Abstract
Molecular imaging probes have potential for in vivo identification of apoptosis and other intracellular processes. TcapQ, a cell-penetrating, near-infrared fluorescent peptide probe designed to be optically silent through intramolecular fluorescence quenching and activated by effector caspases, has been previously described and validated in vitro. Herein, using NMDA-induced apoptosis of retinal ganglion cells (RGCs), representing an in vivo rat model of glaucoma, we assessed the ability of TcapQ to image single-cell apoptosis through effector caspase activity. Following intravitreal injection, intracellular TcapQ activation occurred specifically in RGCs, identified individual apoptotic cells, showed a clear dose-response relationship with NMDA, and colocalized with TUNEL labeling in the retina. There was a significant diminution of probe activation following pretreatment with a specific inhibitor of caspase-3. Stereospecificity was also exhibited by the lack of intracellular fluorescence upon administration of the noncleavable isomer, dTcapQ. TcapQ has potential utility in detecting and monitoring single-cell apoptosis in glaucoma in vivo.
Keywords: caspase, molecular imaging, near-infrared fluorescence
Apoptosis occurs in normal development in a wide range of tissues, and activation of the apoptotic pathway is involved in a number of neurodegenerative diseases (1). Thus, the ability to identify cells in which this pathway has been activated has potential utility in laboratory investigation as well as in clinical diagnosis and management of neurodegenerative diseases. Two major intracellular apoptotic pathways have been identified—one activated predominately by death receptor ligands, the other triggered by various forms of severe cell stress (intrinsic pathway) (2, 3). In the latter, these cellular stresses promote the expression and intracellular redistribution of proapoptotic proteins (e.g., Bax and Bak), with subsequent permeabilization of the mitochondrial outer membrane to cytochrome c and proapoptotic proteins, and eventual activation of the caspase cascade of protease activities that mediate apoptosis (3, 4). Despite the availability of several methods to identify apoptotic cells in vitro (5), there remains a strong need for noninvasive molecular imaging methods that will enable researchers and clinicians to identify apoptotic cells in vivo (6).
Glaucoma, an optic neuropathy characterized by selective retinal ganglion cell (RGC) death with associated optic nerve head cupping and vision loss, remains one of the leading causes of blindness (7). RGC death in glaucoma has been characterized as involving the apoptotic pathway of cell death (8, 9). In the current clinical management of glaucoma, the status of RGCs is assessed by detecting their functional or anatomical loss as evidenced by perimetric evaluation of the visual field or imaging of the optic disc/nerve fiber layer, respectively. Though this strategy is well validated and useful, the ability to assess various indicators of the status of RGCs in vivo before their death would be a significant advance.
One strategy to assess apoptosis in vivo has been through the use of probes targeting extracellular or cell membrane targets, such as the binding of reporter-labeled annexin V to phosphatidylserine exposed on the outer leaflet of the plasma membrane of apoptotic cells (10–13). This strategy has been used to identify apoptotic RGCs in vivo (10, 14–16). One potential drawback to this strategy is that it may not adequately distinguish apoptotic from necrotic forms of cell death in vivo without a secondary marker of membrane integrity. In addition, there may be a limited number of extracellular or cell membrane targets available for binding to labeled probes such as annexin V, which may negatively impact signal to noise of the final images.
The caspase family of proteases has been shown to play a critical role in the final common pathway to apoptotic cell death and is therefore a logical target for molecular imaging probes (17). The in vitro and preliminary in vivo validation of a cell-penetrating effector caspase imaging probe, TcapQ, has been previously described (18, 19). This probe utilizes a modified Tat peptide cell-penetrating moiety, which confers a surprisingly selective uptake by RGCs following intravitreal injection (20). Thus, the cell-penetrating sequence serves both as a targeting sequence for selective cell accumulation of the probe and as a mediator of cell penetration. Herein, selective RGC degeneration was induced by intravitreal injection of NMDA, a well-established and highly reproducible animal model of glaucoma (21), as a clinically relevant system to assess the ability of TcapQ to identify RGC apoptosis in vivo.
Results
TcapQ Activation in NMDA-Treated Eyes Is Dose Dependent.
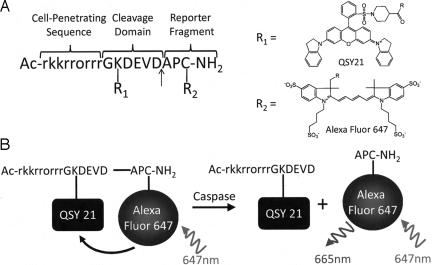
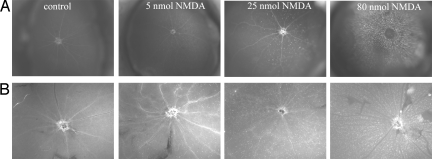
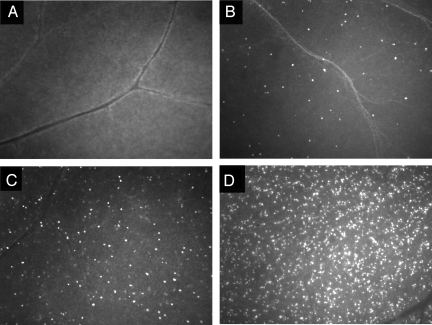
Fig. 1 shows the structure of TcapQ and a schematic of probe cleavage by effector caspases. The intravitreal NMDA rat model of retinal neurodegeneration has been described previously in detail (22–25). The pretreatment duration of 6 h was chosen to limit apoptosis to RGCs as much as possible. Subsequently, 2 h following intravitreal injection of TcapQ, there was a clear NMDA dose-dependent activation of TcapQ in the retina as shown in both intact eyecups and retinal flatmounts imaged by fluorescence microscopy (Fig. 2). Increasing numbers of fluorescent-labeled cell bodies were noted as the NMDA injectate was increased from 5 nmol to 80 nmol. Higher-power fluorescence microscopy clearly showed fluorescence corresponding to probe activation in a pattern consistent with labeling of cell bodies in the inner retina (Fig. 3).
Fig. 1.
Structure and cleavage of TcapQ cell-penetrating activatable peptide probe. (A) Chemical structure of TcapQ showing putative structure of Alexa Fluor 647. Arrow denotes cleavage site mediated by effector caspases. (B) Schematic of TcapQ cleavage by effector caspases.
Fig. 2.
Imaging of NMDA-induced apoptosis in rat retina. Fluorescence images were obtained 2 hrs following intravitreal injection of TcapQ, which had been injected 6 hrs after the indicated dose of intravitreal NMDA or PBS (control). Punctate fluorescent foci represent intracellular TcapQ activation in eyecups (A) and flatmount retinas (B). Increasing numbers of cells showing probe activation were observed as the dose of NMDA increased. (Magnification: ×4.)
Fig. 3.
Higher magnification images of retinal flatmounts from rat eyes treated with intravitreal NMDA and subsequently injected with TcapQ. Fluorescence microscopic photographs of representative retinal flatmounts from PBS-pretreated eyes (A), and NMDA-pretreated eyes with injectate content of 5 nmol (B), 25 nmol (C), and 80 nmol (D). With increasing NMDA doses, a higher frequency of intracellular probe activation is noted. (Magnification: ×10.)
Computer-assisted counting of fluorescent cells in retinal flatmounts, which showed activation of TcapQ, confirmed the expected dose-dependent relationship (Fig. 4). In particular, there was a large increase in the number of cells exhibiting TcapQ activation as the NMDA injectate was increased from 25 nmol to 80 nmol (Figs. 2 and 4). Control rat eyes in which TcapQ was injected following pretreatment with PBS showed only rare fluorescent cells, which, when present, typically corresponded to the peripheral injection site (Figs. 2–4). Colabeling of cell bodies with DAPI in both flatmounts and vertical retinal sections confirmed that probe activation, as demonstrated by the resulting fluorescence, took place in the cytosolic compartment (data not shown).
Fig. 4.
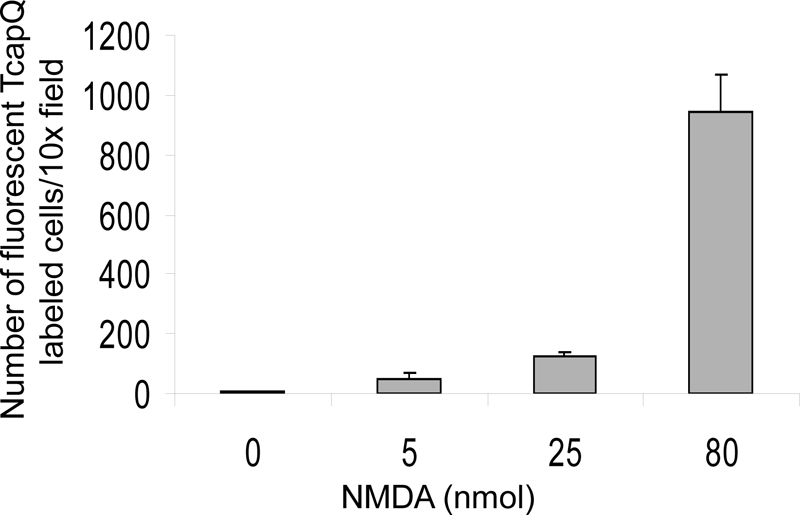
Quantification of probe activation in PBS- and NMDA-pretreated eyes. NMDA-induced apoptosis causes a dose-dependent increase in TcapQ activation in rat retina. Cells with probe activation were quantified in 8 standardized fields from each retina at 10× magnification using image analysis software (mean number of cells ± SEM).
Confirmation of Probe Activation in RGCs Using Retrograde Labeling with FluoroGold.
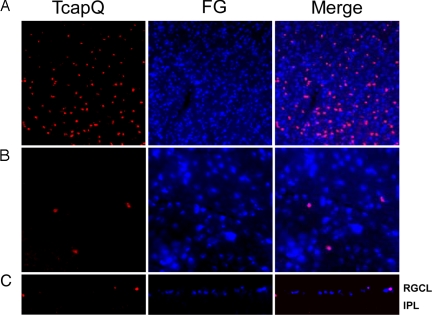
To further confirm that TcapQ activation was seen primarily in RGCs, RGCs were labeled in retrograde using FluoroGold (FG) injected into the superior colliculus. At 4–7 days following FG injection, rats underwent sequential intravitreal injections of 25 nmol NMDA and TcapQ as described previously. Examination of retinal flatmounts and vertical retinal sections confirmed that the vast majority of cells labeled with TcapQ were RGCs (Fig. 5), although there were also occasional labeled cell bodies in the inner nuclear layer if tested at the higher NMDA concentrations.
Fig. 5.
Intracellular TcapQ activation in retrogradely labeled retinal ganglion cells. RGCs were retrogradely labeled by injection of FluoroGold (FG) 7 days before induction of RGC apoptosis. Retinal flatmounts were prepared after 6 h of exposure to 25 nmol NMDA and subsequent TcapQ injection (A and B). Vertical retinal sections were then prepared from the same retinae (C). Intracellular probe activation (red) and FG labeled RGCs (blue) are confirmed to be colocalized (pink) in merged overlay images from retinal flatmounts at low (A) and high (B) magnification. (C) Vertical retinal sections confirm probe activation in retrogradely labeled RGCs in the RGC layer (RGCL). IPL, inner plexiform layer. (Magnification: A and C, ×10; B, ×40.)
Colocalization of Activated TcapQ and TUNEL Staining.
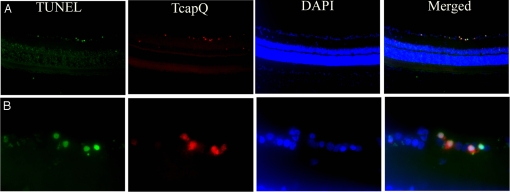
To confirm that probe activation was primarily restricted to retinal cells undergoing apoptosis, vertical retinal sections also were interrogated by fluorescence TUNEL assay. Vertical retinal sections revealed a high correspondence between TcapQ activation and TUNEL, with the size and location of the vast majority of double-labeled cells being consistent with RGCs (Fig. 6).
Fig. 6.
TcapQ activation corresponding to retinal ganglion cell apoptosis in the NMDA model. TUNEL assay was performed on frozen vertical retinal sections from eyes exposed to 80 nmol NMDA with subsequent intravitreal injection of TcapQ. Retinas were prepared and examined by fluorescence microscopy to identify apoptotic TUNEL labeled cells (green), TcapQ activated cells (red), and DAPI stained cell nuclei (blue). As with TUNEL labeling, TcapQ activation was primarily limited to cell bodies in the RGC layer consistent with RGCs. DAPI staining reveals the retinal architecture. Merged images confirm that TcapQ activation colabels TUNEL-positive cells. (Magnification: A, ×10; B, ×40.)
Confirmation of the Specificity of Probe Activation Using Effector Caspase Inhibitors.
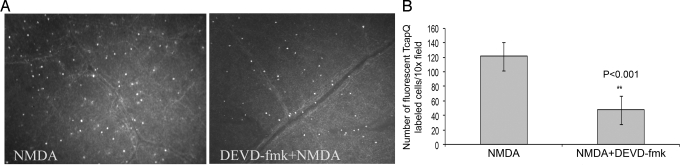
To demonstrate that probe activation was mediated by activated effector caspases, intravitreal injection of the caspase-3 inhibitor DEVD-fmk was performed before and along with TcapQ. These experiments were performed with only the 25 nmol injectate. There was a discernable diminution in visible signals in the presence of the caspase-3 inhibitor (Fig. 7A), and quantitation of the number of fluorescent cells demonstrating TcapQ activation confirmed a significant decrease (Fig. 7B; t test, P < 0.001). Conversely, when intravitreal injection of the caspase-1 inhibitor Z-YVAD-fmk was performed before and along with TcapQ, there was no significant change in the number of TcapQ labeled cells (data not shown).
Fig. 7.
Inhibition of caspase-3 activity results in a significant decrease in TcapQ activation following intravitreal injection of NMDA. Eyes (n = 4) were pretreated with either 25 nmol NMDA alone or with 25 nmol NMDA plus 2 mM DEVD-fmk, and subsequent injection of TcapQ. (A) Fluorescence microscopy of retinal flatmounts reveals a marked decrease in TcapQ activation in the presence of caspase-3 inhibitor (Right). (B) Bar graph shows the mean (±SEM) number of fluorescent TcapQ-labeled RGCs in retinal flatmounts from eyes treated with NMDA alone or NMDA + DEVD-fmk. Statistical significance of NMDA + DEVD-fmk compared with NMDA alone indicated by asterisks (**P < 0.001).
Injection of Noncleavable dTcapQ to Rule Out Nonspecific Probe Activation.
To ensure that the fluorescence detected in retinal cell bodies was not the result of nonspecific cleavage or other nonspecific mechanisms of dequenching of the fluorophore, intravitreal injection of NMDA was followed by injection of a noncleavable isomer, dTcapQ. This probe is identical to that of TcapQ, except that the entire peptide, including the DEVD cleavage sequence, consists entirely of nonnative d, as opposed to l, amino acids. In vitro experiments have previously confirmed that the all-d probe is not activated by effector caspases (19). Eyes in which dTcapQ were injected following NMDA injection showed little to no fluorescent labeling in the retina (data not shown), consistent with stereospecific probe activation by effector caspases in vivo.
Discussion
These studies provide validation that the activatable, near-infrared, cell-penetrating peptide TcapQ can be used to identify apoptotic RGCs in a clinically relevant in vivo model of glaucoma. Activatable probes contain enzyme-specific cleavage sites that when cleaved result in a signal denoting the activity of the specified enzyme. Exploitation of optical quenching strategies in the basal state further reduces background activity. Peptide-based imaging agents have a number of advantages, including high target specificity, relative ease of synthesis, and the potential for conjugation to various imaging moieties (26). Previously, the targeting of peptide-based imaging agents has been limited to extracellular or cell-surface targets because of the barrier function of the cellular membrane. However, efficient delivery of imaging probes to the cell interior using cell-penetrating peptides has greatly expanded potential applications for molecular imaging (26–31), similar to advances with cell-penetrating peptides in therapy (32–35). Delivery of optically quenched, activatable, peptide-based imaging probes to the intracellular compartment makes selective retention and signal amplification possible, improving sensitivity and signal-to-noise ratios, as well as increasing the number of biochemical processes that can be assessed.
Use of an activatable probe to identify apoptosis in vivo in a specific tissue or cell type depends significantly on 2 important factors. First, the probe must be delivered by a route that ensures access to the target tissue. Although, an intravascular route is desirable for ease of access, this was not possible for delivery to the retina as we have previously shown that the targeting moiety used in the TcapQ probe, a modified Tat peptide, does not cross the blood-retinal or blood-aqueous barrier following intravascular injection (20). Though more invasive, the intravitreal route has the advantage of selective delivery of probe to the target tissue without subsequent diffusion throughout the intravascular volume and loss of effective concentration at the target site. Such intravitreal injections are easily performed in multiple species and are routinely used in humans for delivery of therapeutic agents (36–38). Second, the probe must be able to cross the barrier constituted by the cellular membrane to gain access to the intracellular compartment. For this probe, a modified Tat peptide sequence has been used as the targeting moiety. A number of studies using Tat-derived peptides have demonstrated transport and intracellular delivery of molecular imaging as well as therapeutic agents into various cell types and tissues (19,36, 39–42). In addition, we have previously shown the utility of this peptide for enhancing the intracellular accumulation of a conjugated fluorophore in relevant target cells, in this case, RGCs (20).
Several strategies were used to confirm the specificity of probe activation to apoptotic RGCs in the retina. Probe activation was shown to exhibit a dose-response relationship to NMDA, as previously demonstrated for RGC cell death in this model (22–24). Activation of probe, with the resulting fluorescence, was isolated to cell bodies, as evidence by double labeling with DAPI, a nuclear marker. Extracellular probe activation was not noted, and the background level of fluorescence exhibited in tissue sections was nominal. Vertical retinal sections confirmed that the vast majority of cells with activated probe were located in the RGC layer and were consistent with RGCs, as would be expected based on the model of induced apoptosis we used. To provide additional confirmation that TcapQ activation took place primarily in RGCs, retrograde labeling of RGCs was performed using FluoroGold. As anticipated, the vast majority of cells displaying TcapQ activation were shown to be RGCs. Cell bodies displaying probe activation reliably double-labeled with TUNEL, confirming that these cells were in fact undergoing apoptosis. To demonstrate specificity of probe activation to effector caspase activity, 2 separate methods were used. Pre- and coinjection with an effector caspase inhibitor resulted in a significant decrease in probe activation, as anticipated. In addition, the lack of activation of the noncleavable dTcapQ control probe also provided firm evidence that the probe was not nonselectively activated in this model.
The model used in this study, NMDA-induced apoptosis of RGCs, has been used as one of many animal models of glaucoma (21). It has the advantage of being highly reproducible with a well-established dose-response relationship between NMDA exposure/concentration and RGC degeneration. The ability of the probe to detect apoptosis in this in vivo model highlights the potential clinical and research applications of this probe in the study of glaucoma, a condition characterized by the selective apoptotic cell death of RGCs, with subsequent vision loss. Though the overall status of RGCs can be assessed via imaging techniques such as disc topography and nerve fiber layer analysis, which are altered as a result of RGC cell death and degeneration of RGC axons, these techniques are limited to identification of the sequelae of RGC cell death. Perimetric tests, such as automated static perimetry, enable assessment of the functional sequelae of RGC cell death (i.e., visual field defects), but are not well correlated with changes in the physiology of RGCs before degeneration. The use of activatable probes to assess intracellular processes within RGCs in vivo may provide a means to move beyond these limitations.
The eye is a useful setting for the development and eventual utilization of activatable fluorescent probes, such as TcapQ, in that it is a readily accessible organ and the resulting fluorescence can be identified in vivo using optical imaging techniques. The ability to image fluorescent labeling of RGCs through the use of specialty confocal scanning laser microscopy, although not widely available, has been previously reported in both mice and rats (15). The activatable nature of the TcapQ probe, with quenching of fluorescence in its unactivated state, is advantageous in that it results in negligible background fluorescence, and thus enhanced signal-to-noise ratios. This is in contrast to the use of fluorescent-labeled cell-surface probes, such as fluorophore-conjugated annexin V, which are fluorescent in their native state, potentially increasing background due to nonspecific binding and vascular contamination of tissues. Cleavage of the effector caspase cleavage sequence in TcapQ, which occurs only intracellularly, results in separation of the fluorophore and the quencher molecule. This not only results in dequenching of the fluorophore, but also separates the fluorophore from the cell-penetrating moiety, effectively trapping the fluorophore within the intracellular compartment. The activation of multiple probe constructs by active enzyme within a single cell also allows for amplification of the signal.
In summary, we have validated the utility of a peptide based, cell-penetrating, activatable probe for the near-infrared detection of apoptosis in RGCs. Our findings in this clinically relevant model suggest that this probe, TcapQ, may have utility in identifying RGC apoptosis in vivo in animal models of glaucoma. Future studies using this probe will focus on in vivo imaging of RGC apoptosis using confocal scanning laser ophthalmoscopy in the NMDA model as well as other models of glaucoma. It is anticipated that this strategy may have potential for use in humans in the diagnosis and management of glaucoma.
Materials and Methods
Animals.
Male Brown Norway rats weighing 200 to 300 g each were purchased from Charles River Laboratories. All animal experiments were approved by the Animal Studies Committee at Washington University. All experiments were performed in triplicate.
Activatable Cell-Penetrating Peptide Probe (TcapQ).
This activatable peptide probe consists of an all d-amino acid-modified Tat cell-penetrating peptide, an l-amino acid effector caspase recognition sequence (DEVD), a quencher (QSY-21), and a fluorophore (Alexa Fluor-647) (Fig. 1A). Upon cleavage of the effector caspase recognition sequence and subsequent loss of fluorescent quenching, fluorescence from the retained intracellular fluorophore is detectable via fluorescence imaging (Fig. 1B). A second probe, dTcapQ, contained all d-amino acids, which should not be cleaved by effector caspases, and served as a control for nonspecific probe activation. Peptides Ac-rkkrrorrrGK(QSY21)DEVDAPC(AF647)-NH2 (TcapQ) and Ac-rkkrrorrrgk(QSY21)devdapc(AF647)-NH2 (dTcapQ) were synthesized, purified, and characterized as described (18, 19). Stock solutions of purified peptides were formulated in milliQ water at various concentrations and stored at −20 °C.
Procedure of in Vivo Intravitreal Injection.
To establish the NMDA model in rats, intravitreal injection was performed as previously reported (43). Rats were anesthetized by i.p. injection (1 mL/kg) of a mixture containing 1 mL ketamine (100 mg/mL) and 0.15 mL xylazine (100 mg/mL), and the pupil was dilated with 1% tropicamide drops. Intravitreal injections were performed under a microscope with a microsyringe and a 30-gauge needle, which was inserted ≈1 mm behind the cornea limbus. In control experiments, eyes (n = 6 retinas each; 3 independent experiments) were pretreated by injecting 2.5 μL of 0.1 M PBS (pH 7.4) or 0.125 mM TcapQ alone, whereas in experimental groups, eyes (n = 6 retinas each; 3 independent experiments) were pretreated by injecting 2 μL of 2.5 mM, 12.5 mM, and 40 mM NMDA (Sigma; corresponding to 5, 25, and 80 nmoles, respectively) prepared in PBS. Four hours later, 2.5 μL of 0.125 mM TcapQ were injected into the vitreous, and 2 h following injection of the probe, eyes were enucleated and processed. Any animal with visible lens damage, vitreous hemorrhage, or retinal detachment was not included in the analysis.
For inhibition of apoptotic RGCs in the NMDA model, the following 2 specific caspase inhibitors were used: the caspase-1 inhibitor Z-YVAD-fmk and the caspase-3 inhibitor Z-DEVD-fmk (both 2 mM in 2% DMSO; EMD Chemicals, Inc.). These inhibitors have been used and tested extensively to study the apoptotic pathways in retinal degeneration models (44, 45). In separate experiments (n = 6 retinas each; 3 independent experiments), 2 μL of 12.5 mM NMDA plus 2 μL of 2 mM of caspase-1 or caspase-3 inhibitor were injected into the vitreous, followed by TcapQ as described. To assess for nonspecific probe activation, we also injected 2.5 μL of 0.125 mM dTcapQ (19), following 25 nmol NMDA pretreatment as described.
Detection of Probe Activation.
To visualize labeled RGCs in the rat retinas, rats were deeply anesthetized and then perfused through the left ventricle with 0.5% nitrite containing 100 U/mL heparin followed by 4% paraformaldehyde in PBS (pH 7.4). After perfusion, eyes were enucleated and corneas and lenses removed. Eyecups were postfixed in the same fixative for 2 h, then washed 3 times in PBS. Images were taken with a fluorescence stereo microscope (Leica MZ16) using filter sets for far-red (Leica TX, 560 nm excitation filter/610 nm barrier filter, and Leica Cy5, 650 nm excitation filter/738 nm barrier filter) fluorescence. Images of TcapQ probe-labeled retinal apoptotic cells were captured with a cooled CCD camera (Retiga EXI Fast1394; Qimaging Camera) controlled by Q Capture Pro-5.1 software (Qimaging Camera). For the flatmount retina imaging, fixed and washed retinas were dissected from the choroids, divided by 4 radial cuts, mounted on slides, and then coverslipped with Slow-Fade Gold antifade reagent (Molecular Probes). All fluorescence images were obtained with a Leica MZ16F microscope and photographed with color slide filters (TRIC or Cy5 filters). Some flatmount slides were examined by laser confocal microscopy (LSM 510 Zeiss) at 40×. Images were captured with Zeiss LSM software.
TUNEL Apoptosis Assay.
Apoptosis in retinal cells was detected by TUNEL as described previously (43). Eyes enucleated after TcapQ injection were fixed with 4% paraformaldehyde, processed to generate eyecups, and transferred to PBS containing 20% sucrose overnight. Eyecups were then placed in optimal cutting temperature (OCT) medium (TissueTek; Miles) and quick frozen using 2-methylbutane over dry ice. Transverse 10 μm-thick cryostat sections were cut and placed onto slides (SuperFrost Plus; Fisher Scientific). Apoptotic cell death was detected by a TdT-mediated dUTP nick-end labeling (TUNEL) assay (DeadEndTM Fluorometric TUNEL System ; Promega Corp.) according to the protocol provided by the manufacturer. Tissue sections were examined with a microscope (Olympus) equipped with epifluorescence. Digital images were recorded (SPOT; Diagnostic Instruments) and images compiled (Photoshop version 5.5 and 7.0; Adobe Systems).
Retrograde Labeling of Retinal Ganglion Cells.
Retinal ganglion cells were identified in rats by retrograde labeling with FluoroGold (Fluorochrome, Inc.) bilaterally injected into the superior collicular brachium (46). FG (5%, 2.4 μL/injection) diluted in saline was microinjected bilaterally into the superior colliculi of anesthetized rats immobilized in a stereotaxic apparatus. FG is taken up bilaterally by the axon terminals of the RGCs and transported retrogradely to their somata in the retina, where the marker persists for at least 3 weeks without significant fading or leakage. One week after FG application, intravitreal injections of NMDA and TcapQ647 were performed, and retinal flatmounts and frozen sections processed as described previously.
Counting TcapQ-Labeled Cells in Retinal Flatmounts.
Retinal flatmounts were viewed as described to identify TcapQ-labeled cells. Labeled RGCs were counted with a 10× superwide field objective along 4 radii in 4 directions (i.e., superior, temporal, inferior, and nasal areas) centered on the position of the optic nerve head. Two fields were counted along each radius, yielding a total of 8 fields per retina. The selected fields were located at approximately the same distance from the optic disk to account for variation in RGC density as a function of distance from the optic disk. Quantification of fluorescent RGCs was performed using Scion image analysis software (Scion Corp.). The counting process was performed by an experienced observer who was blinded to the procedure that had been performed.
Histological Analysis.
For histological analysis, flatmounted retinas were prepared as described, and retinas were mounted with coverslips using Vectashield mounting medium with DAPI (Vector Laboratories) before fluorescence microscopy. After photographing retinas with DAPI staining, retinas were carefully removed from slides and processed for routine histology. Serial frozen sections (10 μm) were collected and mounted onto slides (SuperFrost Plus; Fisher Scientific). The slides were viewed using an LSM 510 confocal microscope (Zeiss) at 40×. Images were captured with Zeiss LSM software.
Statistical Analysis.
One-tailed Student's t test was used for statistical analysis. The data were expressed as means ± SEM from 4–5 animals. P values <0.05 were considered statistically significant.
Acknowledgments.
We thank colleagues of the Molecular Imaging Center for helpful discussions, and Belinda McMahan and Zelma Jones for technical assistance. This research was supported by a Horncrest Physician Scientist Award and a Career Development Award from Research to Prevent Blindness (RPB) (E.M.B.), National Institutes of Health Grants R21 EY017636 (E.M.B.), CA 82841, and P50 CA94056 (D.P.-W.), and awards to the Department of Ophthalmology and Visual Sciences from RPB and NIH (P30 EY 02687).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Marks N, Berg MJ. Recent advances on neuronal caspases in development and neurodegeneration. Neurochem Int. 1999;35:195–220. doi: 10.1016/s0197-0186(99)00061-3. [DOI] [PubMed] [Google Scholar]
- 2.Reed J. Apoptosis-regulating proteins as targets for drug discovery. Trends Mol Med. 2001;7:314–319. doi: 10.1016/s1471-4914(01)02026-3. [DOI] [PubMed] [Google Scholar]
- 3.Riedl S, Salvesen G. The apoptosome: Signalling platform of cell death. Nat Rev Mol Cell Biol. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- 4.Opferman J, Korsmeyer S. Apoptosis in the development and maintenance of the immune system. Nat Immunol. 2003;4:410–415. doi: 10.1038/ni0503-410. [DOI] [PubMed] [Google Scholar]
- 5.Krysko D, Vanden Berghe T, D'Herde K, Vandenabeele P. Apoptosis and necrosis: Detection, discrimination and phagocytosis. Methods. 2008;44:205–221. doi: 10.1016/j.ymeth.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Schoenberger J, Bauer J, Moosbauer J, Eilles C, Grimm D. Innovative strategies in in vivo apoptosis imaging. Curr Med Chem. 2008;15:187–194. doi: 10.2174/092986708783330647. [DOI] [PubMed] [Google Scholar]
- 7.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nickells R. Apoptosis of retinal ganglion cells in glaucoma: An update of the molecular pathways involved in cell death. Surv Ophthalmol. 1999;43:S151–161. doi: 10.1016/s0039-6257(99)00029-6. [DOI] [PubMed] [Google Scholar]
- 9.Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999;18:39–57. doi: 10.1016/s1350-9462(98)00014-7. [DOI] [PubMed] [Google Scholar]
- 10.Cordeiro M, et al. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proc Natl Acad Sci USA. 2004;101:13352–13356. doi: 10.1073/pnas.0405479101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonge H, et al. Preliminary in vivo evaluation of a novel 99mTc-labeled HYNIC-cys-annexin A5 as an apoptosis imaging agent. Bioorg Med Chem Lett. 2008;18:3794–3798. doi: 10.1016/j.bmcl.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 12.Keen HG, et al. Imaging apoptosis in vivo using 124I-annexin V and PET. Nucl Med Biol. 2005;32:395–402. doi: 10.1016/j.nucmedbio.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Quinti L, Weissleder R, Tung CH. A fluorescent nanosensor for apoptotic cells. Nano Lett. 2006;6:488–490. doi: 10.1021/nl0524694. [DOI] [PubMed] [Google Scholar]
- 14.Guo L, et al. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest Ophthalmol Vis Sci. 2005;46:175–182. doi: 10.1167/iovs.04-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maass A, et al. Assessment of rat and mouse RGC apoptosis imaging in vivo with different scanning laser ophthalmoscopes. Curr Eye Res. 2007;32:851–861. doi: 10.1080/02713680701585872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitz-Valckenberg S, et al. Real-time in vivo imaging of retinal cell apoptosis after laser exposure. Invest Ophthalmol Vis Sci. 2008;49:2773–2780. doi: 10.1167/iovs.07-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashe PC, Berry MD. Apoptotic signaling cascades. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:199–214. doi: 10.1016/S0278-5846(03)00016-2. [DOI] [PubMed] [Google Scholar]
- 18.Bullok K, Piwnica-Worms D. Synthesis and characterization of a small, membrane-permeant, caspase-activatable far-red fluorescent peptide for imaging apoptosis. J Med Chem. 2005;48:5404–5407. doi: 10.1021/jm050008p. [DOI] [PubMed] [Google Scholar]
- 19.Bullok KE, et al. Biochemical and in vivo characterization of a small, membrane-permeant, caspase-activatable far-red fluorescent peptide for imaging apoptosis. Biochemistry. 2007;46:4055–4065. doi: 10.1021/bi061959n. [DOI] [PubMed] [Google Scholar]
- 20.Barnett EM, Elangovan B, Bullok KE, Piwnica-Worms D. Selective cell uptake of modified Tat peptide-fluorophore conjugates in rat retina in ex vivo and in vivo models. Invest Ophthalmol Vis Sci. 2006;47:2589–2595. doi: 10.1167/iovs.05-1470. [DOI] [PubMed] [Google Scholar]
- 21.Pang IH, Clark AF. Rodent models for glaucoma retinopathy and optic neuropathy. J Glaucoma. 2007;16:483–505. doi: 10.1097/IJG.0b013e3181405d4f. [DOI] [PubMed] [Google Scholar]
- 22.Inomata Y, et al. Thioredoxin inhibits NMDA-induced neurotoxicity in the rat retina. J Neurochem. 2006;98:372–385. doi: 10.1111/j.1471-4159.2006.03871.x. [DOI] [PubMed] [Google Scholar]
- 23.Lam TT, Abler AS, Kwong JM, Tso MO. N-methyl-D-aspartate (NMDA)-induced apoptosis in rat retina. Invest Ophthalmol Vis Sci. 1999;40:2391–2397. [PubMed] [Google Scholar]
- 24.Manabe S, Gu Z, Lipton SA. Activation of matrix metalloproteinase-9 via neuronal nitric oxide synthase contributes to NMDA-induced retinal ganglion cell death. Invest Ophthalmol Vis Sci. 2005;46:4747–4753. doi: 10.1167/iovs.05-0128. [DOI] [PubMed] [Google Scholar]
- 25.Tezel G, Wax M. Inhibition of caspase activity in retinal cell apoptosis induced by various stimuli in vitro. Invest Ophthalmol Vis Sci. 1999;40:2660–2667. [PubMed] [Google Scholar]
- 26.Bullok K, et al. Permeation peptide conjugates for in vivo molecular imaging applications. Mol Imaging. 2006;5:1–15. [PubMed] [Google Scholar]
- 27.Costantini D, Hu M, Reilly R. Peptide motifs for insertion of radiolabeled biomolecules into cells and routing to the nucleus for cancer imaging or radiotherapeutic applications. Cancer Biother Radiopharm. 2008;23:3–24. doi: 10.1089/cbr.2007.0430. [DOI] [PubMed] [Google Scholar]
- 28.Fischer R, Fotin-Mleczek M, Hufnagel H, Brock R. Break on through to the other side—biophysics and cell biology shed light on cell-penetrating peptides. Chembiochem. 2005;6:2126–2142. doi: 10.1002/cbic.200500044. [DOI] [PubMed] [Google Scholar]
- 29.Goun EA, Pillow TH, Jones LR, Rothbard JB, Wender PA. Molecular transporters: Synthesis of oligoguanidinium transporters and their application to drug delivery and real-time imaging. Chembiochem. 2006;7:1497–1515. doi: 10.1002/cbic.200600171. [DOI] [PubMed] [Google Scholar]
- 30.Jiang T, et al. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc Natl Acad Sci USA. 2004;101:17867–17872. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messerli S, et al. A novel method for imaging apoptosis using a caspase-1 near-infrared fluorescent probe. Neoplasia. 2004;6:95–105. doi: 10.1593/neo.03214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dietz G, Bahr M. Delivery of bioactive molecules into the cell: The Trojan horse approach. Mol Cell Neurosci. 2004;27:85–131. doi: 10.1016/j.mcn.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Langedijk JP, Olijhoek T, Schut D, Autar R, Meloen RH. New transport peptides broaden the horizon of applications for peptidic pharmaceuticals. Mol Divers. 2004;8:101–111. doi: 10.1023/b:modi.0000025653.26130.ce. [DOI] [PubMed] [Google Scholar]
- 34.Lindgren M, Hallbrink M, Prochiantz A, Langel U. Cell-penetrating peptides. Trends Pharmacol Sci. 2000;21:99–103. doi: 10.1016/s0165-6147(00)01447-4. [DOI] [PubMed] [Google Scholar]
- 35.Wender PA, Galliher WC, Goun EA, Jones LR, Pillow TH. The design of guanidinium-rich transporters and their internalization mechanisms. Adv Drug Delivery Rev. 2008;60:452–472. doi: 10.1016/j.addr.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dietz GP, Kilic E, Bahr M. Inhibition of neuronal apoptosis in vitro and in vivo using TAT-mediated protein transduction. Mol Cell Neurosci. 2002;21:29–37. doi: 10.1006/mcne.2002.1165. [DOI] [PubMed] [Google Scholar]
- 37.Koh HJ, et al. Intraocular properties of urokinase-derived antiangiogenic A6 peptide in rabbits. J Ocul Pharmacol Ther. 2004;20:439–449. doi: 10.1089/jop.2004.20.439. [DOI] [PubMed] [Google Scholar]
- 38.Lau J, Dang M, Hockmann K, Ball AK. Effects of acute delivery of endothelin-1 on retinal ganglion cell loss in the rat. Exp Eye Res. 2005;82:319–326. doi: 10.1016/j.exer.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Bullok K, Dyszlewski M, Prior J, Pica C, Sharma V, Piwnica-Worms D. Characterization of novel histidine-tagged Tat-peptide complexes dual labeled with 99mTc-tricarbonyl and fluorescein for scintigraphy and fluorescence microscopy. Bioconjug Chem. 2002;13:1226–1237. doi: 10.1021/bc025573a. [DOI] [PubMed] [Google Scholar]
- 40.Denicourt C, Dowdy SF. Protein transduction technology offers novel therapeutic approach for brain ischemia. Trends Pharmacol Sci. 2003;24:216–218. doi: 10.1016/S0165-6147(03)00074-9. [DOI] [PubMed] [Google Scholar]
- 41.Harbour J, Worley L, Duanduan M, Cohen M. Transducible peptide therapy for uveal melanoma and retinoblastoma. Arch Ophthalmol. 2002;120:1341–1346. doi: 10.1001/archopht.120.10.1341. [DOI] [PubMed] [Google Scholar]
- 42.Polyakov V, et al. Novel Tat-peptide chelates for direct transduction of technetium-99m and rhenium into human cells for imaging and radiotherapy. Bioconjug Chem. 2000;11:762–771. doi: 10.1021/bc000008y. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Cheng M, Chintala SK. Kainic acid-mediated upregulation of matrix metalloproteinase-9 promotes retinal degeneration. Invest Ophthalmol Vis Sci. 2004;45:2374–2383. doi: 10.1167/iovs.03-1239. [DOI] [PubMed] [Google Scholar]
- 44.Kermer P, Klocker N, Labes M, Bahr M. Inhibition of CPP32-like proteases rescues axotomized retinal ganglion cells from secondary cell death in vivo. J Neurosci. 1998;18:4656–4662. doi: 10.1523/JNEUROSCI.18-12-04656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perche O, Doly M, Ranchon-Cole I. Caspase-dependent apoptosis in light-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48:2753–2759. doi: 10.1167/iovs.06-1258. [DOI] [PubMed] [Google Scholar]
- 46.Neufeld AH, Sawada A, Becker B. Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglion cells in a rat model of chronic glaucoma. Proc Natl Acad Sci USA. 1999;96:9944–9948. doi: 10.1073/pnas.96.17.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]