Abstract
In mammalian testes, such as rats, the mechanism(s) that regulate blood–testis barrier (BTB) restructuring at stages VIII–IX of the seminiferous epithelial cycle of spermatogenesis to facilitate the transit of preleptotene/leptotene spermatocytes is not known. This is due to the lack of information on the regulatory proteins at the BTB. Herein, focal adhesion kinase (FAK), a nonreceptor protein tyrosine kinase, is shown to structurally interact with occludin and ZO-1 to form a functional protein complex at the BTB. Its expression at the BTB in the seminiferous epithelium is stage specific, being lowest at stage VIII–IX tubules, analogous to the expression pattern of occludin. Using primary Sertoli cells cultured in vitro with an established tight junction (TJ) permeability barrier that mimics the BTB in vivo, the knockdown of FAK by RNAi led to a transient disruption of the TJ barrier. This was accompanied by a loss of association between occludin and ZO-1, likely the result of reduced occludin phosphorylation at Tyr and Ser residues, but not Thr, which in turn led to a redistribution of occludin at the Sertoli–Sertoli cell interface, moving from cell membrane into cell cytosol, thereby disrupting the BTB. These findings suggest that a similar mechanism is in place in the testis in vivo to regulate BTB restructuring to facilitate the transit of primary spermatocytes. Furthermore, FAK was shown to be a molecular target of cadmium because its knockdown would desensitize Sertoli cells to cadmium-induced TJ barrier disruption. In summary, FAK is a unique regulator of BTB dynamics in the testis.
Keywords: basal ectoplasmic specialization, cell–cell interaction, spermatogenesis, tight junction
In adult rat testes, at stages VIII–IX of the seminiferous epithelial cycle of spermatogenesis, primary preleptotene spermatocytes are in transit at the blood–testis barrier (BTB) while differentiating into leptotene and zygotene spermatocytes (1) so that diplotene spermatocytes can enter meiosis in the adluminal compartment behind the BTB. Although this event has been known to exist for decades (2), the mechanism(s) that regulates BTB dynamics remains largely unknown. In mammals, the BTB is created by adjacent Sertoli cells in the seminiferous epithelium near the basement membrane via coexisting specialized tight junction (TJ), basal ectoplasmic specialization [basal ES, a testis-specific atypical adherens junction (AJ) type], and desmosome-like junction (3, 4). Studies from the past decade have identified several integral membrane protein complexes that constitute the BTB in rodent testes, such as the occludin–ZO-1 complex at the TJ and the N-cadherin–β-catenin complex at the basal ES (for reviews, see refs. 5 and 6); however, the regulatory proteins that control the Sertoli cell TJ permeability barrier remain unexplored. This information, if known, would be of great importance to investigators in the field. First, the BTB, unlike other blood–tissue barriers such as the blood–brain barrier, is not a static barrier because it must restructure to allow the passage of primary spermatocytes (≈8–10 μm in diameter) while maintaining the immunological barrier to sequester postmeiotic germ cell development from the systemic circulation. Second, the BTB confers a “fence” function to finely regulate the passage of biomolecules, water, hormones, and other substances from the basal to the adluminal compartment. However, the regulatory mechanisms involved in these events are not known owing to the lack of information regarding the putative regulators at the BTB.
Focal adhesion kinase (FAK), a nonreceptor protein tyrosine kinase, is a known modulator of the integrin-based signal transduction pathway (7) usually restricted to the cell–extracellular matrix interface at the focal contact (an anchoring junction also known as focal adhesion complex) (8), regulating cell adhesion, apoptosis, differentiation, cell motility/movement, cell cycle progression, and the TJ permeability barrier in different epithelia and/or endothelia (9, 10). Mice lacking FAK died at embryonic stage (11), illustrating its physiological significance. Even though focal contacts are not found in the seminiferous epithelium of adult rat testes, we have reported the presence of FAK in the epithelium of rat testes by immunohistochemistry (IHC) consistent with its localization at the BTB, likely at the site of the basal ES (12). We sought to investigate whether FAK is an integrated component of the BTB via its structural interactions with some of the known BTB protein complexes, so that it could modulate local junction restructuring events to facilitate primary spermatocyte transit during spermatogenesis. We also performed functional studies to examine whether Sertoli cell–specific knockdown of FAK would affect the TJ permeability barrier function and integral membrane protein distribution at the cell–cell interface.
Results
Stage-Specific Expression of FAK at the BTB in the Seminiferous Epithelium of Adult Rat Testes.
FAK in adult rat testes was localized by IHC [supporting information (SI) Fig. S1]. FAK staining was concentrated near the basement membrane in the seminiferous epithelium in all stages of the epithelial cycle, consistent with its localization at the BTB (Fig. S1Aa). Its expression, however, greatly diminished at stage VIII (Fig. S1A b and f–h vs. c–e and i–k), at the time the BTB undergoes restructuring to facilitate the transit of primary preleptotene spermatocytes (1). Considerably weaker staining was detected in the adluminal compartment at all stages of the epithelial cycle. When the anti-FAK antibody was substituted by rabbit IgG, no specific staining was detected (Fig. S1A l and m), and the antibody specificity was also confirmed by immunoblot analysis (Fig. S1B).
Changes in the Expression and Localization of FAK and BTB-Associated Proteins During CdCl2-Induced BTB Disruption in Rat Testes.
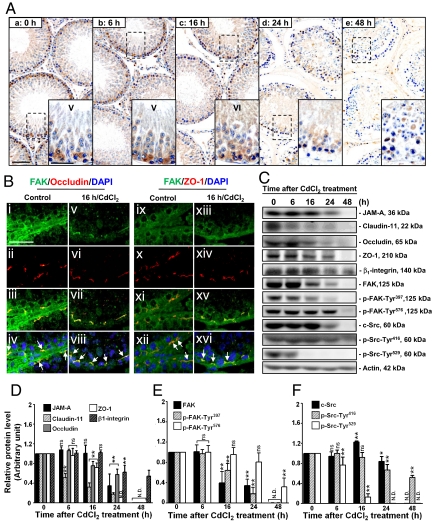
We next examined changes in the expression and localization of FAK using an established in vivo model for studying BTB dynamics, in which treatment of rats with CdCl2 [3 mg/kg body weight (b.w.), i.p.] is known to induce BTB disruption by ≈7–16 h, whereas the TJ barrier of the microvessels in the interstitium is not disrupted until ≈20–24 h (6, 13). Considerably less FAK was detected at the BTB by 16 h after CdCl2 treatment when the BTB was disrupted (although some germ cells were still found in the epithelium), and FAK was hardly detectable by 24–48 h (Fig. 1A). In a study using dual-labeled immunofluorescent analysis, it was shown that FAK indeed colocalized with 2 TJ proteins: occludin (Fig. 1B i–iv) and ZO-1 (Fig. 1B ix–xii) at the BTB. Even though there was a significant decline in the levels of occludin and ZO-1 by 16 h, FAK remained colocalized with them (Fig. 1B v–viii vs. i–iv; Fig. 1B xiii–xvi vs. ix–xii; see also Fig. S2). These findings were consistent with immunoblotting data when the steady-state protein levels of FAK and other BTB proteins were examined (Fig. 1 C–F).
Fig. 1.
A study to assess changes in the localization and levels of FAK and/or BTB-associated proteins in rat testes during CdCl2-induced BTB disruption. (A) Cross-sections of testes embedded in paraffin from control (0 h, a) and CdCl2-treated (3 mg/kg b.w., i.p.; b–e) rats were stained with an anti-FAK antibody (see Table S1). Strong staining for FAK in the epithelium near the basement membrane, consistent with its localization at the BTB, is noted in control (a) and after 6 h of treatment (b), but a gradual and considerable decline was detected from 16 through 48 h, when the BTB was disrupted (6) (c–e). Insets correspond to magnified boxed areas. (B) Colocalization of FAK with occludin (i–iv) and ZO-1 (ix–xii) (arrows in iv, viii, xii, and xvi). By 16 h after CdCl2 treatment, diminished staining of FAK, occludin, and ZO-1 was detected at the BTB; however, their colocalization was still evident (v–viii and xiii–xvi; for a lower magnification, see Fig. S2). (C) Representative immunoblots illustrating changes in the steady-state levels of BTB-associated proteins (e.g., JAM-A, claudin-11, occludin, and ZO-1), hemidesmosome protein (e.g., β1-integrin), and protein kinases (e.g., FAK and c-Src) after CdCl2 treatment. (D–F) Composite immunoblot results normalized against actin. Relative protein levels at 0 h were arbitrarily set at 1, against which statistical analysis was performed. Each bar is the mean ± SD of n = 3. *P < 0.05; **P < 0.01; ns, not significantly different by ANOVA; N.D., not detectable. (Scale bar, 100 μm in Aa, applies to A b–e; 50 μm in Aa Inset and Bi, applies to Insets in A b–e and B ii–xvi, respectively.)
Changes in Interaction Between FAK and the Occludin–ZO-1 Complex During CdCl2-Induced BTB Disruption.
Because FAK was shown to colocalize with occludin and ZO-1 to the same site at the BTB, we next used coimmunoprecipitation (Co-IP) to investigate whether FAK structurally interacted with either occludin or ZO-1. FAK was shown to interact with both occludin and ZO-1 (Fig. 2). Because it is established that occludin forms a stoichiometric 1:1 ratio with ZO-1 (14) to constitute TJ fibrils, these observations suggest that FAK is an integrated component of TJ fibrils at the BTB. Furthermore, there was a transient increase in the interaction of FAK with the occludin–ZO-1 complex at ≈6–16 h during CdCl2-induced BTB disruption, but this interaction considerably diminished by 48 h, when the BTB showed irreversible damage (Fig. 2 vs. Fig. 1). These results thus confirmed the existence of an FAK–occludin–ZO-1 protein complex at the BTB, with FAK likely serving as a regulatory protein.
Fig. 2.
A study by Co-IP to assess changes in protein–protein interactions between FAK and occludin or ZO-1 during CdCl2-induced BTB disruption. (A) Testis lysates (≈700 μg of protein) from rats treated with CdCl2 at specified time points were subjected to Co-IP with anti-occludin or anti-ZO-1 antibodies, and the blot was probed with anti-FAK antibody. A transient increase in the interaction of occludin–FAK and ZO-1–FAK was detected, being highest at the time the BTB was being disrupted, at ≈16 h (6); however, the association between FAK and occludin and/or ZO-1 virtually disappeared by 48 h, when the BTB was irreversibly disrupted. (B) Densitometric analyses of data such as those shown in A. Relative association with FAK at time 0 h was arbitrarily set as 1. Each bar is the mean ± SD of n = 3. *P < 0.05; **P < 0.001; ns, not significantly different by ANOVA; N.D., not detectable.
Sertoli Cell–Specific Knockdown of FAK by RNAi Disrupts the TJ Permeability Barrier and Occludin–ZO-1 Association.
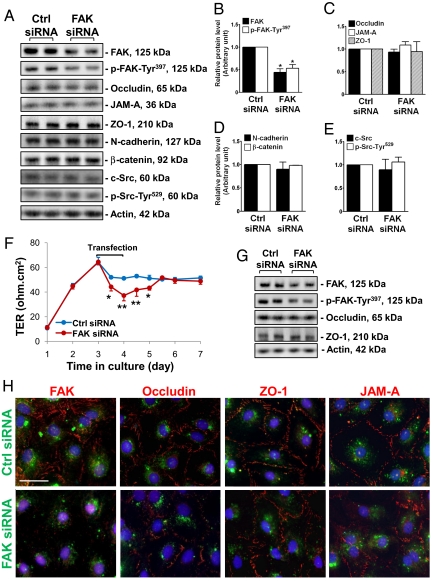
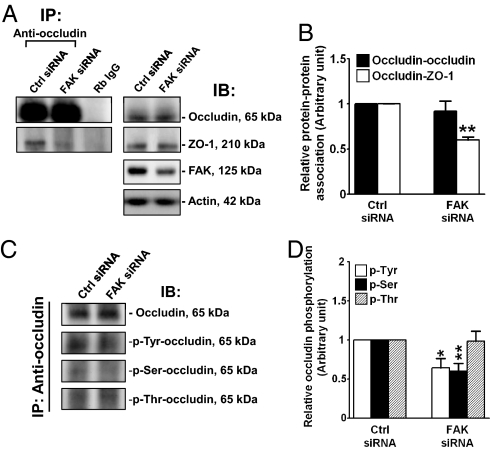
To test the hypothesis that FAK is crucial to maintaining BTB integrity, we sought to silence FAK by RNAi in an in vitro Sertoli cell system to examine its effects on TJ barrier function. Primary Sertoli cells cultured in vitro are known to form a functional TJ barrier by ≈2–3 days that mimics the BTB in vivo, manifested by its ability to resist the passage of an electrical current across the cell epithelium (15) and the presence of ultrastructures, such as TJ and basal ES, when examined by electron microscopy (16). Thus, this in vitro model was used herein. On day 4, the Sertoli cell epithelium was transfected with FAK-specific siRNA vs. nontargeting siRNA duplexes for 24 h. Thereafter duplexes were removed, and cells were cultured for an additional 48 h and harvested for immunoblot analysis. It was noted that ≈50% of FAK was silenced, with a concomitant decline in p-FAK-Tyr397 in cells transfected with FAK siRNA duplexes vs. control duplexes (Fig. 3 A and B). No changes were detected in the steady-state levels of TJ (e.g., occludin, JAM-A, and ZO-1), basal ES (e.g., N-cadherin and β-catenin), or regulatory (e.g., c-Src and p-Src-Tyr529) (Fig. 3 A–E) proteins at the BTB. Knockdown of FAK in Sertoli cells induced a disruption of the TJ barrier function compared with cells transfected with control duplexes (Fig. 3F). In a parallel experiment, cells cultured under the same conditions as shown in Fig. 3F were harvested 24 h after RNAi, at the time of TJ barrier disruption for immunoblot analysis, which illustrated that a knockdown of FAK did not alter the levels of occludin or ZO-1 (Fig. 3G). We next examined changes in the distribution of FAK and 3 TJ proteins (occludin, ZO-1, and JAM-A) at the Sertoli–Sertoli cell interface. In cells transfected with FITC-labeled nontargeting siRNA duplexes (green), FAK (red) was found at the cell–cell interface and cytosol, and some FAK also associated with cell nuclei (blue, DAPI), whereas almost all of the occludin, ZO-1, and JAM-A was restricted to the Sertoli–Sertoli cell interface (Fig. 3H, Top). After FAK knockdown, FAK staining in Sertoli cells was considerably weakened; most of the occludin and some of the JAM-A, but not ZO-1, redistributed from the cell–cell interface to cell cytosol (Fig. 3H, Bottom), illustrating that a loss of protein–protein interaction between occludin and ZO-1 might have occurred. To confirm this possibility, Co-IP was performed, which demonstrated a loss of association between occludin and ZO-1 after FAK knockdown, whereas the protein levels of occludin and ZO-1 remain unaltered (Fig. 4 A and B). Because FAK is a protein tyrosine kinase, and it is known that the phosphorylation status of occludin determines whether it is assembled into TJ fibrils (17, 18), we next determined whether FAK knockdown would affect the phosphorylation status of occludin. Using an antioccludin as the precipitating antibody to isolate occludin from cell lysates (Fig. 4C, Top), samples were used for immunoblotting using specific anti-phospho-Tyr, -Ser, or -Thr antibodies. Although the steady-state level of occludin was not altered in FAK-silenced cells, there was a significant decrease in occludin phosphorylation at Tyr and Ser, but not Thr, residues (Fig. 4 C and D).
Fig. 3.
A study to assess the effects of FAK knockdown by RNAi on Sertoli cell BTB function in vitro. (A) Representative immunoblots illustrate that ≈50% knockdown of FAK in Sertoli cells cultured at 0.4 × 106 cells/cm2 were transfected with FAK-specific duplexes vs. control (Ctrl) siRNA duplexes on day 4; cells were harvested 3 days thereafter. It was noted that p-FAK-Tyr397 level was reduced after FAK knockdown, but not other BTB-associated proteins. (B–E) Densitometric analyses of immunoblotting data normalized against actin with the target proteins in controls arbitrarily set at 1. Each bar is the mean ± SD of n = 3. *P < 0.001 by Student's t test. (F) A study to assess the effects of FAK knockdown on the Sertoli cell TJ barrier in vitro. Sertoli cells were plated at 1.2 × 106 cell/cm2 on Matrigel-coated bicameral units, and cells were transfected with different duplexes on day 3. A transient but statistically significant decrease in transepithelial electrical resistance (TER) was detected between 12 and 48 h in FAK siRNA–transfected cells vs. controls. (G) Immunoblots using lysates from cells cultured under the same conditions as in F and harvested 24 h after transfection, at the time BTB integrity was perturbed. A decline in FAK and p-FAK-Tyr397 protein levels, but not occludin and ZO-1, was detected. (H) To investigate changes in protein distribution at the Sertoli–Sertoli cell interface, Sertoli cells cultured at 0.05 × 106 cells/cm2 on Matrigel-coated coverslips were transfected with FITC-labeled nontargeting siRNA duplexes (green, control, Top) or FAK-specific siRNA duplexes (green, Bottom) and stained for FAK (red) and 3 TJ markers: occludin, ZO-1, and JAM-A (red). FAK knockdown was shown to induce mislocalization of occludin and JAM-A, but not ZO-1, at the cell–cell interface. (Scale bar, 30 μm, which applies to all micrographs.) Micrographs are representative results of 4 experiments.
Fig. 4.
A study by Co-IP to assess the effects of FAK knockdown on occludin–ZO-1 association and the phosphorylation status of occludin in Sertoli cells. On day 4, Sertoli cells cultured at 0.4 × 106 cells/cm2 on Matrigel-coated dishes were transfected with nontargeting (Ctrl) or FAK-specific siRNA duplexes for 24 h and were harvested 48 h thereafter. (A) Sertoli cell lysates (≈250 μg protein) were subjected to Co-IP with an anti-occludin antibody, and the blots were probed with anti-occludin or anti-ZO-1 antibodies. Co-IP with rabbit (Rb) IgG served as the negative control (Left). These samples were also used for immunoblotting to illustrate that the FAK knockdown reduced the levels of FAK but not occludin and ZO-1 (Right). (B) Densitometric analyses of Co-IP data of n = 3 with the protein–protein association in controls arbitrarily set at 1. (C) Sertoli cell lysates (≈250 μg of protein) were subjected to Co-IP with an anti-occludin antibody to immunoprecipitate occludin, which was then used for immunoblot analysis by probing with anti-phospho-Tyr, -Ser, or -Thr antibodies. Blots were re-probed with an anti-occludin antibody to confirm equal protein loading. (D) Densitometric analyses of composite data normalized against the occludin level, with the target proteins in controls arbitrarily set at 1. Each bar is the mean ± SD of 3 to 4 experiments. *P < 0.05; **P < 0.01 by Student's t test.
Can Sertoli Cell–Specific Knockdown of FAK by RNAi Alter the Susceptibility of BTB to CdCl2-Induced TJ Barrier Disruption?
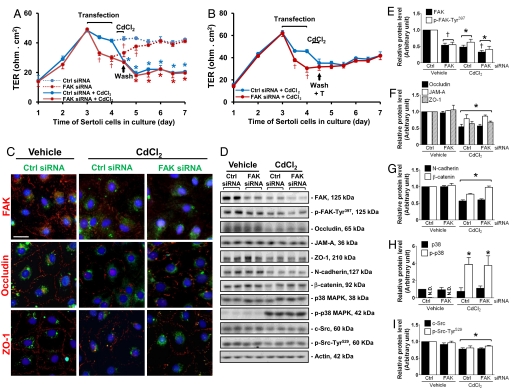
To further validate the physiological significance of FAK in maintaining BTB integrity, we next investigated whether the knockdown of FAK would affect the Sertoli cell BTB susceptibility to CdCl2-induced TJ barrier disruption. Treatment of Sertoli cells with CdCl2 (0.1 μM) for 8 h was shown to induce TJ barrier disruption in control cells transfected with nontargeting siRNA duplexes (Fig. 5A). Although the knockdown of FAK in Sertoli cells would induce disruption of the TJ permeability barrier, however, there was no additive effect of CdCl2 in worsening the BTB integrity in cells that were previously transfected with FAK siRNA duplexes (Fig. 5A). It is important to note that FAK-silenced cells did not respond to CdCl2 immediately, such as by 8 h after CdCl2 treatment (FAK siRNA vs. FAK siRNA + CdCl2) when compared with the control group (Ctrl siRNA vs. Ctrl siRNA + CdCl2). Taken together, these data suggest that the loss of FAK might have rendered Sertoli cells less susceptible to CdCl2-induced BTB disruption (Fig. 5A). In addition, the presence of testosterone at 2 × 10−7 M was shown to “reseal,” at least in part, the CdCl2-disrupted BTB in both groups (Fig. 5B). These findings are consistent with results of (i) the fluorescent microscopy study shown in Fig. 5C, which examined changes in protein distribution in Sertoli cells transfected with FAK siRNA vs. control siRNA duplexes and with or without CdCl2 treatment, illustrating that CdCl2 did not worsen occludin distribution in cells after FAK knockdown and did not alter ZO-1 localization; and (ii) immunoblotting data shown in Fig. 5 D–I. Additionally, activation of p-38 MAPK was detected after CdCl2 treatment in both control siRNA and FAK siRNA groups (Fig. 5H).
Fig. 5.
A study to assess the effects of FAK knockdown on the susceptibility of the Sertoli cell TJ barrier to CdCl2. (A) Sertoli cells were transfected with nontargeting (Ctrl, blue) or FAK (red) siRNA duplexes on day 3, and TER was measured every 12 h thereafter. (A) One day after transfection, cells were treated with either vehicle (0.9% NaCl, dotted lines) or CdCl2 (0.1 μM) for 8 h, then washed twice with F12/DMEM to remove CdCl2. Cells that had been transfected with control siRNA duplexes displayed a significant disruption in TJ barrier function after exposure to CdCl2 (Ctrl siRNA + CdCl2 vs. Ctrl siRNA), whereas no further disruption was observed in FAK knockdown cells after CdCl2 treatment (FAK siRNA vs. FAK siRNA + CdCl2). The extent of TJ barrier disruption in cells transfected with Ctrl siRNA + CdCl2 was similar to that of FAK siRNA-transfected cells + CdCl2; this trend persisted until the end of the experiment. (B) Sertoli cells were treated as shown in A, except that testosterone (T, 2 × 10−7 M) was included in F12/DMEM at the specified time point to facilitate barrier “resealing.” *P < 0.05, Ctrl siRNA vs. Ctrl siRNA + CdCl2, FAK siRNA vs. FAK siRNA + CdCl2; †P < 0.05, Ctrl siRNA vs. FAK siRNA, Ctrl siRNA + CdCl2 vs. FAK siRNA + CdCl2 by Student's t test. (C) Sertoli cells cultured on Matrigel-coated coverslips were transfected with FITC-labeled siRNA duplexes (green, Ctrl or FAK). Approximately 48 h thereafter, cells were treated with vehicle or CdCl2 (3 μM, 3 h) and stained for FAK, occludin, and ZO-1 (red). After CdCl2 exposure, mislocalization of occludin and ZO-1 was detected in cells transfected with Ctrl siRNA, consistent with the declining TER shown in B, illustrating a TJ barrier disruption (C, Left vs. Center), whereas FAK-silenced cells treated with CdCl2 did not display any further damages compared with FAK-silenced cells without CdCl2 exposure (see Fig. 3H, Bottom vs. Fig 4C, Right) or cells transfected with Ctrl siRNA and treated with CdCl2 (C, Center). (Scale bar, 30 μm, which applies to all micrographs.) (D) Immunoblotting using lysates of Sertoli cells transfected with siRNA duplexes and, 72 h after transfection, treated with vehicle or CdCl2 (3 μM) for 16 h to assess changes in the steady-state levels of BTB-associated proteins. (E–I) Densitometric analyses of immunoblotting data normalized against actin. Relative protein levels in Ctrl siRNA without CdCl2 treatment were arbitrarily set at 1. Because p-p38 MAPK was barely detectable in samples without CdCl2 treatment, it was compared with total p38 MAPK at respective groups, which was arbitrarily set at 1. Each bar is the mean ± SD of n = 3. *Significantly different from respective vehicle (P < 0.05); †significantly different from respective Ctrl siRNA (P < 0.05) by ANOVA.
Discussion
FAK Is a Regulatory Component of the Occludin–ZO-1 Protein Complex at the BTB.
FAK is a modulator of integrin-based signaling pathways, structurally linked to integrin at the focal contact (a cell-matrix anchoring junction type). Because focal contact is absent in the seminiferous epithelium, there are few reports in the literature exploring the significance of FAK in spermatogenesis. Recent studies, however, have shown that the ES is composed of proteins usually restricted to focal contact (e.g., integrins, c-Src, vinculin, and paxillin) (12). More important, studies have shown that BTB disruption and/or restructuring induced by environmental toxicants (e.g., cadmium) or cytokines (e.g., TGF-β3 and TNFα) could be manipulated by using specific inhibitors against JNK, ERK, and/or p38 MAPK (19), implicating FAK as the possible upstream modulator of these MAP kinases. Although it was suggested that α6β4-integrin is a basal ES protein at the BTB (20), we failed to localize either α6-, β4-, or β1-integrin at the BTB using commercially available antibodies (unpublished observations) (21). As such, it is unlikely that FAK mediates its effects at the BTB via an integrin-based protein complex.
FAK was first localized in the testis almost exclusively to the BTB by IHC (12). The expression of FAK in the seminiferous epithelium is stage-specific analogous to occludin (22), implicating its presence is crucial to BTB function. Unexpectedly, FAK was shown to be structurally associated with occludin, but not JAM-A and claudin-11 (data not shown). Most importantly, we have demonstrated that FAK is a regulator of BTB integrity. For instance, the knockdown of FAK by RNAi in Sertoli cells with an established TJ barrier caused a loss of phosphorylated Tyr and Ser residues in occludin, leading to its redistribution from the Sertoli–Sertoli cell interface into the cytosol. This FAK knockdown also induced a significant loss of occludin–ZO-1 interaction, possibly as a result of changes in occludin's phosphorylation status. Collectively, these alterations destabilized the barrier, perturbing the TJ barrier. These findings are consistent with earlier reports illustrating that highly phosphorylated occludin is recruited to TJ fibrils to confer barrier function (17, 18), but there is a small pool of less-phosphorylated occludin in the basolateral region of epithelial cells (e.g., Madin-Darby canine kidney cells) that is not assembled into TJ fibrils (17, 23). In short, the knockdown of FAK failed to maintain the pool of phosphorylated occludin that could be assembled into TJ fibrils. These findings also suggest that occludin is a substrate of FAK. In contrast to p-FAK-Tyr397 or p-FAK-Tyr576, which are restricted to the apical ES (12), FAK confined to the BTB is still likely to be activated in a residue other than Tyr397 or Tyr576 before exerting its effects on the occludin–ZO-1 complex. In fact, recent studies have shown there are multiple potential Tyr, Ser, and Thr phosphorylation sites in FAK mapped by mass spectrometry (24).
Is the FAK–Occludin-ZO-1 a Target of Cadmium Toxicity in Rat Testes?
The testis is vulnerable to environmental toxicants, such as cadmium. The BTB is one of the initial structural targets of cadmium in the seminiferous epithelium (25), and its disruption occurs before the endothelial TJ barrier disruption in microvessels in the interstitium (13, 25). Herein it was shown that FAK is one of the likely molecular targets of cadmium in the Sertoli cell epithelium, given that its knockdown apparently renders the Sertoli cell TJ barrier less susceptible to cadmium toxicity. This is supported by 2 lines of evidence. First, the curves depicting the TER after CdCl2 treatment, both in the control and after FAK knockdown, displayed a similar pattern (Fig. 5 A and B), and a similar reduction of BTB proteins was also noted in both experimental conditions (see Fig. 5F). It is noted that a loss of FAK by RNAi alone could perturb the TJ barrier, and if these FAK-silenced cells were not rendered less susceptible to cadmium toxicity, an “additive destructive effect” of FAK knockdown plus CdCl2 treatment on the TER and/or the levels of BTB proteins would have been expected. Second, after CdCl2 treatment, an absence of ZO-1 mislocalization in FAK-silenced Sertoli cells was noted (Fig. 5C). Although the significance of this observation remains to be investigated, ZO-1 (an adaptor) may play a crucial role in conferring the loss of susceptibility of these Sertoli cells to cadmium toxicity, possibly by recruiting other proteins to the BTB to eliminate the “additive destructive effect” on the TJ barrier function. These findings must be further evaluated so that cadmium-induced testicular injury could possibly be managed by “shielding” FAK from toxicants via the use of inhibitors. In short, we have identified a unique FAK–occludin–ZO-1 regulatory protein complex at the BTB in rat testes. Fig. 6 depicts a model by which FAK regulates BTB dynamics based on this report.
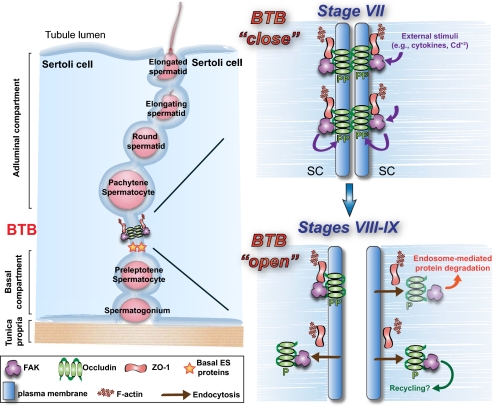
Fig. 6.
Regulation of BTB restructuring by the FAK–occludin–ZO-1 protein complex. (Left) Schematic drawing of the testis. During the epithelial cycle, such as at stages I–VII (Top Right), FAK maintains the optimal phosphorylation status of occludin for its localization at TJ fibrils at the BTB. However, external stimuli, such as cytokines released by Sertoli or germ cells in stages VIII–IX, or toxicants (e.g., cadmium), induce dephosphorylation in occludin, causing its dissociation from ZO-1 and redistribution, likely mediated via endocytosis, destabilizing the BTB (Bottom Right). Testosterone likely facilitates the formation of “new” TJ fibrils at the base of a migrating spermatocyte before the dissolution of “old” TJ fibrils at the apical region of the spermatocyte in transit (26).
Materials and Methods
Animals.
The use of Sprague-Dawley rats for the studies reported herein was approved by the Rockefeller University Laboratory Animal Use and Care Committee (protocol numbers 06018 and 09016). The use of adult rats (300 g b.w.) to obtain samples from the cadmium in vivo model was detailed elsewhere (6, 13) (see also SI Materials and Methods).
Transfection of Sertoli Cells with siRNA Duplexes.
In experiments to knockdown FAK in Sertoli cells by RNAi, cells (0.4 × 106 cells/cm2) were transfected with FAK-specific siRNA (100 nM) duplexes or control duplexes (100 nM) on day 4 by using 4 μL of TransIT-TKO transfection reagent (Mirus Bio) as described previously (27). Silencing of FAK was obtained by using a mixture (1:1) of rat Ptk2 (protein tyrosine kinase 2, also known as FAK) duplexes of 5′-GAUGUUGGUUUAAAGCGAU (s130268) and 5′-GAAUCUACUUGAUGUUAUU (s130269) (Silencer Select Predesigned siRNA; Ambion) vs. nontargeting siRNA duplex (Silencer Select Negative Control 1 siRNA; Ambion). After 72 h, cells were harvested to analyze changes in the steady-state levels of FAK (to examine the efficiency of knockdown) and other BTB-associated proteins by immunoblot analysis, or changes in occludin–ZO-1 association and occludin phosphorylation status by Co-IP (see SI Materials and Methods). In selected experiments, cells were treated with either vehicle (0.9% NaCl) or CdCl2 (3 μM) for an additional 16 h to examine whether FAK knockdown would render any changes on the susceptibility of these cells to cadmium treatment. To examine changes in cellular distribution of BTB-associated proteins in Sertoli cells after RNAi, Sertoli cells were plated on Matrigel-coated coverslips at 0.05 × 106 cells/cm2, and transfection was performed approximately 48 h after cell plating as described above, except that 80 nM of siRNA duplexes were used, and duplexes were fluorescently labeled by using a LabelIT siRNA Tracker FITC kit (Mirus Bio) according to the manufacturer's instruction to track the transfected cells. Two days after transfection, cells were fixed with 2% paraformaldehyde in PBS, permeabilized with Triton X-100, blocked with BSA, and incubated with the corresponding primary and secondary antibodies (Table S1) for immunofluorescent microscopy. In some experiments, after the knockdown of FAK by RNAi, Sertoli cells were further treated with vehicle (0.9% NaCl) vs. CdCl2 (3 μM) for 3 h to examine whether the transient loss of FAK would affect the distribution of FAK and BTB-associated proteins; and cells were processed for fluorescent microscopy.
General Methods and Statistical Analysis.
Primary Sertoli cell cultures, assessment of Sertoli TJ permeability barrier by TER measurement, IHC, and fluorescent microscopy were performed essentially as described earlier (21, 26, 27) and detailed in SI Materials and Methods. Statistical analyses were performed by one-way ANOVA to be followed by Tukey's honest significant test or by Student's t test using the GraphPad Prism software package (version 5; GraphPad Software).
Supplementary Material
Acknowledgments.
This work was supported in part by Grants R01 HD056034, R03 HD051512, and U54 HD029990 Project 5 (all to C.Y.C.) from the National Institute of Child Health and Human Development, National Institutes of Health. E.R.S. was supported by a Fellowship (06/51281–6) from Fundação de Amparo à Pesquisa do Estado de São Paulo.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813113106/DCSupplemental.
References
- 1.Russell LD. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat. 1977;148:313–328. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- 2.Clermont Y. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–235. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- 3.Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochem Biophys Acta. 2008;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin, TX: Landes Bioscience/Springer Science; 2008. pp. 186–211. [Google Scholar]
- 5.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- 6.Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: An in vivo study. J Cell Sci. 2004;117:783–798. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- 7.Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: Roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- 8.Parsons JT. Focal adhesion kinase: The first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 9.Parsons JT, Slack-Davis J, Tilghman R, Roberts WG. Focal adhesion kinase: Targeting adhesion signaling pathways for therapeutic intervention. Clin Cancer Res. 2008;14:627–632. doi: 10.1158/1078-0432.CCR-07-2220. [DOI] [PubMed] [Google Scholar]
- 10.Broussard JA, Webb DJ, Kaverina I. Asymmetric focal adhesion disassembly in motile cells. Curr Opin Cell Biol. 2008;20:85–90. doi: 10.1016/j.ceb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Furuta Y, et al. Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene. 1995;11:1989–1995. [PubMed] [Google Scholar]
- 12.Siu MKY, Mruk DD, Lee WM, Cheng CY. Adhering junction dynamics in the testis are regulated by an interplay of β1-integrin and focal adhesion complex (FAC)-associated proteins. Endocrinology. 2003;144:2141–2163. doi: 10.1210/en.2002-221035. [DOI] [PubMed] [Google Scholar]
- 13.Wong CH, Mruk DD, Siu MKY, Cheng CY. Blood-testis barrier dynamics are regulated by α2-macroglobulin via the c-Jun N-terminal protein kinase pathway. Endocrinology. 2005;146:1893–1908. doi: 10.1210/en.2004-1464. [DOI] [PubMed] [Google Scholar]
- 14.Furuse M, et al. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janecki A, Jakubowiak A, Steinberger A. Regulation of transepithelial electrical resistance in two-compartment Sertoli cell cultures: In vitro model of the blood-testis barrier. Endocrinology. 1991;129:1489–1496. doi: 10.1210/endo-129-3-1489. [DOI] [PubMed] [Google Scholar]
- 16.Siu MKY, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–25047. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- 17.Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita S. Possible involvement of phosphorylation of occludin in tight junction formation. J Cell Biol. 1997;137:1393–1401. doi: 10.1083/jcb.137.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsukamoto T, Nigam S. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am J Physiol. 1999;276:F737–F750. doi: 10.1152/ajprenal.1999.276.5.F737. [DOI] [PubMed] [Google Scholar]
- 19.Wong CH, Cheng CY. The blood-testis barrier: Its biology, regulation and physiological role in spermatogenesis. Curr Topics Dev Biol. 2005;71:263–296. doi: 10.1016/S0070-2153(05)71008-5. [DOI] [PubMed] [Google Scholar]
- 20.Mulholland D, Dedhar S, Vogl A. Rat seminiferous epithelium contains a unique junction (ectoplasmic specialization) with signaling properties both of cell/cell and cell/matrix junctions. Biol Reprod. 2001;64:396–407. doi: 10.1095/biolreprod64.1.396. [DOI] [PubMed] [Google Scholar]
- 21.Yan HHY, Mruk DD, Wong EWP, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:8950–8955. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li MWM, et al. TNFα reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol. 2006;190:313–329. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- 23.Cordenonsi M, et al. Occludin dephosphorylation in early development of Xenopus laevis. J Cell Sci. 1997;110:3131–3139. doi: 10.1242/jcs.110.24.3131. [DOI] [PubMed] [Google Scholar]
- 24.Grigera PR, et al. FAK phosphorylation sites mapped by mass spectrometry. J Cell Sci. 2005;118:4931–4935. doi: 10.1242/jcs.02696. [DOI] [PubMed] [Google Scholar]
- 25.Setchell BP, Waites GMH. Changes in the permeability of the testicular capillaries and of the “blood-testis barrier” after injection of cadmium chloride in the rat. J Endocrinol. 1970;47:81–86. doi: 10.1677/joe.0.0470081. [DOI] [PubMed] [Google Scholar]
- 26.Yan HHY, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008;22:1945–1959. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:9657–9662. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.