Abstract
p90 ribosomal S6 kinase (RSK1) is an effector of both Ras/MEK/MAPK and PI3K/PDK1 pathways. We present evidence that RSK1 drives p27 phosphorylation at T198 to increase RhoA-p27 binding and cell motility. RSK1 activation and p27pT198 both increase in early G1. As for many kinase–substrate pairs, cellular RSK1 coprecipitates with p27. siRNA to RSK1 and RSK1 inhibition both rapidly reduce cellular p27pT198. RSK1 overexpression increases p27pT198, p27-cyclin D1-Cdk4 complexes, and p27 stability. Moreover, RSK1 transfectants show mislocalization of p27 to cytoplasm, increased motility, and reduced RhoA-GTP, phospho-cofilin, and actin stress fibers, all of which were reversed by shRNA to p27. Phosphorylation by RSK1 increased p27pT198 binding to RhoA in vitro, whereas p27T157A/T198A bound poorly to RhoA compared with WTp27 in cells. Coprecipitation of cellular p27-RhoA was increased in cells with constitutive PI3K activation and increased in early G1. Thus T198 phosphorylation not only stabilizes p27 and mislocalizes p27 to the cytoplasm but also promotes RhoA-p27 interaction and RhoA pathway inhibition. These data link p27 phosphorylation at T198 and cell motility. As for other PI3K effectors, RSK1 phosphorylates p27 at T198. Because RSK1 is also activated by MAPK, the increased cell motility and metastatic potential of cancer cells with PI3K and/or MAPK pathway activation may result in part from RSK1 activation, leading to accumulation of p27T198 in the cytoplasm, p27:RhoA binding, inhibition of RhoA/Rock pathway activation, and loss of actomyosin stability.
Keywords: p27 phosphorylation, PI3K, actin cytoskeleton
The Cdk inhibitor, p27, is a key regulator of cell proliferation and differentiation that binds and inhibits cyclin E-Cdk2 (1) and promotes assembly of D-type cyclin-Cdks (2–4). p27 levels fall during G1 progression through controlled translation (5, 6) and timed proteolysis (7), and microRNAs (miRNAs) may regulate its synthesis (8). Multiple mechanisms regulate p27 proteolysis and cellular localization (1). Tyrosine phosphorylation of p27 in early G1 leads to loss of its inhibitory action toward Cdk2, facilitating Cdk2 activation and the subsequent phosphorylation of p27 at T187 by Cdk2, which promotes SCFSKP2-dependent p27 proteolysis (9, 10). Tyrosine phosphorylation of p27 is also required for activation of assembled cyclin D1–Cdk4–p27 complexes to promote G1 progression (4, 10, 11). In early G1, mitogens drive p27 export, and the cytoplasmic Kip1-ubiquitination-promoting-complex (KPC) mediates p27 degradation (12).
p27 contains nuclear export (13) and nuclear localization signals (NLS) (14), and its localization is cell cycle regulated. p27 contains 2 ACG kinase consensus motifs surrounding threonine-157 (T157) and threonine-198 (T198) and PI3K signaling regulates p27 localization (1, 15–18). PI3K is frequently activated in cancers by upstream receptor tyrosine kinases, PTEN deletion, or mutations of genes encoding PI3K and effector kinases (19). Although p27 is largely nuclear in normal cells, many human cancers exhibit cytoplasmic p27 mislocalization (1). Protein kinase B (PKB or AKT) and SGK1 are downstream PI3K effectors that phosphorylate the p27NLS at T157 (15–18), and their constitutive activation mislocalizes p27 to the cytoplasm (15, 18, 20) by impairing its nuclear import (15).
p90 ribosomal S6 kinase (RSK) is a serine/threonine kinase activated downstream of both MAPK and PI3K. RSK1 phosphorylates transcription factors, signaling kinases, and proapoptotic proteins to regulate cell survival and proliferation (21). RSK1 was shown to phosphorylate p27 in vitro (22), but the action of endogenous RSK1 on cellular p27 was not previously demonstrated and the in vivo relevance to p27 function remained unclear.
p27 binds cytoskeleton components to regulate cell shape and motility. p27-null mouse embryonic fibroblasts (MEFs) have decreased motility compared with wild-type MEFs. Reexpression of wild-type p27 or a mutant unable to bind cyclin-Cdks restored cell motility, thus the motility effects of p27 may be independent of its cell cycle function (23). p27 was shown to bind RhoA, inhibiting RhoA-GEF binding and RhoA-dependent ROCK1 activation, leading to increased cell motility (23). Cytoplasmic p21 also disrupts actin stress fiber stability by binding and inhibiting ROCK1 (24). Many cancers show cytoplasmic mislocalization of p27 (1). Although cytoplasmic p27 may disrupt actin stress fiber stability to promote motility and metastasis, the signaling pathways that regulate p27 effects on the cytoskeleton are not fully elucidated.
Present data suggest that RSK1 regulates p27 localization and RhoA-dependent cytoskeleton stability. Cellular RSK1 binds p27, and RSK1 overexpression mislocalizes p27 to the cytoplasm and increases cell motility. The increased cell motility and loss of stress fibers and RhoA-GTP in RSK1-transfectants are reversed by p27 knockdown. RSK1-mediated T198 phosphorylation of p27 increases binding to RhoA in vitro. These data support a model in which RSK1 phosphorylates p27, promoting p27-RhoA binding and RhoA inhibition. RSK1 may contribute to loss of actin stress fibers and increase cell motility after Ras/MAPK and PI3K activation in human cancers.
Results
RSK1 Activation and p27pT198 Increase in Early G1.
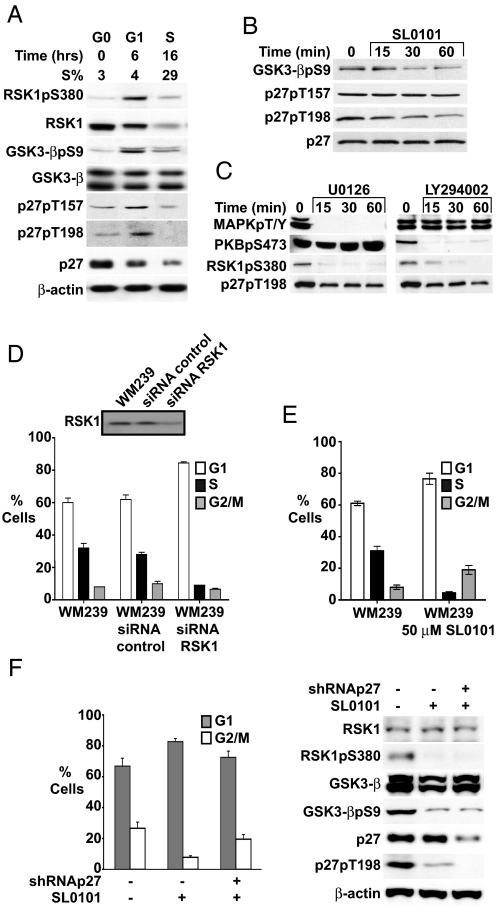
Transfected HA-RSK1 and FLAG-p27 coprecipitate in 293T cells, and RSK1 has been shown to phosphorylate p27 in vitro (22). To investigate further the in vivo relevance of RSK1 in p27 regulation, the cell cycle dependence of cellular RSK1 activation and p27 phosphorylation at putative RSK1 sites was assayed across the cell cycle. RSK1 activity, detected as activated RSK1pS380, was minimal in G0, peaked in G1, and decreased in S phase. RSK1 levels decreased during G1. Phosphorylation of GSK3-β, a known RSK1 substrate, followed RSK1 activation (Fig. 1A). p27 phosphorylated at T157 (p27pT157) and T198 (p27pT198) were minimal in G0, peaked in G1 as total p27 decreased, and fell with S phase entrance (Fig. 1A).
Fig. 1.
RSK1 is activated in early G1 and RSK inhibition decreases p27pT198. (A) WM35 was arrested in G0 and released into cycle, and cell cycle distribution and proteins were assayed at intervals thereafter. (B and C) WM239 cells were treated with 50 μM SL0101 (B), with 5 μM U0126 or 10 μM LY294002 (C) for times indicated and proteins were blotted. (D) WM239 cells were transfected with RSK1 siRNA or control oligonucleotides and cell cycle profiles were analyzed at 24 h. (Inset) RSK1 levels. (E) WM239 cells were mock treated or SL0101 treated for 24 h and cell cycle was analyzed by flow cytometry. Mean percentage cells ± SEM is graphed. (F) WM35 cells were infected with p27shRNA (+) or mock infected (−). One week later, 50 μM SL0101 was added for 48 h followed by analysis of cell cycle distribution and pRSK1, p27, and p27pT198 levels.
RSK1 Inhibition Rapidly Reduces p27pT198.
Because RSK1 activation coincides with p27pT157 and p27pT198 appearance in G1, we tested effects of RSK1 inhibition on these phosphorylated forms of cellular p27. The specific RSK1 inhibitor SL0101 (25) rapidly reduced GSK3-β phosphorylation and p27pT198 levels, whereas total p27 was unchanged (Fig. 1B). Of note, p27pT157 levels were not affected by RSK1 inhibition. In vitro action of recombinant RSK1 on His-p27WT was modestly reduced on the p27T157A substrate, and decreased by 50% toward p27T198A (Fig. S1). Thus although mutations converting T157 and T198 to alanine each reduce p27 phosphorylation by RSK1, the kinase shows a preference for T198 in vitro and only p27pT198 was affected by RSK1 inhibition in WM239 cells. RSK1 is activated by both MAPK and PI3K/PDK1. Both the MEK inhibitor U0126 and the PI3K inhibitor LY294002 inhibited RSK1 and rapidly decreased p27pT198 (Fig. 1C), without changing total p27 levels. Thus both direct and indirect RSK1 inhibition reduces p27pT198.
Prolonged RSK inhibition with SL0101 and RSK1 knockdown both caused G1 arrest (Fig. 1 D and E). This was at least in part p27 dependent because prior shRNA-mediated p27 knockdown impaired G1 arrest by SL0101 (Fig. 1F). Prior data also showed that RSK1–2 inhibition impairs cell proliferation (25, 26).
RSK1 Overexpression Increases p27pT198 and p27 Stability and Confers TGF-β Resistance.
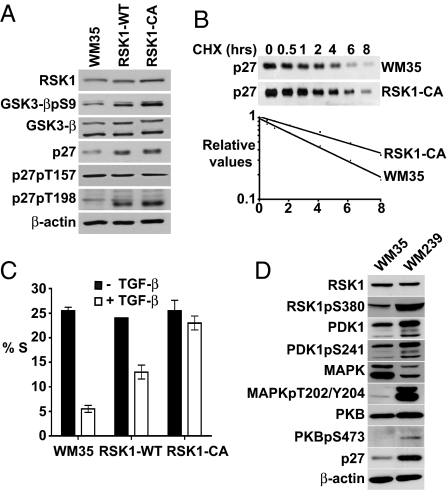
RSK1-WT- or hemagglutinin epitope (HA)-tagged RSK1-transfected WM35 clones (Fig. S2A) and WM35 populations transduced with pBabe RSK1-WT or constitutively active pBabe RSK1-CA showed increased RSK1, phospho-GSK3-β, p27 levels, with an even greater increase in p27pT198 (Fig. 2A). Although both T157 and T198 are in putative RSK1 consensus motifs in p27, only p27pT198 was increased in RSK1-WT and RSK1-CA cells (Fig. 2A), whereas p27pT157 was not. p27 stability was increased in RSK1-CA compared with WM35 (Fig. 2B).
Fig. 2.
RSK1 overexpression increases p27 levels, p27pT198, and p27 stability and confers TGF-β resistance. (A) Analysis of proteins in WM35, RSK1-WT, and RSK1-CA. (B) (Upper) Cycloheximide (CHX) chase of p27 in WM35 and RSK1-CA. (Lower) Linear regression of p27 protein decay. (C) Flow cytometry of WM35 and RSK1 transfectants treated with (□) or without (■) TGF-β for 48 h. Mean % S phase ± SEM is graphed. (D) Protein levels in WM35 and WM239 cells.
Transforming growth factor β (TGF-β) is a potent mediator of G1 arrest. Although multiple redundant cell cycle effects cause TGF-β arrest in normal cells, p27 is essential for TGF-β arrest in WM35 melanoma cells (27). RSK1-WT and RSK1-CA overexpression impaired G1 arrest by TGF-β (Fig. 2C). Additional RSK1 clones also showed increased p27 and TGF-β resistance (Fig. S2B). RSK1 levels and activity were compared in TGF-β-sensitive WM35 and resistant WM239 melanoma lines. PI3K is activated in WM239 by PTEN loss (15), and these cells have increased p27 and activation of PI3K-dependent PDK1, PKB, and RSK1 (Fig. 2D). WM239 also showed greater pMAPK than WM35. Both PI3K and MEK/MAPK activation would increase RSK1pS380 in TGF-β resistant WM239.
Although cyclin E, Cdk2, and Cdk4, and cyclin E-bound p27 levels were unchanged, p27, cyclin D1, and Cdk4-cyclin D1-p27 complexes were increased in RSK1-WT- and RSK1-CA-infected cells (Fig. S3) and in WM35-RSK1–3. The increased cyclin D1 is due in part to its stabilization by RSK1-mediated GSK3-β inhibition (Fig. 2A) because GSK3-β promotes cyclin D1 degradation (28). Accumulation of p27 in cyclin D1-Cdk4 complexes would shift the equilibrium away from binding and inhibition of cyclin E-Cdk2, contributing to TGF-β resistance. The accumulation of p27 in Cdk6 complexes after Ras transfection (29) may be due in part to Ras/PI3K-dependent RSK1 activation.
Cellular RSK1 Binds p27 and RSK1 Mislocalizes p27 to Cytoplasm.
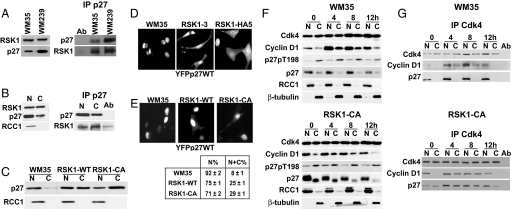
Because many kinase-substrate pairs interact, we assayed if cellular p27 and RSK1 coprecipitate. In both WM35 and WM239 cells, RSK1 coprecipitated with cellular p27 (Fig. 3A). p27-RSK1 complexes were more abundant in the cytoplasm than the nucleus in WM239 (Fig. 3B Right). RSK1-overexpressing cells showed abundant cytoplasmic cellular p27, whereas p27 was predominantly nuclear in parental WM35 cells (Fig. 3C). Transfected YFP-p27WT localized to nuclei in WM35 cells but was both nuclear and cytoplasmic in RSK1–3, RSK1-HA5, RSK1-WT, and RSK1-CA cells (Fig. 3 D and E).
Fig. 3.
RSK1 binds cellular p27, and RSK1 overexpression increases cytoplasmic p27. (A) (Left) RSK1 and p27 levels in asynchronous WM35 and WM239 cells. (Right) Cellular p27 precipitates show associated RSK1. (B) RSK1 and p27 (Left) and p27-bound RSK1 (Right) in nuclear (N) and cytosolic (C) WM239 fractions. Ab = antibody-only control. (C) p27 in N and C fractions of indicated lines. (D and E) Fluorescence microscopy of YFP-p27 in WM35 and RSK1 clones. (E Lower) Quantitation of cells with nuclear only (N%) or nuclear and cytoplasmic YFPp27WT (N+C%) fluorescence. (F and G) WM35 and RSK1-CA cells were synchronized in G0, then released into cycle and collected at intervals. Nuclear (N) and cytoplasmic (C) fractions were assayed by Western blotting for cellular Cdk4-bound cyclin D1 and p27.
The localization of cellular p27pT198 and p27-cyclin D1-Cdk4 complexes was assayed in WM35 and in RSK1-CA cells released from serum starvation. Although p27 was largely nuclear in WM35, RSK1-CA had increased cytoplasmic p27 at all time points. In contrast, p27pT198 was predominantly cytoplasmic in both lines (Fig. 3F). In parental WM35, p27-cyclin D1-Cdk4 complexes detected in early to mid-G1 were predominantly nuclear. With constitutive RSK1 overexpression, p27-cyclin D1-Cdk4 complexes were increased (Fig. S3C) and detected in both nucleus and cytoplasm (Fig. 3G).
RSK1 Overexpression Inhibits RhoA and Increases Cell Motility in a p27-Dependent Manner.
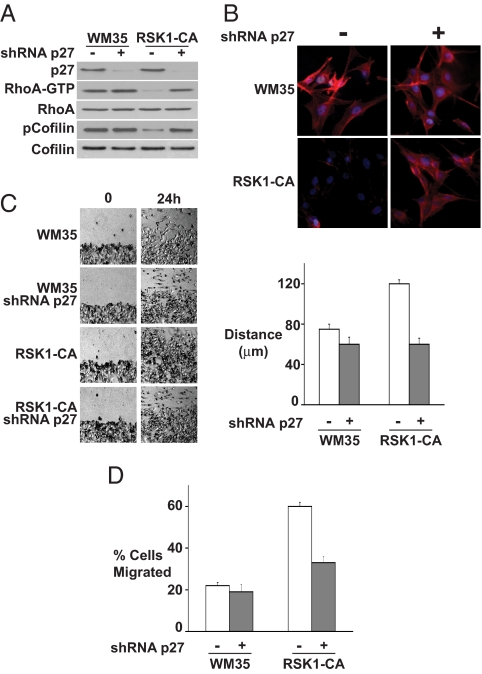
Because RSK1 increases cytoplasmic p27, we assayed the potential influence of RSK1-mediated T198 phosphorylation of p27 on cell motility. RSK1-CA showed decreased RhoA-GTP and decreased phosphorylation of the RhoA/ROCK1 substrate, cofilin, compared with WM35 (Fig. 4A). In RSK1-CA, p27 knockdown restored RhoA-GTP and p-cofilin to levels seen in WM35. To further evaluate RhoA pathway integrity, actin stress fibers were visualized. In early G1, actin stress fibers were dramatically reduced in RSK1-CA compared with WM35. p27 knockdown restored phalloidin staining of actin stress fibers in RSK1-CA to levels similar to those in WM35 (Fig. 4B). Thus RSK1 mediates p27-dependent RhoA pathway inhibition and loss of actin stress fibers.
Fig. 4.
RhoA inhibition and increased cell motility in RSK1-CA cells are reversed by p27 knockdown. WM35 was mock infected (−) or infected with lentiviral shRNA to p27 (+) before all assays. (A) GTP-bound RhoA and indicated proteins in asynchronous WM35 and RSK1-CA. (B) Actin stress fibers shown by phalloidin staining (red) 4 h after release from G0. DAPI stains cell nuclei (blue). (C) (Left) Photomicrographs show cell migration after wounding of cell monolayer. (Right) Mean migration distances (±SEM). (D) Mean cell numbers (±SEM) that migrated through a transwell chamber within 15 h.
RSK1-CA cells also showed increased cell migration and motility, which were reversed by p27 knockdown. Cell migration was assayed after “wounding” of a confluent cell monolayer. At 24 h after wounding, RSK1-CA migration was significantly greater than WM35 (Fig. 4C). RSK1-CA also had a 3-fold greater migration in a modified Boyden chamber transwell assay compared with WM35 (Fig. 4D). p27 shRNA dramatically reduced migration in both wound healing and transwell assays (Fig. 4 C and D). Thus RhoA inhibition in RSK1-CA cells contributes to increased cell motility, in large part due to p27 misregulation.
RSK1 Increases p27 Binding to RhoA.
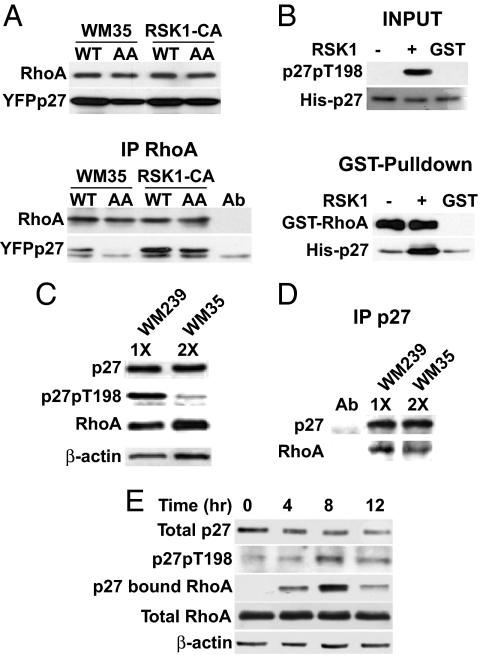
To investigate further how RSK-1 increases p27-dependent cell motility, YFPp27WT and YFPp27T157A/T198A (p27AA) were transiently transfected, and their binding to cellular RhoA was assayed. Although p27AA was modestly reduced in WM35 compared with p27WT (Fig. 5A Upper), RhoA-bound p27AA was less than would be expected due to differences in p27WT and p27AA levels alone (Fig. 5A Lower). RSK1-CA cells showed increased RhoA-p27 complexes, but less p27AA bound RhoA than p27WT (Fig. 5A). RhoA-p27AA complexes were reduced in both lines compared with RhoA-p27WT (Fig. 5A). The binding of nonphosphorylatable p27 (p27AA) to RhoA was reduced but not abolished, suggesting that p27 phosphorylation regulates the extent or stability of p27-RhoA interaction.
Fig. 5.
p27 T198 phosphorylation increases RhoA binding. (A) WM35 and RSK1-CA were transfected with YFP-p27WT (WT) or YFP-p27T157A/T198A (AA). Cellular RhoA and YFP-p27 (Upper) and RhoA-bound YFPp27 (Lower) are shown. (B) His-p27 was treated (+) or not (−) with RSK1 in vitro, then recovered and incubated with GST-RhoA or control (GST). RhoA-bound His-p27 was detected by GST pull-down. His-p27 and p27pT198 inputs are shown. (C and D) Cellular p27 was titrated in WM35 and WM239 to allow precipitation of equal amounts of p27. p27 in WM35 was roughly half that in WM239. (C) Western blots show equal p27 in 10 μg of WM239 lysate (1X) and 20 μg of WM35 lysate (2X). Input p27pT198, RhoA, and β-actin levels are shown. (D) Equal p27 amounts were precipitated and resolved, and associated RhoA was blotted. (E) WM35 cells were synchronized in G0 and released into cycle at 0 h. At 4-h intervals thereafter, total p27, p27pT198, RhoA, and β-actin were blotted and p27-bound RhoA was detected.
To test how p27 phosphorylation by RSK1 affects p27-RhoA binding in vitro, recombinant His-p27WT was reacted with RSK1 kinase. Reaction mixtures were then boiled to inactivate RSK1 and heat-stable His-p27WT was recovered and incubated with GST-RhoA. RSK1 treatment increased p27pT198 phosphorylation (Fig. 5B, input), and increased p27 binding to GST-RhoA in vitro (Fig. 5B, GST-pulldown).
Both p27pT198 and p27-RhoA Complexes Increase in Early G1.
Endogenous p27-RhoA complexes were also detected in WM239. The PTEN-deleted WM239 line shows activation of PI3K effectors (Fig. 2D), higher p27 levels than in WM35, and cytoplasmic p27 mislocalization (15). Cell lysates of WM35 and WM239 were titrated to obtain equal amounts of p27. To ensure precipitation of equal amounts of p27, 2 times more WM35 lysate (2×) was used than WM239 lysate (1×); see Fig. 5C, β-actin blot. Although the input of total lysate for WM239 was half that for WM35, p27pT198 was greater in the WM239 lysate input (Fig. 5C). When equal amounts of cellular p27 were precipitated, WM239 showed higher p27-bound RhoA than WM35 (Fig. 5D).
To further evaluate p27-RhoA binding across the cell cycle, WM35 cells were synchronized by serum starvation and then released at time = 0 h and lysates were recovered at the intervals shown. Although total p27 levels fell during G1 progression, both p27pT198 and p27-bound RhoA increased, peaking at 8 h and then falling in late G1 (Fig. 5E).
Discussion
RSK1 is an effector of both the Ras-PI3K and Ras-MAPK pathways and is phosphorylated by both PDK1 and MAPK. It plays roles in transcription, translation, and mitogenesis (21). Prior evidence has implicated RSK1 as a regulator of p27. RSK1 was shown to phosphorylate p27 in vitro and when overexpressed after transfection, RSK1-p27 complexes were detected in 293 cells (22). Here we demonstrate that cellular p27 and RSK1 coprecipitate and that RSK1 activation and p27 phosphorylation at T198 show similar kinetics in G1. Moreover, specific inhibition of RSK1 rapidly reduced p27pT198 levels and caused a p27-dependent G1 arrest.
p27 can be phosphorylated at T198 in vitro by a number of kinases, including RSK1, AKT, and AMPK (22, 30–32). p27 phosphorylation at T198 appears to increase p27 stability. p27T198A has a shorter t1/2 than p27WT in transfected fibroblasts and in p27T198A knock-in MEFs (33). In addition, nutrient-deprived cells showed AMPK-dependent elevation of p27pT198 and increased p27 stability (31), and transfected p27T198D was stable and p27T189A unstable in MCF-7 cells (31). Because T198 phosphorylation stabilizes p27, the increased p27 stability in RSK1-CA cells is consistent with the notion that RSK1 phosphorylates cellular p27 at T198.
The effect of T198 phosphorylation on p27 localization appears to be cell-type dependent and influenced by coordinate activation of different signaling pathways. In quiescent cells, under conditions that stimulate autophagy and AMPK activation, stable p27pT198 accumulates in cell nuclei (31). p27pT198 was elevated in RSK1-CA compared with the parental WM35 melanoma lines used herein and was predominantly cytoplasmic, and levels increased in early G1 and declined in late G1. In primary human breast cancers with activated PKB/AKT, p27pT198 is detected only in the cytoplasm (32), and transfected p27T198D localizes to the cytoplasm (22, 31). Thus in contrast to effects of AMPK, activation of RSK1 and PKB/AKT both appear to increase p27pT198 and shift p27 to the cytoplasm. Although concomitant phosphorylation of p27 at T157 would reduce nuclear p27 import (15, 20), none of the RSK1 transfectants showed an increase in p27pT157, and RSK1 inhibition did not reduce p27pT157 levels.
Our prior work indicated that p27 phosphorylation by PKB/AKT mediates an increase in cyclin D1-Cdk4-p27 complexes and support a model in which AGC-mediated phosphorylation of p27 increases cyclin D1-Cdk4-p27 assembly, whereas tyrosine phosphorylation of p27 is required for kinase activation (4). Here we show that constitutive RSK1-overexpressing cells have increased cyclin D1-Cdk4-p27 complexes and, in contrast to WM35 where cyclin D1-Cdk4-p27 complexes are predominantly nuclear, these complexes are present in both nucleus and cytoplasm in RSK1-CA. It is not clear whether this represents accumulation of nonfunctional cytoplasmic p27-cyclin D1-Cdk4 or whether this reflects constitutive complex formation (and potentially activation) overwhelming the nuclear import mechanism. The latter may be more likely because other lines with constitutive Ras (29) or PKB/AKT activation (34) not only exhibit increased cyclin D1-Cdk4/6-p27 complexes but also show higher cyclin D1-Cdk activity. Stable cytoplasmic p27pT198 may acquire an oncogenic gain of function to promote cyclin D1-Cdk4 assembly and also to regulate cancer cell motility.
It has been proposed that p27 may contribute to oncogenic progression through an increase in cell motility. Nagahara et al. (35) showed that transduction of TAT-p27 in HepG2 hepatocellular carcinoma cells promotes cell migration. The C-terminal portion of cytoplasmic p27 was subsequently found to be required for activation of migration, but the mechanism thereof remained unclear (36). Besson et al. (23) showed that p27 and RhoA interact both in vitro and in RhoA-transfected cells, and p27 interferes with RhoA activation by impairing the interaction between RhoA and its activator, the guanosine-nucleotide exchange factor (GEF).
The present data provide evidence that T198 phosphorylation of p27 may facilitate the interaction of p27 with RhoA. RSK1-CA cells not only exhibited an increase in cytoplasmic p27 they also showed increased RhoA-bound p27. p27T157A/T198A bound poorly to cellular RhoA compared with p27WT, and phosphorylation of p27 by RSK1 increased p27 binding to RhoA in vitro, suggesting that p27 phosphorylation positively regulates p27–RhoA interaction. We also demonstrate the presence of endogenous RhoA in cellular p27 complexes. Cellular p27–RhoA complexes were increased, as was the extent of p27 phosphorylation at T198 in the PTEN-deleted WM239 line compared with WM35. Moreover, T198 phosphorylation of p27 and cellular p27–RhoA complexes increased with similar kinetics during early G1, while total p27 levels fell. RSK1-CA cells showed reduced actin stress fibers, reduced RhoA-GTP and p-cofilin, and an increase in cell migration and motility, all of which were reversed by knockdown of p27. These data support a model in which RSK1-mediated phosphorylation of p27 at T198 increases p27–RhoA interaction to inhibit the RhoA pathway and reduce actin cytoskeleton stability. This may play a role in G1 to prepare cells for the shape changes that must occur during later phases of the cell cycle. In addition, constitutive phosphorylation of p27 at T198 in cancer cells would increase p27 stability and mislocalize p27 to the cytoplasm to promote cell migration and motility.
The effect of p27 on cell motility and migration has been a subject of controversy. In fibrosarcoma cells, p27 was shown to reduce cell motility by binding and inhibiting stathmin, a protein that promotes microtubule depolymerization (37). Because p27 phosphorylation at T198 appears to promote its interaction with RhoA, the binding of p27 to stathmin may also be regulated by phosphorylation via cell-type dependent signaling.
Cytoplasmic mislocalization of p27 has been reported in many human tumors and is most commonly associated with adverse patient outcome (1). In up to 40% of primary human breast cancers, cytoplasmic p27 is seen in association with PKB/AKT activation (15–17). In human breast cancers, p27pT198 was detected exclusively in the cytoplasm (33). Cytoplasmic p27 was associated with poor prognosis in acute myelogenous leukemia (AML) (38) and in pancreatic (39), ovarian (40), prostate (41), and breast carcinomas (15). The notion that excess cytoplasmic p27 may actively contribute to malignant progression is supported by observations that expression of cytoplasmic p27 (NESp27) increased cell motility in vitro and increased metastasis in a melanoma xenograft model (42). Expression of p27ΔNLS, a mutant that localizes to the cytoplasm, decreased RhoA activation and increased cell motility in MCF-7 cells (43). In knock-in animals, p27CK- localized to cytoplasm and enhanced proliferation of progenitor cell populations to promote tumorigenesis (44).
Multiple effectors downstream of PI3K cooperate to regulate p27. The present data, together with prior observations, support a role for RSK1 in this process. RSK1 activation in early G1 is temporally linked to the increase in p27pT198. Drug-mediated RSK inhibition rapidly decreased p27pT198 and cells expressing constitutively activated RSK1 (RSK1-CA) showed increased, predominantly cytoplasmic p27pT198, whereas mutation of T198 attenuated in vitro phosphorylation by RSK1. Oncogenic MAPK- and PI3K-mediated RSK1 activation may constitute one mechanism underlying cytoplasmic p27 mislocalization, leading to reorganization of the actomyosin cytoskeleton and increased cellular motility and metastatic potential in human cancers. Therapeutic inhibition of RSK may not only inhibit cell proliferation (25, 26) but also oppose molecular mechanisms that drive cancer cell metastasis.
Materials and Methods
Cell Culture.
WM35, its RSK1-transfectants, and WM239 cells were cultured as described by Liang et al. (15). WM35 and RSK1-CA were starved (0.1% FBS) for 48 h, stimulated with 5% FBS, and then recovered for protein and flow cytometry. Cells were treated with 10 μM LY294002 (Biomol Research Labs), 5 μM UO126 (Promega), or 50 μM SL0101 (25) for indicated times. TGF-β (2.5 ng/mL) treatment was 48 h.
Antibodies.
Antibodies to phosphorylated and total RSK1, GSK3-β, PKB, PDK1, and MAPK were from Cell Signaling; to Cdk4, p27, Cdk2, total GSK3-β, RhoA, p-cofilin, total cofilin, and RCC1 were from Santa Cruz Biotechnology; to p27 were from Transduction Laboratories; to p27pT157 and p27pT198 (31) were from R & D; to cyclin D1 and Cdk4 were from Lab Vision; and to β-actin were from Sigma. Cyclin E1 antibodies E12 and E172 were from E. Harlow (Harvard University, Boston, MA). p27 was precipitated with SC-19 (Santa Cruz Biotechnology) and p27-bound RhoA was detected with α-RhoA mAb from Abcam.
Immunoprecipitation and Western Blotting.
Precipitation and blotting used 200 and 50 μg of protein lysate, respectively, as described by Liang et al. (15). Antibody-alone controls were run with all immunoprecipitations, and β-actin verified equal loading. Cellular p27pT198 was detected either by direct blotting of 50–100 μg of lysate with the anti-p27pT198 or by precipitation with p27pT198 antibody (500 μg of lysate per immunoprecipitation) and immunoblotting with total p27 antibody. To detect cellular p27-bound RhoA, p27 precipitates from 1.5 mg of WM239 and 3 mg of WM35 lysates were resolved and RhoA blotted (Abcam). Nuclear/cytoplasmic fractionation was as in Connor et al. (13). Equal cell volumes of nuclear and cytosolic fractions were used for Western blots and for Cdk4 immunoprecipitation blots. Nuclear RCC1 served as fractionation control.
Plasmids and Transfections.
pcDNA3-RSK1 (45) or pMT2-RSK1HA (46) was transfected into WM35 with Lipofectamine PLUS (GIBCO) and clones were selected. RSK1-WT and RSK1-CA were cut from pLPCX-RSK1WT and pLPCX-RSK1CA (47), respectively, with SnaBI and EcoRI, and ligated into pBABE-puro. pBABE-RSK1-WT or pBABE-RSK-1CA were transfected into Phoenix-AMPHO cells. WM35 was infected with virus and selected with puromycin.
Three different siRNA oligonucleotides to RSK1 or nontargeting siRNA controls (Dharmacon) were transfected with Lipofectamine 2000 (Invitrogen). Cells were assayed at 24 h. YFP-p27T57A/T198 was prepared by site-directed mutagenesis of YPFp27WT and transient transfection was as in ref. 15. Fluorescence microscopy was at 24 h after transfection, unless otherwise specified.
Lentiviral shRNA Production and Infection.
Lentivirus vector encoding p27 shRNA was prepared and cells infected as described in ref. 18.
Flow Cytometry.
BrdU labeling and flow cytometry were as described in ref. 15.
Cycloheximide Chase.
Cells were treated with 100 μg/mL cycloheximide and lysed at 0, 0.5, 1, 2, 4, 6, and 8 h. β-Actin blots verified equal loading. p27 was quantitated by densitometry in multiple ECL exposures and p27 t1/2 was measured by linear regression.
RSK1 Kinase Assays.
RSK1 (2.5 ng; Millipore/Upstate) was incubated with 5 μg of His-p27 in 20 μL of kinase buffer (25 mM Tris, pH 7.5, 5 mM glycerol 2-phosphate, 01 mM sodium vanadate, 10 mM MgCl2, 2 mM DTT) at 30 °C for 15 min with 10 μCi of [γ-32P]ATP, products were resolved, and radioactivity was quantified by PhosphorImager. Substrate and kinase concentrations used provided a linear increase in RSK1 action on p27 over time.
Migration Assays.
A linear scratch/wound was made on cell monolayers with a sterile pipette. Photomicrographs were taken of live cells (10× objective) over time and distance migrated was measured by using Photoshop CS3 software (1 μm = 1 pixel).
Invasion Assays.
Cells (105) were seeded in the upper chamber of a 1% gelatin-coated transwell membrane (Corning). At 15 h, cells were fixed in 90% ethanol (10 min) and stained with 1% crystal violet (10 min). Cells in the lower chamber were eluted with 10% acetic acid for 10 min and cell number was determined by OD at 595 nm.
Actin Stress Fiber Staining.
Cells were grown on glass coverslips for 24 h and serum starved (0.1% FBS) 24 h, then medium with 5% FBS was added for 4 h. Cells were fixed in 4% paraformaldehyde for 20 min at 22 °C and permeablized with 0.1% Triton X-100 for 5 min at 22° C. Actin stress fibers were visualized by TRITC-conjugated phalloidin staining with a Chemicon staining kit (Chemicon International). Nuclei were DAPI stained as in ref. 18.
Rho-GTP Pull-Down Assays.
GTP-bound Rho was assayed with Rho assay reagent (Millipore) by lysis in Rho buffer (25 mM Hepes, pH 7.5, 150 mM NaCl, 1% Igepal CA630, 10 mM MgCl2, 1 mM EDTA, 10% glycerol) plus protease inhibitors. Five hundred micrograms of cleared lysate was incubated with 25 μg of Rho assay slurry (contains Rhotekin RBD agarose) or GST-bound Sepharose at 4 °C for 1 h. Beads were washed 3 times in Rho buffer and resuspended in 2× Laemmli buffer, and GTP-bound RhoA was blotted.
GST Pull-Down Assay.
His-p27 was incubated with 100 ng of GST-RhoA (Chemicon International) or GST-bound Sepharose in 500 μL of Rho buffer plus protease inhibitors at 4 °C for 2 h. Beads were rinsed 3 times in Rho buffer and proteins were blotted.
Supplementary Material
Acknowledgments.
This work was supported by National Cancer Institute Grant 1R01CA105118-01 to J.M.S. and by National Institutes of Health/National Cancer Institute predoctoral fellowship 5F31CA113284 to M.D.L. The Doris Duke Charitable Foundation supports J.M.S., F.H., and S.A.W.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805057106/DCSupplemental.
References
- 1.Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: Prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 2.LaBaer J, et al. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- 3.Cheng M, et al. The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 1999;18:1571–1583. doi: 10.1093/emboj/18.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larrea MD, et al. Phosphorylation of p27Kip1 regulates assembly and activation of cyclin D1-Cdk4. Mol Cell Biol. 2008;28:6462–6472. doi: 10.1128/MCB.02300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hengst L, Reed SI. Translational control of p27Kip1 accumulation during the cell cycle. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- 6.Millard SS, et al. Enhanced ribosomal association of p27(Kip1) mRNA is a mechanism contributing to accumulation during growth arrest. J Biol Chem. 1997;272:7093–7098. doi: 10.1074/jbc.272.11.7093. [DOI] [PubMed] [Google Scholar]
- 7.Pagano M, et al. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 8.Gillies JK, Lorimer IAJ. Regulation of p27Kip1 by miRNA 221/222 in glioblastoma. Cell Cycle. 2007;6:2005–2009. doi: 10.4161/cc.6.16.4526. [DOI] [PubMed] [Google Scholar]
- 9.Chu I, et al. p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell. 2007;128:281–294. doi: 10.1016/j.cell.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimmler M, et al. Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell. 2007;128:269–280. doi: 10.1016/j.cell.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 11.James M, Ray A, Leznova D, Blain SW. Differential modification of p27Kip1 controls its cyclin D-cdk4 inhibitory activity. Mol Cell Biol. 2007;28:498–510. doi: 10.1128/MCB.02171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamura T, et al. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27(Kip1) at G1 phase. Nat Cell Biol. 2004;6:1229–1235. doi: 10.1038/ncb1194. [DOI] [PubMed] [Google Scholar]
- 13.Connor MK, et al. CRM1/Ran-mediated nuclear export of p27(Kip1) involves a nuclear export signal and links p27 export and proteolysis. Mol Biol Cell. 2003;14:201–213. doi: 10.1091/mbc.E02-06-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng Y, Hirano K, Hirano M, Nishimura J, Kanaide H. Minimal requirements for the nuclear localization of p27Kip1, a cyclin-dependent kinase inhibitor. Biochem Biophys Res Commun. 2000;274:37–42. doi: 10.1006/bbrc.2000.3098. [DOI] [PubMed] [Google Scholar]
- 15.Liang J, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8:1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- 16.Viglietto G, et al. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med. 2002;8:1136–1144. doi: 10.1038/nm762. [DOI] [PubMed] [Google Scholar]
- 17.Shin I, et al. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8:1145–1152. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- 18.Hong F, et al. mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol Cell. 2008;30:701–711. doi: 10.1016/j.molcel.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 19.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 20.Sekimoto T, Fukumoto M, Yoneda Y. 14-3-3 suppresses the nuclear localization of threonine 157-phosphorylated p27(Kip1) EMBO J. 2004;23:1934–1942. doi: 10.1038/sj.emboj.7600198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujita N, Sato S, Tsuruo T. Phosphorylation of p27Kip1 at threonine 198 by p90 ribosomal protein S6 kinases promotes its binding to 14-3-3 and cytoplasmic localization. J Biol Chem. 2003;278:49254–49260. doi: 10.1074/jbc.M306614200. [DOI] [PubMed] [Google Scholar]
- 23.Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 2004;18:862–876. doi: 10.1101/gad.1185504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S, Helfman DM. Cytoplasmic p21Cip1 is involved in Ras-induced inhibition of the ROCK/LIMK/cofilin pathway. J Biol Chem. 2004;279:1885–1891. doi: 10.1074/jbc.M306968200. [DOI] [PubMed] [Google Scholar]
- 25.Smith JA, et al. Identification of the first specific inhibitor of p90 ribosomal S6 kinase (RSK) reveals an unexpected role for RSK in cancer cell proliferation. Cancer Res. 2005;65:1027–1034. [PubMed] [Google Scholar]
- 26.Clark DE, et al. The serine/threonine protein kinase, p90 ribosomal S6 kinase, is an important regulator of prostate cancer cell proliferation. Cancer Res. 2005;65:3108–3116. doi: 10.1158/0008-5472.CAN-04-3151. [DOI] [PubMed] [Google Scholar]
- 27.Donovan JC, Rothenstein JM, Slingerland JM. Non-malignant and tumor-derived cells differ in their requirement for p27Kip1 in transforming growth factor-beta-mediated G1 arrest. J Biol Chem. 2002;277:41686–41692. doi: 10.1074/jbc.M204307200. [DOI] [PubMed] [Google Scholar]
- 28.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, et al. Disruption of TGF-beta growth inhibition by oncogenic ras is linked to p27Kip1 mislocalization. Oncogene. 2000;19:5926–5935. doi: 10.1038/sj.onc.1203991. [DOI] [PubMed] [Google Scholar]
- 30.Nacusi LP, Sheaff RJ. Akt1 sequentially phosphorylates p27kip1 within a conserved but non-canonical region. Cell Div. 2006;1:11. doi: 10.1186/1747-1028-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang J, et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 32.Motti ML, De Marco C, Califano D, Fusco A, Viglietto G. Akt-dependent T198 phosphorylation of cyclin-dependent kinase inhibitor p27(kip1) in breast cancer. Cell Cycle. 2004;3:e89–e95. [PubMed] [Google Scholar]
- 33.Kossatz U, et al. C-terminal phosphorylation controls the stability and function of p27kip1. EMBO J. 2006;25:5159–5170. doi: 10.1038/sj.emboj.7601388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciarallo S, et al. Altered p27(Kip1) phosphorylation, localization, and function in human epithelial cells resistant to transforming growth factor beta-mediated G(1) arrest. Mol Cell Biol. 2002;22:2993–3002. doi: 10.1128/MCB.22.9.2993-3002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagahara H, et al. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med. 1998;4:1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- 36.McAllister SS, Becker-Hapak M, Pintucci G, Pagano M, Dowdy SF. Novel p27(kip1) C-terminal scatter domain mediates Rac-dependent cell migration independent of cell cycle arrest functions. Mol Cell Biol. 2003;23:216–228. doi: 10.1128/MCB.23.1.216-228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baldassarre G, et al. p27(Kip1)-stathmin interaction influences sarcoma cell migration and invasion. Cancer Cell. 2005;7:51–63. doi: 10.1016/j.ccr.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 38.Min YH, et al. Cytoplasmic mislocalization of p27Kip1 protein is associated with constitutive phosphorylation of Akt or protein kinase B and poor prognosis in acute myelogenous leukemia. Cancer Res. 2004;64:5225–5231. doi: 10.1158/0008-5472.CAN-04-0174. [DOI] [PubMed] [Google Scholar]
- 39.Fukumoto A, et al. Prognostic significance of localized p27Kip1 and potential role of Jab1/CSN5 in pancreatic cancer. Oncol Rep. 2004;11:277–284. [PubMed] [Google Scholar]
- 40.Rosen DG, et al. Subcellular localization of p27kip1 expression predicts poor prognosis in human ovarian cancer. Clin Cancer Res. 2005;11:632–637. [PubMed] [Google Scholar]
- 41.Li R, et al. Biological correlates of p27 compartmental expression in prostate cancer. J Urol. 2006;175:528–532. doi: 10.1016/S0022-5347(05)00151-5. [DOI] [PubMed] [Google Scholar]
- 42.Denicourt C, Saenz CC, Datnow B, Cui XS, Dowdy SF. Relocalized p27(KiP1) tumor suppressor functions as a cytoplasmic metastatic oncogene in melanoma. Cancer Res. 2007;67:9238–9243. doi: 10.1158/0008-5472.CAN-07-1375. [DOI] [PubMed] [Google Scholar]
- 43.Wu FY, et al. Reduction of cytosolic p27(Kip1) inhibits cancer cell motility, survival, and tumorigenicity. Cancer Res. 2006;66:2162–2172. doi: 10.1158/0008-5472.CAN-05-3304. [DOI] [PubMed] [Google Scholar]
- 44.Besson A, et al. Discovery of an oncogenic activity in p27Kip1 that causes stem cell expansion and a multiple tumor phenotype. Genes Dev. 2007;21:1731–1746. doi: 10.1101/gad.1556607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schouten GJ, et al. I kappa B alpha is a target for the mitogen-activated 90 kDa ribosomal S6 kinase. EMBO J. 1997;16:3133–3144. doi: 10.1093/emboj/16.11.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grove JR, et al. Regulation of an epitope-tagged recombinant Rsk-1 S6 kinase by phorbol ester and Erk/Map kinase. Biochemistry. 1993;32:7727–7738. doi: 10.1021/bi00081a018. [DOI] [PubMed] [Google Scholar]
- 47.Eisenmann KM, VanBrocklin MW, Staffend NA, Kitchen SM, Koo HM. Mitogen-activated protein kinase pathway-dependent tumor-specific survival signaling in melanoma cells through inactivation of the proapoptotic protein bad. Cancer Res. 2003;63:8330–8337. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.