Abstract
Osteopontin is a phosphorylated glycoprotein secreted to the mineralizing extracellular matrix by osteoblasts during bone development. It is believed to facilitate the attachment of osteoblasts and osteoclasts to the extracellular matrix, allowing them to perform their respective functions during osteogenesis. Several other functions have been suggested for this protein, and its up-regulation is associated with various disease states related to calcification, including arterial plaque formation and the formation of kidney stones. Although expression of this gene has been demonstrated in multiple tissues, its regulation is not well understood. Our previous studies on the roles of the retinoblastoma protein (pRB) and p300/CBP in the regulation of osteoblast differentiation revealed a link between osteopontin induction and the synthesis of alkaline phosphatase. In this paper, we describe results specifically linking induction of osteopontin to the enzymatic activity of alkaline phosphatase in the medium, which results in the generation of free phosphate. This elevation of free phosphate in the medium is sufficient to signal induction of osteopontin RNA and protein. The strong and specific induction of osteopontin in direct response to increased phosphate levels provides a mechanism to explain how expression of this product is normally regulated in bone and suggests how it may become up-regulated in damaged tissue.
As cells undergo terminal differentiation, various markers are induced in an ordered and sequential manner, but required steps in the induction process are not always clear because individual events in the sequence are not easily separable. The biology of the DNA tumor virus oncogene, adenovirus E1A, points to the cellular E1A targets, retinoblastoma protein (pRB) and p300/CBP, as important regulators of gene expression during terminal differentiation (1–4). We have taken advantage of E1A genetics to explore the roles of the pRB and p300/CBP protein families in expression of early and late markers during osteoblast differentiation. The MC3T3-E1 cell line is derived from newborn mice calvaria (5). It is an established cell line, but the cells maintain much of the tightly linked controls between proliferation and differentiation that usually are seen only in primary cells (6). Treatment with ascorbic acid stimulates these cells to differentiate along the osteoblast line (6–8). Induced cells deposit a collagenous extracellular matrix, accompanied by the activation of specific genes associated with the osteoblast phenotype, such as alkaline phosphatase, osteocalcin, and osteopontin. If a source of organic phosphate such as β-glycerol phosphate is present, a discrete zone of hydroxyapatite-containing mineral is formed within the collagen fibrils. The sequence from induction to mineralization proceeds in a tightly regulated order over a span of 2 to 3 weeks (see schematic in Fig. 1), which permits a detailed analysis of the order of events.
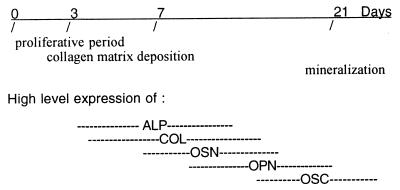
Figure 1.
Temporal expression pattern of markers typical of osteoblast development in MC3T3-E1 cells. The cells progress through three general phases: (i) proliferation, (ii) extracellular matrix deposition and maturation, and (iii) mineralization. High-level expression of genes thought to contribute to the differentiated state of osteoblasts occurs at discrete time points during the differentiation process. Alkaline phosphatase (ALP), α1-collagen (COL), and osteonectin (OSN) are expressed at high levels near the end of the proliferative period and during the period of extracellular matrix deposition and maturation. Genes expressed at or near the time of mineralization include osteopontin (OPN) and osteocalcin (OSC).
We previously have characterized two MC3T3-E1 cell lines stably expressing the wild-type E1A 12S-cDNA splice product (E1A 12S.WT) (9). The 12S-cDNA product contains all of the cell cycle and differentiation-regulating functions of the E1A gene. The E1A proteins directly bind and inhibit the functions of the pRB and p300/CBP protein families. E1A expression can therefore be used as a physiological inhibitor of their functions. The E1A-expressing MC3T3-E1 cells failed completely to mineralize in response to ascorbic acid. Expression of the early-stage marker, alkaline phosphatase, an outer cytoplasmic membrane-localized enzyme, was barely detectable and was not induced in response to ascorbic acid. However, induction of typical later-stage markers, including type I collagen and osteocalcin, was relatively normal, indicating that their expression is regulated through pRB/p300-independent pathways. The major late-stage product affected by E1A 12S.WT expression coordinately with alkaline phosphatase was osteopontin.
In this paper, we describe results specifically linking induction of osteopontin to the enzymatic activity of alkaline phosphatase, which results in the generation of free phosphate in the extracellular medium. Active transport of the phosphate across the cell membrane signals induction of osteopontin at the RNA and protein levels. The ability of cells to transport phosphate has long been recognized as a requirement for mineralization in bone. The strong and specific induction of osteopontin in direct response to increased phosphate levels indicates that the phosphate signal is part of a tightly coordinated program in which osteopontin expression is tied to expression of alkaline phosphatase, which in turn is positively regulated by the central control proteins of the pRB and p300/CBP families.
Materials and Methods
Materials.
Penicillin and streptomycin were purchased from Mediatech (Herndon, VA). Ascorbic acid; β-glycerol phosphate; sodium phosphate mono- and dibasic; Tris[hydoxymethyl]aminomethane (Trizma) -base, -sulfate, and -phosphate; phosphonoformic acid (foscarnet); alizarin red S; acetyl-CoA; and protease inhibitors were obtained from Sigma. Sodium sulfate was purchased from ICN, and radiochemicals were obtained from NEN.
Cell Culture.
Low-passage MC3T3-E1 cells were a gift from Roland Baron (Yale University, New Haven, CT). Cells were maintained in αMEM (Irvine Scientific) plus 10% FBS (Summit Biotechnology, Ft. Collins, CO) as described previously (9). Differentiation was induced by addition of 50 μg/ml final concentration ascorbic acid and 10 mM final concentration β-glycerol phosphate to standard growth medium. Where indicated, calf intestinal alkaline phosphatase (New England Biolabs) was added to the medium at a final enzyme concentration ranging from 0.5 to 1.0 units/ml. The inducing agents were replaced with each medium change (3–4 days). Isolation of the stable transfectant lines has been described previously (9). F9 cells, purchased from American Type Culture Collection (CRL 1720), were grown in DMEM supplemented with 15% FBS and penicillin and streptomycin on 0.1% gelatin (Sigma)-coated plates. NIH 3T3 cells, kindly provided by Scott Shore (Temple University), were grown in DMEM supplemented with penicillin and streptomycin and 10% FBS.
Northern Blots.
Total cell RNA was prepared by using Trizol Reagent (GIBCO/BRL) or Tri Reagent (Sigma) according to manufacturer's recommendations. RNA (20 μg) was loaded per lane and separated by electrophoresis through a 1% formaldehyde-agarose gel. The RNA was transferred to a Hybond-N nylon membrane (Amersham Life Science) and crosslinked by UV irradiation and baking at 80°C. 32P-labeled probes were prepared with a random prime labeling kit (Boehringer Mannheim). Between successive probes, blots were stripped by treatment with boiling 0.1% SDS.
Immunoblotting.
Preconfluent MC3T3-E1 cells were treated with 10 mM sodium phosphate for 48 h and harvested in PBS. pH concentrations in a range from 6.5 to 8.0 were tested and found to be equally effective. Most experiments were done at pH 6.5. Cells were lysed, and proteins were separated and transferred to Immobilon P membrane (Millipore) and visualized as described previously (10). Larry Fisher (National Institute of Dental Research) kindly provided the osteopontin-specific antibody LF-123 (11).
Promoter Expression Assay.
MC3T3-E1 cells were transfected with 5 μg of DNA by using Lipofectamine reagent according to the prescribed protocol (GIBCO/BRL). The osteopontin promoter chloramphenicol acetyltransferase (CAT) construct, pSV2arCAT (−777 to +79), described previously (12), was kindly provided by David Denhardt (Rutgers University). Preconfluent cells were exposed to DNA and Lipofectamine for 6 h then treated with either 10 mM sodium phosphate or sodium sulfate and incubated for an additional 40 h. CAT activity in 100 μg of total cell lysate was assayed as described by Gorman et al. (13) and quantified with a Fuji phosphorimager.
Plasmids and Probes.
The E1A and bone-related plasmids have been described previously (9). The osteopontin plasmid, mop3, used for Northern probes was kindly provided by Marian Young (National Institute of Dental Research) and described in ref. 11.
Results
Osteopontin Is Induced in Direct Response to Extracellular Levels of Phosphate in Ascorbic Acid-Stimulated MC3T3-E1 Cells.
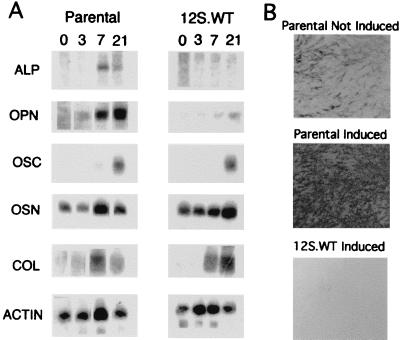
Our previous studies suggested that induction of osteopontin expression in response to ascorbic acid is specifically linked with increased extracellular levels of alkaline phosphatase. MC3T3-E1 cells expressing E1A.12S.WT fail to express alkaline phosphatase and osteopontin, although expression of other markers of osteoblast differentiation is relatively normal (ref. 9 and Fig. 2A). E1A expression represses alkaline phosphatase activity even below basal levels (ref. 9 and Fig. 2B). Osteopontin expression responds to exogenously added alkaline phosphatase in the absence of expression of the endogenous gene (9). This observation indicates that induction of osteopontin is tied to some property of the enzyme, as opposed to dependence on the actual transcriptional mechanisms required for alkaline phosphatase expression. To examine the mechanisms underlying the effect of the extracellular enzyme, we have considered the possibilities that the enzyme itself acts as a rate-limiting positive signal for osteopontin expression, or that enzyme hydrolysis depletes an inhibitor or generates a positive effector in the medium.
Figure 2.
Characterization of the 12S.WT E1A-expressing cell line. (A) Northern blot characterization of differentiation markers in MC3T3-E1 cell lines. Cells were cultured with differentiation media (see Materials and Methods) and total RNA was prepared at days 0, 3, 7, and 21. The same blot was hybridized sequentially with cDNA probes to alkaline phosphatase (ALP), osteopontin (OPN), osteocalcin (OSC), osteonectin (OSN), α1-collagen (COL), and β-actin (actin), and is described in more detail in ref. 9. (B) In situ analysis of alkaline phosphatase activity. The cells were cultured in a six-well plate and stained with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (BCIP/NBT) as described previously (9). Parental-not induced, growth medium; parental-induced, differentiation medium; 12S.WT-induced, differentiation medium. [Reproduced from ref. 9 with permission of John Wiley & Sons, Inc. (copyright 1998 Wiley–Liss, Inc.), http://www.interscience.wiley.com/jpages/0730-23121.]
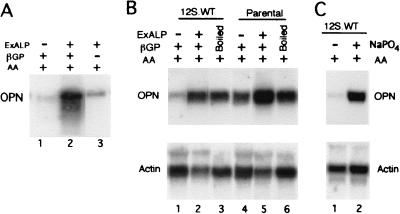
A model in which a hydrolysis product is a required positive signal predicts that the induction of osteopontin, irrespective of the actual process of mineralization, would depend not just on the addition of exogenous alkaline phosphatase but also on the presence in the medium of the organic phosphate source. We first examined the induction of osteopontin expression in 12S.WT-expressing cells, which lack endogenous alkaline phosphatase activity (Fig. 2B), in the presence of exogenously added alkaline phosphatase with and without added β-glycerol phosphate substrate (Fig. 3A). Cells were treated for 21 days, RNA was extracted, and osteopontin expression was probed on Northern blots. Osteopontin is not induced by normal induction medium in these cells (Fig. 3A, lane 1), but is induced strongly if alkaline phosphatase is supplied directly to the culture medium (Fig. 3A, lane 2). If β-glycerol phosphate is omitted from the differentiation medium, induction rises only slightly above the background level (lane 3). Thus, addition of the enzyme alone is not sufficient for induction of osteopontin; apparently a hydrolysis product of β-glycerol phosphate is a required positive effector.
Figure 3.
Northern blot analysis of osteopontin expression after addition of exogenous alkaline phosphatase (ExALP) or sodium phosphate. (A) The 12S.WT.G1 cell line was cultured for 21 days with growth medium supplemented with ExALP, β-glycerol phosphate (βGP), or ascorbic acid (AA) as stated above each lane. (B) The 12S.WT.G1 and parental (MC3T3-E1) cell lines were cultured with growth medium and AA, supplemented with ExALP and βGP or a solution in which ExALP and βGP were incubated and then boiled to inactive the enzyme (Boiled), as stated above each lane. (C) The 12S.WT.G1 cell line was cultured for 21 days in the presence of AA and in the presence or absence of 10 mM sodium phosphate (NaPO4). Blots were hybridized sequentially with cDNA probes to osteopontin (OPN) and β-actin.
To test directly whether hydrolysis of the phosphate substrate is sufficient for the alkaline phosphatase effect, β-glycerol phosphate was incubated with alkaline phosphatase in the absence of cells or medium. This solution then was boiled to inactivate the enzyme. Cell cultures were incubated in the presence of this enzyme-inactivated solution throughout the differentiation period, in parallel with cells incubated in the presence of exogenously added alkaline phosphatase. At day 21, osteopontin RNA levels were analyzed by Northern blotting (Fig. 3B). A comparison of lanes 1 and 4 illustrates again the failure of induction in 12S.WT-expressing cells in the absence of alkaline phosphatase. Lane 3 compared with lane 2 indicates that the hydrolyzed β-glycerol phosphate was as effective as exogenously added alkaline phosphatase and β-glycerol phosphate were in inducing expression of osteopontin. The induced levels were comparable to those observed in parental cells under similar conditions (compare lanes 2 and 3 to lanes 5 and 6). An actin probe was used as a control for variations in RNA loading.
These results suggest that phosphate released from hydrolysis of the organic substrate is a positive effector for osteopontin expression and argue against depletion of an inhibitor as a required step. This suggestion was tested directly by incubating ascorbic acid-induced 12S.WT-expressing cells in the presence of added sodium phosphate without exogenous alkaline phosphatase. The basal medium contained 1 mM phosphate salts. Induction medium contained 10 mM β-glycerol phosphate. Various concentrations of sodium phosphate were tested in cells harvested at time points ranging from 11 to 21 days after induction in the absence of β-glycerol phosphate. Sodium phosphate (4 mM) was the minimal concentration that consistently induced a detectable increase in osteopontin expression, whereas concentrations in the area of 10 mM were maximally effective at any time point. A typical experiment is shown in Fig. 3C. Incubation in 10 mM sodium phosphate for 14 days after ascorbic acid induction in the absence of β-glycerol phosphate is sufficient to induce osteopontin expression to the approximate level seen with exogenously added alkaline phosphatase (Fig. 3B, lane 2) or in normally induced parental cells incubated with the standard addition of 10 mM β-glycerol phosphate in the differentiation medium (Fig. 3B, lane 4). The induction does not appear to follow a linear dose–response; rather, it seems that concentrations below 4 mM are ineffective, and concentrations very near 10 mM are required for strong induction (data not shown).
Properties of the Phosphate Signal Resulting in Increased Osteopontin RNA Levels.
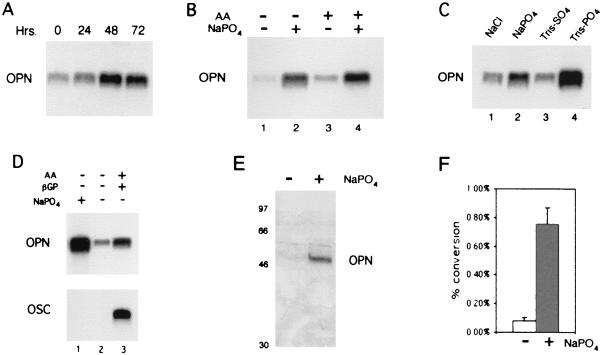
In the previous experiments, cells were exposed to increased phosphate levels from the start of the differentiation process (day 0) to the time of harvesting. To determine the minimal time necessary for the osteopontin response to the phosphate signal, the 12S.WT-expressing cells were grown in the presence of ascorbic acid and treated with sodium phosphate at 0, 24, 48, and 72 h before harvesting for RNA analysis at day 11. The results (Fig. 4A) indicate that cells respond to the phosphate signal with increased levels of osteopontin RNA within 24 h and reach a maximum within 48 h.
Figure 4.
Properties of phosphate-signal-regulated osteopontin expression. (A) Northern blot (NB) analysis of 12S.WT.G1 cells grown in the presence of ascorbic acid (AA) and treated with 10 mM sodium phosphate (NaPO4) for 0, 24, 48, and 72 h before harvesting the cells at day 11 for RNA isolation. (B) NB analysis of the parental cells grown in the absence (lanes 1 and 2) or presence (lanes 3 and 4) of AA and then treated (lanes 2 and 4) with 10 mM sodium phosphate for 48 h. The cells were harvested for RNA analysis at day 11. (C) NB analysis of the parental cells given growth medium for 4 days postconfluency and then treated with 10 mM NaCl, 10 mM sodium phosphate, 10 mM Tris-sulfate (TrisSO4), or 10 mM Tris-phosphate (TrisPO4) for 60 h and harvested for RNA analysis. (D) NB analysis of preconfluent MC3T3-E1 (lanes 1 and 2) and day 21 (lane 3) probed with osteopontin (OPN) and osteocalcin (OSC). Treatment conditions are stated above each lane. (E) Western blot analysis of osteopontin in response to 10 mM sodium phosphate. Preconfluent MC3T3-E1 were treated in the presence or absence of 10 mM sodium phosphate for 48 h and analyzed by PAGE. Membranes were probed with the osteopontin-specific antibody LF-123. (F) CAT assay analysis of the osteopontin promoter in response to 10 mM sodium phosphate. Transiently transfected cells were treated with either sodium sulfate (white bar) or sodium phosphate (shaded bar). The graph shows the percentage conversion of chloramphenicol to acetylated forms.
Ascorbic acid is an important inducer of osteoblast differentiation. The comparatively rapid response of osteopontin to phosphate prompted us to ask whether ascorbic acid is required directly for osteopontin induction or whether induction will occur independently of ascorbic acid as long as alkaline phosphatase activity or free phosphate levels rise in the medium. Because this experiment was done in the absence of ascorbic acid, this question can be addressed with the parental cell line. One set of MC3T3-E1 cells was treated with ascorbic acid in the absence of β-glycerol phosphate; another set was untreated. At day 9, both sets received 10 mM sodium phosphate, and incubation was continued for 48 h. Cells were harvested and probed for osteopontin expression by Northern analysis (Fig. 4B). Comparison of lane 4 with lane 2 shows that osteopontin is induced to a similar level in either the presence or absence of ascorbic acid, as long as the cells receive the phosphate signal. This result indicates that ascorbic acid does not provide any direct signal required for induction of osteopontin. When phosphate levels are elevated directly, bypassing the requirement for ascorbic acid-dependent induction of alkaline phosphatase, osteopontin expression is induced in MC3T3-E1 cells in the absence of any signal other than the effect of confluency.
We also have examined the effect of salts other than phosphate and the effect of treating with organic, rather than inorganic, phosphate salts. The Na+ is unlikely to be having an effect, because the medium contains 150 mM NaCl. MC3T3-E1 cells, at day 4 postconfluency, were treated with various salts as indicated in Fig. 4C and harvested 3 days later. In the presence of 10 mM sodium phosphate (lane 2) osteopontin expression was induced as described above, whereas mock treatment (10 mM NaCl) had no effect (lane 1). The organic phosphate salt, Tris-phosphate, induced osteopontin very effectively (lane 4). However, Tris-sulfate had no effect on osteopontin levels (lane 3). Sodium sulfate also had no effect (data not shown). Thus, osteopontin seems to be regulated specifically in response to phosphate levels rather than to any less specific change in ionic concentration in the environment of the cells.
Previous experiments were all performed on confluent cells. Confluency alone is sufficient to activate some aspects of the differentiation process in MC3T3-E1 cells (6, 14). To determine whether any signal at all other than a sufficient rise in phosphate levels is required for induction of osteopontin, we assayed the effect of the phosphate signal in actively proliferating cells. Proliferating MC3T3-E1 cells were treated with 10 mM sodium phosphate and harvested 48 h later. The results (Fig. 4D, Upper, lane 1 versus lane 2) indicate that osteopontin expression is induced in direct response to the phosphate signal in the absence of any other stimulus associated with confluency.
Other than osteopontin, no other gene product characteristically induced in differentiating osteoblasts that we tested was inhibited concomitantly with the inhibition of alkaline phosphatase in the 12S.WT-expressing MC3T3-E1 cells (Fig. 2). Thus, it seems that within this differentiation program, osteopontin is specifically designed to respond to phosphate levels. To examine this idea directly, we probed for induction of osteocalcin, another late marker of osteoblast differentiation. When the same blot (Fig. 4D, Lower) was hybridized with an osteocalcin probe, no signal was detected in lane 1. A lane containing RNA from normally induced cells harvested at day 21 (lane 3) is included to verify the activity of the osteocalcin probe. Similar experiments showed no influence of phosphate on expression of α-1 collagen or osteonectin (data not shown).
If phosphate were not a normal physiological signal for induction of osteopontin gene expression, it would be possible that phosphate induces osteopontin RNA levels in some manner that is not reflected at the protein level. To address the question of protein expression, cells were treated again as described in Fig. 4D. Phosphate-treated and untreated cells were analyzed by Western blotting with osteopontin-specific antibody LF-123 (11). The results (Fig. 4E) indicate that osteopontin protein levels increase markedly within 48 h of exposure of the cells to the phosphate signal. Thus, increased extracellular phosphate concentration up-regulates osteopontin expression at the RNA level, and the increased RNA level is reflected directly at the protein level in the cell.
Osteopontin is believed to be regulated primarily at the transcriptional level (15). To determine specifically whether the change in RNA levels seen in response to phosphate is reflective of changes at the transcriptional level, promoter expression was assayed in a CAT reporter assay. A CAT reporter construct containing base pairs −777 to +79 of the mouse osteopontin gene was transiently transfected into MC3T3-E1 cells, which were subsequently treated with sodium phosphate. The results (Fig. 4F) indicate that osteopontin promoter expression is induced sharply in phosphate-treated cells as compared with control cells. Promoter expression increased approximately 9-fold in response to phosphate, consistent with the Northern blots, which show an approximate 8-fold increase averaged over multiple experiments.
Increased Osteopontin Expression in Response to the Phosphate Signal Is Blocked by the Phosphate-Transport Inhibitor Foscarnet.
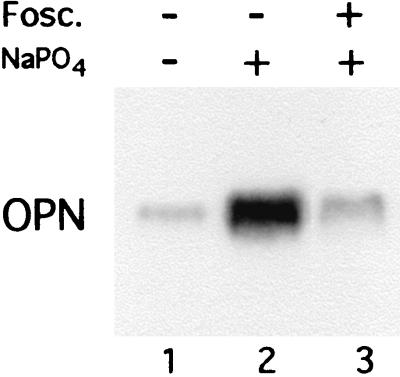
Inorganic phosphate enters cells through a Na+-dependent transport system present in the plasma membrane. In osteoblasts, this transport is mediated by an enzyme also identified as the gibbon ape leukemia virus receptor-1 (16, 17). The phosphate analog foscarnet (phosphonoformic acid) is a competitive inhibitor of sodium phosphate cotransport and is known to inhibit the formation of calcium phosphate crystals (18) and hydroxyapatite (19). We examined the effect of foscarnet on expression of osteopontin in the presence of elevated levels of inorganic phosphate added to the cell medium. The results (Fig. 5) show that foscarnet completely inhibits the induction of osteopontin in response to the phosphate signal (lane 3 as compared with lane 2). This finding suggests that the effect of phosphate on osteopontin gene expression requires that the phosphate actually enter the cell through normal transporter mechanisms and argues against the possibility that it results from a nonspecific disturbance in the cell environment.
Figure 5.
The phosphate transport inhibitor foscarnet blocks up-regulation of osteopontin in response to phosphate. MC3T3-E1 cells 4 days postconfluency were pretreated with 300 μM foscarnet (lane 3) for 3 h and then treated with 10 mM sodium phosphate (NaPO4) for 72 h (lanes 2 and 3) in the continued presence of foscarnet (lane 3) and analyzed by Northern analysis.
Phosphate Is a Sufficient Signal for Induction of Osteopontin Gene Expression in Proliferating Cells and in Cells Outside of the Osteoblast Lineage.
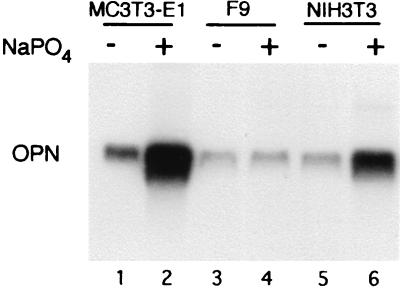
Osteopontin is expressed under various conditions in several types of tissue, including kidney, smooth muscle, and transformed cells. To determine the effect of phosphate on the expression of osteopontin in cells other than osteoblast precursors, the same experiment was performed in NIH 3T3 cells, which do not retain an ability to differentiate into osteoblast-like cells. The results (Fig. 6, lanes 5 and 6) show an induction of osteopontin in response to increased phosphate levels similar to the effect seen in MC3T3-E1 cells. Thus, the regulation of osteopontin by phosphate is not restricted to cells in the osteoblast lineage. The effect of phosphate is not indiscriminate, however; F9 cells, a murine embryonal carcinoma cell line, do not show increased osteopontin expression in the presence of increased phosphate levels (Fig. 6, lanes 3 and 4).
Figure 6.
Northern blot analysis of osteopontin (OPN) expression in various cell types. Preconfluent MC3T3-E1, NIH 3T3, and F9 cells were treated with 10 mM sodium phosphate (NaPO4) for 48 h and harvested for RNA analysis.
Discussion
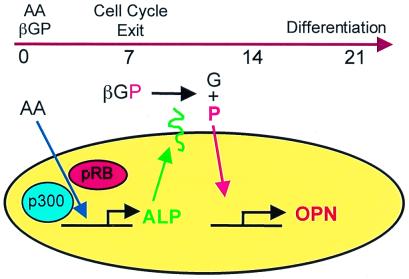
The ability of cells to actively transport phosphate is recognized as a requirement for mineralization in bone, but the underlying reasons are not well understood. Phosphate is not generally thought to act as a specific signal for induction of gene expression, although it has been implicated as a negative regulator of expression of the osteoblast phosphate transporter gibbon ape leukemia virus receptor-1 (16). Our results indicate that osteopontin expression is strongly regulated at the RNA level in response to elevated extracellular phosphate levels. In bone, this signal is an integral part of a tightly coordinated program that ensures that mineralization is not induced before osteoblasts cease proliferation (illustrated schematically in Fig. 7). The phosphate signal ties osteopontin expression to alkaline phosphatase, which in turn is positively regulated by the cell growth control proteins of the pRB and p300 families (9). In the differentiating cell, these signals ensure that induction of the early differentiation marker alkaline phosphatase does not begin until antiproliferation signals are enacted, and expression of osteopontin is not induced until alkaline phosphatase is active at the cell surface.
Figure 7.
Schematic representation of the coordination of alkaline phosphatase activity and osteopontin levels in osteoblasts. Alkaline phosphatase (ALP) activity is induced by ascorbic acid (AA) in a process that requires the p300/CBP and pRB families. ALP then is localized to the plasma membrane and oriented such that its catalytic subunit is ectoplasmic. Within the extracellular environment, ALP cleaves a phosphate (P) from β-glycerol phosphate (βGP) to leave free glycerol (G). The free phosphate enters the cell through a Na-dependent phosphate transporter, which is subject to inhibition by foscarnet. Transport of the phosphate results in the up-regulation of osteopontin (OPN) RNA levels. During the differentiation process, the requirement for the pRB and p300 families ensures that induction of the early differentiation marker, alkaline phosphatase, does not begin until proliferation has shut down, and expression of osteopontin is not induced until alkaline phosphatase is active at the cell surface.
The ability of phosphate to signal induction of osteopontin gene expression depends on the function of the phosphate transport system. The ability of the phosphate analog foscarnet to inhibit induction of osteopontin suggests that the extracellular phosphate must be transported into the cell to exert its effect on osteopontin, so that the actual response is mediated by the rise in the intracellular phosphate level. Numerous factors other than ascorbic acid (and hence an increase in alkaline phosphatase) are known to stimulate osteopontin expression at the RNA level in osteoblasts. These factors include transforming growth factor-β, retinoic acid, endothelin, bone morphogenic protein, and vitamin D (15). Several of these factors also are known to induce alkaline phosphatase and, presumably, elevated phosphate levels. Therefore, it is likely that the phosphate signal for osteopontin induction is a feature shared by these agents. However, the 5′ upstream regulatory region of the osteopontin gene contains elements similar to various known cis-acting elements, consistent with the possibility that multiple signals act on the osteopontin gene. For example, there are apparently functional response elements for the vitamin D receptor (20) and the estrogen receptor-related α receptor (21) in the osteopontin promoter.
A variety of cells of other tissue types express phosphate cotransporter enzymes on their cell surface. Similarly, osteopontin is expressed in various cells other than those in the connective tissue lineages (22). Interestingly, osteopontin protein levels are up-regulated during cell injury and are associated with the pathology of many diseased tissues. For example, osteopontin has been detected in kidney (23), particularly the distal tubules, and is elevated by ischemic injury (24). Osteopontin is also a major matrix component of kidney stones (25–27). Arterial smooth muscle cells express osteopontin, which is associated with the calcification of arterial plaque in atherosclerosis (28, 29). The phenotype of osteopontin-null mice is consistent with a role for osteopontin in wound healing (30). Osteopontin also is expressed in many types of cells that are subject to high turnover (31), as well as in transformed cells (32, 33).
The precise biochemical function of osteopontin is not known in either bone or other tissues but functional motifs have offered clues (34–36). Experiments prompted by the presence of an arginine-glycine-aspartate (RGD) integrin-binding motif in osteopontin support the suggestion that osteopontin facilitates the attachment of osteoblasts and osteoclasts to the extracellular matrix (35, 37). Osteopontin can act as a cytokine (38), and exposure of cells to purified osteopontin leads to downstream events that include calcium mobilization (39, 40) and possible activation of the calcium ATPase pump (41). There is evidence that osteopontin binds calcium (42, 43), leading to the hypothesis that osteopontin has a normal physiological role in inhibiting crystal growth in urine and in other biological fluids by binding to calcium on the crystal faces (44). Similarly, osteopontin is thought to modulate hydroxyapatite crystal elongation during bone formation (45). One scenario suggested by our results is that osteopontin is induced in tissues under conditions where phosphate levels rise significantly above the normal physiological level. In bone, this rise is part of the normal mineralization process. In cells other than bone, this rise may occur when normal regulation of phosphate is aberrant, in conditions such as tissue damage or cardiac, vascular, or renal insufficiency. The tight link between osteopontin expression and phosphate levels, combined with the ability of osteopontin to modulate calcium levels, suggests that the various biological activities attributed to osteopontin may be related to an overall role in regulating calcium localization or transport in conditions of high cellular phosphate levels.
Acknowledgments
We thank Roland Baron, Larry Fisher, Marion Young, and David Denhardt for gifts of cell lines, antibodies, and plasmids. We also thank Charlie Grubmeyer, Peter Dallas, Xavier Graña, Scott Shore, and Steve Popoff for helpful discussions. We thank Tobin Beck for assistance with graphics and illustrations. G.R.B., Jr., was partially supported by a training grant (T30-CA09214) from the National Institutes of Health and by a Daniel Swern Fellowship from Temple University. This work was supported by a grant from the National Cancer Institute (CA53592), by a Biomedical Science grant from the Arthritis Foundation, and by the Temple University Research Enterprise Program (all to E.M.).
Abbreviations
- pRB
retinoblastoma protein
- CAT
chloramphenicol acetyltransferase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.140021997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.140021997
References
- 1.Moran E. Semin Virol. 1994;5:327–340. [Google Scholar]
- 2.Beck G R, Jr, Zerler B, Moran E. In: Human Tumor Viruses. McCance D J, editor. Washington, DC: Am. Soc. Microbiol.; 1998. pp. 51–86. [Google Scholar]
- 3.Mayol X, Grana X. Prog Cell Cycle Res. 1997;3:157–169. doi: 10.1007/978-1-4615-5371-7_13. [DOI] [PubMed] [Google Scholar]
- 4.Giordano A, Avantiagatti M L. J Cell Physiol. 1999;181:218–230. doi: 10.1002/(SICI)1097-4652(199911)181:2<218::AID-JCP4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 5.Sudo H, Kodama H, Amagi Y, Yamamoto S, Kasai S. J Cell Biol. 1983;96:191–198. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quarles D, Yohay D A, Lever L W, Caton R, Wenstrup R J. J Bone Miner Res. 1992;7:683–692. doi: 10.1002/jbmr.5650070613. [DOI] [PubMed] [Google Scholar]
- 7.Franceschi R T, Iyer B S, Cui Y. J Bone Miner Res. 1994;9:843–854. doi: 10.1002/jbmr.5650090610. [DOI] [PubMed] [Google Scholar]
- 8.Towler D A. In: Principles of Bone Biology. Bilezikian J, Raisz L G, Rodan G A, editors. San Diego: Academic; 1996. pp. 1173–1188. [Google Scholar]
- 9.Beck G R, Jr, Sullivan E C, Moran E, Zerler B. J Cell Biochem. 1998;68:269–280. doi: 10.1002/(sici)1097-4644(19980201)68:2<269::aid-jcb13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 10.Dallas P D, Cheney I W, Liao D-W, Bowrin V, Byam W, Pacchione S, Kobayashi R, Yaciuk P, Moran E. Mol Cell Biol. 1998;18:3596–3603. doi: 10.1128/mcb.18.6.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher L W, Stubbs J T, III, Young M F. Acta Orthop Scand. 1995;66, Suppl. 266:61–65. [PubMed] [Google Scholar]
- 12.Craig A M, Denhardt D T. Gene. 1991;100:163–171. doi: 10.1016/0378-1119(91)90362-f. [DOI] [PubMed] [Google Scholar]
- 13.Gorman C M, Moffat L F, Howard B H. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franceschi R T, Iyer B S. J Bone Miner Res. 1992;7:235–246. doi: 10.1002/jbmr.5650070216. [DOI] [PubMed] [Google Scholar]
- 15.Denhardt D T, Noda M. J Cell Biochem Suppl. 1998;30–31:92–102. [PubMed] [Google Scholar]
- 16.Kavanaugh M P, Miller D G, Zhang W, Law W, Kozak S L, Kabat D, Miler D. Proc Natl Acad Sci USA. 1994;91:7071–7075. doi: 10.1073/pnas.91.15.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer G, Bonjour J-P, Caverzasio J. Endocrinology. 1997;138:5202–5209. doi: 10.1210/endo.138.12.5561. [DOI] [PubMed] [Google Scholar]
- 18.Fleisch H A, Russel R G, Bisaz S, Muhlbauer R C, Williams D A. Eur J Clin Invest. 1970;1:12–18. doi: 10.1111/j.1365-2362.1970.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 19.Williams G, Sallis J D. Biochem J. 1979;184:181–184. doi: 10.1042/bj1840181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noda M, Vogel R L, Craig A M, Prahl J, DeLuca H F, Denhardt D T. Proc Natl Acad Sci USA. 1990;87:9995–9999. doi: 10.1073/pnas.87.24.9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanacker J-M, Delmarre C, Guo X, Laudet V. Cell Growth Differ. 1998;9:1007–1014. [PubMed] [Google Scholar]
- 22.Brown L F, Berse B, Van de Water L, Papadopoulos-Sergiou A, Perruzzi C A, Manseau E J, Dvorak H F, Senger D R. Mol Biol Cell. 1992;3:1169–1180. doi: 10.1091/mbc.3.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez C A, Hoyer J R, Wilson P D, Waterhouse P, Denhardt D T. Lab Invest. 1993;69:355–363. [PubMed] [Google Scholar]
- 24.Pandanilam B J, Martin D R, Hammerman M R. Endocrinology. 1996;137:2133–2140. doi: 10.1210/endo.137.5.8612558. [DOI] [PubMed] [Google Scholar]
- 25.Kohri K, Nomura S, Kitamura Y, Nagata T, Yoshioka K, Iguchi M, Yamate T, Umekawa T, Suzuki Y, Sinohara H, et al. J Biol Chem. 1993;268:15180–15184. [PubMed] [Google Scholar]
- 26.McKee M D, Nanci A, Khan S R. J Bone Miner Res. 1995;10:1913–1929. doi: 10.1002/jbmr.5650101211. [DOI] [PubMed] [Google Scholar]
- 27.Persy V P, Verstrepen W A, Ysebaert D K, De Greef K E, De Broe M E. Kidney Int. 1999;56:601–611. doi: 10.1046/j.1523-1755.1999.00581.x. [DOI] [PubMed] [Google Scholar]
- 28.Fitzpatrick L A, Severson A, Edwards W D, Ingram R T. J Clin Invest. 1994;94:1597–1604. doi: 10.1172/JCI117501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giachelli C M, Schwartz S M, Liaw L. Trends Cardiovasc Med. 1995;5:88–95. doi: 10.1016/1050-1738(95)00005-T. [DOI] [PubMed] [Google Scholar]
- 30.Liaw L, Birk D E, Ballas C B, Whitsitt J S, Davidson J M, Hogan B L M. J Clin Invest. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodan G. Ann NY Acad Sci. 1995;760:1–5. doi: 10.1111/j.1749-6632.1995.tb44614.x. [DOI] [PubMed] [Google Scholar]
- 32.Smith J H, Denhardt D T. J Cell Biochem. 1987;34:13–22. doi: 10.1002/jcb.240340103. [DOI] [PubMed] [Google Scholar]
- 33.Senger D R, Peruzzi C A, Gracey C F, Papadoupoulos A, Tenen D G. Cancer Res. 1989;48:5770–5774. [PubMed] [Google Scholar]
- 34.Franzén A, Heinegård D. Biochem J. 1985;232:715–724. doi: 10.1042/bj2320715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oldberg A, Franzén A, Heingård D. Proc Natl Acad Sci USA. 1986;83:8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butler W T, Ridall A L, McKee M D. In: Principles of Bone Biology. Bilezikian J, Raisz L G, Rodan G A, editors. San Diego: Academic; 1996. pp. 155–166. [Google Scholar]
- 37.Reinholt F P, Hultenby K, Oldberg C, Heinegård D. Proc Natl Acad Sci USA. 1990;87:4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patarca R, Saavedra R A, Cantor H. Crit Rev Immunol. 1993;13:225–246. [PubMed] [Google Scholar]
- 39.Zimolo Z, Wesolowski G, Tanaka H, Hyman J L, Hoyer J R, Rodan G A. Am J Physiol. 1994;266:C376–C381. doi: 10.1152/ajpcell.1994.266.2.C376. [DOI] [PubMed] [Google Scholar]
- 40.Denhardt D T, Lopez C A, Rollo E E, Hwang S M, An X R, Walther S E. Ann NY Acad Sci. 1995;760:127–142. doi: 10.1111/j.1749-6632.1995.tb44625.x. [DOI] [PubMed] [Google Scholar]
- 41.Miyauchi A, Alvarez J, Greenfield E M, Teti A, Grano M, Colucci S, Zambonin-Zallone A, Ross F P, Teitelbaum S L, Cheresh D, et al. J Biol Chem. 1991;266:20369–20374. [PubMed] [Google Scholar]
- 42.Chen Y, Bal B S, Gorski J P. J Biol Chem. 1992;267:24871–24878. [PubMed] [Google Scholar]
- 43.Singh K, Deonarine D, Shanmugam V, Senger D R, Mukherjee A B, Chang P L, Prince C W, Mukherjeee B B. J Biochem (Tokyo) 1993;114:702–707. doi: 10.1093/oxfordjournals.jbchem.a124240. [DOI] [PubMed] [Google Scholar]
- 44.Hoyer J R, Otvos L, Jr, Urge L. Ann NY Acad Sci. 1995;760:257–265. doi: 10.1111/j.1749-6632.1995.tb44636.x. [DOI] [PubMed] [Google Scholar]
- 45.Boskey A L, Maresca M, Ullrich W, Doty S B, Butler W T, Prince C W. Bone Miner. 1993;22:147–159. doi: 10.1016/s0169-6009(08)80225-5. [DOI] [PubMed] [Google Scholar]