Abstract
Androgen receptor (AR) signaling regulates the development and homeostasis of male reproductive organs, including the prostate. Deregulation of AR and AR coregulators, expression, or activity is involved in the initiation of prostate cancer and contributes to the transition of the disease to hormone-refractory stage. The ubiquitous βArrestin proteins are now recognized as bona fide adapters and signal transducers with target effectors found in both the cytosol and nucleus. Here, we provide evidence that βArrestin2 forms a complex with AR and acts as an AR corepressor in androgen-dependent prostate cancer cells. Accordingly, the forced overexpression of βArrestin2 diminishes, and knockdown of βArrestin2 expression with RNAi increases the androgen-induced prostate-specific antigen (PSA) gene expression. βArrestin2 serves as an adapter, bringing into close proximity the Mdm2 E3 ligase and AR, thereby promoting AR ubiquitylation and degradation. Human prostate tissues evidence an inverse relationship between the expression of βArrestin2 and AR activity: glands that express high levels of βArrestin2 exhibit low expression of PSA, and those glands that express low levels of βArrestin2 evidence elevated PSA levels. We conclude that βArrestin2 acts as a corepressor of AR by serving as a scaffold for Mdm2 leading to the AR ubiquitylation and degradation.
Keywords: beta-arrestin, ubiquitin, Mdm2, testosterone, androgen deprivation therapy
Prostate cancer accounts for approximately one-third of all male cancer cases in the United States, and 186,320 cases were diagnosed in 2008 (1). The cancer often presents as an androgen-dependent (AD), hormone-sensitive disease that can be successfully managed with targeted therapies that aim to inhibit function of the androgen receptor (AR). Although these therapies are initially effective, a significant portion of the cancer patients develop advanced androgen-independent (AI), hormone-refractory disease (2). Not surprising then, effective management of prostate cancer remains elusive and an estimated 28,660 American lives were claimed by the disease in 2008 alone (1). The deregulation of expression and activity of AR, and AR-interacting partners, is thought to be involved in the progression of the prostate cancer to advanced disease (3, 4).
The AR is a member of the nuclear hormone receptor superfamily of ligand-controlled transcription factors, and it regulates expression of multiple genes that are involved in the normal development and malignant transformation of the prostate (4–6). Recent evidence suggests that the AR also participates in the transition of the prostate cancer to AI disease (7). Indeed, approximately one-third of AI prostate carcinomas show amplification and overexpression of the wild-type AR, suggesting it adjusts to capture the low levels of circulating androgen (8, 9). In another one-third of AI cancer, the AR is mutated allowing it to become activated by other steroids or even anti-androgens (2, 10). In the remaining one-third of AI prostate cancers, no discernable AR mutations or amplifications are detected, suggesting existence of additional AR-regulatory mechanisms. The AR protein undergoes several types of post-translational modifications, including phosphorylation (11), acetylation (12), SUMOylation (13), and ubiquitylation (14). However, the functional consequences of these AR protein alterations vis-á-vis prostate cancer initiation and/or progression, and the mechanisms involved remain to be established.
βArrestins are cytosolic adapter proteins that were originally discovered based on their character to mediate the agonist-dependent G protein-coupled receptor (GPCR) desensitization (15). Now, however, the βArrestins are acknowledged as regulators of agonist-induced GPCR (as well as other types of cell surface receptors) internalization (16), and to act as bona fide adapters and signal transducers (17). Exemplar ubiquitous βArrestin2 mediates internalization of the β2-adrenergic receptor by serving multiple functions, including the binding to endocytic proteins such as clathrin and AP2 (18, 19) and E3 ligases (20, 21) that ubiquitylate both the βArrestin2 itself and β2-adrenergic receptor. Hence, βArrestin2 serves as an adapter, bringing into close proximity E3 ligases and their receptor substrates. Here, we hypothesized that βArrestins interact with and regulate the AR function by a ubiquitylation-dependent mechanism. Accordingly, we present data to demonstrate that βArrestin2 regulates the AR transcriptional activity by forming a trimeric complex containing AR, βArrestin2, and the E3 ligase Mdm2, leading to AR ubiquitylation and down-regulation.
Results
βArrestin2 Forms a Complex with AR.
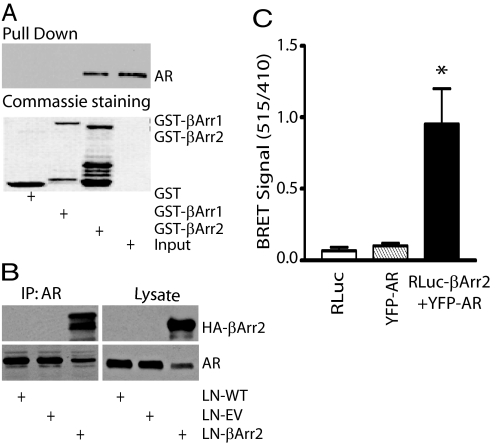
G protein-coupled receptors and their downstream effectors regulate prostate cancer initiation and progression (5, 6), and existing evidence implicates the GPCR effector βArrestin proteins as regulators of cell proliferation and survival (17). We hypothesized that the functionally versatile βArrestins impact prostate cancer by interacting with partner proteins (22) that regulate specific mitogenic signaling networks. To address this possibility, we used in vitro pull-down assays with purified GST-βArrestin1 and GST-βArrestin2 proteins conjugated to agarose beads together with LNCaP (used as a model for AD prostate cancer) cell extracts. Protein complexes were resolved by SDS/PAGE, followed by the staining of gel with Commassie blue. We observed that GST-βArrestin2, but not GST-βArrestin1, precipitated with it a protein that migrated on SDS/PAGE with an apparent molecular mass of ≈100 kDa. Because LNCaP cells express AR, and the AR has a calculated molecular mass of 110 kDa, we queried whether the βArrestin2-binding partner was indeed AR. The pull-down assay was repeated and protein complexes were fractionated on SDS-PAGE, transferred to nitrocellulose filter and subjected to Western blot analysis using anti-AR antibodies. Fig. 1A shows the selective pull-down of AR with GST-βArrestin2, but not GST-βArrestin1.
Fig. 1.
AR forms a complex with βArrestin2. (A) AR binding to βArrestin1 and βArrestin2. Purified GST, GST-βArr1, or GST-βArr2 proteins were incubated with LNCaP cell lysate in the presence of glutathione-Sepharose beads. Protein-bead complexes were washed and analyzed for AR binding by Western blot analysis (Upper) using anti-AR antibodies. Lower, Coomassie blue stain to show purified proteins. Input lane contained 1% of the total cell lysate. (B) Coimmunoprecipitation of AR and βArrestin2. LNCaP lysates were subjected to immunoprecipitation with anti-AR antibodies and immunoblotting with anti-HA IgG to detect βArrestin2 (Upper). The filter was stripped of IgG and reprobed with anti-AR antibodies to show the AR immunoprecipitation (Lower). Whole cell lysates were probed with anti-HA (Upper) and anti-AR (Lower) antibodies to demonstrate the total protein expression. LN-WT, wild-type LNCaP; LN-EV, empty vector-expressing LNCaP; LN-βArr2, βArrestin2-overexpressing LNCaP. (C) Specific BRET signal between Renilla luciferase-βArrestin2 (RLuc-βArr2) and YFP-AR. HEK293 cells were cotransfected with cDNAs encoding RLuc-βArr2 (5 μg) alone, YFP-AR (5 μg) alone, or in combination. The data shown represent average of results obtained from 2 experiments performed in triplicate (*, P < 0.05 versus control).
We used 2 distinct approaches to confirm existence of the βArrestin2-AR complexes: protein coimmunoprecipitation and bioluminescent resonance energy transfer (BRET) assays. Coimmunoprecipitation of ectopically expressed βArrestin2 and AR in HEK293 (supporting information (SI) Fig. S1) and endogenously expressed AR in LNCaP (Fig. 1B) cells evidenced the complex formation between AR and βArrestin2. Direct contact between the AR and βArrestin2 was further examined by BRET. Yellow fluorescent protein (YFP)-tagged AR and Renilla luciferase-tagged βArrestin2 were coexpressed in the HEK293 cells, and results demonstrate the association between AR and βArrestin2 (Fig. 1C).
Regulation of AR Function by βArrestin2.
To assess the potential effect of βArrestin2 binding on the AR function, we first established the βArrestin2 expression in AD LNCaP cells. Results show that the LNCaP cells expressed less βArrestin2 gene (Fig. S2A) and protein (Fig. S2B) levels, compared with the benign prostate RWPE1 cells. We also found that the LNCaP cells expressed less βArrestin2 protein than the AI PC3 or DU145 cells, suggesting βArrestin2 may exert a specific regulatory function in AR-expressing prostate cells. To address this possibility, we established a polyclonal LNCaP cell line that stably overexpresses βArrestin2 (Fig. S2C). The overexpression of βArrestin2 did not affect the morphology of cells grown in culture medium containing normal or charcoal-stripped serum. We attempted, but repeatedly failed, to generate LNCaP cell lines with (stably) reduced βArrestin2 expression.
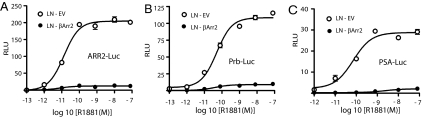
The AR plays a critical role in the initiation and progression of prostate cancer (2–5), and we examined the effect of βArrestin2 on the AR activation using LNCaP cells stably expressing empty vector (LN-EV) or HA-βArrestin2 (LN-βArr2) that were transiently transfected with cDNAs encoding AR response element (ARE)-regulated reporters fused to luciferase gene (i.e., PSA-Luc, ARR2-Luc, or Prb-Luc) together with an SV40-driven Renilla luciferase (SV40-RLuc) reporter. By using the 3 distinct reporters, we observed an androgen dose-dependent increase in the AR activity in LN-EV cells, with an EC50 ≈10−10 M and a maximal response of 10−9 M (Fig. 2 A–C). Forced overexpression of βArrestin1 had minimal effect on the androgen-mediated activation of AR, consistent with the observation that it does not form a complex with the AR (Fig. 1A). Distinctly, the overexpression of βArrestin2 significantly attenuated the potency and efficacy of the androgen-induced AR reporter activation (Fig. 2 A–C). We also examined the effect of βArrestin2 overexpression on basal AR function using the ARE-Luc reporters, and the results show that LN-βArr2 cells have significantly less basal AR activity, compared with the LN-EV control cells (Fig. S3 A–C).
Fig. 2.
Effect of βArrestin2 expression on AR activity. LN-EV or LN-βArr2 cells were cotransfected with firefly luciferase genes driven by AREs derived from probasin (ARR2 and Prb) or PSA promoters, and Renilla luciferase driven by the SV40 promoter. Cells were incubated in starvation medium, and treated with increasing concentrations of R1881 (10−13 to 10−7 M) for 24 h and reporter activities were determined using commercially available kits from Promega. Relative luciferase units were calculated, and each point represents mean ± SD. of normalized luciferase activities obtained from 3 experiments performed in triplicate.
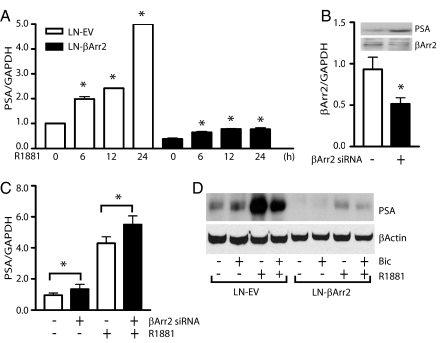
The expression of endogenous PSA gene is controlled by ligand-activated AR, and we compared levels of PSA gene expression in control LN-EV and LN-βArr2 cells. Real-time PCR analysis showed the LN-EV cells express more PSA gene than the LN-βArr2 cells (Fig. 3A), mirroring the AR reporter assay results (Fig. 2). To support the conclusion that βArrestin2 impacts AR function, we used siRNA to knockdown endogenous βArrestin2 expression in the LNCaP cells (Fig. 3B) and determined the basal and androgen-regulated PSA gene and protein levels. The results show increased basal and androgen-induced levels of the PSA gene (Fig. 3C) and protein (Fig. 3B, inset) in cells expressing reduced levels of βArrestin2, compared with the control siRNA-transfected cells. We confirmed the effect of βArrestin2 on PSA protein expression using Western blot (Fig. 3D) and immunocytochemical (Fig. S4) assays. In both cases, we found that the stimulation of LN-EV cells with androgen markedly increased the PSA protein expression, which was inhibited by the anti-androgen bicalutamide (Figs. 3D and S4). The similar treatments of the LN-βArr2 cells did not equally increase the PSA expression (Figs. 3D and S4), and showed that βArrestin2 acts as a corepressor of the AR signaling.
Fig. 3.
Regulation of AR function by βArrestin2. (A) Forced overexpression of βArrestin2 inhibits the PSA gene expression. Total RNA was isolated from LN-EV or LN-βArr2 cells treated, or not, with R1881 (1 nM) for the indicated times, and equal amount of cDNA was subjected to real-time PCR amplification to quantitate expression of PSA and GAPDH genes (*, P < 0.05 versus untreated cells). (B and C) The knockdown of endogenous βArrestin2 expression increases PSA levels. LNCaP cells were transfected with SMARTpool siRNA targeting βArrestin2 gene or scrambled siRNA control. Messenger RNA was isolated and used to synthesize cDNA, and equal amount of the cDNA was used to measure the PSA gene expression with real-time PCR (*, P < 0.05 versus siRNA scrambled-transfected control cells). Inset in B shows the βArrestin2 and PSA proteins expression as determined by immunoblotting. The graphs depicted in A–C represent values normalized to GAPDH level. (D) βArrestin2 attenuates the androgen-regulated PSA protein expression. LN-EV or LN-βArr2 cells were grown in phenol red-free medium in the presence or absence of bicalutamide (10 μM) for 12 h, followed by the addition of R1881 (1 nM) for 24 h. Cell monolayers were lysed and lysates were subjected to Western blot analysis using anti-PSA IgG (Upper). The filter was stripped of IgG and reblotted with anti-βActin antibodies to show the equal protein loading (Lower). Bic, bicalutamide.
βArrestin2-Dependent AR Expression and Subcellular Localization.
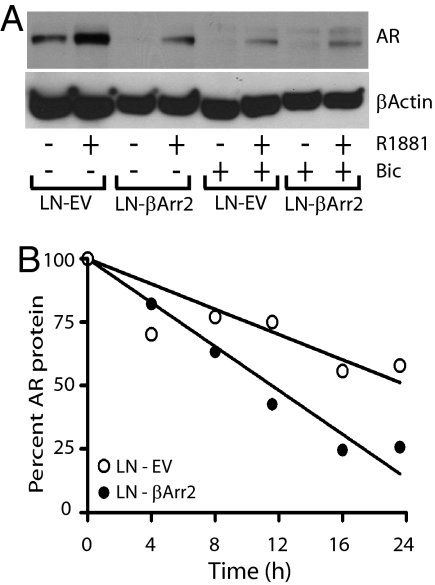
To establish just how βArrestin2 affects the PSA expression (i.e., AR function), we compared the basal and androgen-controlled endogenous AR levels in the LN-EV and LN-βArr2 cells. Appropriately treated cell monolayers were lysed, and AR expression was determined by immunoblotting. Treatment of control cells with R1881 increased the AR expression, and pretreatment with bicalutamide inhibited this R1881 effect (Figs. 4A and S5). Notably, LN-βArr2 cells expressed less basal amounts of the AR protein, and their treatment with R1881 only partially increased the AR expression level (Figs. 4A and S5), showing again that βArrestin2 exerts a corepressor effect on AR.
Fig. 4.
Effect of βArrestin2 on AR expression. Cells were grown in phenol red-free medium and were pretreated, or not, with bicalutamide (10 μM) for 12 h, followed by exposure to R1881 (1 nM) for 24 h. Cell monolayers were lysed and subjected to Western blot analysis to determine expression of AR using anti-AR IgG (Upper). The filter was stripped of IgG and reblotted with anti-βActin antibody (Lower) to show equal protein loading. (B) βArrestin2 regulates AR stability. Asynchronous cells were treated with cycloheximide (50 μg/mL) and at indicated time points, cells were collected and AR protein expression was analyzed by Western blot analysis. The blot was stripped of IgG and reblotted with actin antibodies. The levels of AR were determined by densitometric analysis and normalized to actin levels in each sample. The AR protein levels at each time point, in both LN-EV and LN-βArr2 cells, were quantified and calculated as percentage of the initial normalized AR level (time 0 h). The AR half-lives were calculated using a single linear regression curve fit (Prism 4, GraphPad Software).
Agonist-controlled AR activation requires its translocation to the nucleus and binding to AREs in the promoter region of substrate genes, and we used confocal immunofluorescence microscopy to determine effect of βArrestin2 on the AR localization. Treatment of control LN-EV cells with R1881 induced the AR nuclear accumulation, and that was inhibited when cells were pretreated with bicalutamide (Fig. S6). However, the similar treatment of LN-βArr2 cells evidenced a decrease in nuclear AR expression (Fig. S6), providing possible explanation for the reduced androgen-induced PSA expression in these cells. Also, the LN-βArr2 cells generally expressed less AR protein, compared with the LN-EV cells both at the basal level and after R1881 treatment (Figs. 4A and S6), suggestive of the idea that βArrestin2 may control AR function by regulating stability of the AR protein. Accordingly, LN-EV and LN-βArr2 cells were treated with cycloheximide to inhibit protein synthesis, and AR protein turnover was analyzed over time. Compared with the control LN-EV cells, AR half-life was considerably reduced in the LN-βArr2 cells (Fig. 4B).
Proteasomal AR Degradation via βArrestin2.
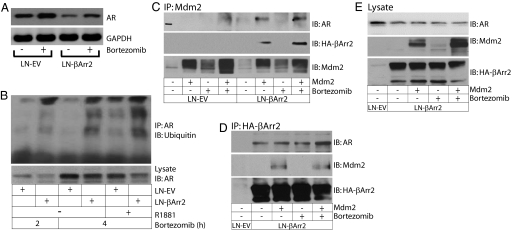
Emerging evidence indicates the targeting of AR for degradation via the ubiquitin-proteasome pathway is mediated in large part by the RING finger-containing E3 ligases (23, 24). We used bortezomib, a proteasome inhibitor, as a tool to investigate possible mechanisms involved in the βArrestin2-dependent AR degradation. The treatment of control LN-EV cells with bortezomib increased accumulation of AR protein (Fig. 5A), suggesting the constitutive AR degradation. Similarly, the decrease in AR protein levels (caused by the forced overexpression of βArrestin2) was reversed upon treatment with bortezomib (Fig. 5A), implying βArrestin2 causes the AR degradation in the proteasome.
Fig. 5.
βArrestin2 promotes the Mdm2-mediated AR ubiquitlylation. (A) LNCaP cells were cultured for 24 h in growth media and treated, or not, with bortezomib (100 nM) for 4 h. Monolayers were lysed and lysates were subjected to AR expression analysis by immunoblotting (Upper). The filter was stripped of IgG and reblotted with anti-GAPDH antibody (Lower) to show the equal protein loading. (B) AR is a target for βArrestin2-dependent ubiquitylation. Cells were pretreated with bortezomib (100 nM) for 2 or 4 h, followed by treatment with R1881 (1 nM), or not, for additional 2 h. Cell lysates were subjected to immunoprecipitation with anti-AR antibody and ubiquitylated species were detected by immunoblotting with anti-ubiquitin antibody. Cell lysates were probed with anti-AR (Lower) IgG to demonstrate the total protein expression. (C–E) Mdm2 forms a complex with βArrestin2 and AR. LNCaP cells were transfected with a cDNA encoding Mdm2 and were pretreated, or not, with bortezomib (100 nM) for 4 h in growth media. Cell lysates were subjected to immunoprecipitation using anti-Mdm2 (C) or anti-HA (D) antibodies and immunoblotted with the indicated anti-AR, anti-HA, and anti-Mdm2 IgGs. (E) Cell lysates (from experiment shown in D) were immunoblotted with indicated IgG to show total expression of the AR, Mdm2, and βArrestin2.
Ubiquitylation is a mechanism to target proteins for proteasomal degradation, and experimental evidence shows that mono-ubiquitylation determines the protein trafficking, whereas poly ubiquitylation leads to proteasomal degradation (25). We tested involvement of βArrestin2 in the possible AR ubiquitylation using control LN-EV and LN-βArr2 cells. The cells were treated with bortezomib in the presence or absence of androgen, and lysates were subjected to AR immunoprecipitation followed by immunoblotting with anti-polyubiquitin antibody. The treatment with bortezomib precipitated a substantial increase in the intensity of high molecular mass smear-like bands in the LN-βArr2, but much less in the LN-EV cells (Fig. 5B). Furthermore, in the presence of bortezomib, the ubiquitylation signal was markedly enhanced after hormone treatment compared with the nonstimulated control LN-EV cells (Fig. 5B), consistent with a previous report (26).
βArrestin2 Scaffolds Mdm2 to AR.
The βArrestins scaffold the Mdm2 E3 ubiquitin ligase to facilitate proteasomal degradation of IGF1 receptor (27), and there is a report indicating that AR undergoes the ubiquitylation modification via Mdm2 (14). We examined whether βΑrrestin2 serves as an adapter to bring into close proximity the Mdm2 and AR. Immunoprecipitation of Mdm2 from protein extracts derived from LN-EV and LN-βArr2 cells evidenced the complex formation between βArrestin2, Mdm2, and AR (Fig. 5C). The Mdm2-AR complex was observed in LN-βArr2 but not in LN-EV cells overexpressing the Mdm2 alone (Fig. 5C), perhaps because of limited protein expression and/or assay sensitivity. Nonetheless, the Mdm2-AR complex was observed in both LN-EV and LN-βArr2 cells that were treated with bortezomib (Fig. 5C). It is reasonable to suggest that because LN-EV cells contain low levels of βArrestin2 (Fig. S2), the sensitivity of the protein complex detection will dramatically increase when proteasomal protein degradation is prevented. In contrast, LN-βArr2 cells have sufficient levels of the βArrestin2 protein to allow the detectable complex formation, even in the absence of bortezomib (Fig. 5C). To confirm the binding of βArrestin2 to Mdm2, we adapted a similar approach: immunoprecipitation of βArrestin2 revealed the binding to both Mdm2 and AR (Fig. 5D). These results suggest that βArrestin2 serves as an adapter that brings together the Mdm2 and AR leading to the AR ubiquitylation.
To add support to the role of βArrestin2 in the Mdm2-mediated AR ubiquitylation, we analyzed its possible influence on the AR degradation. The immunoprecipitation of AR from protein extracts obtained from LN-EV and LN-βArr2 cells revealed an enhanced accumulation of ubiquitylated proteins in the presence of Mdm2 and bortezomib (Fig. S7A). There was an increased ubiquitylation signal in LN-βArr2 compared with LN-EV cells, suggesting the increased expression of βArrestin2 enhances the ubiquitylation and degradation of AR (Fig. S7B). To gain confidence in the conclusion that βArrestin2 is involved in the Mdm2-mediated AR ubiquitylation, we knocked down expression of endogenous βArrestin2 with siRNA (Fig. S7C). The results show that reduction of βArrestin2 expression led to a marked decrease in the AR ubiquitylation (Fig. S7D). Remarkably, the reduction of endogenous Mdm2 expression alone, or in combination with βArrestin2 revealed a substantial increase in the AR protein expression (Fig. S8 A–B). Together, these results establish a role for βArrestin2 in the Mdm2-mediated AR ubiquitylation and degradation.
Expression of βArrestin2 in Human Prostate Tissues.
Immunohistochemical studies were carried out using paraffin-embedded prostate tissue microarrays containing 24 prostatectomy cases and 94 cores. Specificity of the signal was confirmed by using contiguous sections for immunostaining to evaluate the expression of PSA and βArrestin2. The measurement of correlation between the average βArrestin2 and PSA stain scores was calculated using both parametric Pearson correlations and the nonparametric rank-based Spearman correlations coefficients, with grade considered a continuous variable. By using both statistical analyses, we found that βArrestin2 and PSA expression were negatively correlated (Pearson's coefficient = −0.30; P value = 0.0025, and Spearman correlation coefficient = −0.29; P value = 0.004). The results show an inverse relationship between the βArrestin2 and PSA proteins expression, and both proteins were found largely in the epithelial and not stromal prostate cells (Fig. 6). We also examined the expression profile of AR and βArrestin2 and observed that, in general, glands that expressed βArrestin2 did not express AR and those glands that expressed AR did not express βArrestin2 (Fig. 6B). Collectively, these results suggest the inverse relationship between βArrestin2 expression and the expression and activity (as demonstrated with PSA levels) of AR in human prostate tissues.
Fig. 6.
Expression of βArrestin2 and PSA in human prostate tissue. (A) Sections of formalin-fixed paraffin-embedded prostate microarray tissues were immunostained using anti-PSA and anti-βArrestin2 antibodies. (B) Higher magnification of the tissue sections immunostained with anti-PSA, anti-βArrestin2, and anti-AR antibodies.
Discussion
AR belongs to the nuclear receptor superfamily of transcription factors and is responsible for mediating all biological actions of androgens in target tissue. In addition to its implied role in the initiation and progression of prostate cancer, the AR plays a pivotal role in the process of male sexual development and maintenance of male sex characteristics (28). AR dysfunction is also linked to Kennedy's disease, a progressive degenerative condition affecting lower motor neurons (29), and recent results showed that disruption of interaction between the AR and its coregulators ameliorated progression of the disease in animal models (30). In this study, we report identification of the multifunctional βArrestin2 protein as an AR corepressor. We show that βArrestin2 forms a complex with AR and Mdm2 that, in turn, marks the AR with ubiquitin to target it for degradation in the proteasome.
Pathologic growth of the prostate is controlled largely by androgens, and locally advanced and metastatic diseases are treated with endocrine therapies aimed to decrease circulating androgen levels via chemical or physical castration and/or blockade of AR activation with anti-androgens (2–6). A limitation of the hormonal therapies is that they offer only a temporary relief, and the primary predictor of treatment failure is the biochemical transition of the tumor cells from AD to AI state that is characterized by aggressive growth and invasion of distal organs. Androgens exert their effect on target cells by activating the AR, and at the molecular level multiple mechanisms have been implicated in the regulation of AR activity. For example, the AR associates with cofactors that influence the outcome of receptor activation (4). Cofactors can be coactivators or corepressors depending on whether they enhance the AR-mediated transcriptional activity or repress it. Both types of cofactors exert their effects on AR at multiple levels, including protein stability, nuclear translocation, interaction with the transcriptional machinery, or the binding to DNA. Here, we identified the ubiquitous βArrestin2 protein as a corepressor of AR function: overexpression of βArrestin2 dampens, and knockdown of endogenous βArrestin2 expression with siRNA increases the AR-dependent PSA expression.
The AR is a target for posttranslational modifications, including phosphorylation (11), acetylation (12), and SUMOylation (13) that may govern the receptor function. For example, the acetylation of AR at lysine residues 630, 632, and 633 enhances coactivator binding and mutation of these residues causes the AR trafficking defects, misfolding and protein aggregation. Emerging evidence supports a role of the ubiquitin-proteasome degradation system in the transcriptional regulation of AR (14). The current findings indicate that ubiquitous βArrestin2 protein acts as a scaffold to recruit Mdm2 to the AR, thereby allowing the AR ubiquitylation and degradation in the proteosome. Hence, βArrestin2 may bring together AR and Mdm2, leading to AR ubiquitylation. Alternatively, an earlier study showed that Mdm2 mediates AR degradation in an Akt phosphorylation-dependent manner (14), and βArrestin2 regulates the Akt activity (31). Therefore, βArrestin2 may affect the AR ubiquitylation via the regulation of Akt.
The results suggest the selective binding of AR to βArrestin2. Although βArrestin1 and βArrestin2 exhibit high (≈70%) amino-acid homology and share similar functions, emerging evidence suggests the minor differences in their sequence may explain functional disparities. Indeed, the βArrestins have been demonstrated to possess differential binding affinities for target GPCR partners (22), and Lefkowitz and colleagues (20) showed that βArrestin2, but not βArrestin1, specifically regulates the agonist-stimulated ubiquitylation of the β2-adrenergic receptor. The binding of βArrestins to GPCRs is preceded by the receptor phosphorylation, and AR has been demonstrated to be a phospho-protein (32). However, it remains undetermined whether the AR phosphorylation plays a role in the preferential binding to βArrestin2.
The AR has emerged as a key molecular determinant in the progression of human prostate cancer to hormone-refractory state. In some prostate cancers, increased AR expression or activity (via activating mutations) is sufficient to convert the cancer growth from a hormone-sensitive to a hormone-refractory disease (33). More recent results suggest that AR plays both suppressive and proliferative roles in prostate cancer (34): Epithelial luminal cells lacking AR exhibit increased apoptosis, whereas epithelial basal cells lacking AR show the increased tumor growth. Indeed, clinical outcomes demonstrate that in some patients diagnosed with hormone-refractory prostate cancer the AR expression is lost, implying that diminished AR expression associates with progression of the disease. Our results show that βArrestin2 mediates the AR degradation and suggest that up-regulation of βArrestin2 may be responsible for the loss of AR expression (and function) in this subset of clinical cases. Upon validation, these results put forward the idea that increased βArrestin2 expression may concurrently endow these cancers with other mitogenic or survival pathways, thereby liberating them from “dependence” on AR signaling.
In summary, the results show that βArrestin2 acts as a corepressor of AR-dependent gene expression by scaffolding Mdm2 for subsequent AR ubiquitylation and degradation in the proteasome. Elucidation of the pathophysiologic effect of βArrestin2 on the AR expression (and function) in the context of prostate cancer progression may lead to the identification of βArrestin2-selective ‘personalized’ drugs that are effective in the treatment of (a subset of) patients with advanced disease.
Materials and Methods
Details.
For details of experimental procedures see SI Text.
Immunoprecipitation and Immunoblotting Assays.
Appropriately treated cells were lysed in RIPA buffer [150 mM NaCl, 50 mM Tris·HCl, pH 8, 1 mM EDTA, 0.25% (wt/vol) sodium deoxycholate, 0.1% (vol/vol) Nonidet P-40, 1 mM NaF, 1 mM sodium pyrophosphate, 100 μM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 10 μg/mL leupeptin, 10 μg/mL aprotonin, and 0.7 μg/mL pepstatin] at 4°C, 48 h after transfection. Cell extracts were cleared by centrifugation, and the supernatants were incubated at 4°C with the indicated antibody for 2 h. Immune complexes were immobilized on protein A/G-Sepharose beads (Calbiochem) for 3 h, washed 3 times with RIPA lysis buffer, and analyzed after fractionation on SDS-PAGE by Western blot analysis, as described in ref. 32.
Luciferase Assay.
Appropriately transfected cells were equally divided into 24-well plates and allowed to attach. The culture medium was replaced after 24 h with starvation medium [phenol red-free RPMI 1640 containing 0.1% bovine serum albumin (BSA), 1% penicillin/streptomycin, and 10 mM Hepes buffer, pH 7.5], and identical cell populations, in duplicate, were stimulated with R1881 or vehicle control for additional 24–48 h. Luciferase activities in cell lysates were measured using the Dual Luciferase assay system (Promega) and were normalized by the Renilla activities and protein concentrations of the samples. Results are presented as fold change from baseline by dividing the relative luciferase activity of the treated cells over the value obtained for unstimulated cells.
Real-Time PCR.
Total RNA was isolated with TRIzol Reagent, as described by the manufacturer (Invitrogen). The RNA was reverse transcribed to make a cDNA that was amplified by PCR using specific sense and anti-sense primers for the PSA and housekeeping gene GAPDH (see SI Text). Real-time PCR was performed with the BioRad iQ5 Thermocycler using SYBR green reagents (BioRad) according to the manufacturer's instructions.
Immunostaining.
Human prostate tissue microarray slides were obtained from NDRI and consisted of 24 cases and 94 cores. The tissue sections were processed and stained as described in ref. 35. Target retrieval buffer (Dako) was performed for anti-AR following the manufacturer's instructions (see SI Text). Staining for βArrestin2 and PSA was graded as follows: 0, negative (no cells stained); 1, weakly positive (< 10% of cells stained); 2, moderately positive (10%–50% of cells stained); or 3, strongly positive (> 50% of cells stained).
Supplementary Material
Acknowledgments.
We thank our colleagues for sharing valuable reagents. We also thank S. Perumal and B. Baban for technical help with the pull-down and microscopy studies, and A. Sharma for help with the statistical analysis. This work was supported by National Institutes of Health Grants CA129155 and CA131988 (to Y.D.) and GM47417 and CA129626 (to J.L.B.). Y.D. is a Georgia Cancer Coalition Distinguished Scholar.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900258106/DCSupplemental.
References
- 1.Jemal A, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 3.Grossmann ME, Huang HJ, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst. 2001;93:1687–1697. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- 4.Heinlein CA, Chang CS. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 5.Daaka Y. G proteins in cancer: The prostate cancer paradigm. Sci STKE. 2004;2004:re2. doi: 10.1126/stke.2162004re2. [DOI] [PubMed] [Google Scholar]
- 6.Bagchi G, Moniri NH, Daaka Y. Androgen Receptor. Nat Signal Gateway. 2006:AA003790. [Google Scholar]
- 7.Lara PN, et al. Molecular biology of prostate carcinogenesis. Crit Rev Oncol Hematol. 1999;32:197–208. doi: 10.1016/s1040-8428(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 8.Koivisto P, et al. Androgen receptor gene amplification: A possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–319. [PubMed] [Google Scholar]
- 9.Linja MJ, et al. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550–3555. [PubMed] [Google Scholar]
- 10.Quigley CA, et al. Androgen receptor defects: Historical, clinical, and molecular perspectives. Endocr Rev. 1995;16:271–321. doi: 10.1210/edrv-16-3-271. [DOI] [PubMed] [Google Scholar]
- 11.Gioeli D, et al. Androgen receptor phosphorylation: Regulation and identification of the phosphorylation sites. J Biol Chem. 2002;277:29304–29314. doi: 10.1074/jbc.M204131200. [DOI] [PubMed] [Google Scholar]
- 12.Fu MF, et al. p300 and p300/cAMP-response element-binding protein-associated factor acetylate the androgen receptor at sites governing hormone-dependent transactivation. J Biol Chem. 2000;275:20853–20860. doi: 10.1074/jbc.M000660200. [DOI] [PubMed] [Google Scholar]
- 13.Poukka H, et al. Covalent modification of the androgen receptor by small ubiquitin-like modifier 1 (SUMO-1) Proc Natl Acad Sci USA. 2000;97:14145–14150. doi: 10.1073/pnas.97.26.14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin HK, et al. Phosphorylation-dependent ubiquitylation and degradation of androgen receptor by Akt require Mdm2 E3 ligase. EMBO J. 2002;21:4037–4048. doi: 10.1093/emboj/cdf406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson SS, et al. Role of βArrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 17.Shenoy SK, Lefkowitz RJ. Seven-transmembrane receptor signaling through βArrestin. Science Signal. 2005;2005:cm10. doi: 10.1126/stke.2005/308/cm10. [DOI] [PubMed] [Google Scholar]
- 18.Goodman OB, et al. βArrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 19.Laporte SA, et al. The interaction of βArrestin with the AP-2 adaptor is required for the clustering of β2-adrenergic receptor into clathrin-coated pits. J Biol Chem. 2000;275:23120–23126. doi: 10.1074/jbc.M002581200. [DOI] [PubMed] [Google Scholar]
- 20.Shenoy SK, et al. Regulation of receptor fate by ubiquitination of activated β2-adrenergic receptor and βArrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 21.Shenoy SK, et al. Nedd4 mediates agonist-dependent ubiquitination, lysosomal targeting, and degradation of the β2-adrenergic receptor. J Biol Chem. 2008;283:22166–22176. doi: 10.1074/jbc.M709668200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao K, et al. Functional specialization of βArrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci USA. 2007;104:12011–12016. doi: 10.1073/pnas.0704849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang HY, et al. Cloning and characterization of human prostate coactivator ARA54, a novel protein that associates with the androgen receptor. J Biol Chem. 1999;274:8570–8576. doi: 10.1074/jbc.274.13.8570. [DOI] [PubMed] [Google Scholar]
- 24.Poukka H, et al. The RING finger protein SNURF modulates nuclear trafficking of the androgen receptor. J Cell Sci. 2000;113:2991–3001. doi: 10.1242/jcs.113.17.2991. [DOI] [PubMed] [Google Scholar]
- 25.Marchese A, et al. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaughan L, et al. Regulation of androgen receptor and histone deacetylase 1 by Mdm2-mediated ubiquitylation. Nucleic Acids Res. 2005;33:13–26. doi: 10.1093/nar/gki141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Girnita L, et al. βArrestin is crucial for ubiquitination and down-regulation of the insulin-like growth factor-1 receptor by acting as adaptor for the Mdm2 E3 ligase. J Biol Chem. 2005;280:24412–24419. doi: 10.1074/jbc.M501129200. [DOI] [PubMed] [Google Scholar]
- 28.McLachlan RI, et al. The endocrine regulation of spermatogenesis: Independent roles for testosterone and FSH. J Endocrinol. 1996;148:1–9. doi: 10.1677/joe.0.1480001. [DOI] [PubMed] [Google Scholar]
- 29.Spada ARL, et al. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991;352:77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- 30.Yang Z, et al. ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat Med. 2007;13:348–353. doi: 10.1038/nm1547. [DOI] [PubMed] [Google Scholar]
- 31.Beaulieu J-M, et al. An Akt/βArrestin2/PP2A Signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Bagchi G, et al. Androgens transduce the Gαs-mediated activation of protein kinase A in prostate cells. Cancer Res. 2008;68:3225–3231. doi: 10.1158/0008-5472.CAN-07-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen CD, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 34.Niu YJ, et al. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proc Natl Acad Sci USA. 2008;105:12182–12187. doi: 10.1073/pnas.0804700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo R, et al. Expression and function of lysophosphatidic acid LPA1 receptor in prostate cancer cells. Endocrinology. 2006;147:4883–4892. doi: 10.1210/en.2005-1635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.