Abstract
Skeletal muscle is formed via fusion of myoblasts, a well-studied process in Drosophila. In vertebrates however, this process is less well understood, and whether there is evolutionary conservation with the proteins studied in flies is under investigation. Sticks and stones (Sns), a cell surface protein found on Drosophila myoblasts, has structural homology to nephrin. Nephrin is a protein expressed in kidney that is part of the filtration barrier formed by podocytes. No previous study has established any role for nephrin in skeletal muscle. We show, using two models, zebrafish and mice, that the absence of nephrin results in poorly developed muscles and incompletely fused myotubes, respectively. Although nephrin-knockout (nephrinKO) myoblasts exhibit prolonged activation of MAPK/ERK pathway during myogenic differentiation, expression of myogenin does not seem to be altered. Nevertheless, MAPK pathway blockade does not rescue myoblast fusion. Co-cultures of unaffected human fetal myoblasts with nephrinKO myoblasts or myotubes restore the formation of mature myotubes; however, the contribution of nephrinKO myoblasts is minimal. These studies suggest that nephrin plays a role in secondary fusion of myoblasts into nascent myotubes, thus establishing a possible functional conservation with Drosophila Sns.
Keywords: myoblast fusion, sticks and stones
Vertebrate skeletal muscle is a syncytium formed via two phases of myoblast fusion. During the first phase, myoblasts fuse to generate nascent myotubes that serve as scaffolds for further growth; in the second phase, more myocytes fuse into these nascent myotubes to form mature myotubes (1, 2). Myoblast fusion is a highly regulated process in Drosophila, where founder cells serve as the “seeds” in muscle formation and express the membrane protein Kirre, also known as Dumbfounded (Duf) (3, 4). Fusion-competent myoblasts (FCM) express the transmembrane protein Sticks and stones (Sns) (5) which interacts with Kirre/Duf (6), bringing the two muscle cell types together, to initiate a complex intra-cellular program that leads to the fusion of the FCM into the myotubes. There has been sparse evidence demonstrating whether the orthologues of these proteins are conserved in vertebrate myoblasts (7), although recent studies reported a role for Kirrel (Kirre-like) in developing zebrafish skeletal muscle (8).
Nephrin is a protein the function of which has been associated with maintenance of the kidney filtration barrier (9). Mutations in nephrin cause the congenital nephrotic syndrome of the Finnish type (10). Similarly, nephrin-knockout mice (nephrinKO) die at day 2 after birth of severe proteinuria (11–13). Despite the structural similarities with Drosophila Sns, nephrin expression or function in vertebrate skeletal muscle has not yet been reported. In the current study, nephrin was found expressed in developing mouse skeletal muscle and in human fetal muscle cells undergoing fusion. Nephrin expression is also up-regulated in the skeletal muscle of two murine models of human muscular dystrophies, when myogenic repair is needed. Downregulation of nephrin expression in developing zebrafish results in fish unable to swim with abnormal myosepta length. Accordingly, muscle cells isolated from nephrinKO mice exhibit a secondary fusion defect and are unable to form mature myotubes. NephrinKO myoblast cultures maintain MAPK/ERK pathway activity during differentiation, but this activation does not affect expression of myogenin, which appears normal. Pharmacological inhibition of the MAPK/ERK pathway does not restore normal myotube formation, suggesting that this pathway is likely not causative of the observed fusion deficiency. In cell mixing experiments, human myoblasts are able to fuse to nephrinKO nascent myotubes to form hybrid mature myotubes. Conversely, nephrinKO myoblasts provide little or no contribution to developing human myotubes. These studies suggest a new role for nephrin in skeletal muscle, where its expression appears to be necessary for mononucleated myoblasts to fuse into myotubes. These findings also highlight the possible functional conservation between nephrin and Drosophila Sns.
Results
Nephrin Is Expressed in Embryonic, Adult Diseased, or Adult Injured Muscle.
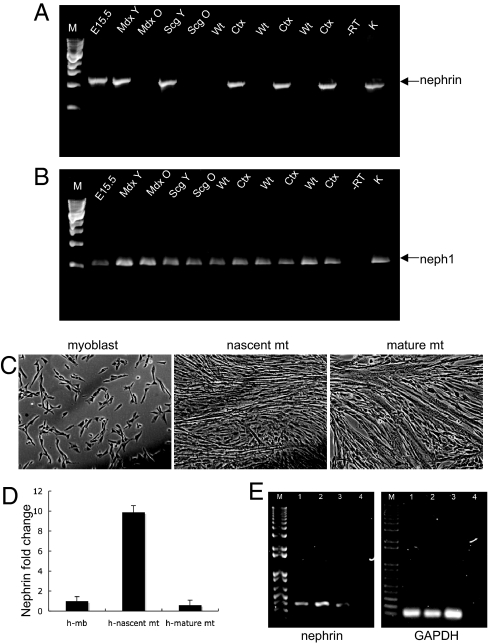
If nephrin is important for fusion of myoblasts into myotubes, its expression should be up-regulated when myoblast fusion is occurring. Indeed, nephrin message was present by nested reverse transcription–polymerase chain reaction (RT-PCR) in embryonic murine skeletal muscle (Fig. 1A, E15.5), in the skeletal muscle of young mice with muscular dystrophy, which exhibit spontaneous muscle degeneration/regeneration (14) (Fig. 1A, mdx Y (dystrophin null); Scg Y (δ-sarcoglycan-null), both 3–4 weeks old) and in wild-type muscle after acute injury with cardiotoxin (Fig. 1A, Ctx). No message was detected in uninjured adult wild-type mouse skeletal muscle (Fig. 1A, Wt), in 4-month old mdx (Fig. 1A, mdx O) or δ-sarcoglycan (Fig. 1A, Scg O) muscles. In multiple independent PCR reactions followed by sequence analysis, the full nephrin message, including most of exon 1a through the 3′UTR was confirmed to be the same transcript as that found in kidney (data not shown). Nephrin was also transiently up-regulated in human fetal skeletal myoblasts (isolated from 17-week-old fetus) undergoing fusion in culture for 2 days (Fig. 1 C–E), after which nephrin expression returned to the low baseline levels (Fig. 1D). Thus, nephrin expression is exquisitely controlled and is present when myoblast fusion is expected to occur. It was also confirmed that one nephrin-associated protein, Neph1 (kirrel), is ubiquitously present in all of the muscles tested, regardless of age, disease, or injury (Fig. 1B).
Fig. 1.
Nephrin mRNA is present in low quantities in vertebrate skeletal muscle. Nested RT-PCR was performed on RNA isolated from the skeletal muscles of different mice. The expected PCR products (1.2 kb) were gel purified, sequenced and confirmed to be nephrin. (A) RT-PCR product resulting from nested PCR, 1.2 kb (arrow). (B) Neph1, a protein associated with nephrin, is present throughout (expected product is 546 bp). Lanes: M, 1-kb markers; 1, embryonic skeletal muscle, E15.5; 2, mdx skeletal muscle, 3 weeks (Mdx Y); 3, mdx skeletal muscle 4 months old (Mdx O); 4, δ-sarcoglycan null 3.5 weeks (Scg Y); 5, δ-sarcoglycan null 1 year (Scg O); 6, 8, and 10, WT gastrocnemius, 3 months; 7, 9, and 11, contralateral WT gastrocnemius 5 days after cardiotoxin injection; 12, no RT; 13, kidney. (C) Human fetal myoblasts (17 weeks) at the indicated stages of fusion. (D) Transient up-regulation of nephrin mRNA in nascent myotubes as determined by real-time quantitative PCR analysis. The fold change value in the myoblasts was set as 1, and the fold change values in nascent and mature myotubes were plotted accordingly. GAPDH was used as a reference gene in the amplification. Mb: myoblasts, Mt: myotubes. (E) Agarose gels of end products of qRT-PCR. Lanes: M, 1kb plus markers; 1, myoblasts; 2, nascent myotubes; 3, mature myotubes; 4, negative control.
Downregulation of Nephrin Expression in Developing Zebrafish.
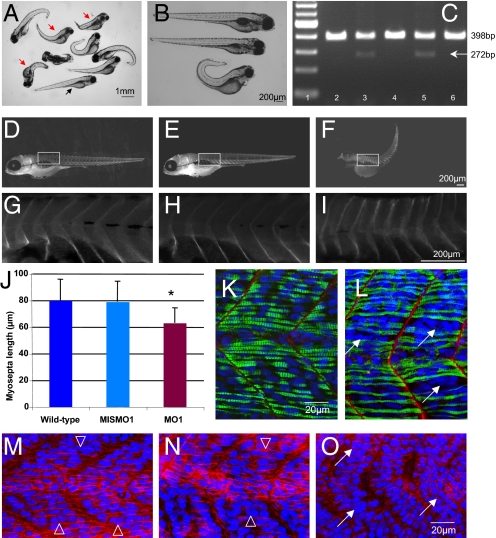
Zebrafish nephrin has been localized to the pronephros as in mammalian kidneys (15). Loss of nephrin by morpholino knockdown results in altered podocyte morphology and nephrosis at 96 hpf. Whether the muscles were affected was not indicated. Nephrin morpholino experiments were therefore conducted. Two morpholinos, one directed against nephrin (MO1) and a mismatched morpholino (MIS-MO1), were injected into fertilized eggs. The morpholino MO1 (kindly provided by I. Drummond) is directed against the transmembrane domain of nephrin and results in an in-frame deletion that prevents localization of nephrin to the membrane (15). The injected embryos were examined at 1 and 4 dpf (days postfertilization). Morpholino disruption of nephrin expression resulted in shorter, slightly curved embryos that were unable to swim properly and that sank to the bottom of the dish (Fig. 2 A and B). Analysis of 120 embryos at 1 dpf injected with the nephrin morpholino MO1 demonstrated that 55% of the embryos were curved, short, and poor swimmers, whereas no embryos displayed this phenotype when injected with the control MIS-MO1. At 4 dpf, 37% of the embryos injected with nephrin morpholino MO1 had died, and 10% were curved and short. The embryos injected with MIS-MO1 appeared to be normal (wild-type) (Fig. 2B). RT-PCR analysis confirmed the splicing defect in the embryos injected with the nephrin morpholino MO1 (Fig. 2C). To study whether the abnormal length of MO1-injected embryos was caused by shorter individual myosepta, the distances between myosepta was determined by microscopic analysis on whole-mount embryos at 96 hpf stained for alpha-dystroglycan (Fig. 2 D–I). Ten embryos each were analyzed for myosepta length in non-injected control, MIS-MO1, and nephrin MO1-injected embryos. Within each embryo, five myosepta were measured starting from the same anatomical position (Fig. 2 D–I, [supporting information (SI) Table S1]. Compared with MIS-MO1–injected embryos, the nephrin MO1-injected zebrafish displayed shorter myoseptum to myoseptum distance, consistent with a muscle defect (Fig. 2J; P < 4E-08 via t test). Comparison using a t test of the myoseptum to myoseptum distance in wild-type compared with MO1-injected embryos was also significant (P < 2.08E-08), whereas no significant difference was observed by comparing wild-type and MIS-MO1–injected embryos (P = 0.75). Finally, whole-mount control, MIS-MO1–injected, or MO1-injected zebrafish embryos were stained at 1dpf using antibodies directed to laminin and myosin heavy chain (MHC, F59) (Fig. 2 K and L) or with anti-β-catenin (Fig. 2 M–O). Wild-type (Fig. 2 K and M) and MIS-MO1-injected embryos (Fig. 2N) displayed regularly aligned nuclei (Fig. 2 M and N open arrowheads) and compacted myofibers (Fig. 2K). In contrast, zebrafish embryos injected with nephin morpholino MO1 exhibited a less organized structure in several (but not all) myosepta, with compacted clusters of nuclei (Fig. 2 L and O, arrows) that were not observed in wild-type or MIS-MO1–injected embryos. Therefore, downregulation of nephrin expression in developing zebrafish results in a phenotype consistent with muscle defects.
Fig. 2.
Nephrin morpholino experiments in zebrafish reveal smaller muscles. (A) Light micrograph of variable phenotypes noted with nephrin morpholino in zebrafish embryos at 4 days post fertilization (dpf). Many of the embryos appear curved and shorter (red arrows) compared with control (black arrow). (B) Light micrograph of wild-type (top) mismatched (middle) and nephrin morpholino embryos (MO1) at 4 dpf. (C) RT-PCR analysis of mismatched and nephrin morpholino-injected embryos confirms the expected splicing defect leading to an in-frame deletion of the transmembrane domain, rendering a protein no longer able to anchor at the membrane; this is manifest by a decrease in RT-PCR product size (from 398 bp to 272 bp). Lane 1, molecular weight markers; lane 2, uninjected wild-type embryo; lane 3, 1.25 ng nephrin morpholino MO1; lane 4, 1.25 ng mismatched morpholino; lane 5, 2.5 ng nephrin morpholino MO1; lane 6, 2.5 ng mismatched morpholino. (D-I) Morphometric analyses performed on whole-mount embryos stained with anti β-dystroglycan antibody confirm that the myosepta to myosepta distance of embryos injected with nephrin morpholino MO1 (F, I) are smaller than those injected with mismatched morpholino (E, H) or wild-type uninjected embryo (D, G). (J) Bar graph of myosepta length measurements from wild-type, mismatched MO1 and nephrin morpholino MO1 injected embryos. Asterisk denotes a statistically significant P value by t test (P ≤ 0.00000004). Individual measurements are listed in Table S1. Whole-mount control (K) and nephrin morpholino (L) embryos stained with anti-laminin (red) and anti-myosin heavy chain antibody (green); nuclei are stained in blue with DAPI. Nephrin morpholinos displayed shorter myosepta and presence of clustered nuclei (white arrows). (M-O) Whole-mount 1 dpf embryos are stained with anti-β-catenin antibody (red), and nuclei are stained in blue with DAPI. (M) Wild-type control; (N) control mismatched morpholino (MISMO1); (O) nephrin morpholino (MO1). Open arrows in (M) and (N) point to nuclei that appear regularly aligned, whereas arrows in (O) point to clustered nuclei.
Assessment of Myocyte Fusion in Nephrin-Knockout Myoblast Cultures.
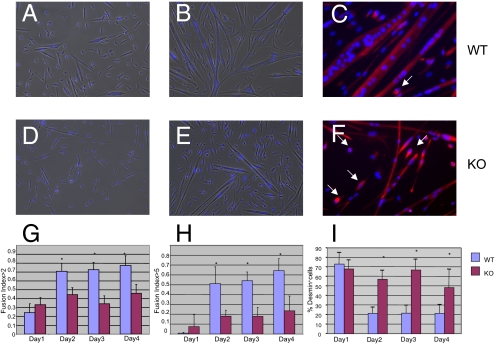
To assess whether nephrin plays a role in myoblast fusion and determine when its expression is necessary, we turned to a murine model. Methods for propagation and differentiation of muscle cells are well established for mouse compared with zebrafish tissue (16–19). Skeletal myoblasts were isolated from neonate nephrinKO and wild-type mice, as nephrinKO mice die within the first 2 days of life because of kidney failure (11). The fusion ability of nephrinKO and wild-type cultures were assessed in vitro. For each culture, equal numbers of mononuclear cells were plated, allowed to differentiate and the fusion indices (FI; number of nuclei in myotubes divided by total number of nuclei) were calculated over 4 successive days. Wild-type and nephrinKO myoblasts began to fuse from day 1 (Fig. 3 A and D), but by day 2 clear differences arose: more of the knockout myocytes remained mononuclear and the overall myotube size was small (Fig. 3 E and F), whereas myotubes in the wild-type cultures were branching and twitching (Fig. 3 B and C, twitching not shown). The nephrinKO myocytes had lower fusion indices (Fig. 3G) and were also inefficient in forming large myotubes (containing five or more nuclei) compared with wild-type myocytes (Fig. 3H). Immunohistochemistry with an antibody against desmin, a myogenic specific marker, demonstrated that the mononuclear cells in the knockout cultures were indeed myogenic, even if they were not fusing (Fig. 3F, arrows). Moreover, the number of desmin-positive mononuclear cells was significantly higher in knockout cultures compared with wild-type cultures at days 2, 3 and 4 in differentiation medium (Fig. 3I). These findings raised the question as to whether nephrinKO mononuclear cells had impaired differentiation potential because of persistent proliferative activity.
Fig. 3.
Myoblasts isolated from nephrinKO neonatal mice are unable to form mature myotubes. Light micrographs of wild-type (A, B) and knockout (D, E) myocytes after 1 day (A, D) and 4 days (B, E) in differentiation medium. Immunofluorescence staining for desmin of wild-type (C) and knockout (F) cultures demonstrates that the mononuclear cells in the nephrinKO cultures are myogenic (white arrows). (G, H) Quantification of the fusion indices for control and nephrinKO myoblasts. The fusion index (FI) is determined by dividing the number of nuclei found within myotubes by the total number of nuclei in a microscopic field (three to four microscopic fields per sample; three independent experiments). (G) Overall FI, counting nuclei in myotubes containing two or more nuclei. (H) FI for myotubes containing five or more nuclei. (I) Percentage of desmin-positive mononuclear cells decreases in wild-type as the FI increases, whereas it remains the same in the nephrinKO cultures. Asterisk indicates significant differences between wild-type (lilac) and knockout (crimson), P < 0.05.
NephrinKO Myocytes Have Constitutively Activated MAPK/ERK Pathway, but This Does Not Seem to Affect Myogenin Expression.
Wild-type and knockout myoblasts were plated at equal numbers and cultured under proliferation conditions. Cell numbers were counted daily for 4 days and although there was no significant difference during the first 2 days, nephrinKO cells subsequently grew much faster than wild-type cells (Fig. S1A). Samples from the cultures were immunostained at day 4 for desmin and the percentage of positive cells was similar in both cultures (Fig. S1B). Cell cycle analyses were also performed using flow cytometry, and control cultures were found to contain half the number of cells in S phase compared with nephrinKO cultures (Fig. S1 C and D).
Studies by others have suggested that the p42/44 (Erk1/Erk2) MAPK pathway is necessary for the activation and proliferation of satellite cells upon injury (20, 21). To address whether the decreased fusion ability of nephrinKO myoblasts could be caused by a persistent activation of Erk1/Erk2, wild-type and nephrinKO myoblasts differentiated cultures were analyzed by Western blot using antibodies directed against total and phospho-p42/44 MAPK (Fig. S1 E–G). Whereas the p42/44 MAPK pathway appeared nearly inactive during differentiation in control cultures, it was persistently active in the nephrinKO samples (Fig. S1E).
To address whether the constitutive activation of the MAPK pathway could result in slowed myogenic differentiation and, consequently, fusion of nephrinKO myocytes, wild-type and nephrinKO myoblast cultures were differentiated over the course of 4 days, and myogenin expression was monitored (Fig. S2). It was found that myogenin expression was similar in wild-type and nephrinKO cultures, suggesting that early myogenic differentiation is not altered by the persistent activation of MAPK/ERK in nephrinKO myoblasts. Next, the MAPK inhibitor PD98059 was applied during myoblast differentiation. Treatment of nephrinKO cells with 50 μM PD98059 markedly blocked the phosphorylation of ERK1/2; however nephrinKO myotubes treated with the inhibitor remained small (Fig. S3), suggesting that the persistent activation of the MAPK pathway is likely not associated with the fusion defect observed in nephrinKO myoblast cultures.
Nephrin Must Be Present on Myoblasts for Secondary Fusion to Occur.
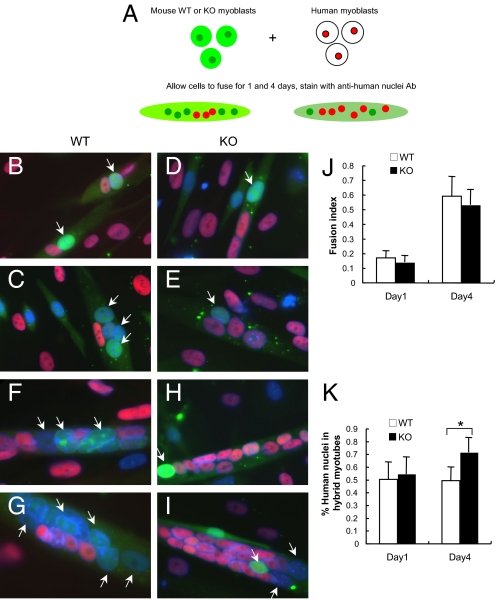
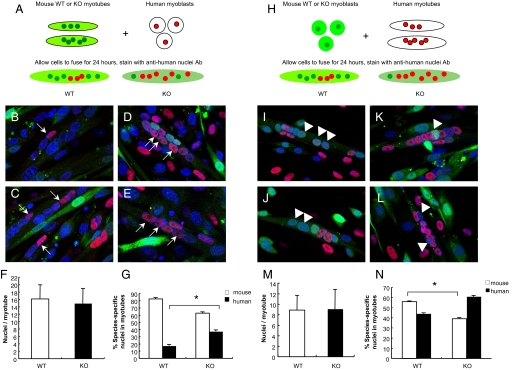
Studies in Drosophila suggest that Sns must be present in fusion-competent myoblasts (FCM), but not in nascent myotubes, for myoblast fusion to occur. Our initial studies did not clarify whether nephrin expression is required on mononuclear cells, nascent myotubes, or both, for myotubes to form. To address this question, coculture experiments were performed by mixing murine myoblasts (wild-type or nephrinKO) with human myoblasts. First, murine wild-type and nephrinKO myoblasts labeled with the green fluorescent dye CellTracker CMFDA (22, 23) were co-cultured with human myoblasts. Fusion was allowed to occur for 1 and 4 days, after which the presence of mouse- and human-derived nuclei to the formed myotubes was quantified (diagrammed in Fig. 4A and Fig. S4). Coculture of wild-type mouse myoblasts with human myoblasts resulted in hybrid myotubes containing a similar proportion of mouse and human-derived nuclei after 1 day (Fig. 4 B and C) and day 4 (Fig. 4 F, G, and J). In contrast, nephrinKO myoblasts initially formed hybrid myotubes with human cells 1 day after differentiation (Fig. 4 D, E, and K), but by day 4 the contribution of human-derived cells was more predominant (Fig. 4 H, I, and K). Thus, consistent with our previous findings, nascent myotube formation is not affected by nephrin depletion, whereas lack of nephrin expression results in little additional contribution of murine knockout myoblasts to hybrid myotubes.
Fig. 4.
NephrinKO myoblasts fuse poorly to human myoblasts compared with control mouse myoblasts. (A) Wild-type or nephrinKO myoblasts were labeled with a green fluorescent dye and mixed with human myoblasts for 1 and 4 days in DM. Myotubes were then fixed and stained with anti-human nuclei antibody (red). Nuclei were stained with DAPI. The myotubes with dual labeling were analyzed for contribution of human and mouse nuclei. Representative images showing the fusion of wild-type (B, C, F, G) or nephrinKO (D, E, H, I) myoblasts to human myoblasts after coculture for 1 day (B-E) and 4 days (F-I). The fusion index (number of fused nuclei, irrespective of their species/total number nuclei) (J), was similar at both days 1 and 4. The contribution of human versus mouse-derived nuclei to the myotubes was also assessed (K). At day 1, there was no statistical difference between the number of wild-type or nephrinKO mouse myoblasts that had fused to human myoblasts; however, at day 4, many more human-derived nuclei were detected within the myotubes of the nephrinKO:human myoblast hybrid cultures compared with wild-type mouse myoblasts:human myoblasts (P = 8.68204 E-11 by t test). White arrows in (B) through (I) point to nuclei of murine origin.
To address whether nephrin expression is necessary in nascent myotubes to allow fusion of myoblasts into them, wild-type or nephrinKO nascent myotubes were co-cultured with human mononuclear cells (Fig. 5A). Both co-cultures gave rise to hybrid myotubes (Fig. 5 B–E), demonstrating efficient cell fusion between murine and human myocytes. The number of total nuclei in the myotubes, regardless of their origin (murine or human), revealed no significant difference between wild-type and knockout co-cultures (Fig. 5F). However, there were more nuclei of human origin in knockout hybrid myotubes than in wild-type myotubes (37.4% vs. 17.5%, Fig. 5G). Thus, wild-type myotubes recruit both murine and human myoblasts, while nephrinKO myotubes recruit primarily human myoblasts. The converse experiment was also performed to confirm whether nephrin must be present on myoblasts to complete fusion (Fig. 5H). Here, wild-type (Fig. 5 I and J) or nephrinKO myoblasts (Fig. 5 K and L) were co-cultured with human nascent myotubes resulting in chimeric myotubes of similar size in both co-cultures (Fig. 5M). However, knockout myoblasts fused with human myotubes less efficiently. Only 39% of nuclei in knockout chimeric myotubes were from murine myotubes compared with 56% in wild-type chimeric myotubes (Fig. 5N), and these percentages were statistically significantly different by t test (P < 0.01). These findings support the conclusion that absence of nephrin in myoblasts results in inefficient formation of secondary (mature) myotubes.
Fig. 5.
Human fetal myoblasts are able to fuse into nephrinKO nascent myotubes, whereas nephrinKO myoblasts fuse less efficiently into nascent human myotubes as compared with wild-type murine myoblasts. (A) Schematic: wild-type or nephrinKO nascent myotubes were labeled with a green fluorescent dye and mixed with human mononuclear myoblasts for 24 hours in DM. Myotubes were then fixed and stained with anti-human nuclei antibody. Myotubes with dual labeling were analyzed for contribution of human and mouse nuclei. (B-E) Representative confocal images showing fusion of human myoblasts (red nuclei, white arrows) with wild-type (B, C) or nephrinKO (D, E) mouse myotubes (green). Nuclei were stained with DAPI (blue). (F) The total number of nuclei in myotubes, regardless of their origin (mouse or human), does not differ in wild-type and knockout myotubes co-cultures. (G) The percentage of human nuclei in myotubes with dual labels was calculated and was found to be significantly increased when human myoblasts were mixed with knockout nascent myotubes compared with wild-type nascent myotubes. *P < 0.01. (H) Schematic representation of mixing of prelabeled mouse myoblasts (wild-type or knockout) with human nascent myotubes. (I-L) Representative confocal images showing the fusion of murine myoblasts (green, white arrowheads) with human myotubes (red nuclei). Nuclei were stained with DAPI (blue). Coculture of wild-type (I, J) or nephrinKO (K, L) murine myoblasts with human nascent myotubes. The total number of nuclei in myotubes, regardless of their origin, does not differ in the co-cultures (M); however, the percentage of mouse nuclei in myotubes was significantly decreased when knockout myoblasts were mixed with human myotubes compared with wild-type myoblasts (N). *P < 0.01 via t test.
Discussion
Nephrin is a cell surface protein expressed in the kidney glomerulus at the epithelial podocytes. Podocytes interdigitate with one another to form a filtration barrier that allows water and ionic salts, but not proteins, to leave the bloodstream (9). Although nephrin function in the kidney is still largely unknown, it seems to be involved in the “outside-in” signaling that maintains the communication between podocyte foot processes and sustains the podocyte barrier function (24, 25). Despite structural similarities to Drosophila Sns, a cell-surface protein expressed by fusion-competent myoblasts, nephrin has been reported as “absent” in skeletal muscle (26). The results reported here indicate that process of myoblast fusion in Drosophila may be replicated by homologous players in mammalian cells.
Nephrin is expressed in skeletal muscle when cell fusion is occurring, during development and during injury or disease that require myofiber regeneration. Overall, the level of nephrin expression is low, and it is present in a narrow window of time. Nephrin is not detected in wild-type adult muscle, whereas it is present in murine embryonic muscle and in the muscle of young, but not old, mice with muscular dystrophy, which undergo a spontaneous phase of myofiber regeneration at ≈2–3 weeks of age (14). Myofiber regeneration requires fusion of mononuclear cells into nascent myofibers, supporting the need for nephrin expression. Consistently, young patients (≈1 year of age) affected by Duchenne Muscular Dystrophy (DMD), express higher levels of nephrin mRNA in their muscle than unaffected individuals or older DMD patients (27, 28). With age, both mice and humans with muscular dystrophy exhibit impaired muscle regeneration capacity accompanied by increased fibrosis, partly because of exhaustion of resident satellite cells (29–31). Therefore, a decline of nephrin expression in aged mice is consistent with the hypothesis of a decline in the number of ‘fusion-competent“ myoblasts in the chronic disease state.
Lack of nephrin in myogenic cells unveils a defect in recruitment of fusion-competent myoblasts to nascent myotubes. Myoblasts from nephrinKO mice are unable to form large myotubes and to maintain a constitutively active MAPK/ERK1/2 pathway, even when in differentiation medium. However, the activated MAPK/ERK1/2 pathway that has been associated with the activation of satellite cells (20, 21) does not seem to interfere with early differentiation of nephrinKO myoblasts or to be the cause of inefficient myoblast fusion. In support of this, it was found that expression of myogenin did not differ in nephrinKO cultures compared with control, and that pharmacological inhibition of MAPK/ERK pathway failed to rescue normal myotube formation in nephrinKO cultures. Our results suggest that nephrin may have a role in vertebrate myoblasts similar to Drosophila Sns. For secondary myotubes to form, nephrin must be present in the mononucleated myoblasts and not in the nascent myotubes. Conversely, human myoblasts fuse efficiently to nephrinKO nascent myotubes, suggesting that nephrin expression on nascent myotubes is not necessary for wild-type myoblasts to fuse.
Nephrin may act in concert with other transmembrane proteins with similar motifs likely to be important for vertebrate muscle fusion. For example, CDO and BOC are members of the Ig superfamily and have been found to regulate fusion (32–34). Mannose receptors have been shown to regulate myogenic motility preceding myoblast fusion (35). Whether these or other membrane-associated molecules interact with nephrin to result in myoblast fusion is currently unknown.
Nephrin in the vertebrate kidney is part of a complex network of proteins that are necessary for the structural and signaling integrity of the podocyte slit diaphragm (9). Future work must be done to determine other structural partners for nephrin in skeletal muscle and factors that modulate the expression of nephrin. Studies suggest that nephrin expression can be increased in the kidney by PPAR-γ agonists, such as pioglitazone, a drug for diabetes (36). Perhaps similar treatment with myoblasts can also increase nephrin expression and thereby increase myoblast fusion. Thus, with more knowledge of the regulation of nephrin in the skeletal muscle, it may become possible to improve the efficiency of cell-based therapies for skeletal muscle diseases such as Duchenne Muscular Dystrophy.
Materials and Methods
RNA Isolation and RT-PCR Amplification of Nephrin mRNA.
Total RNA was isolated from primary myocyte cultures using Qiagen RNeasy kit (Qiagen) and from gastrocnemius muscles of C57Bl6, mdx5cv, delta-sarcoglycan null mice as previously described (37). Detailed methods, including cardiotoxin injury, primer sequences, and RT/PCR amplification conditions can be found in the SI Materials and Methods.
Zebrafish Morpholino Experiments.
Zebrafish experiments were performed using previously described procedures (38), with 1.25 and 2.5 ng of nephrin morpholino or mismatched morpholino oligonucleotides. Morpholino sequences, RT-PCR, and whole-mount immunostaining analyses are detailed in SI Materials and Methods.
Primary Muscle Cell Culture and Fusion Experiments.
Primary muscle cultures were derived from the limb muscles of 1–2-day-old wild-type and nephrinKO neonates as described (18). Differentiation experiments were performed by plating the myocytes at a density of 8 × 104 cells/well in 12-well plates. Detailed information on culture conditions and determination of the fusion indices is described in SI Materials and Methods.
Cell Cycle Analysis.
Flow-cytometry analyses of the cell cycle were performed according to a previously described protocol (39). Additional details including Western blot analyses of ERK1/2 proteins can be found in SI Materials and Methods.
Co-Culturing Experiments of Murine and Human Myoblasts.
Murine myoblasts were stained with 5 μM CellTracker green CMFDA (Molecular Probes). Labeled mouse and human myoblasts were plated together in equal numbers and allowed to differentiate for 1 and 4 days in differentiation medium. Cells were then stained with anti-human nuclei antibody (Millipore, clone 235–1, MAB 1281). Additional information can be found in SI Materials and Methods.
Co-Cultures of Myoblasts with Nascent Myotubes.
To form nascent myotubes, mouse or human myoblasts at ≈70% confluency were switched to differentiation medium (DM) for 24 hours. Meanwhile, 20–30% confluent myoblasts were cultured in DM for 24 hours to produce differentiated, mononucleated cells. After 24 hours, murine myoblasts or myotubes were labeled with 5 μM CellTracker green CMFDA. Murine and human cells were trypsinized, plated in 12-well plates at equal cell numbers, and co-cultured for another 24 hours. Cells were then fixed, permeabilized, and stained with anti-human nuclei antibody (Millipore) as detailed in SI Materials and Methods.
Supplementary Material
Acknowledgments.
The authors thank Ian Drummond for the nephrin morpholino (MO1). The authors also thank the MRDDRC flow cytometry facility, the MRDDRC sequencing facility and MDDRC Imaging Core for confocal microscopy, all supported by a grant from the National Institute of Health (5P30HD018655). The authors express their gratitude to the members of the Gussoni and Kunkel laboratories for helpful discussions and critical review of this manuscript. This work was supported by funding from the Muscular Dystrophy Association to E.G. (MDA 3589 and MDA 4146), the National Institutes of Health (RO1NS047727 to E.G. and 5P50NS040828 to L.M.K.). L.M.K. is an Investigator supported by the Howard Hughes Medical Institute (HHMI). R.L.S. was supported by a Mentored Clinical Scientist Development Award from the National Heart, Lung, and Blood Institute (NIH-K08HLL04216). P.H. is supported by a Scientist Development Grant from American Heart Association (0730285N) and by the Joshua Frase Foundation. R.K. is supported by the NIH grant DK 55001. The authors have no conflicts of interest to declare.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904398106/DCSupplemental.
References
- 1.Horsley V, Pavlath GK. Forming a multinucleated cell: Molecules that regulate myoblast fusion. Cells Tissues Organs. 2004;176:67–78. doi: 10.1159/000075028. [DOI] [PubMed] [Google Scholar]
- 2.Richardson BE, Nowak SJ, Baylies MK. Myoblast fusion in fly and vertebrates: New genes, new processes and new perspectives. Traffic. 2008;9:1050–1059. doi: 10.1111/j.1600-0854.2008.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abmayr SM, Balagopalan L, Galletta BJ, Hong SJ. Cell and molecular biology of myoblast fusion. Int Rev Cytol. 2003;225:33–89. doi: 10.1016/s0074-7696(05)25002-7. [DOI] [PubMed] [Google Scholar]
- 4.Chen EH, Olson EN. Unveiling the mechanisms of cell-cell fusion. Science. 2005;308:369–373. doi: 10.1126/science.1104799. [DOI] [PubMed] [Google Scholar]
- 5.Bour BA, Chakravarti M, West JM, Abmayr SM. Drosophila SNS, a member of the immunoglobulin superfamily that is essential for myoblast fusion. Genes Dev. 2000;14:1498–1511. [PMC free article] [PubMed] [Google Scholar]
- 6.Galletta BJ, Chakravarti M, Banerjee R, Abmayr SM. SNS: Adhesive properties, localization requirements and ectodomain dependence in S2 cells and embryonic myoblasts. Mech Dev. 2004;121:1455–1468. doi: 10.1016/j.mod.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Krauss RS. Evolutionary conservation in myoblast fusion. Nat Genet. 2007;39:704–705. doi: 10.1038/ng0607-704. [DOI] [PubMed] [Google Scholar]
- 8.Srinivas BP, Woo J, Leong WY, Roy S. A conserved molecular pathway mediates myoblast fusion in insects and vertebrates. Nat Genet. 2007;39:781–786. doi: 10.1038/ng2055. [DOI] [PubMed] [Google Scholar]
- 9.Tryggvason K. Nephrin: Role in normal kidney and in disease. Adv Nephrol Necker Hosp. 2001;31:221–234. [PubMed] [Google Scholar]
- 10.Kestila M, et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 11.Hamano Y, et al. Determinants of vascular permeability in the kidney glomerulus. J Biol Chem. 2002;277:31154–31162. doi: 10.1074/jbc.M204806200. [DOI] [PubMed] [Google Scholar]
- 12.Putaala H, et al. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: Inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet. 2001;10:1–8. doi: 10.1093/hmg/10.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Rantanen M, et al. Nephrin TRAP mice lack slit diaphragms and show fibrotic glomeruli and cystic tubular lesions. J Am Soc Nephrol. 2002;13:1586–1594. doi: 10.1097/01.asn.0000016142.29721.22. [DOI] [PubMed] [Google Scholar]
- 14.Watchko JF, O'Day TL, Hoffman EP. Functional characteristics of dystrophic skeletal muscle: Insights from animal models. J Appl Physiol. 2002;93:407–417. doi: 10.1152/japplphysiol.01242.2001. [DOI] [PubMed] [Google Scholar]
- 15.Kramer-Zucker AG, Wiessner S, Jensen AM, Drummond IA. Organization of the pronephric filtration apparatus in zebrafish requires Nephrin, Podocin and the FERM domain protein Mosaic eyes. Dev Biol. 2005;285:316–329. doi: 10.1016/j.ydbio.2005.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- 17.Yablonka-Reuveni Z, et al. The transition from proliferation to differentiation is delayed in satellite cells from mice lacking MyoD. Dev Biol. 1999;210:440–455. doi: 10.1006/dbio.1999.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neville C, et al. Skeletal muscle cultures. Methods Cell Biol. 1997;52:85–116. [PubMed] [Google Scholar]
- 20.Shefer G, et al. Skeletal muscle cell activation by low-energy laser irradiation: A role for the MAPK/ERK pathway. J Cell Physiol. 2001;187:73–80. doi: 10.1002/1097-4652(2001)9999:9999<::AID-JCP1053>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Jones NC, et al. The p38alpha/beta MAPK functions as a molecular switch to activate the quiescent satellite cell. J Cell Biol. 2005;169:105–116. doi: 10.1083/jcb.200408066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munoz-Barroso I, et al. Dilation of the human immunodeficiency virus-1 envelope glycoprotein fusion pore revealed by the inhibitory action of a synthetic peptide from gp41. J Cell Biol. 1998;140:315–323. doi: 10.1083/jcb.140.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaroszeski MJ, Gilbert R, Heller R. Detection and quantitation of cell-cell electrofusion products by flow cytometry. Anal Biochem. 1994;216:271–275. doi: 10.1006/abio.1994.1041. [DOI] [PubMed] [Google Scholar]
- 24.Lehtonen S. Connecting the interpodocyte slit diaphragm and actin dynamics: Emerging role for the nephrin signaling complex. Kidney Int. 2008;73:903–905. doi: 10.1038/ki.2008.69. [DOI] [PubMed] [Google Scholar]
- 25.Uchida K, et al. Decreased tyrosine phosphorylation of nephrin in rat and human nephrosis. Kidney Int. 2008;73:926–932. doi: 10.1038/ki.2008.19. [DOI] [PubMed] [Google Scholar]
- 26.Kuusniemi AM, et al. Tissue expression of nephrin in human and pig. Pediatr Res. 2004;55:774–781. doi: 10.1203/01.PDR.0000117842.10241.2C. [DOI] [PubMed] [Google Scholar]
- 27.Haslett JN, et al. Gene expression comparison of biopsies from Duchenne muscular dystrophy (DMD) and normal skeletal muscle. Proc Natl Acad Sci USA. 2002;99:15000–15005. doi: 10.1073/pnas.192571199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haslett JN, et al. Gene expression profiling of Duchenne muscular dystrophy skeletal muscle. Neurogenetics. 2003;4:163–171. doi: 10.1007/s10048-003-0148-x. [DOI] [PubMed] [Google Scholar]
- 29.Yablonka-Reuveni Z, Anderson JE. Satellite cells from dystrophic (mdx) mice display accelerated differentiation in primary cultures and in isolated myofibers. Dev Dyn. 2006;235:203–212. doi: 10.1002/dvdy.20602. [DOI] [PubMed] [Google Scholar]
- 30.Webster C, Blau HM. Accelerated age-related decline in replicative life-span of Duchenne Muscular Dystrophy myoblasts: Implications for cell and gene therapy. Somatic Cell Molec Genet. 1990;16:557–565. doi: 10.1007/BF01233096. [DOI] [PubMed] [Google Scholar]
- 31.Pagel CN, Partridge TA. Covert persistence of mdx mouse myopathy is revealed by acute and chronic effects of irradiation. J Neurol Sci. 1999;164:103–116. doi: 10.1016/s0022-510x(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 32.Kang JS, et al. BOC, an Ig superfamily member, associates with CDO to positively regulate myogenic differentiation. EMBO J. 2002;21:114–124. doi: 10.1093/emboj/21.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wegorzewska M, Krauss RS, Kang JS. Overexpression of the immunoglobulin superfamily members CDO and BOC enhances differentiation of the human rhabdomyosarcoma cell line RD. Mol Carcinog. 2003;37:1–4. doi: 10.1002/mc.10121. [DOI] [PubMed] [Google Scholar]
- 34.Cole F, et al. Positive regulation of myogenic bHLH factors and skeletal muscle development by the cell surface receptor CDO. Dev Cell. 2004;7:843–854. doi: 10.1016/j.devcel.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Jansen KM, Pavlath GK. Mannose receptor regulates myoblast motility and muscle growth. J Cell Biol. 2006;174:403–413. doi: 10.1083/jcb.200601102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benigni A, et al. Transcriptional regulation of nephrin gene by peroxisome proliferator-activated receptor-gamma agonist: Molecular mechanism of the antiproteinuric effect of pioglitazone. J Am Soc Nephrol. 2006;17:1624–1632. doi: 10.1681/ASN.2005090983. [DOI] [PubMed] [Google Scholar]
- 37.Eisenberg I, et al. Distinctive patterns of microRNA expression in primary muscular disorders. Proc Natl Acad Sci USA. 2007;104:17016–17021. doi: 10.1073/pnas.0708115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guyon JR, et al. The dystrophin associated protein complex in zebrafish. Hum Mol Genet. 2003;12:601–615. [PubMed] [Google Scholar]
- 39.Darzynkiewicz Z, Juan G. In: Current Protocols in Cytometry. Robinson J., editor. Vol. 1. New York: Wiley; 1997. pp. 7.5.1–7.5.24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.