Abstract
Pharmacologic inhibitors of the prostaglandin-synthesizing COX-2 oncogene prevent the development of premalignant human colon adenomas. However, resistance to treatment is common. In this study, we show that the adenoma prevention activity of the COX-2 inhibitor celecoxib requires the concomitant presence of the 15-hydroxyprostaglandin dehydrogenase (15-PGDH) tumor suppressor gene, and that loss of 15-PGDH expression imparts resistance to celecoxib's anti-tumor effects. We first demonstrate that the adenoma-preventive activity of celecoxib is abrogated in mice genetically lacking 15-PGDH. In FVB mice, celecoxib prevents 85% of azoxymethane-induced tumors >1 mm in size, but is essentially inactive in preventing tumor induction in 15-PGDH-null animals. Indeed, celecoxib treated 15-PGDH null animals develop more tumors than do celecoxib naive WT mice. In parallel with the loss of tumor prevention activity, celecoxib-mediated suppression of colonic PGE2 levels is also markedly attenuated in 15-PGDH-null versus WT mice. Finally, as predicted by the murine models, humans with low colonic 15-PGDH levels also exhibit celecoxib resistance. Specifically, in a colon adenoma prevention trial, in all cases tested, individuals who developed new adenomas while receiving celecoxib treatment were also found as having low colonic 15-PGDH levels.
Keywords: colon cancer, 15-PGDH
Colon cancer is the second leading cause of cancer-related death in the United States (1). Strategies for preventing colon cancer have focused on preventing development of colonic adenomas, the pre-malignant tumors that are the precursors of invasive colon cancers (1). Pharmacologic approaches have targeted the inhibition of COX-2, an enzyme that mediates conversion of arachidonic acid to bioactive prostaglandins (PGs), and whose expression is markedly increased in colon cancers (1, 2). Celecoxib, a potent COX-2 inhibitor, decreases colon adenoma development in individuals with familial adenomatous polyposis (3). In individuals with non-familial sporadic colon adenomas, celecoxib reduces by 33%–45% the risk of developing future adenomas, and by 57%–64% the risk of developing adenomas with advanced histology (4, 5). Nonetheless, a significant proportion of individuals exhibit resistance to the colon tumor prevention activity of celecoxib.
Recently, we described that 15-hydroxyprostaglandin dehydrogenase (15-PGDH), a PG-degrading enzyme, functions as an endogenous inhibitor of the colonic COX-2 pathway and as a colon tumor suppressor gene (6, 7). 15-PGDH is highly expressed in normal colon mucosa, but expression is ubiquitously lost in human colon cancers (6, 8). Knocking out the murine 15-PGDH gene markedly sensitizes normally resistant C57BL/6J mice to colon tumor induction by the carcinogen azoxymethane (AOM) (7). In this study, we examined the potential interactions between pharmacologic regulation of colonic PGs by celecoxib and the genetic regulation of colonic PGs by 15-PGDH, and the potential that loss of 15-PGDH as an interacting partner could provide a resistance mechanism accounting for the failure of some individuals to benefit from celecoxib treatment.
Results and Discussion
15-PGDH Is Required for Celecoxib Prevention of Murine Colon Tumors.
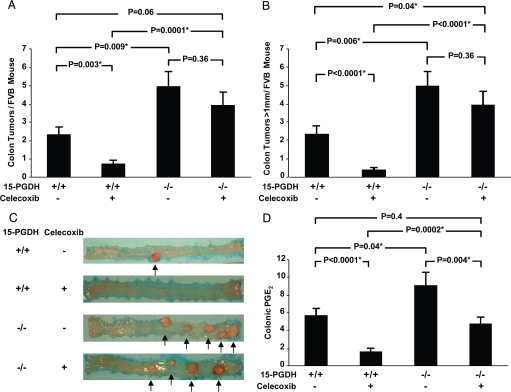
To conduct this study, we selected FVB mice because, at baseline, this mouse strain is sensitive to AOM-induced colon tumors (9). In 15-PGDH WT FVB mice, AOM induced 2.3 ± 0.4 tumors per mouse colon (Fig. 1 A and C), which on histologic review were all adenomatous lesions. As in human trials, administering dietary celecoxib protected WT FVB mice against colon tumor development, reducing adenoma development to 0.7 ± 0.3 tumors per mouse (P = 0.003; Fig. 1 A and C). Furthermore, whereas nearly all tumors arising in control mice exceeded 1 mm in size, tumors in celecoxib-treated mice rarely reached this size (2.2 mm ± 0.4 vs. 0.3 mm ± 0.1; P < 0.0001; Fig. 1B).
Fig. 1.
Celecoxib resistance in 15-PGDH knockout mice. (A) AOM induced colon tumor development in 15-PGDH+/+ FVB mice untreated (−) (n = 16) or treated (+) (n = 12) with celecoxib, versus FVB 15-PGDH−/− mice untreated (n = 13) or treated (n = 17) with celecoxib. P values represent comparisons of tumor numbers between groups, with asterisks indicating statistically significant values. Error bars designate SEM. (B) AOM induced development of large colon tumors (diameter >1 mm) in the same mice cohorts in A. (C) Gross morphology of representative colons from AOM-treated 15-PGDH+/+ and −/− mice administered celecoxib-containing (+) or celecoxib-free (−) diets. Arrows designate colon tumors. (D) Colonic mucosal PGE2 levels (in ng/mg protein) in 15-PGDH+/+ FVB mice untreated (−) (n = 10) or treated (+) (n = 9) with celecoxib, versus FVB 15-PGDH−/− mice untreated (n = 12) or treated (n = 11) with celecoxib.
Dietary celecoxib could thus almost completely protect WT FVB mice from developing colon tumors. However, further investigation revealed that the ability of celecoxib to protect mice from colon tumors was crucially dependent upon the concomitant activity of 15-PGDH, and that this protection could be abrogated by breeding 15-PGDH-knockout alleles into the FVB strain. Thus, celecoxib-treated 15-PGDH-null mice developed 5.5 times more colon adenomas than did their celecoxib-treated 15-PGDH WT litter-mates (3.9 ± 0.8 vs. 0.7 ± 0.3; P = 0.0001; Fig. 1 A and C). Moreover, all colonic adenomas arising in celecoxib-treated knockout mice were large, each exceeding 1 mm in size; whereas large tumors were nearly completely absent in celecoxib-treated 15-PGDH WT mice (3.9 large tumors ± 0.8 in knockout mice vs. 0.3 large tumors ± 0.1 in WT mice; P = 0.0001; Fig. 1 B and C). By several measures, 15-PGDH-knockout mice were almost completely resistant to the colon tumor prevention effects of celecoxib. For example, tumor development in the knockout mice did not significantly differ between celecoxib-treated versus untreated animals (3.9 ± 0.8 vs. 4.9 ± 0.8, respectively; P = 0.36; Fig. 1A). Moreover, colon tumor development in celecoxib-treated 15-PGDH-knockout mice actually exceeded that in drug-untreated WT mice [tumors >1 mm, 3.9 ± 0.8 vs. 2.2 ± 0.4, respectively (P = 0.04); tumors of any size, 3.9 ± 0.8 vs. 2.3 ± 0.4, respectively (P = 0.06); Fig. 1 A and B]. We conclude that celecoxib-mediated colon tumor prevention requires concurrent presence of the PG-inactivating activity of colonic 15-PGDH, and that 15-PGDH loss confers in vivo resistance to this drug effect.
15-PGDH Is Required for Celecoxib Reduction of Colonic PGE2.
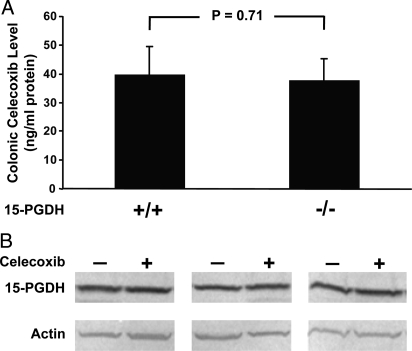
To further investigate the mechanism of celecoxib's dependence on 15-PGDH, we determined PGE2 levels in colonic mucosa of FVB mice under different experimental conditions. Consistent with the role of 15-PGDH in mediating PG degradation, 15-PGDH gene knockout essentially doubled FVB colonic PGE2 levels (9.1 ng/mg protein ± 1.5 in knockouts vs. 5.70 ng/mg protein ± 0.8 in controls; P = 0.04; Fig. 1D). Celecoxib treatment of 15-PGDH WT mice markedly lowered PGE2 levels to 1.6 ng/mg protein ± 0.4 (P < 0.001; Fig. 1D). In contrast, in 15-PGDH-knockout mice, the biochemical activity of celecoxib was much attenuated, with the drug lowering PGE2 levels to only 4.7 ng/mg protein ± 0.8. This level was 3 times the level achieved in drug-treated WT mice (P = 0.0002), and was not significantly different from the PGE2 level of drug-free WT mice (P = 0.4; Fig. 1D). In overview, the efficacy of celecoxib in lowering colonic PGE2 levels in these different models closely paralleled the drug's anti-tumor activity (Fig. 1D vs. Fig. 1 A and B), and mice that lacked 15-PGDH equally acquired resistance to celecoxib's biochemical activity of lowering colonic PGE2 and to celecoxib's phenotypic effect of preventing colon tumors. Celecoxib resistance of 15-PGDH-null mice was not caused by any change in drug absorption or catabolism, as tissue levels of celecoxib were indistinguishable between drug-treated 15-PGDH WT and null mice (39.5 ng/mg protein ± 9.7 vs. 37.7 ng/mg protein ± 7.6; P = 0.7; Fig. 2A). Additionally, celecoxib did not regulate 15-PGDH levels, as Western blot analysis showed equal colonic 15-PGDH amounts in celecoxib-treated versus untreated WT mice (Fig. 2B). Rather, the effective depletion of colonic PGE2 required the COX-2 inhibitor drug to act in concert with the independent PG-degrading activity of 15-PGDH.
Fig. 2.
Celecoxib and 15-PGDH tissue levels in murine colonic mucosa. (A) Tissue levels of celecoxib were determined by MS in tissue homogenates of colonic mucosa obtained from 15-PGDH WT (+/+) (n = 20) or knockout (−/−) (n = 26) mice administered 2 weeks of a celecoxib-supplemented diet. Error bars designate SEM. Mice cohorts correspond to those of Fig. 1D. (B) Western analysis of 15-PGDH expression determined in colon mucosa from 3 sets of mice receiving 2 weeks of a control diet (−) or 2 weeks of a celecoxib-containing (+) diet. Actin protein levels serve as a loading control.
Low 15-PGDH Levels Are Associated with Celecoxib Resistance in Humans with Recurrent Colon Adenomas.
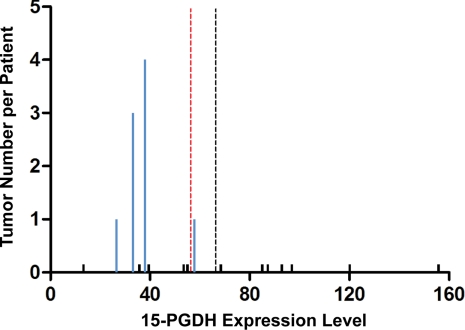
These observations in mice suggested that humans with lower levels of colonic 15-PGDH might also be resistant to the colon tumor prevention activity of celecoxib. To test this hypothesis, we examined frozen biopsy specimens of rectal mucosa obtained from 16 individuals at the time of their enrollment in the Adenoma Prevention with Celecoxib (APC) trial (5). These individuals were all at high risk for colon adenoma development based on having had multiple colon adenomas and/or adenomas greater than 5 mm in diameter before their enrollment. Following clearing of their colonic adenomas by colonoscopy, each individual received 36 months of daily treatment with celecoxib (5). Measurement by real-time PCR of 15-PGDH transcript levels in these pre-treatment biopsy samples showed a 12-fold variation from lowest to highest 15-PGDH mRNA level among these 16 unrelated individuals (median, 56.4; mean, 66.2; range, 13.3–155.7; Fig. 3). Repeat colonoscopy after 36 months revealed that 4 of these individuals had proven to be resistant to celecoxib treatment, as evidenced by the development of new adenomas that all arose at new locations (Fig. 3). In total, 9 new adenomas were detected in these patients (Fig. 3). 15-PGDH levels among individuals who developed new adenomas were lower than 15-PGDH levels among individuals who remained adenoma-free (P = 0.04; Fig. 3). This can be further appreciated by noting that all 4 individuals with new adenomas exhibited 15-PGDH levels below the cohort mean (P = 0.03; Fig. 3). The relationship of low 15-PGDH level to celecoxib resistance becomes even stronger if analyzed in terms of the numbers of new adenomas that individuals developed, with 8 of the 9 adenomatous polyps that recurred during celecoxib treatment arising in individuals with colonic 15-PGDH values lower than the cohort median (P = 0.01), and with all 9 new adenomas arising in individuals with colonic 15-PGDH levels lower than the cohort mean (P = 0.001; Fig. 3).
Fig. 3.
Celecoxib resistance in humans with low levels of 15-PGDH. Shown on the x axis are pretreatment 15-PGDH transcript levels measured by real-time PCR in RNA from rectal mucosal biopsies of 16 individuals enrolled in the APC trial (5). Bar heights on the y axis indicate number of recurrent adenomas detected in each individual at the completion of 36 months of celecoxib treatment, with blue bars denoting individuals with recurrent disease and minimal black bars indicating individuals with zero recurrences. The median level of 15-PGDH is denoted by the dashed red line, and the mean level is denoted by the dashed black line.
In summary, we find that 15-PGDH activity can determine sensitivity or resistance to the colon tumor preventive activity of celecoxib. Gene knockout of 15-PGDH confers near-complete resistance to celecoxib-related colon tumor prevention in mice. More significantly, low levels of colonic 15-PGDH are associated with failure of celecoxib colon tumor prevention in man. These findings elucidate a previously unsuspected pharmacogenetic interaction that bears on the differences among individuals in the efficacy of celecoxib treatment for prevention of colorectal adenomas. These observations imply that measurement of 15-PGDH may be clinically useful in selecting patients who are most likely to benefit from treatment with COX-2 inhibitors for colon tumor chemoprevention, an observation that should further be tested in future prospective clinical trials. Hence, these observations also suggest that it will be of value to identify genetic markers that can predict individuals with low expression of 15-PGDH. In addition, these observations further document the role of 15-PGDH as a key suppressor of tumor development in the colon. Moreover, they suggest that agents capable of inducing or reactivating 15-PGDH expression may provide new approaches for colon adenoma and cancer prevention. Finally, because drugs that inhibit COX activity are among the most common medications prescribed for relief of pain and inflammation, it will be of clear future interest to determine if low 15-PGDH levels also impart clinical resistance to these therapeutic activities.
Materials and Methods
Mouse Breeding.
Mouse studies were conducted in the Case Animal Resource Center (Cleveland, OH) under a protocol approved by the institutional animal care and use committee. 15-Hydroxy-PG dehydrogenase-knockout mice on a C57BL/6J background were generated as described previously (7, 10) and were bred to generation F8 onto an FVB/N (Jackson Laboratory) background, with genotyping done as previously described (7). 15-PGDH+/− mice generation F8 were intercrossed and siblings of 15-PGDH+/+ and −/− genotypes were selected out for studies with AOM and celecoxib. Eight- to 12-week-old mice were administered AOM by i.p. injection once weekly for 6 weeks at a 10-mg/kg dose (Sigma). Mice were euthanized 24 weeks after the last AOM injection. After euthanasia, the colons were opened longitudinally, rinsed with ice-cold PBS solution, and examined under a dissecting microscope to identify all tumors. Tumors were resected, fixed in 10% neutral buffered formalin, and paraffin-embedded for histologic examination. All mice received an AIN-76A diet (Harlan Teklad). The diet of celecoxib-treated mice was supplemented with 1,250 mg/kg of this agent (LKT Laboratories) (11). In AOM studies, celecoxib supplementation was initiated starting the day of first AOM injection and continued throughout the lifetime of the mouse. In studies of colonic PG levels, celecoxib was administered to a cohort of 8- to 12-week-old mice continuously for 2 weeks, at which time mice were euthanized.
PGE2 Analyses.
Following 2 weeks of control or celecoxib-supplemented diet, mice were euthanized. The colons were opened and washed with ice-cold PBS solution, and the colon mucosa was then gently scraped, snap-frozen in liquid nitrogen, and stored at −80 °C. PGE2 analyses were performed as previously described (7). To extract PGE2 from mouse colon epithelial tissue, frozen mucosal samples (25–50 mg) were ground to a fine powder in a liquid nitrogen-cooled mortar (Fisher Scientific). Samples were then transferred to sealed microcentrifuge tubes, and 3 times the volume of ice-cold PBS buffer containing 0.1% butylated hydroxytoluene and 1 mM EDTA were added. The sample was then homogenized in an ultrasonic processor (Misonix) at 0 °C for 3 min. A 100-μL aliquot of the homogenate was transferred to a glass tube (13 × 100 mm) and subjected to extraction of eicosanoids by using a modified version of the method of Kempen et al. (12). Briefly, 20-μL aliquots of 1 N citric acid and 10 μL of deuterated PGE2 (100 ng/mL) were added to the samples. Eicosanoids were then extracted with 1 mL of hexane:ethyl acetate (1:1, vol./vol.) and vortex-mixed for 2 min. Samples were centrifuged at 1,800 × g for 10 min at 4 °C. The upper organic layer was collected, and the organic phases from 3 extractions were pooled and then evaporated to dryness under a stream of nitrogen at room temperature. All extraction procedures were performed at minimum light levels under cold conditions (4 °C). Samples were then reconstituted in 100 μL of methanol:ammonium acetate buffer (10 mM, pH 8.5; 70:30, vol./vol.) before liquid chromatography (LC)/tandem MS (LC/MS/MS) analysis. The protein concentration was determined by a Bradford protein assay (Bio-Rad).
PGE2 was measured with reverse-phase LC electrospray ionization MS. LC/MS/MS analyses were performed with a Quattro Ultima tandem mass spectrometer (Micromass) equipped with an Agilent HP 1100 binary pump HPLC inlet. PGE2 was separated by using a Luna 3 μm Phenyl-Hexyl 2 × 150 mm LC column (Phenomenex). The mobile phase consisted of 10 mM ammonium acetate (pH 8.5) and methanol. The flow rate was 250 μL/min with a column temperature of 50 °C. The sample injection volume was 25 μL. Samples were kept at 4 °C during the analysis. The mass spectrometer was operated in the electrospray negative-ion mode with a cone voltage of 100 V, a cone gas flow rate of 117 L/h, and a devolution gas flow rate of 998 L/h. The temperature of the desolvation region was 400 °C, and the temperature of the source region was 120 °C. Fragmentation for all compounds was performed by using argon as the collision gas at a collision cell pressure of 2.10 × 10−3 torr. The collision energy was 19 V. PGs were detected by using electrospray negative ionization and multiple-reaction monitoring of the transition ions for the PGE2 (351.2 > 271.2) and 13,14-dihydro-15-keto-PGE2 (351.2 > 333.1). This method produces excellent linearity and a lower limit of quantification of 10 ng/mL, which is more than adequate to assess endogenous eicosanoid metabolism in small (25–30 mg) amounts of tissue. The results were expressed as nanograms of eicosanoid per milligram of protein.
Determination of Celecoxib Levels in Mouse Tissues by LC/MS/MS.
Following 2 weeks of control or celecoxib-supplemented diet, mice were euthanized. The colons were opened and washed with ice-cold PBS solution. Mucosa from the distal 6 cm of mouse colon was then gently scraped, snap-frozen in liquid nitrogen, and stored at −80 °C for analysis. Approximately 10 mg of frozen tissue was ground to a fine powder using a liquid nitrogen-cooled mortar (Fisher Scientific). The samples were then transferred to microcentrifuge tubes and 3 volumes of ice-cold PBS buffer were added before further homogenization of the sample with an ultrasonic tissues processor (Misonix). An aliquot (100 μL) of homogenate was transferred to a glass tube (13 × 100 mm). To the homogenate, 2 mL of hexane:ethyl acetate (1:1, vol./vol.) was added; the mixture was vortex-mixed for 5 min and then centrifuged at 1800 × g at 5 °C for 5 min. The extraction was repeated twice and the upper organic layer was collected, pooled, and evaporated to dryness under a stream of nitrogen at room temperature. The sample was then reconstituted in 200 μL of methanol: 10 mM ammonium acetate, pH 8.5 (1:1, vol./vol.). The celecoxib level in the samples was determined by LC/MS/MS. The LC/MS/MS was operated under the same conditions as described for measurement of PGE2 with minor changes. Briefly, 10 μL of the sample was injected on a Luna 3 μm phenyl-hexyl 2 × 150 mm analytical column (Phenomenex). Celecoxib was detected and quantified by operating the mass spectrometer in electrospray negative ion mode and monitoring the transition m/z 380.2 > 316.1. Quantification was done by comparing the sample peak areas to a standard curve and concentration of celecoxib was normalized by protein concentration. Preliminary studies in which celecoxib was spiked into control normal mouse samples demonstrated that, under these conditions, drug recovery consistently exceeded 95% over a wide concentration range.
15-PGDH Western Blot Analysis.
Western assay of 15-PGDH was done as described previously, using conditions in which the assay is in the linear range (6). Mucosa was collected from the distal 6 cm of the colon by scraping and snap-freezing in liquid nitrogen, and was stored at 80 °C. Tissue lysates were prepared by pipetting in radioimmunoprecipitation buffer (Upstate Biotechnology; 50 mM Tris-HCL/1% Nonidet P-40/0.25% Na-deoxycholate/150 mM NaCl/1 mM EDTA/1 mM PMSF) supplemented with protease inhibitor mixture (Roche), separated on 12% SDS/PAGE Ready Gels (Pierce; 30 μg per lane), and transferred to Immobilon polyvinylidene difluoride membrane (Millipore). The blots were blocked with 5% milk, probed with monoclonal anti-PGDH antibody at a 1:200 dilution (equaling 9.5 μg/mL) (6) and anti-actin antibody (Sigma-Aldrich) at a 1:2,000 dilution, developed by using horseradish peroxidase-conjugated anti-mouse TrueBlot antibodies (eBioscience), visualized by using an Enhanced Chemiluminescence Plus detection kit (Amersham Biosciences), following the manufacturer's instructions, and then scanned on a PhosphorImager (Molecular Dynamics).
Human Subjects.
We performed an IRB-approved prospective, randomized trial of the NSAID celecoxib for prevention of sporadic colorectal adenomas (5). In this study, known as the APC trial (5), 2,035 patients with a history of colorectal adenomas were randomized to receive placebo, celecoxib 200 mg twice daily, or celecoxib 400 mg twice daily, and were followed for 36 months. Celecoxib at either dose significantly reduced the incidence of adenomas detected at the end of the 36-month surveillance interval. A subset of patients in the APC trial also underwent a separate pretreatment endoscopic procedure before the initiation of celecoxib use, during which biopsy specimens of normal rectal mucosa were obtained. Mucosal specimens (2 mm diameter) were flash-frozen in liquid nitrogen and stored at −80 °C until assay for 15-PGDH.
Human 15-PGDH Transcript Measurement.
RNA was isolated from colon mucosal biopsies using an RNAqueous kit (Ambion) following the manufacturer's recommended protocol with minor alterations. Briefly, approximately 10 mg of frozen tissue was ground to a fine powder using a liquid nitrogen-cooled mortar. The samples were then transferred to microcentrifuge tubes containing 400 μL of lysis/binding solution. Each sample was then passed through a 26-gauge needle several times until no longer viscous, and then loaded onto a filter cartridge for the subsequent washing steps. RNA from each sample was eluted from the filter cartridge using 40 μL of elution solution followed by another elution with 20 μL of elution solution, for a pooled elution volume of 60 μL. Concentration and quality of the RNA samples were determined using a ND-1000 Spectrophotometer (NanoDrop), and 500 ng of sample was used for cDNA synthesis and subsequent real-time PCR assays for 15-PGDH. cDNA was synthesized using AMV Reverse Transcriptase (Roche) following the manufacturer's recommended protocol. Real-time PCR measurement of 15-PGDH was performed using the human 15-PGDH TaqMan Probe/Primer kit Hs00168359_m1 from Applied Biosystems and 1× IQ Supermix from Bio-Rad, and detected in an Icycler optical module (Bio-Rad) (6). A 25-μL reaction mix contained a 1:20 dilution of primer/probe in 1× Supermix (Bio-Rad). Thermal cycling was initiated at 95 °C for 10 min, followed by 50 cycles of 95 °C for 15 sec and 60 °C for 1 min. Cytokeratin 20 (Krt20), a marker of colonic epithelial cell mass, was used as the endogenous control and was amplified using the human Krt20 TaqMan primer/probe kit Hs00300643_m1 from Applied Biosystems and 1× IQ Supermix, with PCR initiated at 95 °C for 10 min, followed by 50 cycles of 95 °C for 15 sec and 60 °C for 1 min. The level of 15-PGDH RNA was determined as the ratio of 15-PGDH:Krt20 = 2 exp (CTPGDH − CTKrt20). Plotted values of 15-PGDH represent numerical averages of 15-PGDH levels assayed from 3 independent reverse transcription reactions. Additionally, for each reverse transcription reaction, CTKrt20 and CTPGDH were determined as the average values obtained from 3 independent real-time PCR reactions.
Statistical Analyses.
PG and celecoxib levels were log-transformed to be approximately normally distributed for analysis with a generalized linear regression model with contrasts, generating 2-sided P values. Tumor numbers in AOM-treated mice were analyzed using a negative binomial generalized linear model with contrasts, generating 2-sided P values. Results of all mouse studies in the manuscript are presented as means ± SEM. In humans, associations between presence or absence of tumor relapse and 15-PGDH level (treated as a continuous variable) was analyzed using a negative binomial generalized model with contrasts, generating a 2-sided P value (P = 0.04). Comparison of median 15-PGDH levels in individuals who showed relapse versus those who did not was also done using a Wilcoxon signed-rank test, generating a 1-sided P value (P = 0.04) for the pre-specified model that individuals with lower 15-PGDH levels would show resistance to celecoxib. Association between presence or absence of relapse in individuals and 15-PGDH dichotomized by the population mean was done using a Poisson generalized linear model with contrasts, generating a 2-sided P value (P = 0.03). Association between numbers of recurrent adenoma tumors in celecoxib-treated individuals and 15-PGDH levels dichotomized by the population median or population mean was analyzed using a Poisson generalized linear model with contrasts, generating 2-sided P values (P = 0.01 and P = 0.001, respectively).
Acknowledgments.
Supported by Public Health Service awards CA116867 (to S.D.M.) and CA127306 (to S.D.M.) and contract N01–95015 (to M.M.B.), and by Ministry of Science & Technology of Korea grant R01–2007-000–21103–0 (to S.J.M.). S.D.M. is an investigator of the Howard Hughes Medical Institute. We thank Angela Dotson for helpful technical assistance.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Markowitz SD. Aspirin and colon cancer–targeting prevention? N Engl J Med. 2007;356:2195–2198. doi: 10.1056/NEJMe078044. [DOI] [PubMed] [Google Scholar]
- 2.Cha YI, DuBois RN. NSAIDs and cancer prevention: targets downstream of COX-2. Annu Rev Med. 2007;58:239–252. doi: 10.1146/annurev.med.57.121304.131253. [DOI] [PubMed] [Google Scholar]
- 3.Steinbach G, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 4.Arber N, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 5.Bertagnolli MM, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 6.Yan M, et al. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers. Proc Natl Acad Sci USA. 2004;101:17468–17473. doi: 10.1073/pnas.0406142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myung SJ, et al. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci USA. 2006;103:12098–12102. doi: 10.1073/pnas.0603235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Backlund MG, et al. 15-Hydroxyprostaglandin dehydrogenase is down-regulated in colorectal cancer. J Biol Chem. 2005;280:3217–3223. doi: 10.1074/jbc.M411221200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nambiar PR, et al. Preliminary analysis of azoxymethane induced colon tumors in inbred mice commonly used as transgenic/knockout progenitors. Int J Oncol. 2003;22:145–150. [PubMed] [Google Scholar]
- 10.Coggins KG, et al. Metabolism of PGE2 by prostaglandin dehydrogenase is essential for remodeling the ductus arteriosus. Nat Med. 2002;8:91–92. doi: 10.1038/nm0202-91. [DOI] [PubMed] [Google Scholar]
- 11.Williams CS, et al. Celecoxib prevents tumor growth in vivo without toxicity to normal gut: lack of correlation between in vitro and in vivo models. Cancer Res. 2000;60:6045–6051. [PubMed] [Google Scholar]
- 12.Kempen EC, Yang P, Felix E, Madden T, Newman RA. Simultaneous quantification of arachidonic acid metabolites in cultured tumor cells using high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Anal Biochem. 2001;297:183–190. doi: 10.1006/abio.2001.5325. [DOI] [PubMed] [Google Scholar]