Abstract
Cell fusion is involved in many critical developmental processes, including zygote formation and organogenesis of placenta, bone, and skeletal muscle. In adult tissues, cell fusion has been shown to play an active role in tissue regeneration and repair, and its frequency of occurrence is significantly increased during chronic inflammation. Fusion between tumor cells and normal cells, or among tumor cells themselves, has also been speculated to contribute to tumor initiation, as well as phenotypic evolution during cancer progression and metastasis. Here, we show that dual metastasis organotropisms can be acquired in the same cell through in vitro or in vivo spontaneous fusion between bone- and lung-tropic sublines of the MDA-MB-231 human breast cancer cell line. The synkaryonic hybrids assimilate organ-specific metastasis gene signatures from both parental cells and are genetically and phenotypically stable. Our study suggests cell fusion as an efficient means of phenotypic evolution during tumor progression and additionally demonstrates the compatibility of different metastasis organotropisms.
Keywords: breast cancer, cell fusion, nuclear reprogramming, genomic instability, centrosome
The development of cancer is believed to be driven by the progressive accumulation of numerous genetic and epigenetic alterations that gradually allow early hyperplasia to become highly malignant tumors (1, 2). Likewise, metastasis organotropism—the capability of tumor cells to colonize specific target organs—is thought to emerge via acquisition of distinct sets of organ-specific metastasis genes in metastatic variants that are most adapted to different target organ microenvironments through Darwinian selection (3). Indeed, genomic profiling of metastatic variants selected in vivo in mouse models of breast cancer has unveiled 2 separate sets of genes that promote metastasis to bone and lung, respectively (4, 5), although it is unclear whether such distinct organ-specific metastasis gene signatures can coexist in the same cell to give rise to tumor cells capable of colonizing both organs. An alternative theory of metastasis progression has also been proposed that argues for rapid acquisition of metastatic phenotypes through fusion between tumor cells or between tumor cells and certain normal cells, such as macrophages (6–9), rather than requiring the progressive accumulation of independent genetic or epigenetic alterations in a single cell lineage. Given that a 1-cm3 tumor of ≈109 cells is estimated to harbor as many as 105 proliferating hybrid cells produced by spontaneous cell fusion (6, 10), the contribution of cell fusion to the phenotypic evolution of tumors cannot be overlooked.
In this study, we used a well-characterized model system of organ-specific breast cancer metastasis to show that spontaneous cell fusion between bone-tropic and lung-tropic cancer cells, both in vitro and in vivo, generates stable hybrids with dual metastasis tropism to both organs. In addition to directly demonstrating the role of cell fusion in the rapid acquisition of complex metastasis properties, our study also discovered a surprisingly high level of chromosomal and phenotypic stability in hybrids during long-term passage in vitro and in vivo, despite the existence of amplified numbers of centrioles in these cells as a consequence of cell fusion. These results suggest a potentially important role of cell fusion in the progression and phenotypic diversity of cancer.
Results
Spontaneous Cell Fusion Generates Synkaryonic Hybrids That Inherit Chromosomal Abnormalities of Parental Cells.
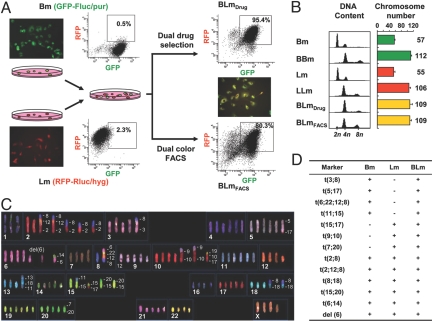
To examine the genetic and phenotypic consequences of cell fusion between tumor cells with distinct metastasis characteristics, we used 2 previously reported sublines of the human breast cancer cell line MDA-MB-231: the bone-metastatic SCP2 and the lung-metastatic LM2 (4, 5). These 2 cell lines were renamed as Bm and Lm, respectively, to facilitate the presentation of results (Fig. 1A). To isolate spontaneously fused cells from the coculture of these 2 cell lines, we labeled Bm with a GFP-Fluc (firefly luciferase) fusion protein expression construct with a puromycin resistance marker and Lm with RFP-Rluc (Renilla luciferase) and hygromycin as markers. These 2 cell lines were cocultured for 1 day without any fusogenic reagents and hybrid cells were selected by either dual drug selection or dual color fluorescence-activated cell sorting (FACS) (Fig. 1A). After 4 rounds of cell sorting, we obtained a relatively pure GFP+/RFP+ population (80.3%, named as BLmFACS) whereas only 1 round of puromycin/hygromycin dual drug selection gave rise to a population with 95.4% GFP+/RFP+ cells (named as BLmDrug) (Fig. 1A). We also isolated BBm and LLm hybrids (resulting from self-fusion of Bm and Lm cells) by using the same approach. The fused cells are synkaryons with enlarged nuclear and cell sizes (Fig. S1 A–C), but with growth doubling times similar to the parental cell lines (Bm 34.6h, Lm 36.0h, BLmFACS 36.2h, and BLmDrug 34.2h). Flow cytometric DNA content analysis and karyotyping showed nearly doubled DNA content and chromosome numbers in hybrids compared with the parental cell lines (Fig. 1B). Spectral karyotyping (SKY) further revealed that BLm hybrids adopted all major chromosomal abnormalities (translocations and deletions) of both parental cell lines (Figs. 1 C and D and S1D).
Fig. 1.
Spontaneous fusion hybrids are hyperploid and inherit chromosomal abnormalities from both parental cell lines. (A) Experimental design for using FACS or drug selection to isolate spontaneous fusion hybrids (BLmFACS or BLmDrug) from the coculture of Lm and Bm cells that were differentially labeled with various markers. The fluorescence dot plots for BLmDrug and BLmFACS represent the results after 1 round of drug selection and 4 rounds of FACS, respectively. (B) DNA content and chromosome numbers of the parental and hybrids evaluated by flow cytometric (propidium iodide staining) and karyotyping (Giemsa staining) methods (n = haploid chromosome number of MDA-MB-231). (C) Representative SKY image showing the chromosomal composition of BLmDrug. (D) Summary of chromosomal translocations and deletions in Bm, Lm, and BLmDrug.
To test whether spontaneous cell fusion also occurs in vivo, we injected an equal mixture of Bm and Lm cells s.c. into nude mice. When the tumor diameter reached 10 mm, a single-cell suspension was made from the tumor by mincing and digestion with collagenase A. The cells were plated directly into dual drug selective media at a very low density (104 cells/10-cm dish) to select for surviving colonies for 14 days. By using this approach, the frequency of in vivo spontaneous fusion resulting in viable progenies was estimated to be 2.0 ± 0.8 × 10−5 in s.c. tumors, very similar to the in vitro fusion frequency of 2.6 ± 0.7 × 10−5 (see SI Materials and Methods, data reported as the mean ± SD), and is consistent with previously reported frequencies in other tumor models (11).
Fusion Between Bone- and Lung-Tropic Metastatic Cells Produced Dual-Tropic Hybrids.
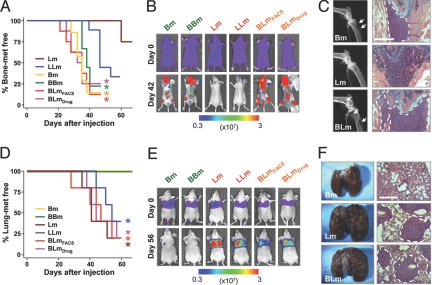
Parental Bm, Lm, and hybrids BBm, LLm, BLmDrug, and BLmFACS were injected into nude mice through either left cardiac ventricles or tail veins to test their bone or lung metastasis abilities, respectively. Metastatic progression was measured by weekly bioluminescence imaging (BLI) and analyzed with Kaplan-Meier curves (Fig. 2). Bm and BBm formed aggressive bone metastases but no lung metastasis. Conversely, Lm and LLm displayed high metastasis potential to lung and a much weaker ability to form bone metastasis. In contrast, BLmDrug and BLmFACS showed strong metastasis ability to both bone and lung, indicating that BLm cells have inherited metastasis phenotypes from both parental cell lines. Formation of massive metastases in both bone and lung by the hybrid cells were confirmed by X-ray and histological analyses of the lesions (Fig. 2 C and F). The acquisition of dual metastasis organotropism was also confirmed when bone and lung metastasis assays were performed with 2 hybrid clones (BLmDrug-i.v.#1 and BLmDrug-i.v.#2) obtained from in vivo fusion by Bm and Lm cells in s.c. tumors as described above (Fig. S2).
Fig. 2.
BLm hybrid cells are highly metastatic to both bone and lung. (A and D) Kaplan-Meier curves show the metastasis propensities of the parental and hybrids to bone (A) or the lung (D). *, P < 0.05 with log-rank test when compared with Lm (A) and Bm (D) (n = 8 in A or n = 5 in D). (B and E) BLI images of representative mice from each experimental group showing development of metastases in the hindlimbs (B) or the lungs (E). (C) X-ray radiography of the hindlimbs and H&E staining of the femurs from mice injected with parental and BLm cells. Osteolytic bone lesions are marked with arrows, and metastases are marked with dotted lines. (F) Photographs and H&E staining of the lung from mice injected with parental and BLm cells. Metastasis nodules are indicated with dotted lines in the H&E images. (Scale bar, 500 μm in C and F.)
Interpretation of the finding that hybrid cells can form aggressive metastases in both organs may be complicated by the fact that the hybrids may contain a very small fraction of unfused parental cells, which may separately form metastases in bone and lung. This possibility was ruled out by 2 pieces of evidence. First, firefly luciferase and Renilla luciferase BLI performed on consecutive days showed exactly matched metastasis locations in the mice injected with BLmDrug (Fig. S3). This result suggested that most, if not all, of metastases were derived from hybrids with dual markers instead of rare singly labeled parental cells that survived the drug selection. A more rigorous test was performed by analyzing single cell isolates from BLm. By using single-cell sorting, we isolated 21 single cell clones from BLmDrug. We randomly picked 5 clones (#6, #7, #8, #12, and #18) to test their tissue-specific metastasis abilities (Fig. S4). All of the clones were bone metastatic, although clones #6 and #7 were not as strong as the pooled BLmDrug population. All of the clones except clone #7 were also lung metastatic, with clone #18 showing an even more aggressive phenotype than the pooled population. Taken together, these results indicated that both bone and lung metastasis phenotypes can be efficiently acquired in a single tumor cell through cell fusion.
Bone and Lung Metastasis Gene Signatures Are Coexpressed by Hybrids.
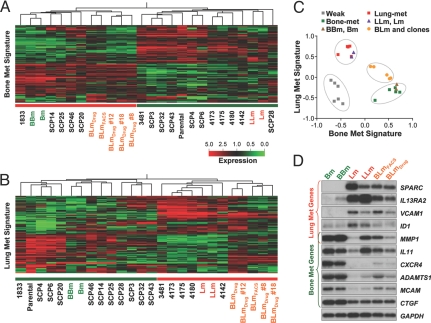
As cell fusion did not confer growth advantages, we reasoned that the acquired dual metastasis tropism may be because of the coexpression of tissue-specific metastasis genes that have been previously defined for both parental cell lines (4, 5). We performed microarray analyses on various cell lines derived from our current study and used the previously identified bone and lung metastasis gene signatures to perform unsupervised clustering of these cell lines as well as several previously characterized MDA-MB-231 sublines with different metastasis organotropisms (Fig. 3 A and B). Both signatures were able to segregate various cell lines into a lowly metastatic group and a highly metastatic group. BLm and its single cell-derived clones were consistently clustered into the strongly metastatic groups based on either signature, although not all of the genes in each signature retained their expression level in the BLm cells. As expected, the self-fusion hybrids, BBm and LLm, always clustered together with their parental cell lines of origin, Bm and Lm. When the expression profiles of MDA-MB-231 variants and hybrids were scored for their similarity to the lung and bone metastasis signatures, the hybrids were positive for both signatures, albeit with scores slightly lower than those from the sublines with only one metastasis organ tropism (Fig. 3C). Northern blot analysis confirmed the coexpression of typical bone metastasis genes and lung metastasis genes in the BLm cells and BLmDrug clones (Figs. 3D and S5). These results indicate that the dual metastasis organ tropism displayed by BLm reflects the coexpression of distinct sets of tissue-specific metastasis genes and that fusion as a cellular process itself did not significantly alter gene expression or metastatic behavior.
Fig. 3.
Coexpression of both bone and lung metastasis signatures in the BLm hybrid cells. (A and B) Hierarchical clustering of the BLm cells and MDA-MB-231 sublines is shown with different metastatic abilities by using the bone-metastasis gene signature (A) or the lung-metastasis gene signature (B). Cell lines under the red line are strongly metastatic, whereas those under the green line are weakly metastatic (4, 5). “Parental” refers to the original MDA-MB-231 cell line acquired from ATCC and used to generate all other sublines. Cell lines started with “SCP” were single-cell clones of MDA-MB-231. MDA-MB-231 derivatives 1833, 3481, 4173, 4175, 4180, and 4142 were obtained by in vivo selection (4, 5). In both heatmaps, the BLm lines clustered together with the strongly metastatic sublines. (C) The metastasis signature correlation score analysis shows the grouping of the weakly metastatic cell lines (negative for both signatures), cell lines with a single metastasis tropism (positive for either one, but not both, of the signatures), and BLm cells (positive for both signatures). Cell lines used in this analysis are the same as in A and B. (D) Northern blot showing BLm cells coexpress representative lung- and bone-metastatic signature genes. MMP1 is a shared gene for both signatures.
Chromosomal and Phenotypic Stability of Hybrids.
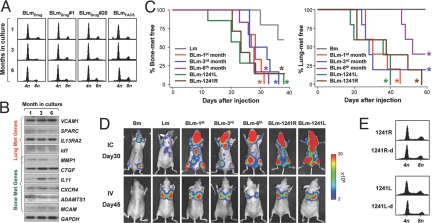
Cell fusion may result in dramatic chromosomal instability and hybrid cells may gradually lose extra chromosomes through reductive mitosis (12). We tested the long-term genomic and phenotypic stability of BLm by culturing the cells continuously for nearly a year (up to the time of manuscript preparation). Remarkably, BLmFACS, BLmDrug and its clones maintained the nearly combined genome size (Fig. 4A) and the expression of the characteristic metastasis genes (Fig. 4B) throughout the duration of the experiment. In addition, the tissue-specific metastasis ability to both bone and lung was maintained in BLmDrug cells that were cultured for 1, 3, and 6 months (Fig. 4 C and D). To test whether hybrids also maintained the chromosomal and phenotypic stability in vivo during metastasis assays in mice, we isolated tumor cells (1241R and 1241L) from bone metastases formed by BLmDrug and confirmed their sustained strong metastatic ability to both organs (Fig. 4 C and D). DNA content analysis of 1241R, 1241L and their secondary in vivo derivatives (1241R-d and 1241L-d) consistently revealed a combined genome size (Fig. 4E).
Fig. 4.
Genomic and phenotypic stability of hybrid BLm cells during long-term in vitro culture and in vivo passage. (A) DNA content histograms of the pooled BLmDrug and its single cell derived clones measured after 1, 3, and 6 months of culture (n = haploid number of chromosomes in MDA-MB-231). (B) Expression of selected genes from the lung and bone metastasis signatures in the pooled BLmDrug at 3 time points during the long-term in vitro culture. (C) Kaplan-Meier curves showing bone metastasis (Left) and lung metastasis (Right) developed after injection of BLmDrug cultured in vitro for 1, 3, or 6 months, or isolated from bone metastases formed by BLmDrug (1241L, 1241R). *, P < 0.05 with log-rank test when compared with Lm or Bm (n = 8 for bone metastasis assays and n = 5 for lung metastasis assays). (D) Representative mice from experimental groups in (C) with hindlimb bone metastases on day 30 after injection and lung metastases on day 45 after injection. (E) Stable DNA content of BLmDrug after 1 round (1241L and 1241R) or 2 rounds (1241R-d, 1241L-d) of in vivo passage as bone metastasis (n = haploid chromosome number of MDA-MB-231).
Discussion
Although cell fusion is generally considered to be a highly specialized event that only occurs in limited scenarios during development (13, 14), a broader role for cell fusion in repair and regeneration of adult tissues has been increasingly recognized since the discovery of cell fusion as one of the underlying mechanisms of the so-called “transdifferentiation” phenomenon—the ability of one lineage of differentiated cells, such as bone marrow cells, to give rise to a different lineage of cells, such as liver or neural cells (15–21). In recent years, cell fusion has also become an important research platform to study epigenetic reprogramming, cell fate manipulation and pluripotency (14, 22, 23). In cancer research, cell fusion has been speculated to play important roles in several aspects of tumor progression, including generation of cancer stem cells (8), acquisition of metastasis ability (7), and multidrug resistance (11). Cancer cells can have considerably high rate (up to 1%) of fusion in vivo in experimental tumor models (10). Recent findings that chronic inflammation dramatically increases the frequency of cell fusion between hematopoietic cells with various cell lineages (24, 25) may help explain the high fusogenicity of tumor cells, as inflammation is often associated with the tumor microenvironment (26). Fusion between cancer cells may be further facilitated by fusogenic viruses (27, 28) and entosis (29). Despite the technical difficulty in directly detecting cell fusion in cancer patients, several clinical observations support the existence and potential importance of cell fusion in cancer development. First, in kidney cancer patients who were also recipients of previous bone marrow transplantation, the renal cell carcinoma cells were found to contain genetic material from both the recipients and the donor (30, 31). Secondly, premature chromosome condensation, a consequence of fusion of cells at different stages of cell cycle, has been observed in a large number of tumor types (32–35). Finally, binucleated and multinucleated cells are frequently observed in many types of tumors (9), and increased DNA content and aneuploidy in tumor cells, which may result from cell fusion, is associated with poor prognosis and metastasis (36–38). Indeed, we estimated that cell fusion accounts for 65–88% of the hyperploid cells in our current experimental setting (see calculation in SI Materials and Methods and Fig. S6 and Table S1).
Compared with mutation-based phenotypic evolution during cancer progression, the main advantage of cell fusion is its efficiency in generating drastic genomic changes and phenotypic heterogeneity (11). Although fusion of 2 genetically identical tumor cells may not benefit tumor cell progression, fusion between tumor cells with distinct phenotypes can generate hybrids with diversified phenotypes to allow better adaptation to various secondary environments during metastasis or to survive different therapeutic challenges. In this study, we derived spontaneous fusion hybrids from the mixed population of 2 breast cancer sublines with distinct organ-specific metastasis propensities (Bm and Lm) and found that the hybrids (BLm) efficiently acquired the ability to metastasize to both organs (Fig. 2). Similar results were observed when hybrid cells were obtained from the fusion of Lm and SCP28, another bone-tropic metastatic subline of MDA-MB-231 (Fig. S7).
The gain of dual tropism in the BLm cells is not simply due to larger cell size and increased mechanical arrests in capillary beds of distant organs, because hybrids of identical parental cells (LLm and BBm) did not show significant change in metastasis propensity. Instead, dual metastasis organotropism of hybrids is likely because of the expression of both sets of genes important for metastasis to each organ. It should be noted that the modest but statistically insignificant (P = 0.0849, log-rank test) increase of bone metastatic potential by LLm as compared with Lm (Fig. 3D) may be because of elevated expression of 2 bone metastasis genes, MMP1 and IL11. The ability of hybrid cancer cells to retain the genomic, transcriptomic, and phenotypic characteristics of both parents without a significant bias (Figs. 3 and S7C) is distinctively different from the dramatic nuclear reprogramming of differentiated somatic cells to the embryonic state after their fusion with embryonic stem (ES) cells (22, 23). The difference in transcriptional reprogramming in hybrids may be in part because of the difference in epigenetic regulation between the somatic/ES cell hybrids and the BLm hybrids. Although ES cells may reprogram the epigenetic regulation landscape (e.g., methylation pattern of the OCT4 promoter) to a predominant ES state after fusion with fibroblasts (22), coexistence of epigenetic regulatory mechanisms from both fusion partners in the BLm hybrids may explain the moderately elevated expression of both sets of organ-specific metastasis genes in the hybrids (Fig. 3 A and D). Although the level of expression of these genes in the hybrid are modest compared with the generally higher level of organotropic gene expression in Bm or Lm cells, it is sufficient to promote metastasis of hybrids to both bone and lung.
The fact that cell fusion can generate tumor cells with dual metastasis tropism to bone and lung indicates that different metastasis organotropism and the related metastasis signatures can coexist in the same tumor cell to facilitate simultaneous metastasis to multiple organs (4, 5, 39). Hybrid cells with multiple metastasis organotropism may confer several advantages for disseminated tumor cells to proliferate and survive therapeutic treatments. First, tumor cells with multiple metastasis organotropism will be able to colonize several different organs when they are disseminated systemically through blood circulation, whereas tumor cells with only a single metastasis organotropism can only colonize one organ and will be lost when they are distributed to an inhospitable microenvironment. Therefore, fusion between tumor cells with different metastatic ability can increase the efficiency of metastatic dissemination. Secondly, because metastases in different organs often have different responses to therapeutic treatments (40), tumor cells with multiple metastasis organotropisms can survive in a different organ and then quickly re-colonize the organ that has positively responded to therapies. Finally, cell fusion could also lead to combinatorial advantage in other phenotypes, like chemoresistance, and may therefore, confer additional survival advantage for hybrid cells.
We also found that hybrid cells resulted from spontaneous fusion are remarkably robust in maintaining their genetic and phenotypic stability, at least for several months in our current experiment (Figs. 4 and S7 G and H). The chromosome stability after cell fusion was also observed in various other experimental systems, including fusion beween HeLa and fibroblasts (41), between mouse mammary tumor cells (11, 42), or between ES cells and fibroblasts (22). Cell fusion automatically leads to increased number of centrosomes in the hybrids. Supernumerary centrosomes can cause multipolar spindles in mitosis which will result in either aborted mitosis or daughter cells with imbalanced chromosome numbers (12, 43). However, the near complete retention of all chromosomes from parent cell lines in our hybrids and their remarkable chromosomal stability during long-term passage suggested that they may have intrinsic mechanism(s) to undergo bipolar segregation of chromosomes despite the existence of additional centrosomes resulting from cell fusion. Indeed, when we quantified the number of centrosomes in postprophase mitosis in Bm, Lm, and various BLm cell lines by staining the cells with the centrosome marker pericentrin, all cells tested displayed a similar distribution of centrosome numbers: >90% of cells in meta/ana/telophase harbor 2 centrosomes as microtubule-organizing centers; <10% of the cells have more than 2 centrosomes, which may be accounted for by the basal level of centrosome amplification present in MDA-MB-231 cells (Fig. S8). This result suggests that BLm hybrids are able to maintain bipolar cell division despite initially gaining additional centrosomes after cell fusion. When we stained cells for both the centrosome marker (pericentrin) and a centriole marker (centrin) and examined the centriole distribution in the cells with 2 centrosomes during mitosis (Fig. S9), we observed 3 major configurations: (i) 2 standard centrosomes each containing 2 centrioles; (ii) 1 or both centrosomes containing >2 centrioles (coalesced centrioles); and (iii) centrioles dispersed in the cytoplasm without pericentrolar materials (orphan centrioles) in addition to the centrioles associated with the 2 centrosomes. Therefore, we envision 3 corresponding scenarios that may have allowed hybrids to divide successfully despite supernumerary centrioles introduced by cell fusion: (i) hybrids retain only 2 structurally and functionally intact centrosomes by discarding extra centrioles; (ii) multiple centrioles coalesce into 2 spindle, similar to what was observed in neuroblastoma (43, 44); and (iii) multipolar mitosis is suppressed by keeping extra centrioles in a pericentriolar material-free, inactive status (orphan centrioles). Interestingly, when these 3 configurations were quantified, a significantly higher percentage (50.0%) of cells with orphan centrioles was seen in BLmDrug compared with Bm (22.1%) and Lm (19.4%), perhaps representing a vestige of the fusion events responsible for the formation of BLmDrug. Orphan centrioles might therefore be an important approach for hybrids to maintain relative chromosomal stability following cell fusion.
Overall, our study demonstrates a role for cell fusion in the rapid acquisition of complex malignant traits such as metastasis organotropism in tumor cells. Genomic and phenotypic characteristics of both parental cells were assimilated in the resultant hybrids without an overall nuclear reprogramming toward a predominant fusion partner and were stably maintained in hybrids after long-term passage in vitro and in vivo. These observations, together with recent findings of the involvement of cell fusion in many physiological and pathological conditions, suggest a potentially important role of cell fusion in cancer progression that warrants further investigation.
Materials and Methods
Cell Culture.
SCP2 (Bm) and LM2 (Lm) sublines were derived from the parental cell line MDA-MB-231 (ATCC) (4, 5) and were generously provided by Joan Massaugé (Sloan-Kettering Institute, New York). These sublines and their fusion variants were maintained in DMEM with10% FBS and antibiotics supplemented with appropriate selective drugs. H29 cells, a packaging cell line used for retrovirus production, were maintained in DMEM supplemented with 10% FBS, 1% glutamine, and antibiotics.
Generation of Hybrids by Spontaneous Cell Fusion.
Bm and Lm cells were labeled with different fluorescence, bioluminescence, and drug selection makers before fusion. The retroviral construct pMSCV-hyg-hrl-mrfp-ttk was generated by subcloning the coding sequence of the Rluc-RFP-TK triple fusion reporter from pcDNA3.1-CMV-hrl-mrfp-ttk (45) to the BglII and HpaI sites of pMSCV-hyg (Clontech). Retroviruses were generated from the H29 packing cell lines and used to transduce Lm cells. Bm cells were first labeled with the TK-GFP-Fluc triple reporter encoded in retroviruses produced from SFG-NESTGL (46) (tk-gfp-fluc) and then stably transfected with pPUR (Clontech) to become resistant to puromycin. To obtain fusion hybrids, 106 RFP-Rluc/hyg labeled Lm cells were mixed with 106 GFP-Fluc/pur labeled Bm cells in a 10-cm dish. After 24 h, the coculture was either selected with 1.5 mg/mL hygromycin B and 0.3 μg/mL puromycin to obtain dual resistant cells, or subjected to FACS to purify RFP/GFP double-positive cells. For analysis of DNA content, 106 cells were fixed with 70% ethanol, treated with 0.12 mg/mL RNase A (Sigma), stained with 8 μg/mL propidium iodide (Sigma), and analyzed by flow cytometry.
Karyotype Analysis.
To obtain metaphase spreads to quantify chromosome numbers, the cell culture was treated with 0.1 μg/mL Colcemid (GIBCO) for 1 h. The cells were harvested, treated with 0.075 M KCl for 10 min, fixed with a methanol/acetic acid mixture for 30 min, and dropped on precooled slides. Air dried slides were stained with Giemsa solution (GIBCO). SKY was performed by the SKY/FISH facility in the Roswell Park Cancer Institute as described (47).
Tumor Xenografts and Analysis.
All procedures involving mice, such as housing and care, and all experimental protocols were approved by Institutional Animal Care and Use Committee (IACUC) of Princeton University. For intracardiac injections, 105 cells in PBS were injected into the left cardiac ventricle of 4-week-old, female nude mice (NCI) as described (4). For i.v. injection, 2 × 105 cells in PBS were injected into the tail vein of nude mice as described (5). Development of metastases in bone and lung was monitored by BLI with the IVIS Imaging System (Xenogen) as described (4, 5). BLI analysis was performed with Living Image software (Xenogen) by measuring photon flux of the region of interest. Metastasis status was recorded as positive when BLI signal was higher than that of the basal BLI signal of total injected cells on day 0, and used for constructing Kaplan-Meier curves. X-ray radiography analysis of bone lesions was performed by using procedures as described (4). For the orthotopic xenograft model, mammary fat pad injections and tumor size measurements were performed following the procedure described previously (5).
Accession Number.
Microarray data reported herein have been deposited at the National Center for Biotechnology Information Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) with the accession no. GSE14244.
Statistical Analysis.
Results were reported as mean ± SD. Kaplan-Meier curves were created by using Stata 7.0 software (Stata Corporation). Log-rank test was used to calculate the statistic significance of difference between metastasis curves. Other comparisons were performed by using unpaired 2-sided Student's t test without equal variance assumption or nonparametric Mann-Whitney test.
Additional experimental procedures and discussion, including measurement of spontaneous fusion frequency, histological and immunofluorescence analyses, microarray analysis, Northern blot analysis, and methods to estimate the contribution of cell fusion to hyperploidy are listed in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank C. DeCoste for assistance on FACS; Roswell Park Cancer Institute for SKY analysis; G. Hu and M. Yuan for technical assistance in statistical analysis and animal experiments; G. Hu, Y. Wei, M.A. Blanco, and members of our laboratories for helpful discussions; T.A. Guise and K.S. Mohammad for training and technical advice in bone histology; J. Massagué (Sloan-Kettering Institute, New York) for Lm and Bm cell lines; R. Blasberg (Sloan-Kettering Institute, New York) for SFG-NESTGL plasmid; S.S. Gambhir (University of California, Los Angeles) for triple-reporter plasmids; and J. L. Salisbury (Mayo Clinic, Rochester, MN) for centrin antibody. Y.K. is a Champalimaud Investigator and was funded by grants from the Department of Defense, The American Cancer Society, The National Institutes of Health, and the New Jersey Commission on Cancer Research. X.L. is a recipient of a Harold W. Dodds Fellowship from Princeton University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE14244).
This article contains supporting information online at www.pnas.org/cgi/content/full/0900108106/DCSupplemental.
References
- 1.Chin L, Gray JW. Translating insights from the cancer genome into clinical practice. Nature. 2008;452:553–563. doi: 10.1038/nature06914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Fidler IJ. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 4.Kang Y, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 5.Minn AJ, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duelli D, Lazebnik Y. Cell fusion: A hidden enemy? Cancer Cell. 2003;3:445–448. doi: 10.1016/s1535-6108(03)00114-4. [DOI] [PubMed] [Google Scholar]
- 7.Pawelek JM, Chakraborty AK. Fusion of tumour cells with bone marrow-derived cells: A unifying explanation for metastasis. Nat Rev Cancer. 2008;8:377–386. doi: 10.1038/nrc2371. [DOI] [PubMed] [Google Scholar]
- 8.Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJA. The origin of the cancer stem cell: Current controversies and new insights. Nat Rev Cancer. 2005;5:899–904. doi: 10.1038/nrc1740. [DOI] [PubMed] [Google Scholar]
- 9.Larizza L, Schirrmacher V. Somatic cell fusion as a source of genetic rearrangement leading to metastatic variants. Cancer Metastasis Rev. 1984;3:193–222. doi: 10.1007/BF00048385. [DOI] [PubMed] [Google Scholar]
- 10.Fortuna MB, Dewey MJ, Furmanski P. Cell fusion in tumor development and progression: Occurrence of cell fusion in primary methylcholanthrene-induced tumorigenesis. Int J Cancer. 1989;44:731–737. doi: 10.1002/ijc.2910440430. [DOI] [PubMed] [Google Scholar]
- 11.Miller FR, McInerney D, Rogers C, Miller BE. Spontaneous fusion between metastatic mammary tumor subpopulations. J Cell Biochem. 1988;36:129–136. doi: 10.1002/jcb.240360204. [DOI] [PubMed] [Google Scholar]
- 12.Nigg EA. Centrosome aberrations: Cause or consequence of cancer progression? Nat Rev Cancer. 2002;2:815–825. doi: 10.1038/nrc924. [DOI] [PubMed] [Google Scholar]
- 13.Ogle BM, Cascalho M, Platt JL. Biological implications of cell fusion. Nat Rev Mol Cell Biol. 2005;6:567–575. doi: 10.1038/nrm1678. [DOI] [PubMed] [Google Scholar]
- 14.Singec I, Snyder EY. Inflammation as a matchmaker: Revisiting cell fusion. Nat Cell Biol. 2008;10:503–505. doi: 10.1038/ncb0508-503. [DOI] [PubMed] [Google Scholar]
- 15.Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: A hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- 16.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: Cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 17.Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: Expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 18.Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- 19.Terada N, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez-Dolado M, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes, and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 22.Cowan CA, Atienza J, Melton DA, Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science. 2005;309:1369–1373. doi: 10.1126/science.1116447. [DOI] [PubMed] [Google Scholar]
- 23.Silva J, Chambers I, Pollard S, Smith A. Nanog promotes transfer of pluripotency after cell fusion. Nature. 2006;441:997–1001. doi: 10.1038/nature04914. [DOI] [PubMed] [Google Scholar]
- 24.Johansson CB, et al. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat Cell Biol. 2008;10:575–583. doi: 10.1038/ncb1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nygren JM, et al. Myeloid and lymphoid contribution to non-haematopoietic lineages through irradiation-induced heterotypic cell fusion. Nat Cell Biol. 2008;10:584–592. doi: 10.1038/ncb1721. [DOI] [PubMed] [Google Scholar]
- 26.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duelli DM, et al. A virus causes cancer by inducing massive chromosomal instability through cell fusion. Curr Biol. 2007;17:431–437. doi: 10.1016/j.cub.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 28.Duelli D, Lazebnik Y. Cell-to-cell fusion as a link between viruses and cancer. Nat Rev Cancer. 2007;7(12):968–976. doi: 10.1038/nrc2272. [DOI] [PubMed] [Google Scholar]
- 29.Overholtzer M, et al. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell. 2007;131(5):966–979. doi: 10.1016/j.cell.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 30.Chakraborty A, et al. Donor DNA in a renal cell carcinoma metastasis from a bone marrow transplant recipient. Bone Marrow Transplant. 2004;34:183–186. doi: 10.1038/sj.bmt.1704547. [DOI] [PubMed] [Google Scholar]
- 31.Yilmaz Y, Lazova R, Qumsiyeh M, Cooper D, Pawelek J. Donor Y chromosome in renal carcinoma cells of a female BMT recipient: Visualization of putative BMT-tumor hybrids by FISH. Bone Marrow Transplant. 2005;35:1021–1024. doi: 10.1038/sj.bmt.1704939. [DOI] [PubMed] [Google Scholar]
- 32.Johnson RT, Rao PN. Mammalian cell fusion: Induction of premature chromosome condensation in interphase nuclei. Nature. 1970;226:717–722. doi: 10.1038/226717a0. [DOI] [PubMed] [Google Scholar]
- 33.Atkin NB. Premature chromosome condensation in carcinoma of the bladder: Presumptive evidence for fusion of normal and malignant cells. Cytogenet Cell Genet. 1979;23:217–219. doi: 10.1159/000131329. [DOI] [PubMed] [Google Scholar]
- 34.Kovacs G. Premature chromosome condensation: Evidence for in vivo cell fusion in human malignant tumours. Int J Cancer. 1985;36:637–641. doi: 10.1002/ijc.2910360602. [DOI] [PubMed] [Google Scholar]
- 35.Williams DM, Scott CD, Beck TM. Premature chromosome condensation in human leukemia. Blood. 1976;47:687–693. [PubMed] [Google Scholar]
- 36.Kang HS, Youn YK, Oh SK, Choe KJ, Noh DY. Flow cytometric analysis of primary tumors and their corresponding metastatic nodes in breast cancer. Breast Cancer Res Treat. 2000;63:81–87. doi: 10.1023/a:1006470614782. [DOI] [PubMed] [Google Scholar]
- 37.Isharwal S, et al. Prognostic value of Her-2/neu and DNA index for progression, metastasis and prostate cancer-specific death in men with long-term follow-up after radical prostatectomy. Int J Cancer. 2008;123:2636–2643. doi: 10.1002/ijc.23838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horn LC, Raptis G, Nenning H. DNA cytometric analysis of surgically treated squamous cell cancer of the uterine cervix, stage pT1b1-pT2b. Anal Quant Cytol Histol. 2002;24:23–29. [PubMed] [Google Scholar]
- 39.Gupta GP, Massagué J. Cancer metastasis: Building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Wei Y, Au JL-S. Role of tumor microenvironment in mediating chemoresistance. Cancer growth and progression. In: Meadows GG, editor. Integration/Interaction of Oncologic Growth. Vol 15. New York: Springer; 2005. pp. 285–321. [Google Scholar]
- 41.Stanbridge EJ, et al. Human cell hybrids: Analysis of transformation and tumorigenicity. Science. 1982;215:252–259. doi: 10.1126/science.7053574. [DOI] [PubMed] [Google Scholar]
- 42.Miller FR, Mohamed AN, McEachern D. Production of a more aggressive tumor cell variant by spontaneous fusion of two mouse tumor subpopulations. Cancer Res. 1989;49:4316–4321. [PubMed] [Google Scholar]
- 43.Brinkley BR. Managing the centrosome numbers game: From chaos to stability in cancer cell division. Trends Cell Biol. 2001;11:18–21. doi: 10.1016/s0962-8924(00)01872-9. [DOI] [PubMed] [Google Scholar]
- 44.Ring D, Hubble R, Kirschner M. Mitosis in a cell with multiple centrioles. J Cell Biol. 1982;94:549–556. doi: 10.1083/jcb.94.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ray P, De A, Min J-J, Tsien RY, Gambhir SS. Imaging tri-fusion multimodality reporter gene expression in living subjects. Cancer Res. 2004;64:1323–1330. doi: 10.1158/0008-5472.can-03-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ponomarev V, et al. A novel triple-modality reporter gene for whole-body fluorescent, bioluminescent, and nuclear noninvasive imaging. Eur J Nucl Med Mol Imaging. 2004;31:740–751. doi: 10.1007/s00259-003-1441-5. [DOI] [PubMed] [Google Scholar]
- 47.Bartos JD, et al. Genomic heterogeneity and instability in colorectal cancer: Spectral karyotyping, glutathione transferase-Ml and ras. Mutat Res. 2004;568:283–292. doi: 10.1016/j.mrfmmm.2004.06.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.