Abstract
Conservation efforts typically focus on maximizing biodiversity in protected areas. The space available for reserves is limited, however, and conservation efforts must increasingly consider how management of protected areas can promote biodiversity beyond reserve borders. Habitat corridors are considered an important feature of reserves because they facilitate movement of organisms between patches, thereby increasing species richness in those patches. Here we demonstrate that by increasing species richness inside target patches, corridors additionally benefit biodiversity in surrounding non-target habitat, a biodiversity “spillover” effect. Working in the world's largest corridor experiment, we show that increased richness extends for approximately 30% of the width of the 1-ha connected patches, resulting in 10–18% more vascular plant species around patches of target habitat connected by corridors than around unconnected but otherwise equivalent patches of habitat. Furthermore, corridor-enhanced spillover into non-target habitat can be predicted by a simple plant life-history trait: seed dispersal mode. Species richness of animal-dispersed plants in non-target habitat increased in response to connectivity provided by corridors, whereas species richness of wind-dispersed plants was unaffected by connectivity and increased in response to changes in patch shape—higher edge-to-interior ratio—created by corridors. Corridors promoted biodiversity spillover for native species of the threatened longleaf pine ecosystem being restored in our experiment, but not for exotic species. By extending economically driven spillover concepts from marine fisheries and crop pollination systems, we show how reconnecting landscapes amplifies biodiversity conservation both within and beyond reserve borders.
Keywords: dispersal, habitat corridors, halos, life-history traits, reserve design
Habitat destruction is the leading cause of biodiversity loss (1). Global efforts to prevent extinctions focus on protecting and augmenting species diversity within reserves, but the amount of protected habitat remains alarmingly small and inadequate for most taxa (2). Reserves would have greater impact if their benefits extended beyond their boundaries into surrounding non-target habitat—a process termed spillover in marine reserves (3) and halos in terrestrial systems (4) (hereafter referred to as spillover).
Spillover effects have most often been examined in terms of ecosystem services provided by protected areas to surrounding non-target areas, for example, higher fish catch in marine systems (e.g., 3–5) and improved crop pollination in terrestrial systems (6, 7). Spillover, however, is likely more general, perhaps extending to biodiversity as a whole (5). Although spillover concepts are prominent in countryside biogeography, which focuses on landscapes heavily altered by humans (e.g., 8), we could find only 2 studies, both from the same landscape, documenting spillover effects for biodiversity in terrestrial systems (7, 8). Importantly, in both marine and terrestrial systems, spillover has been tied to the creation of new reserves, not the configuration of existing reserves. Opportunities for the creation of new reserves may be limited, particularly in many terrestrial ecosystems, and thus the greatest conservation gains may come from increasing the impact of existing reserve networks. If managers can reliably increase biodiversity in both target and non-target habitats through changing the configuration of existing protected areas, they can extend the impact of protected areas (e.g., 8).
Spillover is largely a function of within-patch dynamics, providing a conceptual model for its prediction: patches with higher density of organisms should have higher spillover. This is evident in marine systems, where reserves support increased density of fish species that are normally harvested (9), leading to spillover of these species into the surrounding waters where fishing is allowed (3–5). Similarly in terrestrial systems, spillover of pollinator services exists around forest reserves because these reserves contain greater densities of pollinating insects than surrounding agricultural landscapes (6, 7).
This general conceptual model of spillover can be extended from single species to an entire community of species: just as greater spillover of populations occurs around reserves that harbor larger populations, greater spillover of biodiversity should occur around reserves that harbor larger numbers of species. Furthermore, to provide evidence for a spillover effect, biodiversity levels must decline with distance from target habitat. However, because species differ in the distance they will move into non-target habitat (10–12), basic life-history traits associated with mobility may predict patterns of spillover. This conceptual model has direct management implications: actions that increase biodiversity within patches, such as increasing patch connectivity (13), may increase biodiversity of adjacent non-target habitat by elevating levels of spillover.
To assess management implications for spillover of biodiversity, we must answer 3 questions. First, does biodiversity indeed “spill over” from target (i.e., protected) to non-target (i.e., unprotected) areas? Second, how can landscape management of existing protected areas, such as increasing connectivity or changing patch shape, promote spillover, thus benefiting biodiversity beyond reserve borders? Third, given that spillover is likely unequal among species (14), can we predict which species will most benefit based on readily available information on dispersal?
Here we demonstrate the importance of two of the most often considered properties of protected areas—patch connectivity and patch shape (15, 16)—in promoting biodiversity spillover. Working with plant communities in and around patches of target habitat within fully replicated experimental landscapes, we test the conservation benefits of the most popular management strategy for fragmented landscapes: habitat corridors (15). Corridors are thin strips of habitat used to connect otherwise unconnected habitat patches and increase biodiversity in the patches they connect (13). Our study design tests the mechanisms by which corridors may function by isolating effects of habitat connectivity from effects of patch shape, while controlling for patch area (see Fig. 1 and Materials and Methods). It does so by comparing 3 patch types: patches connected by corridors (i.e., connected patches) and unconnected patches of high edge-to-area ratio (i.e., unconnected high-edge patches) or low edge-to-area ratio (i.e., unconnected low-edge patches; Fig. 1). This aspect of our experimental design is important because corridors not only alter connectivity but also change the shape of patches by increasing edge-to-area ratio, which in turn can alter species composition and possibly change spillover (17–19).
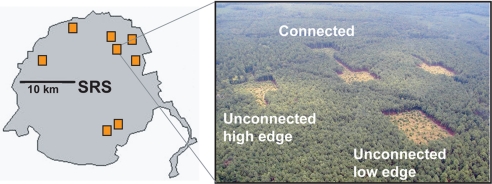
Fig. 1.
Locations of 8 experimental landscapes at the Savannah River Site (SRS) and aerial photograph of one landscape. Each landscape consists of 5 patches of approximately 1 ha of regenerating longleaf pine savanna (target habitat): 2 connected by a 150-m corridor and 3 unconnected and isolated by 150 m of mature plantation forest (non-target habitat). Unconnected patches either had a high or low edge-to-area ratio (high edge and low edge, respectively). Two impacts of corridors are evaluated with this design. Comparing connected patches with unconnected high-edge patches tests for effects of connectivity while controlling for differences in patch shape, as both patch types have similar edge-to-area ratio. Comparing unconnected high-edge and unconnected low-edge patches tests for the changes in patch shape associated with corridor implementation while controlling for connectivity.
We first examine effects of corridors on spillover of total plant species richness. We predict spillover to be greatest around connected patches because these patches harbor the greatest number of species (13). We then sort plants by their mode of dispersal, a fundamental life-history trait that is central to understanding large-scale population dynamics and persistence (21–23), and to predicting spillover in marine reserves (10, 12) and connectivity in terrestrial systems (20). Previous responses of animal- and wind-dispersed species groups within our experimental patches (20) suggest that spillover of both groups will be promoted by corridors, but animal-dispersed species should respond only to connectivity whereas wind-dispersed species should also respond to patch-shape effects. Finally, to more directly examine conservation benefits of corridors, we examine effects of connectivity and patch shape on native plant species of conservation concern in the ecosystem in which we work (longleaf pine savanna) and, separately, on exotic species. We predict corridor-enhanced spillover of native, but not exotic, species because exotic species are generally unaffected by corridors in our experiment (13).
Results
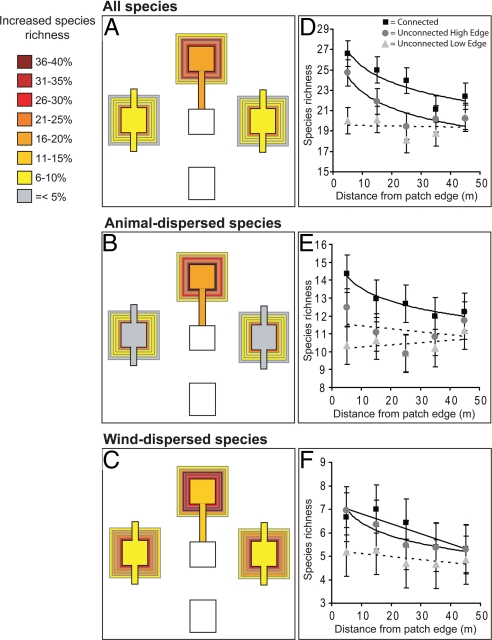
Connectivity increased spillover of plant biodiversity, as species richness was greater in non-target habitat around patches connected by corridors than around those not connected by corridors (Fig. 2 A and D). Patch type (F2,98 = 14.35, P < 0.001) and distance from the patch edge (F4,98 = 4.19, P < 0.01) were both significant predictors of total species richness in non-target habitat. Across distances, non-target habitat surrounding connected patches contained, on average, 13% more species per 100 m2 than unconnected high-edge patches (t = 3.10, df = 10.8, P < 0.01) and 18% more species per 100 m2 than unconnected low-edge patches (t = 5.33, df = 10.8, P < 0.001). In contrast, non-target habitat surrounding unconnected high-edge and low-edge patches did not differ in total species richness (t = 2.23, df = 10.8, P > 0.2).
Fig. 2.
Species richness within target patches and surrounding non-target habitat from 8 experimental landscapes at the Savannah River Site for all plant species (A), animal-dispersed species (B), and wind-dispersed species (C). Values in A-C are relative to unconnected low-edge patches, thereby separating effects of connectivity and patch shape. Within-patch colors represent average species richness increase during annual surveys (2001–2007); colored strips in non-target habitat represent the percent increase in species richness in 2007 relative to unconnected low-edge patches for 10-m increments away from the target habitat patch. Overall richness differences correspond to elevated richness in non-target habitat for more than 9,600 m2 relative to unconnected high-edge patches and more than 16,000 m2 relative to unconnected low-edge patches. Patch type and distance from the target patch were significant predictors (P < 0.05) of overall (D), wind-dispersed (E), and animal-dispersed species richness (F). Points represent means ± 1 SE. Patterns of decay in spillover (significant relationships between richness and distance from patch edge) are denoted by solid lines. Dashed lines representing non-significant relationships are drawn for comparison.
Mirroring the pattern for the full plant community, connectivity increased spillover of animal-dispersed species, as richness of this group was greatest around patches connected by corridors (Fig. 2 B and E). Patch type (F2,98 = 20.77, P < 0.001) and distance from the patch edge (F4,98 = 3.22, P < 0.05) were again both significant predictors of species richness. Across distances, non-target habitat surrounding connected patches contained, on average, 13% more animal-dispersed species per 100 m2 than unconnected high-edge patches (t = 4.3, df = 14.3, P < 0.001) and 19% more animal dispersed species per 100 m2 than unconnected low-edge patches (t = 6.30, df = 14.3, P < 0.001). Non-target habitat surrounding unconnected high-edge and low-edge patches did not differ in the richness of animal-dispersed species (t = 1.97, df = 14.3, P > 0.05).
Changes in patch shape associated with corridors explained spillover of wind-dispersed species, as species richness of this group was greatest around unconnected high-edge patches and connected patches, which are also high-edge (Fig. 2 C and F). Patch type (F2,98 = 7.78, P < 0.001) and distance from the patch edge (F4,98 = 3.34, P < 0.05) were both significant predictors of wind-dispersed species richness. Across distances, non-target habitat surrounding connected patches contained, on average, 20% more wind-dispersed species per 100 m2 than unconnected low-edge patches (t = 3.76, df = 11.9, P < 0.001), but did not differ from non-target habitat surrounding high-edge patches for wind-dispersed species (t = 0.85, df = 11.9, P > 0.40). Non-target habitat surrounding unconnected high-edge patches had, on average, 16% more wind-dispersed species per 100 m2 than non-target habitat around unconnected low-edge patches (t = 2.91, df = 11.9, P < 0.01).
Corridors promoted spillover of native but not exotic plant species. Patch type (F2,98 = 11.91, P < 0.001) and distance from the patch edge (F4,98 = 3.63, P < 0.01) were both significant predictors of native longleaf pine savanna species richness in non-target habitat. Across distance categories, non-target habitat surrounding connected patches contained 20% more native longleaf pine savanna species per 100 m2 than unconnected high-edge patches (t = 2.13, df = 98, P < 0.05). Non-target habitat surrounding unconnected high-edge patches contained, on average, 10% more longleaf pine savanna native species per 100 m2 than unconnected low-edge patches (t = 2.74, df = 98, P < 0.01). In contrast, exotic species were not affected by patch type (F2,98 = 0.64, P > 0.5) or distance from patch edge (F4,98 = 0.69, P > 0.6).
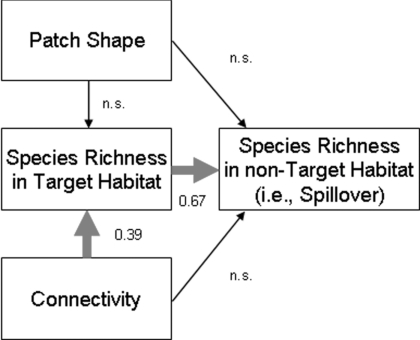
Using structural equation modeling, we tested 2 alternative explanations for the spillover effects we observed: (i) corridors elevated within-patch species richness, which in turn caused spillover; or (ii) corridors directly caused spillover, independent of changes to within-patch species richness. Models clearly supported the first explanation (Fig. 3). In all cases, significant correlations existed between within-patch richness and richness in non-target habitat [full plant community standardized regression weight (SRW), 0.67; P < 0.001; animal-dispersed species SRW, 0.58; P < 0.001; wind-dispersed species SRW, 0.63; P < 0.001]. We found no support for corridors promoting spillover independent of within-patch species richness, either via connectivity or patch-shape mechanisms (maximum SRW, 0.05; minimum P > 0.7).
Fig. 3.
Results of structural equation model show pathway for spillover of plant species richness. Corridors promote spillover by elevating richness within target patches through increased connectivity. Standardized regression weights are presented for significant correlations.
An alternative means of assessing spillover is to examine the rate and shape of biodiversity decay from the edge of the target habitat into the non-target habitat. Because we tested spillover at only 5 distances, we have limited ability to examine differences in the shape of spillover decay among patch types or plant groups, but we nevertheless found strong differences in rates of decay. For all species combined, spillover decayed with distance from connected (χ2 = 24.4, df = 1, P < 0.0001) and unconnected high-edge patches (χ2 = 9.9, df = 1, P < 0.01), but we found no evidence for decay in spillover around unconnected low-edge patches (χ2 = 0.03, df = 1, P > 0.8; Fig. 2D). Decay was driven by elevated spillover around connected patches at distances of 0 to 10 m (F1,98 = 13.3, P < 0.001), 10 to 20 m (F1,98 = 7.2, P < 0.01), and 20 to 30 m (F1,98 = 10.3, P < 0.01) and around unconnected high-edge patches at 0 to 10 m (F1,98 = 6.8, P = 0.01), relative to unconnected low-edge patches (Fig. 2A). Spillover of animal-dispersed species also decayed around connected patches (χ2 = 21.3, df = 1, P < 0.0001), with no evidence for decay around unconnected high-edge (χ2 = 0.4, df = 1, P > 0.5) or low-edge patches (χ2 = 1.1, df = 1, P > 0.3; Fig. 2E). Decay of animal-dispersed species was driven by elevated spillover around connected patches at 0 to 10 m (F1,98 = 22.4, P < 0.001), 10 to 20 m (F1,98 = 7.7, P < 0.01), 20 to 30 m (F1,98 = 10.4, P < 0.01), and 30 to 40 m (F1,98 = 4.5, P < 0.05), relative to distance from unconnected low-edge patches (Fig. 2B). Spillover of wind-dispersed species decayed around connected (F1,3 = 11.6, P < 0.05) and unconnected high-edge patches (χ2 = 40.4, df = 1, P < 0.0001), and there was no evidence for decay around unconnected low-edge patches (F1,3 = 3.6, P > 0.15; Fig. 2F). Decay of wind-dispersed species was driven by elevated spillover around connected patches at distances of 0 to 10 m (F1,98 = 4.0, P < 0.05), 10 to 20 m (F1,98 = 5.7, P < 0.05), and 20 to 30 m (F1,98 = 5.5, P < 0.05), and around unconnected high-edge patches at 0 to 10 m (F1,98 = 5.6, P < 0.05), relative to unconnected low-edge patches (Fig. 2C). If patterns of spillover are similar around the entire periphery of our patches, then connected patches supported elevated richness in more than 9,600 m2 of non-target habitat relative to unconnected high-edge patches and more than 16,000 m2 of non-target habitat relative to unconnected low-edge patches (Fig. 2 A–C).
These results are not an artifact of biodiversity patterns that existed before the initiation of this experiment because (i) patch types did not differ in species richness immediately after creation and connected patches gradually became more species-rich over time (13), (ii) preexisting seed banks did not differ (13), and (iii) levels of biodiversity in the present study were consistently higher near the patch edges we created than in surrounding forest, resulting in a spillover effect that decayed past approximately 30 m (Fig. 2D) and differed by dispersal mode (Fig. 2 E and F).
Discussion
Corridors increased within-patch plant species richness by 20%, and this led to a biodiversity spillover effect around patches connected by corridors. Relative to non-target habitat surrounding unconnected patches, plant species richness around patches connected by corridors was elevated by 10% to 18% (Fig. 2 A and D). This biodiversity spillover around patches was caused by the effects of corridors on species richness within patches, and the increase within patches was not caused by differences in patch area, which was identical across patch types. Likewise, this result was not generated by differences in patch shape because unconnected high-edge patches—those with similar amounts of edge to connected patches—had lower levels of spillover than did connected patches (Fig. 2 A and D). Because of this species enrichment of non-target habitat, the biodiversity benefit of corridors is larger than previously reported (13).
Although spillover effects were generated by connectivity for the full plant community, species responded differently, and these differences were predictable using the basic life-history trait of seed dispersal. The number of animal-dispersed species increased in non-target areas around connected patches (Fig. 2 B and E), whereas the number of wind-dispersed species increased in non-target areas around connected patches and around unconnected patches with high amounts of edge (Fig. 2 C and F). Thus, the richness of animal-dispersed species increased in response to the connectivity that corridors provide, whereas the richness of wind-dispersed species increased in response to changes in patch shape (i.e., increased edge) that typically accompany the presence of corridors. Like for the full plant community, these spillover patterns were a result of how corridors altered within-patch richness.
Species richness declined with distance from patch edge around patches connected by corridors, providing clear support for a biodiversity spillover effect. The rate of spillover decay was influenced primarily by connectivity provided by corridors and to a lesser extent changes in patch-shape (Fig. 2 D–F). We found no evidence for spillover around unconnected low-edge patches; species richness was similar at all distances from those patches, even immediately adjacent to the patch edge. Thus, it appears that plant biodiversity spillover cannot be universally expected (Fig. 2 C–E).
Although connectivity has been suggested to facilitate the spread of exotic species (24), we found no evidence for this effect. Corridors did not influence the diversity of exotic species in target patches (13), resulting in no spillover of exotic species into surrounding non-target habitat. The most parsimonious explanation is that exotic species that become invasive have sufficient movement capabilities that make corridors unnecessary for their spread. Regardless of specific mechanisms, native species as a group responded positively to corridors via spillover, demonstrating an unsuspected benefit of corridors for promoting native biodiversity.
Differences in spillover among patch types were driven by differences in species richness within patches—patches with higher species richness had higher spillover (13, 20) (Fig. 3). Thus, spillover of plant biodiversity appears to be determined by processes operating within target patches, which include seed dispersal and pollination (25, 26). For example, higher species richness of animal-dispersed plants in non-target habitat surrounding connected patches is likely driven by the behaviors of seed dispersing birds along corridors and near edges (25), leading to greater deposition of seeds (26) and greater numbers of bird-dispersed plant species in connected patches (20). Similarly, for wind-dispersed species, changes in landscape structure caused by increased connectivity and edge-to-area ratios may alter wind dynamics and the movement of seeds (20). Our results show that use of a simple life-history trait—dispersal mode—can predict spillover responses.
We observed spillover effects that persisted for 30% the diameter of our target patches, expanding the area where corridors benefited biodiversity by 160%. The magnitude of this effect is almost certainly linked to the scale of our experimental landscapes, which are each approximately 50 ha and contain approximately 1.4 ha target patches. In naturally occurring landscapes, the relative area impacted by spillover would likely vary by patch size. Our results may translate directly to the many landscapes where only small natural areas remain, such as Europe, where 78% of natural areas are <1 km2 (27). It is less clear how our results might translate to landscapes with larger protected areas containing relatively less edge, where area affected by spillover might be proportionally smaller. In larger landscapes, spillover may be unimportant when it is driven by changes in patch shape, as was the case for wind-dispersed species. Connectivity, however, was the major driver of spillover in our study, and should also be important for spillover around large protected areas. Furthermore, within-patch connectivity effects have become more pronounced with time in our experiment (13) and it is plausible to expect future strengthening and extending of spillover effects. Finally, even in very large patches, where population dynamics may be driven internally, spillover effects may promote biodiversity in buffer zones, adding further benefit to their maintenance (28).
Although community-level spillover remains generally unexplored in marine systems, we suspect our results are applicable there as well. First, the creation of marine reserves can increase fish species richness (9, 29), which likely promotes spillover into surrounding waters in a manner analogous to our terrestrial system. Second, there are dispersal correlates: many marine species display a dispersing planktonic phase, akin to plant seeds, and a sedentary adult phase, akin to rooted adult plants (30, 31). Third, ocean currents promote connectivity of isolated reefs, resulting in greater fish density in reefs connected downstream (32), suggesting that connectivity might promote spillover as in our study. Finally, life-history traits, especially planktonic larval duration, are considered of great promise for predicting marine species movement, connectivity, and community responses to management (e.g., 30, 31, 33).
We expect the distance at which spillover occurs from target habitats to be magnified in marine systems, as dispersal distances for marine species are typically 1 to 2 orders of magnitude greater than for terrestrial plants (30, 31). The extent to which this and other differences may influence patterns of biodiversity spillover from marine reserves is difficult to determine, as well replicated studies of marine spillover with proper controls are not yet available (14, 34). Our results thus provide a framework from which to evaluate spillover in both terrestrial and marine systems, strongly suggesting that the management of reserve networks can have large influences on biodiversity spillover.
Our results may have far-reaching implications for reserve management, underscoring the importance of incorporating habitat connectivity into reserve design and placing new emphasis on non-target areas near reserves. Promoting spillover is central to the design of marine reserves, which are frequently created to increase fish catches in surrounding waters (5). Our results extend economically driven spillover concepts from marine to terrestrial reserves and from populations to communities of organisms. By shifting the focus from reserve design effects on single or small sets of species to effects on biodiversity as a whole, our results take spillover to the community level—the level at which virtually all management takes place. In addition, our findings build upon recent observations of halo effects in terrestrial systems for economically important ecosystem services, like pollination (6, 7), and provide a management strategy—i.e., corridors—that magnifies managers' abilities to promote these services.
Materials and Methods
We conducted our research in mature pine plantation forest (i.e., non-target habitat) surrounding patches of open habitat being managed for restoration of longleaf pine savannas (i.e., target habitat), one of the most endangered ecosystems in the southeastern United States. Eight experimental landscapes, all within the Savannah River Site in South Carolina (33.20°N, 81.40°W), were created in 2000 by clearing mature pine forest and restoring the resulting open habitats for longleaf pine savannas with prescribed fire, planting of longleaf pine seedlings, and removal of hardwoods. Each landscape contains a central 100-m × 100-m patch and 4 peripheral patches 150 m from each edge of the central patch. One peripheral patch is connected by a 150-m × 25-m corridor (i.e., connected patch), whereas the remaining 3 peripheral patches are isolated by non-target habitat. Unconnected patches are of equal area to the connected patch plus its corridor and are either low-edge (100 m × 137.5 m), or high-edge (100 m × 100 m with one 75-m × 25-m protrusion extending from each side; Fig. 1). Each landscape contains one duplicate unconnected patch type. Comparisons between connected patches and unconnected high-edge patches provide a test of how corridors impact biodiversity through connectivity, as edge-to-area ratios are similar between these 2 patch types. Comparisons between unconnected high-edge and low-edge patches provide a test of how corridors impact biodiversity through the large amount of edge associated with them.
In October 2007, we established four 50-m × 10-m transects in the non-target pine forest surrounding peripheral patches. Within each of the 8 replicate landscapes, we randomly selected and established transects around one connected, one unconnected high-edge, and one unconnected low-edge patch (24 patches total). Central patches and one randomly selected duplicate unconnected patch in each landscape were not used in this study. Transects originated at patch corners and were oriented from the corner along a line equidistant from the 2 edges. We subdivided each transect into 5 contiguous 10-m × 10-m plots and recorded all vascular plant species rooted in each plot. All subsequent analyses were conducted with plot-level data (i.e., richness/100 m2).
To test for corridor and edge effects, we used split-plot ANOVA. We used a mixed model with the replicate landscape as the random blocking effect. The fixed main-plot effect was patch type (i.e., connected, unconnected high-edge, unconnected low-edge), and the fixed spilt-plot effect was distance from habitat edge (e.g., 0–10 m, 10–20 m). We used independent linear contrasts to test for differences between patch types and patch types within distance categories. We ran separate analyses for total species richness and richness of the following species groups: wind-dispersed, animal-dispersed, longleaf pine savanna native, and exotic species (see ref. 13 for species classification information). Significance of results remained the same after controlling for multiple comparisons.
We fit regression models to help interpret patterns of spillover. We examined species richness (i.e., all species, animal-dispersed species, wind-dispersed species) as a function of distance from patch edge for each patch type using linear and logarithmic regression models, selecting the model with higher r2 for each spillover distribution. We used these 2 models because of their simplicity and because they have been used to understand patterns of spillover around marine protected areas (4) and between groups of fish with varying mobility (10, 12).
We used structural equation modeling to test between 2 alternative explanations for spillover: (i) spillover was produced indirectly by corridors, driven by corridor-induced changes to within-patch species richness levels, or (ii) spillover was produced directly by corridors, independent of how corridors altered within-patch species richness levels. We created 3 models (full plant community, animal-dispersed species, wind-dispersed species) with the AMOS software (version 17.0; AMOS Development). Each model tested whether within-patch and non-target richness (averaged across distances) were controlled by connectivity, patch-shape, and/or soil moisture holding capacity (13, 20), and whether non-target habitat richness was controlled by within-patch richness. To derive within-patch patterns of species richness, we performed an annual census of all vascular plant species occurring in each connected patch plus its corridor, unconnected high-edge, and unconnected low-edge patch (each 13,750 m2) during 2001 through 2007 (methods fully described in ref. 13). Mean values across years were 104.3 species for connected patches, 87.0 species for unconnected low-edge patches, and 94.7 species for unconnected high-edge patches.
Acknowledgments.
We thank the United States Department of Agriculture Forest Service-Savannah River and especially Chris Hobson, Jim Segar, John Blake, and Ed Olson for creating and maintaining our experimental landscapes. Jim Grace, Kevin Gross, and Colin Kremer assisted with data analysis, and Paul Ehrlich provided helpful comments on an earlier manuscript draft. This research was supported by National Science Foundation Grants DEB-9815834, DEB-9907365, DEB-0613701, DEB-0613975, DEB-0614333, and DEB-0733746; and by funds provided to the Department of Agriculture, Forest Service, Savannah River, under Interagency Agreement DE-AI09–00SR22188 with the Department of Energy, Aiken, SC.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.B. is a guest editor invited by the Editorial Board.
References
- 1.Wilcove DS, Rothstein D, Dubow J, Philips A, Losos E. Quantifying threats to imperiled species in the United States. BioScience. 1998;48:607–615. [Google Scholar]
- 2.Rodrigues ASL, et al. Effectiveness of the global protected area network in representing species diversity. Nature. 2004;428:640–643. doi: 10.1038/nature02422. [DOI] [PubMed] [Google Scholar]
- 3.Roberts CM, Bohnsack JA, Gell F, Hawkins JP, Goodridge R. Effects of marine reserves on adjacent fisheries. Science. 2001;294:1920–1923. doi: 10.1126/science.294.5548.1920. [DOI] [PubMed] [Google Scholar]
- 4.McClanahan TR, Mangi S. Spillover of exploitable fishes from a marine park and its effect on the adjacent fishery. Ecol Appl. 2000;10:1792–1805. [Google Scholar]
- 5.Gell FR, Roberts CM. Benefits beyond boundaries: The fishery effect of marine reserves. Trends Ecol Evol. 2003;18:448–455. [Google Scholar]
- 6.Ricketts TH, Daily GC, Ehrlich PR, Michener CD. Economic value of tropical forest to coffee production. Proc Natl Acad Sci USA. 2004;34:12579–12582. doi: 10.1073/pnas.0405147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ricketts TH. Tropical forest fragments enhance pollinator activity in nearby coffee crops. Conserv Biol. 2004;18:1262–1271. [Google Scholar]
- 8.Ricketts TH, Daily GC, Ehrlich PR, Fay JP. Countryside biogeography of moths in a fragmented landscape: Biodiversity in native and agricultural landscapes. Conserv Biol. 2001;15:378–388. [Google Scholar]
- 9.Halpern BS. The impact of marine reserves: Do reserves work and does reserve size matter? Ecol Appl. 2003;13(suppl):S117–S137. [Google Scholar]
- 10.Rakitin A, Kramer DL. Effect of marine reserve on the distribution of coral reef fishes in Barbados. Mar Ecol Prog Ser. 1996;131:97–113. [Google Scholar]
- 11.Kramer DL, Chapman MR. Implications of fish home range size and and relocation for marine reserve function. Environ Biol Fish. 1999;55:65–79. [Google Scholar]
- 12.Abesasmis RA, Russ GR, Alcala AC. Gradients of abundance of fish across no-take marine reserve boundaries: evidence from Philippine coral reefs. Aquat Conserv Mar Freshw Ecosyst. 2006;16:349–371. [Google Scholar]
- 13.Damschen EI, Haddad NM, Orrock JL, Tewksbury JJ, Levey DJ. Corridors increase plant species richness at large scales. Science. 2006;313:1284–1286. doi: 10.1126/science.1130098. [DOI] [PubMed] [Google Scholar]
- 14.Palumbi SR. Marine reserves and ocean neighborhoods: The spatial scale of marine populations and their management. Annu Rev Environ Resour. 2004;29:31–68. [Google Scholar]
- 15.Crooks KR, Sanjayan MA. Connectivity conservation. New York: Cambridge Univ Press; 2006. [Google Scholar]
- 16.Ewers RM, Didham RK. Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev. 2005;81:117–142. doi: 10.1017/S1464793105006949. [DOI] [PubMed] [Google Scholar]
- 17.Laurance WF, Didham RK, Power ME. Ecological boundaries: a search for synthesis. Trends Ecol Evol. 1991;16:70–71. [Google Scholar]
- 18.Ries L, Fletcher RJ, Battin J, Sisk TD. Ecological responses to habitat edges: mechanisms, models, and variability explained. Annu Rev Ecol Evol Syst. 2004;35:491–522. [Google Scholar]
- 19.Ewers RM, Didham RK. Pervasive impact of large-scale edge effects on a beetle community. Proc Acad Nat Sci USA. 2008;105:5426–5429. doi: 10.1073/pnas.0800460105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damschen EI, et al. The movement ecology and dynamics of plant communities in fragmented landscapes. Proc Nat Acad Sci USA. 2008;105:19078–19083. doi: 10.1073/pnas.0802037105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montoya D, Zavala MA, Rodriguez MA, Purves DW. Animal versus wind dispersal and the robustness of tree species to deforestation. Science. 2008;320:1502–1504. doi: 10.1126/science.1158404. [DOI] [PubMed] [Google Scholar]
- 22.Nathan R. Long-distance dispersal of plants. Science. 2006;313:786–788. doi: 10.1126/science.1124975. [DOI] [PubMed] [Google Scholar]
- 23.Ozinga WA, et al. Dispersal failure contributes to plant losses in NW Europe. Ecol Lett. 2009;12:66–74. doi: 10.1111/j.1461-0248.2008.01261.x. [DOI] [PubMed] [Google Scholar]
- 24.Simberloff D, Farr JA, Cox J, Mehlman DW. Movement corridors: conservation bargains or poor investments? Conserv Biol. 1992;6:493–504. [Google Scholar]
- 25.Levey DJ, Bolker BM, Tewksbury JJ, Sargent S, Haddad NM. Effects of landscape corridors on seed dispersal by birds. Science. 2005;309:146–148. doi: 10.1126/science.1111479. [DOI] [PubMed] [Google Scholar]
- 26.Tewksbury JJ, et al. Corridors affect plants, animals, and their interactions in fragmented landscapes. Proc Natl Acad Sci USA. 2002;99:12923–12926. doi: 10.1073/pnas.202242699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaston KJ, Jackson SF, Nagy A, Cantú-Salazar L, Johnson M. Protected areas in Europe. Ann NY Acad Sci. 2008;1134:97–119. doi: 10.1196/annals.1439.006. [DOI] [PubMed] [Google Scholar]
- 28.Gascon C, Williamson GB, da Fonseca GAB. Receding forest edges and vanishing reserves. Science. 2000;288:1356–1358. doi: 10.1126/science.288.5470.1356. [DOI] [PubMed] [Google Scholar]
- 29.McClanahan TR, Arthur R. The effect of marine reserves and habitat on populations of East African coral reef fish. Ecol Appl. 2001;11:559–569. [Google Scholar]
- 30.Carr MH, et al. Comparing marine and terrestrial ecosystems: Implications for the design of coastal marine reserves. Ecol Appl. 2003;13(suppl):S90–S107. [Google Scholar]
- 31.Kinlan BP, Gaines SD. Propagule dispersal in marine and terrestrial environments: a community perspective. Ecol Appl. 2003;13:2007–2020. [Google Scholar]
- 32.Roberts CM. Connectivity and management of Caribbean coral reefs. Science. 1997;278:1454–1457. doi: 10.1126/science.278.5342.1454. [DOI] [PubMed] [Google Scholar]
- 33.Cowen RK, Spongaugle S. Larval dispersal and marine population connectivity. Annu Rev Mar Sci. 2009;1:443–466. doi: 10.1146/annurev.marine.010908.163757. [DOI] [PubMed] [Google Scholar]
- 34.Halpern BS, Regan HM, Possingham HP, McCarthy MA. Accounting for uncertainty in marine reserve design. Ecol Lett. 2006;9:2–11. doi: 10.1111/j.1461-0248.2005.00827.x. [DOI] [PubMed] [Google Scholar]