time of memory assessment, rats remember when an earlier episode occurred (in addition to what and where information). These findings represent a qualitative similarity to human episodic memory.
Results
Experiment 1.
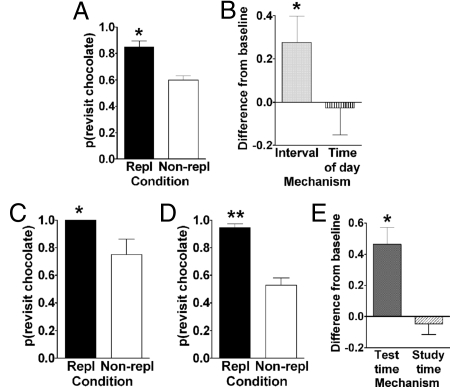
The design of Experiment 1 is shown in Fig. 1A. Morning or afternoon was randomly selected for presentation of first and second helpings, separated by a 2-min delay. Chocolate was available at a randomly selected location during first helpings and sometimes replenished at that location during second helpings. For some rats, the replenishment of chocolate occurred in the morning, and non-replenishment of chocolate occurred in the afternoon; this arrangement was reversed for the remaining rats. To obtain chocolate in second helpings (test phase), the rats needed to remember where they found it during first helpings (study phase) of that same day. Thus, availability of chocolate in their second helpings on any given day depended on whether their first helpings occurred at 7:00 a.m. or 1:00 p.m. on that day. Rats preferentially revisited the chocolate location when it was about to replenish. The probability of revisiting the chocolate location in the first 4 arm entries during second helpings was higher at the replenishment time of day than at the non-replenishment time of day [t (15) = 4.3, P < 0.001; see Fig. 2A]. The probability of revisiting chocolate was above the probability expected by chance in both replenishment [t (15) = 10.2, P < 0.0001; chance = 0.41] and non-replenishment [t (15) = 5.7, P < 0.0001] conditions. Because the delay between first and second helpings was constant for both morning and afternoon sessions, the constant delay provided no information as to the replenishment of chocolate. Thus, the cue of how long ago first helpings occurred could not be used to solve the task. Moreover, differential rates of revisiting chocolate-flavored locations were accomplished while rats accurately avoided revisits to depleted chow-flavored locations [see supporting information (SI) Table S1].
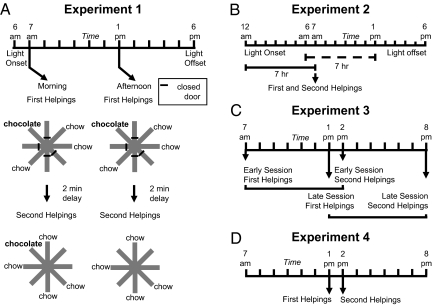
Fig. 1.
Experimental design. (A) Design of Experiment 1. The morning or afternoon was randomly selected for presentation of first helpings (study phase) and second helpings (test phase). The figure shows an example of the accessible arms and flavors in a study phase and the corresponding test phase that would occur after a 2-min retention interval. Chocolate- or chow-flavored pellets were available at 4 randomly selected arms in the study phase; access to the other 4 arms was prevented by closed doors. After a 2-min delay, chow-flavored pellets were available at previously inaccessible locations in the test phase. In the replenishment condition, chocolate replenished at the location that had chocolate in the study phase (shown for the morning session); in the non-replenishment condition, chocolate did not replenish at the other time of day (shown in the afternoon session). Chocolate replenished at second helpings in the test in the morning (7:00 a.m.) session but not in the afternoon (1:00 p.m.) session for half of the rats; these contingencies were reversed (not shown) for the remaining rats. For each rat, 1 session (i.e., first and second helpings) was conducted per day. The same arms were used to illustrate morning and afternoon sessions in the figure to facilitate inspection of presence and absence of chow and chocolate, but these arms were randomly selected in each session for each rat. (B) Phase-shift design of Experiment 2. Light onset occurred at 12:00 a.m. (i.e., 6 h earlier than in Experiment 1), and the first and second helpings occurred at the time of a typical morning session (i.e., starting at 7:00 a.m.). Note that 7 h elapsed between light onset and the study-test sequence (solid horizontal line), which is comparable to the time between the typical light onset and a typical afternoon session (dashed horizontal line) in Experiment 1. The design of the experiment puts predictions for time-of-day and how-long-ago cues in conflict. Thus, a rat would be expected to behave as in its morning baseline (on the basis of time of day) or as in its afternoon baseline (on the basis of how long ago). (C) Transfer-test design of Experiment 3. The time of day at which first helpings occurred was the same as in Experiment 1 (i.e., 7:00 a.m. in early or 1:00 p.m. in late sessions). The introduction of 7-h retention intervals in Experiment 3 produced test phases that occurred at novel times of day (2:00 p.m. in early and 8:00 p.m. in late sessions). Early and late sessions had study times (but not test times) that corresponded to those in Experiment 1. The first 2 sessions in Experiment 3 consisted of 1 replenishment and 1 non-replenishment condition. On subsequent days an early or late session was randomly selected. Differential revisits to the chocolate location are expected if the rats were adjusting revisit rates on the basis of the time of day at which the study episode occurred; revisit rates are expected to be equal in early and late sessions if the rats used time of day at which the test phase occurred. Study and test phases were as in Experiment 1, except that they were separated by 7-h delays (shown by horizontal brackets). (D) Conflict-test design of Experiment 4. The study and test phases occurred at 1:00 p.m. and 2:00 p.m., respectively. These times correspond to the typical time of day at which late-session first helpings and early-session second helpings occurred in Experiment 3. The design of the experiment put predictions for time of day at study and time of day at test in conflict. Thus, a rat would be expected to behave as in its early-session, second-helpings baseline (on the basis of test time of day) or as in its late-session, second-helpings baseline (on the basis of study time of day).
Fig. 2.
(A) Rats preferentially revisit the chocolate location when it is about to replenish in Experiment 1. The probability of a revisit to the chocolate location in the first 4 choices of a test phase is shown for replenishment (Repl) and non-replenishment (Non-repl) conditions; replenish and non-replenish sessions were presented in random order. *P < 0.001 difference between conditions. (B) Rats used time of day, rather than an interval, to adjust revisit rates in Experiment 2. Rats treated the study-test sequence as a morning session, suggesting the use of time of day rather than an interval-timing mechanism. The figure plots the difference between observed and baseline revisit rates. For the bar labeled interval, the baseline was the probability of revisiting chocolate in the afternoon; thus, the significant elevation above baseline shown in the figure suggests that the rats did not use an interval mechanism. For the bar labeled time of day, the baseline was the probability of revisiting chocolate in the morning; thus, the absence of a significant elevation above baseline is consistent with the use of time of day. The horizontal line corresponds to the baseline revisit rate to the chocolate location from Experiment 1. Positive difference scores correspond to evidence against the hypothesis indicated on the horizontal axis. *P < 0.04 different from baseline. (C and D) Rats preferentially revisited the chocolate location when it was about to replenish when the study, but not the test, time of day was familiar in Experiment 3. The probability of a revisit to the chocolate location in the first 4 choices of a test phase is shown for first replenishment and first non-replenishment conditions (c; initial) and for subsequent sessions (D; terminal). *P < 0.04 and **P < 0.0001 difference between conditions. (E) Rats remembered the time of day at which the study episode (i.e., first helpings) occurred in Experiment 4. Rats treated the novel study-test sequence as a late-session test phase, suggesting memory of the time of day at study rather than discriminating time of day at test. The figure plots the difference between observed and baseline revisit rates. For the bar labeled test time, the baseline was the probability of revisiting chocolate in the second helpings of the early session (test phase) in Experiment 3; thus, the significant elevation above baseline suggests that the rats did not use the time of day at test to adjust revisit rates. For the bar labeled study time, the baseline was the probability of revisiting chocolate in the second helpings of the late session (test phase) in Experiment 3; thus, the absence of a significant elevation above baseline is consistent with memory of the time of day at study. The horizontal line corresponds to the baseline revisit rate to the chocolate location from Experiment 3 (terminal). Positive difference scores correspond to evidence against the hypothesis indicated on the horizontal axis. *P < 0.001 different from baseline. (A–E) Error bars indicate SEM. (A, C, and D) The probability expected by chance is 0.41.
Experiment 2.
Experiment 1 suggests that the adjustment of revisits to the location recently baited with chocolate in Fig. 2A may be based on time of day (i.e., morning vs. afternoon). As noted above, the delay between first and second helpings in Experiment 1 did not provide information about chocolate replenishment. However, these data do not tell us whether the rats used a remaining interval to solve this task. Rats could have timed the interval between light onset in the colony and the daily session and used this cue to adjust revisit rates. As noted in Fig. 1A, light onset occurred at 6:00 a.m. in Experiment 1. Because morning sessions occurred 1 h after light onset and afternoon sessions occurred 7 h after light onset, an alternative explanation for the chocolate-revisit data shown in Fig. 2A is that rats used these intervals to guide revisits to the chocolate location. In Experiment 2, we put predictions for circadian time-of-day and interval hypotheses in conflict by shifting the light onset by 6 h (to 12:00 a.m.), using the same rats. The first helpings occurred at 7:00 a.m. in this experiment, which was 7 h after 12:00 a.m., as shown in Fig. 1B. A circadian oscillator is unaffected by a single manipulation of light onset, whereas an interval would be affected in this case (21, 22, 26). According to the time-of-day hypothesis, if rats used circadian time of day, then they should revisit chocolate at the same rate that usually occurs in a morning session. Alternatively, according to the interval hypothesis, if the rats timed 7 h from light onset, then they should revisit chocolate at the same rate that usually occurs in an afternoon session.
Rats adjusted revisit rates on the basis of the circadian time of day at which the session occurred rather than using the interval between light onset and the session. Fig. 2B shows data from this experiment relative to baseline data from Experiment 1 according to interval and time-of-day hypotheses; the baseline data come from Fig. 2A. Observed revisit rates were significantly different from the baseline for the interval hypothesis [t (15) = 2.3, P < 0.04], suggesting that the rats did not time the interval between light onset and study-test sessions. The observed data were not reliably different from the time-of-day hypothesis [t (15) = −0.03, P > 0.8], consistent with the proposal that the rats adjusted their revisit rates to chocolate on the basis of the time of day at which sessions occurred.
Experiment 3.
The phase-shift manipulation of light onset in Experiment 2 suggests that the rats were using circadian time of day to adjust revisit rates to the chocolate location in morning and afternoon sessions. However, these data do not tell us whether the rats were using the time of day at their first helpings or the time of day at their second helpings to produce this adjustment in revisits. It is important to note that the 2-min separation between the time of day at first and second helpings is too small for the rats to discriminate on the basis of circadian time of day (24, 27). Yet, documenting remembering is critically important to establish the existence of episodic-like memory. Remembering would be implicated if the rats used the features (what-where-when) of their first helpings to adjust revisits; by contrast, remembering would not be implicated if they used the features of their second helpings. Thus, it is important to determine whether the rats were, at the time of their second helpings, remembering the time of day at which their first helpings occurred. An alternative hypothesis is that at the time of their second helpings the rats may discriminate circadian time of day and adjust revisit rates on the basis of this information without remembering the time of day at their first helpings. Consequently, in Experiment 3 we substituted a 7-h delay for the 2-min delay, using the same rats. The time of day at which the rats obtained their first helpings was the same as in Experiment 1 (i.e., 7:00 a.m. for early and 1:00 p.m. for late first helpings). However, the introduction of the 7-h delay between first and second helpings produced second helpings that occurred at novel times (2:00 p.m. for early and 8:00 p.m. for late sessions; Fig. 1C). Thus, the time of first helpings was at a familiar time of day (i.e., from Experiment 1), and the time of second helpings was at a novel time of day. If rats adjusted their revisit rates on the basis of the time of day at which their first helpings occurred, then they should continue to differentially revisit chocolate locations more in the replenishment condition than in the non-replenishment condition. Alternatively, if the rats were adjusting revisit rates on the basis of the time of day at which their second helpings were available, then there is no basis for the rats to know that a higher revisit rate should occur at the novel times of 2:00 or 8:00 p.m. It is important to note that the strongest version of these predictions comes from the very first transfer test to novel early and late test times (i.e., before receiving feedback from exposure to the new test times). Consequently, the critical data for evaluating the above hypotheses come from the very first early and late sessions.
The rats were more likely to revisit the chocolate location in the replenishment condition than in the non-replenishment condition, consistent with memory of the time of day at first helpings; thus, rats remembered their first-helpings episode (i.e., what, where, and when memories). Importantly, this difference was observed on the very first replenish and non-replenish trials [i.e., before feedback with the new test times; t (15) = 2.24, P = 0.04; see Fig. 2C]. Thus, at the time of second helpings, the rats remembered the time of day at which their first helpings (study phase) occurred and adjusted chocolate revisit rates accordingly. The probability of revisiting chocolate was above the probability expected by chance [replenish: all rats revisited, SEM = 0; non-replenish: t (15) = 3.0, P < 0.01]. As expected, with additional training the rate of revisiting chocolate continued to be higher in the replenishment condition compared with the non-replenishment condition [t (15) = 6.8, P < 0.0001; see Fig. 2D]. The probability of revisiting chocolate was above the probability expected by chance [replenish: t (15) = 19.2, P < 0.0001; non-replenish: t (15) = 2.3, P < 0.05].
Experiment 4.
To provide a converging line of evidence for memory of the time at which the first helpings occurred, the same rats were subjected to a novel conflict test that dissociated predictions based on time of day at first and second helpings. Because early and late sessions overlapped in time in Experiment 3 (see Fig. 1C), we were able to begin a session in Experiment 4 with a late opportunity for first helpings (1:00 p.m.) and end with an early opportunity for second helpings (2:00 p.m.; Fig. 1D). Consequently, we could determine whether the rats adjusted revisits at second helpings on the basis of the time of day at first helpings or the time of day at second helpings. According to the study-time hypothesis, if rats remember when their first helpings occurred (i.e., study phase) at their second helpings, then they should revisit chocolate at the same rate that usually occurs at a late second helping (i.e., 8:00 p.m., which is the usual time of day at second helpings after their 1:00 p.m. first helpings). Alternatively, according to the test-time hypothesis, if rats use time of day at their second helpings (i.e., test phase) to adjust revisit rates, then they should revisit chocolate at the same rate that usually occurs at an early second helping (i.e., 2:00 p.m., which is the usually time of day at second helpings after their 7:00 a.m. first helpings). It is important to note that rats had never received this sequence of 1:00 p.m. and 2:00 p.m. and that the data were collected before any feedback occurred with respect to replenishment or non-replenishment of chocolate.
The rats adjusted chocolate revisit rates on the basis of the time of day at which their first helpings occurred rather than using the time of day at which their second helpings occurred (consistent with remembering their first-helping episodes). Fig. 2E shows data from this experiment relative to baseline data according to the study-time and test-time hypotheses; baseline data come from Fig. 2D. The observed data were significantly different from the baseline for the test-time hypothesis [t (15) = 4.2, P < 0.001], suggesting that the rats did not use the time of day at second helpings (test phase) to adjust chocolate revisit rates. The observed data were not reliably different from the study-time hypothesis [t (15) = −0.7, P > 0.5]. Thus, at the time of second helpings, the rats remembered the time at which the first helpings occurred.
Discussion
In a series of manipulations, we documented that the rats remembered when the study episode occurred. First, we ruled out timing the interval between light onset and the feeding opportunities by shifting light onset to an earlier time that put interval and time-of-day predictions in conflict. Presumably, the estimate of time of day comes from an endogenous circadian oscillator. A characteristic feature of such a system is that adjustment to phase shifts of the light cycle is gradual (22, 27); thus, the representation of time of day would be unaffected by a single manipulation of light onset. The rats did not use the interval between light onset and the session, which suggests that they used time of day. Second, we sought evidence that the rats remembered the time of day at first helpings, rather than using the time of day at second helpings, by introducing a 7-h delay between first and second helpings. The time of day at first, but not at second, helpings was familiar to the rats from the earlier experiment. The rats continued to revisit at a higher rate in the replenishment condition compared with the non-replenishment condition; thus, we infer that the rats used time of first helpings rather than time of second helpings to adjust revisit rates. Third, we provided a novel test for the rats by beginning a session with a late opportunity for first helpings and ending the session with an early opportunity for second helpings. Thus, we could determine whether the rats adjusted revisits at second helpings on the basis of remembering the time of day at first helpings or by using the time of day at second helpings. At the time of memory assessment, the rats remembered the time of day from the study episode (i.e., time of day at which first helpings occurred). These experiments suggest that rats remember what-where-when under conditions in which how-long-ago cues were made irrelevant to performance. Importantly, the relative strength of memories that decay over time cannot explain our results because the delay between first and second helpings was constant in each experiment.
It is unlikely that the rats solved the task at first helpings by prospectively forming an action plan to visit (or refrain from visiting) the chocolate location. First, an action plan to refrain from visiting the chocolate location predicts below-chance levels of revisiting the chocolate location in the non-replenishment condition. However, rats revisited the chocolate location in the non-replenishment condition at rates reliably above chance (Figs. 2 A, C, and D). Thus, this hypothesis is rejected. Second, an action plan based on the failure to encode the chocolate location at first helpings predicts chance levels of revisiting the chocolate location in the non-replenishment condition. This hypothesis is also rejected by the observation that chocolate revisit rates were above chance in the non-replenishment condition. Third, we and others (13–16) have changed the value of a distinctive flavor after the completion of first helpings. In each of these tests of what-where-when memory, the rats flexibly adjusted their revisit rates to take into account the current desirability of the distinctive food; this result is incompatible with the execution of an action plan that was formed at first helpings. Fourth, in earlier studies (13–15), the replenishment status of the distinctive flavor could not be predicted at first helpings, yet the rats discriminated what, where, and when.
In a series of elegant experiments, Roberts et al. (17) unconfounded time of day at study and the cue of how long ago the study episode occurred. The data of Roberts et al. suggest that how-long-ago dominates when multiple temporal cues are available, which may be a form of overshadowing under conditions of cue competition (28). Thus, we sought to evaluate what-where-when memories under conditions in which how-long-ago cues were irrelevant to predicting replenishment. When how-long-ago was rendered irrelevant in the present studies, we found evidence for remembering when an earlier study episode occurred, in addition to what and where knowledge about the episode.
People can describe when earlier events occurred using calendar-date-time systems (i.e., a representational system that retains the time of occurrence of earlier events) (19). Our data suggest that, at the time of memory assessment, rats remember when specific events occurred in time. Moreover, these experiments provide insight into the type of temporal representational systems that rats may use to support episodic-like memory, namely a timing system that retains the time of occurrence of earlier events.
Methods
General Methods.
Sixteen male Long-Evans rats encountered chocolate, at a daily unique location (study), which sometimes replenished later (test). The replenishment depended on the time at which chocolate had been initially encountered. Optimal performance is to revisit the chocolate location at the test when replenishment is imminent but to reduce this tendency when the chocolate replenishment is not forthcoming. Chow-flavored locations from first helpings never replenished at second helpings. Thus, solving this task requires knowledge about what and where events occurred in addition to information about when the critical events occurred because chocolate was available at a daily-unique location. Because the interval between study and test was constant within each experiment, how long ago a chocolate encounter occurred could not be used to predict replenishment. Detailed methods appear in SI Methods.
Supplementary Material
Acknowledgments.
We thank C. R. Gallistel for insightful comments on an earlier version of the manuscript. This work was supported by National Institute of Mental Health Grant R01MH080052 (to J.D.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904360106/DCSupplemental.
References
- 1.Friedman WJ. Memory for the time of past events. Psychol Bull. 1993;113:44–66. [Google Scholar]
- 2.Roberts WA. Are animals stuck in time? Psychol Bull. 2002;128:473–489. doi: 10.1037/0033-2909.128.3.473. [DOI] [PubMed] [Google Scholar]
- 3.Tulving E. How many memory systems are there? Am Psychol. 1985;40:385–398. [Google Scholar]
- 4.Tulving E. What is episodic memory? Curr Dir Psychol Sci. 1993;2:67–70. [Google Scholar]
- 5.Tulving E. In: Organization of Memory. Tulving E, Donaldson W, editors. New York: Academic; 1972. pp. 381–403. [Google Scholar]
- 6.Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395:272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- 7.Clayton NS, Dickinson A. Memory for the content of caches by scrub jays (Aphelocoma coerulescens) J Exp Psychol Anim Behav Process. 1999;25:82–91. [PubMed] [Google Scholar]
- 8.Clayton NS, Dickinson A. Motivational control of caching behaviour in the scrub jay, Aphelocoma coerulescens. Anim Behav. 1999;57:435–444. doi: 10.1006/anbe.1998.0989. [DOI] [PubMed] [Google Scholar]
- 9.Clayton NS, Dickinson A. Scrub jays (Aphelocoma coerulescens) remember the relative time of caching as well as the location and content of their caches. J Comp Psychol. 1999;113:403–416. doi: 10.1037/0735-7036.113.4.403. [DOI] [PubMed] [Google Scholar]
- 10.Clayton NS, Yu KS, Dickinson A. Scrub jays (Aphelocoma coerulescens) form integrated memories of the multiple features of caching episodes. J Exp Psychol Anim Behav Process. 2001;27:17–29. [PubMed] [Google Scholar]
- 11.Clayton NS, Yu KS, Dickinson A. Interacting cache memories: Evidence for flexible memory use by Western scrub-jays (Aphelocoma californica) J Exp Psychol Anim Behav Process. 2003;29:14–22. [PubMed] [Google Scholar]
- 12.de Kort SR, Dickinson A, Clayton NS. Retrospective cognition by food-caching Western scrub-jays. Learn Motiv. 2005;36:159–176. [Google Scholar]
- 13.Babb SJ, Crystal JD. Discrimination of what, when, and where: Implications for episodic-like memory in rats. Learn Motiv. 2005;36:177–189. [Google Scholar]
- 14.Babb SJ, Crystal JD. Discrimination of what, when, and where is not based on time of day. Learn Behav. 2006;34:124–130. doi: 10.3758/bf03193188. [DOI] [PubMed] [Google Scholar]
- 15.Babb SJ, Crystal JD. Episodic-like memory in the rat. Curr Biol. 2006;16:1317–1321. doi: 10.1016/j.cub.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 16.Naqshbandi M, Feeney MC, McKenzie TLB, Roberts WA. Testing for episodic-like memory in rats in the absence of time of day cues: Replication of Babb and Crystal. Behav Process. 2007;74:217–225. doi: 10.1016/j.beproc.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Roberts WA, et al. Episodic-like memory in rats: Is it based on when or how long ago? Science. 2008;320:113–115. doi: 10.1126/science.1152709. [DOI] [PubMed] [Google Scholar]
- 18.Ferkin MH, Combs A, DelBarco-Trillo J, Pierce AA, Franklin S. Meadow voles, Microtus pennsylvanicus, have the capacity to recall the ‘what’, ‘where’, and ‘when’ of a single past event. Anim Cogn. 2008;11:147–159. doi: 10.1007/s10071-007-0101-8. [DOI] [PubMed] [Google Scholar]
- 19.Gallistel CR. The Organization of Learning. Cambridge, MA: MIT Press; 1990. [Google Scholar]
- 20.Suddendorf T, Corballis MC. Mental time travel and the evolution of the human mind. Genet Soc Gen Psychol Monogr. 1997;123:133–167. [PubMed] [Google Scholar]
- 21.Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi JS, Turek FW, Moore RY, editors. Handbook of Behavioral Neurobiology: Circadian Clocks. New York: Plenum; 2001. [Google Scholar]
- 23.Crystal JD. Elements of episodic-like memory in animal models. Behav Process. 2009;80:269–277. doi: 10.1016/j.beproc.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Pizzo MJ, Crystal JD. Time-place learning in the eight-arm radial maze. Learn Behav. 2004;32:240–255. doi: 10.3758/bf03196025. [DOI] [PubMed] [Google Scholar]
- 25.Pizzo MJ, Crystal JD. The influence of temporal spacing on time-place discrimination. Learn Behav. 2006;34:131–143. doi: 10.3758/bf03193189. [DOI] [PubMed] [Google Scholar]
- 26.Crystal JD. In: Encyclopia of Animal Behavior. Clayton NS, Moore J, Breed M, editors. New York: Elsevier; in press. [Google Scholar]
- 27.Johnson CH. An Atlas of Phase Response Curves for Circadian and Circatidal Rhythms. Nashville, TN: Vanderbilt University; 1990. [Google Scholar]
- 28.De Houwer J, Beckers T, Vandorpe S. Evidence for the role of higher-order reasoning processes in cue competition and other learning phenomena. Learn Behav. 2005;33:239–249. doi: 10.3758/bf03196066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.