Abstract
This article describes a self-powered system that uses chemical reactions—the thermal excitation of alkali metals—to transmit coded alphanumeric information. The transmitter (an “infofuse”) is a strip of the flammable polymer nitrocellulose patterned with alkali metal ions; this pattern encodes the information. The wavelengths of 2 consecutive pulses of light represent each alphanumeric character. While burning, infofuses transmit a sequence of pulses (at 5–20 Hz) of atomic emission that correspond to the sequence of metallic salts (and therefore to the encoded information). This system combines information technology and chemical reactions into a new area—“infochemistry”—that is the first step toward systems that combine sensing and transduction of chemical signals with multicolor transmission of alphanumeric information.
Keywords: alphanumeric characters, atomic emission, combustion
This article describes a system that transmits information from a sender to a receiver in the form of coded pulses of light generated entirely by chemical reactions; electronics are not a part of transmission. It is the first step toward systems that integrate the transmission of alphanumeric information (usually accomplished by using electronic or photonic systems) and sensing/transducing (often accomplished by using chemistry). This system encodes the information as a spatial pattern of metallic salts on a string of flammable polymer (a “fuse”); this fuse emits pulses of light in the visible or infrared spectrum when it burns. This research lies at a new interface in science between information technology and chemistry; we call this area “infochemistry,” and the specific type of transmitter of information described in this article, an “infofuse.”
The generation of proteins from information in the genome is the prototypical biological example of infochemistry (1, 2). Transmission of information in the cell occurs between the DNA (the sender) and the ribosome (the receiver), in the form of mRNA (the transmitted message); it is very local. Techniques such as site-directed mutagenesis (3) make it possible to encode information in a string of nucleotides, and to generate a variety of useful “outputs”: nucleic acid sequences coded in RNA and DNA, proteins, and ultimately biological function. A corresponding nonbiological, synthetic system that encodes, transmits, and detects information without electrical power does not exist. Systems that include signal flares, smoke signals, and pH indicators arguably use chemistry to transmit information, but these systems are very simple or very slow. There are a number of reports of systems of synthetic molecular logic, which transduce the presence (or absence) of chemical, electrical, or optical reactants of a chemical reaction into information (4, 5). These systems, however, are slow (the reactions or analyses can take minutes-hours), and when information stored in metastable states, it decays over time. The infofuse we describe here transmits data over a distance through the light generated by thermally emissive metal atoms—a multicolor binary code—at pulse rates (5–20 Hz) that are practical for real (if length-limited) alphanumeric messages.
Results and Discussion
A strip of combustible material (nitrocellulose, NC) (6) provides the energy required by the system. Combustion, when initiated at one end, moves along the strip at a constant velocity and generates a moving zone of intense heat. We call this structure a “fuse,” although the function of a fuse—to provide a chemical timer for a detonation—is substantially simpler than the use we describe here. NC burns with a hot (T ≈1,000 °C) flame, generates minimal smoke or residue, and is easy (and relatively safe) to handle (7). We code information in the system by applying small spots of thermally emissive salts (as metal perchlorates or nitrates) along the fuse.
Fuses burned most reliably when in a vertical orientation and held at the bottom with a small alligator clip. The rate of heat transfer determined the rate of burning of the fuse; when the flame front of the fuse was close to another surface, the fuse burned more slowly than when it was free-standing. For fuses with thicknesses ≈100 μm and widths ≈1 mm, the rate of burning was ≈2.7 cm/s; fuses thinner than these burned faster (≈50-μm-thick fuses burned at ≈4 cm/s). The standard deviation of the rate of burning (measured with 10-cm fuses) was ≈10% of the mean.
We patterned emissive salts as aqueous solutions with either a micropipettor (≈200 nL per spot) or a desktop inkjet printer (8, 9) and dried them at 60 °C. Burning an infofuse transmits a sequence of pulses of light, the wavelengths and order of which encode information (Fig. 1). We detected this light by using either (i) a color CCD camera equipped with a notch filter (OD >2) to absorb emission from adventitious sodium in the nitrocellulose, or (ii) a fiber optic cable coupled to a spectrometer. We did not use a notch filter when detecting the light with a spectrometer. The distance between the detector and burning infofuse was typically 2 m, but we could detect the signal at a distance of 30 m in daylight.
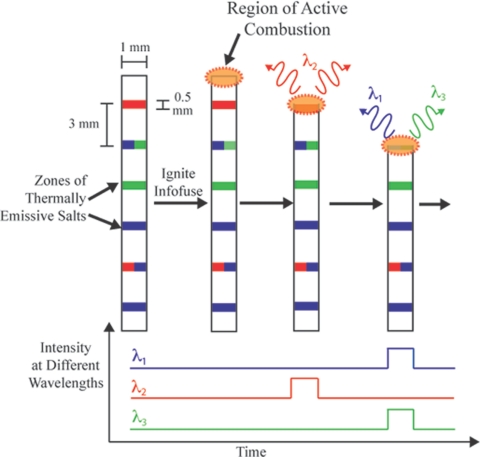
Fig. 1.
Schematic diagram outlining the operation of an infofuse: ignition generates a propagating hot zone. As this zone passes through consecutive zones that contain thermally emissive salts, it emits light at wavelengths characteristic of thermally excited states of species resulting from each salt. The sequence and composition of these patterned salts encodes the information written on the fuse.
NC film burned with a yellow-orange flame. The emission spectrum of this flame (Fig. 2) showed only sharp atomic peaks at 589 nm (adventitious Na), and 767 and 770 nm (adventitious K). The intensity of each peak from potassium was ≈50% of the intensity of the peak from sodium. NC creates almost no char or soot while burning; the intensity of incandescence at λ < 1,100 nm was therefore less than our spectrometer could detect (as opposed to burning cotton string, which showed broad incandescent emission with intensity greater than atomic emission from adventitious sodium). This feature maximized the signal-to-noise of infofuses in the visible and near infrared.
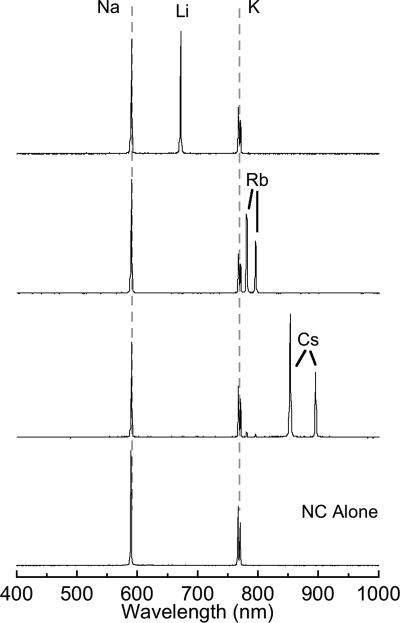
Fig. 2.
Emission spectra of 3 unique alkali metal perchlorate salts deposited onto burning films of NC. The gray dashed lines indicate the background emission of sodium and potassium from the NC that does not interfere with the emission from the other alkali metals.
Fig. 2 and supporting information (SI) Fig. S1 show that the emission spectra of metallic salts deposited onto NC films had either atomic (alkali metals) or molecular (alkaline earth or transition metals) emission lines in the blue (copper), green (barium), yellow (sodium), red (lithium, strontium, calcium), or near-infrared (potassium, rubidium, and cesium) regions of the electromagnetic spectrum (10). The intensity of emission from intentionally deposited sodium or potassium nitrate was ≈10–15 times that of the emission from adventitious sodium or potassium in the NC. The spectra of alkali metals constitute a convenient set of sharp, nonoverlapping signals for transmitting coded messages. The broad linewidths and spectral overlap that the alkaline earth metals have with the encoding alkali metals limits their applicability for transmitting information with infofuses.
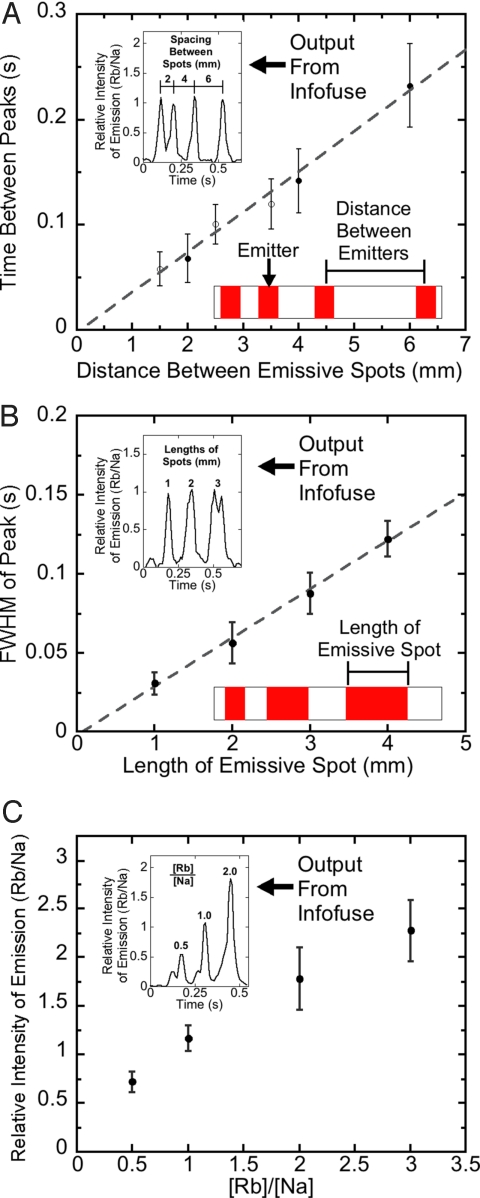
Chemical systems have a number of addressable variables. In addition to the wavelength of emission, pulses from infofuses have 3 parameters that can encode information: (i) the temporal length of a pulse, (ii) the time between successive pulses, and (iii) the intensity of emission from a pulse. We demonstrated these characteristics by using an infofuse patterned with rubidium nitrate (Fig. 3): the length of the emissive spot along the fuse determined the full width at half-maximum of the peaks, the physical spacing between spots on the fuse determined the temporal spacing between peaks, and although the correlation between the concentration of emissive salt and observed intensity was not linear, the amount of rubidium salt determined the intensities of the peaks. The spacing between emissive spots, combined with the rate of burning, determines the rate of transmission of data. With the formulation of infofuses described here, the smallest distance between emissive spots that gave pulses of light that we could resolve reliably was 1.5 mm; with interspot distances <1.5 mm, the propagating flame front excited more than one spot of thermal emitters simultaneously. Designing fuses with smaller flame fronts (such as strips of nitrocellulose thinner than those described here) will be important for increasing the rate of transmission.
Fig. 3.
Dependence of output on physical parameters of printed emissive spots. (A) The distance between the centers of sequential spots on infofuses determined the times measured between sequential peaks. The filled circles represent spots patterned by hand; the open circles represent spots patterned by inkjet printing. (B) The length of inkjet-printed emissive spots on infofuses determined the FWHM of the temporal emissive signal. (C) The ratio of RbNO3 to NaNO3 determined the ratio of intensities of emission from Rb (IRb) and Na (INa). Each datum is the mean of at least 7 measurements; the error bars are the standard deviations of those means. (Insets) Plots of IRb/INa as a function of time.
To encode information in the intensity of the pulses, the intensity of the signal must be reproducible. Two types of error compromise this reproducibility: (i) differences in the deposited volume of the aqueous solution of emissive salts, and (ii) variable sensitivity of the detector as a function of the position of the flame front; the detector is more sensitive at the center of its field of view than at its edges. To compensate for these errors, we used the emission of light at λ = 589 nm from sodium (codeposited with RbNO3) as an internal standard (11).
Any system of infochemistry must be able to store and transmit arbitrary patterns of information. To demonstrate this capability with infofuses, we selected a set of 40 characters—26 letters (A–Z), 10 digits (0–9), and 4 special symbols (. ! ? @)—using emission from the 3 alkali metals (Li, Rb, Cs) that are distinct from emissions from Na and K in the NC. Eight (23) unique binary (1, 0) combinations exist for 3 emitters. To reduce error in decoding information due to variations in the rate of burning of the fuse (from inhomogeneities in the physical dimensions of the manually cast film of nitrocellulose) we did not use the combination in which all 3 emitters were “0” simultaneously; with this omission, 3 elements yield 7 (23 − 1) unique emissions on thermal excitation. (Although the rate of burning of fuses more precisely fabricated than those described here will be more uniform, other factors that can influence the rate of burning, such as proximity to a surface that can accept thermal energy, will prevent the “all-0” combination of emitters from being useful in transmitting information.) Thus, 2 consecutive pulses of light (49 unique combinations) are enough to encode the selected set of 40 characters. [With only 2 emitters, each of the 40 characters would require 4 pulses: (22 − 1)4 = 81. To code one of 40 characters in each pulse would require 7 independent wavelengths.] From these 49 combinations, we constructed an encoding scheme (Fig. S2) for infofuses such that the simplest and least ambiguous code (different emissive wavelengths for consecutive pulses of light) represented the most common letters (E, T, A) (see ref. 12) in the English language; combinations with greater probability for ambiguity represented decreasingly common letters.
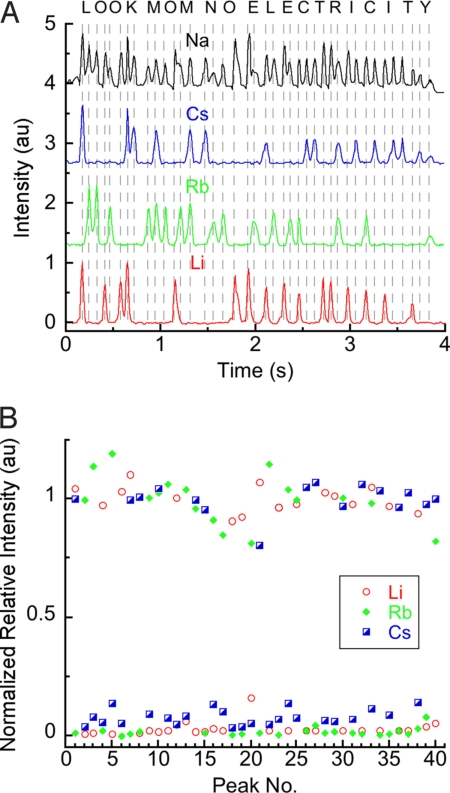
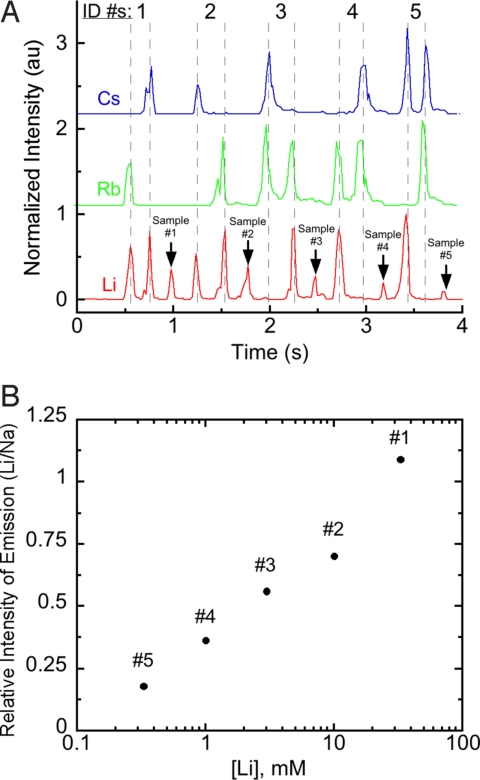
We used our encoding scheme from Fig. S2 to encode and transmit the phrase “LOOK MOM NO ELECTRICITY” (Fig. 4) with a pulse frequency of ≈11 Hz. As the image of the flame front progressed from a region of higher sensitivity of the detector to a region of lower sensitivity, the apparent intensity of emission tended to decrease with time. In addition to this global trend, differences in the quantity of emissive material on the fuse and the rate of combustion of the NC caused local variations in the intensities of peaks. We improved the average error in intensities from 27% to 7% by processing the intensities of the peaks with a 3-step procedure: (i) dividing the intensity of emission at the encoding wavelengths by the intensity of emission from sodium at each peak to give relative intensities (Irel), (ii) separating the 40 peaks into groups of 20 (1–20, 21–40), (iii) normalizing Irel at each peak to the mean of Irel for peaks in its group. In future designs of infofuses, this small amount of error in the corrected intensities should allow for multistate encoding by using the intensity of emission, thereby increasing the density of information contained in each pulse.* We also used an infofuse to sense Li+ (Fig. 5) by transmitting information about its concentration (as the intensity relative to an internal standard). Encoded identifying numbers preceded each of 5 samples.
Fig. 4.
Transmission of a 20-character message with an infofuse. (A) Intensity of emission at the emission wavelengths of Na, Li, Rb, and Cs as a function of time of an infofuse programmed to spell the phrase “LOOK MOM NO ELECTRICITY,” using the encoding scheme from Fig. S2. (B) Normalized, relative intensities of peaks of each pulse processed by comparison with the intensity of emission from the internal standard (sodium perchlorate).
Fig. 5.
Demonstration of sensing Li+ with an infofuse. This infofuse sensed Li and transmitted its emission from 5 samples (dried solutes from a 500-nL drop of aqueous solution that contained a variable concentration of LiClO4 and 10 mM NaClO4). Pulses that encoded identifying numbers (#1–#5, encoded by using the scheme from Fig. S2) preceded each sample. Sample #5 contained ≈20 ng LiClO4 (≈1 ng Li+).
Infofuses represent a first step toward a broader goal of integrating chemistry and information in new ways. Although current infofuses convert the energy released from combustion into light with ≈1% of the efficiency of a battery-operated LED, the combustion of nitrocellulose liberates ≈10 times more energy per unit weight (≈4 MJ/kg) (13) than an alkaline battery. More importantly, the transmission of their information content is linked inexorably to this release of energy through the chemistry of the system. The spatial distribution of the emissive salts encodes the information that pulses of light transmit with 4 characteristics: (i) The emitted signal is isotropic, and observable from any angle. (ii) The emission spectra of the salts contain sharp atomic lines (with better signal-to-noise than semiconductor devices). (iii) The power required is generated by combustion; infofuses are self-powered and transmit information without electricity. (iv) The density of this power, in principle, is higher than that of electrochemical cells, and does not self-discharge over time.
Further improvements—both of infofuses, and directed toward the broader goal of integrating information and chemistry—offer the possibility of lightweight, portable, self-powered systems that can interact with their environment, transmit alphanumeric information, and integrate with modern information technologies, but that do not require any substantial technological infrastructure themselves. Infochemistry can approach the transmission of information in new ways: for example, by moving from binary to base-N encoding schemes that use a number of parameters (such as wavelength and intensity) that allow each “packet” of information to carry more information than a bit. Chemical systems are not bound by the principles of electronics (e.g., the geometrically fixed architectures represented in a circuit), and promise to create new information technologies that are, instead, governed by principles of chemistry. Infochemical systems could, we believe, ultimately be useful for environmental sensing and for diagnostics that sense and process chemical or biochemical inputs and transmit the results optically over a distance.
Materials and Methods
Sheets of nitrocellulose were prepared by pouring ≈50 mL of a 5% (wt/vol) solution of nitrocellulose (in acetone) into a 15 cm × 30 cm polyethylene box, closing the lid, and allowing the solvent to evaporate over 3 days at room temperature. The resulting sheets of nitrocellulose were either optically clear or slightly translucent. The sheets were then cut into strips with width ≈1 mm by using a desktop rotary paper trimmer.
We used 2 methods to pattern strips of nitrocellulose with emissive salts: (i) manual spotting, or (ii) inkjet printing. A solution for each of the combinations of unique emitters (Li, Rb, and Cs) of emissive salts for manual spotting were prepared by dissolving ≈0.5% (wt/vol) of the alkali perchlorates in a stock solution of 0.5% sodium perchlorate in water. Each spot of emitters (≈200 nL) was deposited onto the strip of nitrocellulose with a micropipettor (VWR). After all of the desired spots were deposited onto the fuse, it was dried in an oven at ≈60 °C for 20–30 min until the water from the deposition of emitters had evaporated.
Solutions of atomic emitters for inkjet printing were made with the nitrate salts of alkali metals, which are more soluble than the corresponding perchlorate salts. Aqueous solutions for inkjet printing contained 10% (wt/vol) sodium nitrate, 5–30% (wt/vol) of the encoding alkali nitrate (Li, Rb, or Cs) for transmitting information, and 0.4% (wt/vol) of the surfactant Surfynol 440 (Air Products), which was necessary to print the emitters reliably. A blank inkjet cartridge (MIS Associates) compatible with the desktop inkjet printer we used (Epson Stylus C88+) was loaded with ≈12 mL of this solution. The desired pattern of emitters was drawn in Adobe Illustrator, and printed onto nitrocellulose fuses (taped onto a piece of paper) by using the Epson inkjet printer. Once patterned, the infofuses were dried in an oven at ≈60 °C for 20–30 min.
For spectroscopic detection, a system of 4 lenses collected and focused the light emitted from a burning infofuse. Two 1-inch-diameter fisheye lenses collected light from the entire area occupied by the infofuse; a focusing lens directed the collected light into a 1-mm-diameter multimode optical fiber (Ocean Optics) that was equipped with a collimating lens. Light from this fiber was coupled to a HR2000+ high-resolution CCD spectrometer (Ocean Optics), which was connected to a PC with a USB cable and controlled by software (SpectraSuite) supplied by Ocean Optics. Emission spectra from infofuses were collected with an integration time of 10 ms, at a rate of 100 spectra per second. The distance between the detector and the burning infofuse was typically 2 m.
Supplementary Material
Acknowledgments.
We thank Dr. Coskun Kocabas for assisting in the design of optics for detection of emission. This work was supported by Defense Advanced Research Projects Agency Award W911NF-07-1-0647 and by postdoctoral fellowships from the American Cancer Society (S.W.T) and National Institutes of Health/National Institute of General Medical Sciences (C.N.L.).
Footnotes
The authors declare no conflict of interest.
See Commentary on page 9127.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902476106/DCSupplemental.
A character set ternary (0,1,2) in the corrected intensity of emission, and that uses an intensity between the currently used “1” and “0” for encoding, would increase the number of possible combinations of emitters in each pulse to 26 (33 − 1), whereas the upper and lower thresholds for the intermediate intensity could be at the maximal errors (≈20%, ≈80%) observed in Fig. 4B.
References
- 1.Nelson DL, Cox MM. Lehninger Principles of Biochemistry. 4th Ed. New York: W. H. Freeman; 2004. [Google Scholar]
- 2.Adleman LM. Molecular computation of solutions to combinatorial problems. Science. 1994;266:1021–1024. doi: 10.1126/science.7973651. [DOI] [PubMed] [Google Scholar]
- 3.Kunkel TA, Roberts JD, Zakour RA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 4.De Silva AP, Uchiyama S. Molecular logic and computing. Nat Nanotechnol. 2007;2:399–410. doi: 10.1038/nnano.2007.188. [DOI] [PubMed] [Google Scholar]
- 5.Szacilowski K. Digital information processing in molecular systems. Chem Rev. 2008;108:3481–3548. doi: 10.1021/cr068403q. [DOI] [PubMed] [Google Scholar]
- 6.Akhavan J. The Chemistry of Explosives. 2nd Ed. Cambridge, UK: R Soc Chem; 2004. [Google Scholar]
- 7.Stoner JO. Casting thin-films of cellulose nitrate, polycarbonate, and polypropylene. Nucl Instr Meth Phys Res A. 1995;362:167–174. [Google Scholar]
- 8.Calvert P. Inkjet printing for materials and devices. Chem Mater. 2001;13:3299–3305. [Google Scholar]
- 9.Cho H, Parameswaran MA, Yu H-Z. Fabrication of microsensors using unmodified office inkjet printers. Sensor Actuator B. 2007;123:749–756. [Google Scholar]
- 10.Klapötke TM, Steinhauser G. “Green” pyrotechnics: A chemists' challenge. Angew Chem Int Ed. 2008;47:3330–3347. doi: 10.1002/anie.200704510. [DOI] [PubMed] [Google Scholar]
- 11.Zorn ME, Wilson CG, Gianchandani YB, Anderson MA. Detection of aqueous metals using a microglow discharge atomic emission sensor. Sensor Lett. 2004;2:179–185. [Google Scholar]
- 12.Feynman RP. In: Feynman Lectures on Computation. Hey T, Allen RW, editors. Boulder, CO: Westview Press; 1999. pp. 124–127. [Google Scholar]
- 13.Kubota N. Propellants and Explosives: Thermochemical Aspects of Combustion. 2nd Ed. Weinheim, Germany: Wiley-VCH; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.